Abstract
Children, and particularly infants, have physiological, anatomic, and social factors that increase vulnerability to temperature extremes. We performed a systematic review to explore the association between acute adverse infant outcomes (children 0–1 years) and exposure to high and low ambient temperatures. MEDLINE (Pubmed), Embase, CINAHL Plus, and Global Health were searched alongside the reference lists of key papers. We included published journal papers in English that assessed adverse infant outcomes related to short-term weather-related temperature exposure. Twenty-six studies met our inclusion criteria. Outcomes assessed included: infant mortality (n = 9), sudden infant death syndrome (n = 5), hospital visits or admissions (n = 5), infectious disease outcomes (n = 5), and neonatal conditions such as jaundice (n = 2). Higher temperatures were associated with increased risk of acute infant mortality, hospital admissions, and hand, foot, and mouth disease. Several studies identified low temperature impacts on infant mortality and episodes of respiratory disease. Findings on temperature risks for sudden infant death syndrome were inconsistent. Only five studies were conducted in low- or middle-income countries, and evidence on subpopulations and temperature-sensitive infectious diseases was limited. Public health measures are required to reduce the impacts of heat and cold on infant health.
Keywords:
heat exposure; cold exposure; ambient temperature; SIDS; mortality; neonatal health; infant health 1. Introduction
Climate change is one of the greatest global threats to public health, and indeed an existential threat for humankind and the natural world [1]. In all future temperature predictions, the global temperature is expected to rise [2]. Heatwaves are projected to occur more often and last longer due to climate change and ongoing urbanisation [2]. The frequency of cold days is projected to reduce, but cold spells will continue to occur [2]. High and low temperature exposure as a risk factor for morbidity and mortality is being increasingly established in epidemiological studies [3,4,5,6]. Older persons, people with comorbidities, pregnant women, and children have been identified as at risk from high temperatures [3,4,7,8]. Additionally, low socioeconomic and ethnic minority groups are at a higher risk of temperature-related adverse health outcomes [3,5,6,7,9]. Socioeconomic status may impact on factors such as access to air-conditioning and to healthcare, which may differ across countries, regions and urban versus rural populations [3,5,7,9]. Moreover, populations from a lower socioeconomic backgrounds, and urban areas are more likely to reside in warmer neighbourhoods due to high population density, sparse vegetation, and lack of green spaces, and are less likely to be able to adapt to temperature extremes [3].
Three systematic reviews in the early 2010s that assessed impacts of high temperatures on child health found some evidence that young children, especially infants under the age of 1, are vulnerable to heat. Evidence, however, was limited to very few studies [10,11,12]. There is also evidence that high temperatures cause acute increases in gastrointestinal diseases, malaria, heatstroke, renal disease, asthma, and unintentional injuries in children [10,11,12,13,14]. Studies, however, investigated children as a single age group and did not stratify by smaller age bands. Children have significantly different developmental stages between 0 and 18 years of age, in terms of metabolism, thermoregulation, and behaviours that affect their health [10,11,15,16].
Infants (children aged 0–1) may be more vulnerable to temperature extremes due to physiological, anatomical, and behavioural factors (Figure 1) [15,16,17,18,19,20]. Thermoregulation, the control of internal body temperature, is influenced by many factors, some of which include body surface area to volume ratio, sweating rate, heart and blood vessel responses, and hydration status [16]. Several factors complicate thermoregulation in infants. Infants have immature thermoregulatory mechanisms, a higher metabolic rate (more energy expenditure), and a higher sweating threshold [16,19,20,21]. Additionally, infants have a smaller blood volume and a higher heart rate, which will affect the response of the heart and blood vessels to extreme temperatures [16,19,20,21]. Anatomically, infants, and especially preterm neonates, have a higher body surface to volume ratio which increases exposure to temperature [16]. In addition to these physiological and anatomical factors, infants are particularly vulnerable, as they rely on a caregiver to safeguard them, which includes moving to a cooler or warmer environment if required, dressing appropriately, and keeping hydrated [16,20].
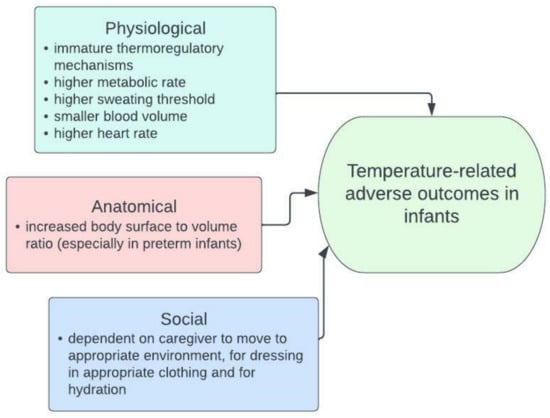
Figure 1.
The factors that could increase temperature vulnerability in infants compared to older children and adults [15,16,17,18,19,20].
In this study, we aimed to explore the association between exposure to high and low ambient temperatures and acute adverse outcomes in infants. We examined the strength for evidence for these associations, and the potential causal pathways. We identify evidence on various adverse outcomes and across settings and socioeconomic circumstances.
2. Methods
A systematic literature review was undertaken to identify epidemiological studies that assessed the impact of short-term exposure to high and/or low ambient temperatures on infants aged 0–1 years old. Medline (Pubmed), Embase, Global Health, and CINAHL Plus were searched in July 2020 (Table S1). Reference lists of included articles were screened for eligibility. Table 1 describes the inclusion and exclusion criteria using the PICOS framework [22]. Searches were restricted to English language and publication between 2000 and 2020.

Table 1.
Inclusion and exclusion criteria using the PECOS framework.
The earliest year of publication (2000) was chosen for the relevance of the impacts of temperature on current populations. There have been significant adaptations over time to reduce the impact of temperature on adverse outcomes, such as improved housing, infrastructure, and technology [23]. Studies that aggregated temperature over longer periods such as a month are prone to bias from seasonality and were excluded as they do not meet the objectives of studying short-term temperature exposure. Lastly, diurnal temperature range and mean day-to-day temperature as exposures were excluded to meet the objectives of assessing the impacts of high and low ambient temperatures, rather than temperature fluctuations
The screening of titles, abstracts, and full texts were performed by a single reviewer (DPL). Data extraction was completed using Microsoft Excel. The Köppen–Geiger climate classification was supplemented, where studies did not have a local climate description [24]. An adapted quality assessment tool was developed to assess the quality of the studies using the Office of Health Assessment and Translation (OHAT) tool [25], which evaluates the risk of bias. Two further questions on analysis and conflicts of interest were added to the tool based on a systematic review that recommended the inclusion of nine domains for observational research: selection, exposure, outcome assessment, confounding, loss to follow-up, analysis, selective reporting, conflicts of interest, and other [26]. OHAT does not specifically consider environmental health study designs such as time series and case-crossover, hence we adapted the tool to tailor it to our research question (See Table S2). The risk of bias in each domain was scored as: (a) definitely low, (b) probably low, (c) probably high or insufficient information, and (d) definitely high [25]. The final grading was given dependent on the individual domain grades. We considered an individual study to have a definitely low risk or probably low risk of bias if all, or most domains were rated as definitely low risk. Similarly, probably high risk and definitely high risk were rated based on the number of domains that scored probably or definitely high risk. Due to the limited number of studies, the risk-of-bias assessment results were not used to exclude studies from the analysis. Following data extraction and critical appraisal, a narrative synthesis was performed. The considerable methodological diversity and statistical heterogeneity of study findings precluded meta-analysis.
3. Results
The study selection process resulted in 26 studies that were included in the review (Figure 2) [27].
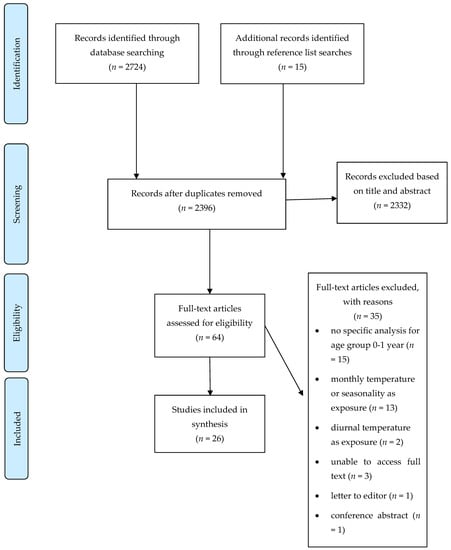
Figure 2.
PRISMA flow diagram that illustrates the study selection process.
The studies cover a limited range of geographical locations and climate zones, with most studies from North America and Asia; only five studies were from low- and middle-income countries (LMICs) [19,28,29,30,31]. Most studies are conducted in urban populations, with five from rural areas, one of which was a combined urban-rural study [28,31,32,33,34]. Nine studies assessed the impact of temperature on all-cause infant mortality and five further studies assessed sudden infant death syndrome (SIDS) (Table 2). There were five studies each on hospital visits or admissions, and infectious diseases, and two studies reported on other conditions in the neonatal period (Table 3).

Table 2.
Summary of study findings that assess temperature effects on all-cause infant mortality and SIDS.

Table 3.
Summary of the study findings that assess temperature effects on hospital visits/admissions, infectious diseases and other neonatal outcomes.
3.1. Temperature and Infant Mortality
There was considerable evidence that high temperatures affected infant mortality; this was shown in several studies in different regions and climate zones (USA, Spain, and South Korea) [15,21,34,35,36,37]. Heatwaves or very hot days (>95th percentile of mean temperature) were associated with increased mortality in infants in California and male infants in France [34,37]. Higher temperatures were not found to be associated with increased infant mortality in Madrid; however, this study had a higher risk of bias as compared to others that examined this outcome.
There was also evidence that low temperatures increase the risk of infant mortality [32,33,38]. Infant mortality rose when maximum temperatures declined below 6 °C in Madrid. Further, neonatal mortality increased when the temperatures were low on the day of birth, specifically in indigenous populations, and rural labourers in Sweden and Italy [32,33].
Apart from SIDS, which we consider separately below, cause-specific mortality was assessed in only two studies, one of which reported an association between very hot days and mortality relating to disorders that originate in the perinatal period in California (22nd week of gestation to first 7 days of life), while no association was detected between the specific causes of mortality and temperature in a study in Spain [21,37].
The only cause of infant death that has been more comprehensively studied in the included literature is SIDS, defined as the sudden death of an infant that remains unexplained even after a case investigation, and usually occurs during sleep [39]. There were inconsistent results for the impact of daily temperature on SIDS. In studies with a lower risk of bias, SIDS cases increased at higher temperature in USA, Canada, and South Korea [36,39,40], but no association was found in another study in USA and in Austria [21,41]. There were two studies with a high risk of bias, one conducted in the USA that found no association and another one in Taiwan that found a protective effect at higher temperatures [42,43].
3.2. Temperature and Infant Morbidity
Higher temperatures were associated with increased hospital visits or admissions for all-cause morbidity in infants and for heat-related morbidity in neonates across many settings (New York, rural Bangladesh, Brisbane, and Ahmedabad) [13,19,28,44]. A 0.6% (95%CI 0.1–1.1%) increased risk per interquartile range increase in temperature was reported in New York and 1.05% (95%CI 1.01, 1.10%) for hot days over 97th percentile was reported in Brisbane [13,44]. Low ambient temperature was associated with increased hospital admissions for respiratory disease in Auckland, however, the evidence is less robust as this study had a high risk of bias [45].
Evidence for the impact of temperature on infectious disease in infants was less rigorous than for other outcome categories, with three out of five studies having a definitely high risk of bias. The three studies with a high risk of bias consistently found an association between low temperature and bronchiolitis [46,47,48]. Two studies, with lower risk of bias, found evidence that Hand, Foot and Mouth Disease (HFMD) incidence in China was higher at lag 0, lag 14, and cumulatively over 14 days with as much as a 2-fold increase in incidence when temperatures were above the 95th percentile [29,30].
3.3. Temperature and Other Neonatal Conditions
Among newborn infants in Japan, outdoor temperature was correlated with time taken for blood to clot, as measured by the International Normalised Ratio (INR) [49]. The clinical relevance of the difference in INR is uncertain [49]. Additionally, the risk of being referred for neonatal jaundice increased with higher temperatures on the day of delivery in Nepal [31].
3.4. Evidence of Effect Modification by Socioeconomic Factors, Race, Rural Versus Urban Areas and Time Periods
There is inconsistent evidence of effect modification in the association between temperature and adverse outcomes by socioeconomic factors and race. Jhun et al. [39]) and Basu et al. [21] found that the effects of high temperatures on risk of SIDS and all-cause mortality were higher in black infants as compared to white infants. Karlsson et al. and Scalone et al. found an increased risk of neonatal mortality in indigenous populations [32,33]. However, three other studies found no evidence for effect modification by socioeconomic factors and race [15,36,44]. There are five studies conducted in rural areas, and all five had a positive association between temperature and adverse infant outcomes [28,31,32,33,34]. Only one study described effect modification by urban environment; a greater percentage increase in infant mortality was evident in Paris during a heatwave as compared to the rest of France [34]. There is no evidence of a difference in magnitude of temperature-infant health outcomes over time, but this is an important issue to consider.
3.5. Quality of Studies
The studies that assessed mortality as an outcome had overall better internal validity compared with the studies that assessed hospital admissions and infectious diseases (Figure 3). This may be due to the study designs such as time-series and case crossover that were used to assess mortality and temperature, which generally scored better for assessment of confounding and analysis as compared to traditional study designs. Many studies had a low score for confounding bias, as the analysis frequently did not take long-term time trends, seasonality and air pollution into consideration. Some studies had no conflict-of-interest disclosures and consequently scored poorly on this question. In addition, studies had exposure detection bias based on misclassification of temperature. Common reasons for misclassification of temperature were large distances from weather stations to the individuals in cohorts, or a lack of detailed information provided about temperature measures.
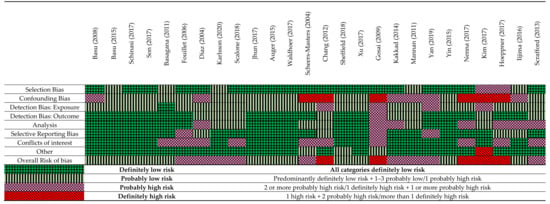
Figure 3.
A summary of the critical appraisal using a modified OHAT Risk of bias tool [15,17,19,21,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,44,45,46,47,48,49,50].
3.6. Key Findings
There was consistency across studies that higher ambient temperatures and heatwaves were associated with an increased risk of all-cause infant mortality, all-cause hospital admissions, and HFMD incidence. Mortality and hospital admissions are major health outcomes. Similarly, while HFMD is a self-limiting acute viral illness, it can sometimes be fatal and can cause serious complications such as meningitis or myocarditis (inflammation of lining of brain and heart, respectively). Further, HFMD is prevalent in Asia and is increasing in some countries with epidemic and pandemic potential [51,52]. The evidence for high temperature effects on these outcomes were robust as these studies had a low risk of bias due to better exposure assessment and/or statistical analyses that adjusted for confounders. These findings are in keeping with two previous systematic reviews in children that identified infants as a subpopulation that are at a high risk of adverse outcomes associated with exposure to higher ambient temperature and heatwaves [10,11].
Only three studies assessed the impact of ambient low temperatures on infant mortality, despite the risk of hypothermia in newborns [53]. Two of the three studies found consistent evidence that low ambient temperatures on the day of birth increased the risk of neonatal mortality, while the third found that infant mortality increased when outdoor temperatures dropped below a 6 °C threshold. Two of these studies gathered data from historical populations in the 1800′s. These data can provide context to physiology and risk to temperature exposure; however, using historical data may have limitations with respect to quantifying risk as there have since been adaptations, such as better housing and insulation, which will likely reduce exposures to cold temperature extremes. Lastly, there is some evidence that low temperatures are associated with bronchiolitis in infants.
Evidence of the relationship between temperature and SIDS is difficult to interpret. It is postulated that SIDS may be related to thermal stress in the infant, supported by evidence implicating heat-related factors such as prone sleeping, overwrapping infants, and elevated indoor room temperatures [39,41]. These factors may occur due to high ambient temperatures or, paradoxically, when cooler temperatures prompt a disproportionate warming of the infant [54]. The seemingly contradictory evidence in this review may be due to several factors. Firstly, SIDS is prone to detection bias; the thoroughness of investigations, suspicion for diagnosis and inclusion of post-mortems vary across settings. Secondly, the studies are heterogenous in design, exposure measurements and risk of bias. Thirdly, SIDS is a rare occurrence, and the number of cases may be insufficient to explore statistically. Finally, the temperature-SIDS association may be modified by other risk factors, for instance, overwrapping and bundling, or air-conditioning use that may vary across populations.
No studies were found on the short-term temperature impacts of infectious diseases such as malaria, dengue, and infectious gastroenteritis. These infections carry a high burden of disease in infants and are known to be associated with temperature [13,55]. Several reasons are hypothesised for the lack of evidence in infectious disease. Firstly, modelling studies and studies that use a wider age group were excluded in the study protocol [52,56,57]. Secondly, there is a lack of epidemiological evidence and good quality records and data-keeping from LMICs, where the burden of infectious diseases is situated. Climate change will increase transmission of many infectious diseases and hence additional evidence on these outcomes is a key research priority [13].
There is limited evidence of effect modification by socioeconomic factors and race in health–temperature associations in infants. Similarly, there is limited evidence of effect modification between urban and rural areas, but we do find evidence of temperature impacts in both urban and rural environments. The limited evidence of effect modification could be due to insufficient power for sub-group analysis, and selection bias, for example, in studies that assess only hospital records.
Only 5 of the 21 included studies were from LMICs, and entire regions such as Africa, the Middle East, and South and Central America are not represented. All five studies found a positive association between temperature and adverse infant outcomes; however, comparisons to high-income countries are limited due to methodological heterogeneity. However, there is evidence, not included in this review, that the impact of climate change on children is not evenly distributed, but occurs in LMICs that are already experiencing a higher burden of disease [21]. A study demonstrated that in Africa, climate change has already doubled the heat-related child mortality compared to what would have been expected without climate change [58]. There is an obvious mismatch between vulnerability to temperature effects and research effort, which needs to be remediated in future work.
The published literature in this review is heterogenous in exposure and outcome assessments, making it difficult to directly compare results across populations. A standardisation of some measures, for example, changes in outcome per degree Celsius change in temperature, may reduce methodological diversity.
4. Limitations of This Review
Only English language studies were included which may limit geographical representation. Numerous studies that assess infants within wider age groups, for example, 0–2 and 0–5 years old, have been excluded. While there is significant heterogeneity in metabolism, temperature regulation, and behaviours in children in varying age groups, the inclusion of a wider age group, for example, children 0–2 years, may provide valuable information [7,11,14,16]. Some studies were excluded due to use of temperature metrics such as diurnal temperature variation. Future research could encompass a range of temperature indices to quantify the temperature–health association more broadly. Additionally, studies that were published prior to 2000, and subsequent to the search in July 2020, were excluded which may provide additional evidence, especially for older studies on the effects of low temperatures on infant health and SIDS. Furthermore, grey literature was excluded which may result in publication bias. Lastly, the use of a single reviewer for study selection may have introduced errors during screening and data extraction.
5. Conclusions
The evidence collected in this review indicates that there are important, sizable, increased risk of mortality and a range of morbidities in infants exposed to high temperatures. In addition, there is some evidence of an increased risk of infant mortality and bronchiolitis with lower temperature exposures. There is, however, limited evidence from LMICs, too few studies that assess subpopulations, and no literature was identified on temperature-sensitive infectious diseases such as dengue and malaria in infants.
Global temperatures and frequency of heat waves are increasing rapidly. In 2020, there were 626 million more person-days of heatwave exposure affecting infants compared to the annual average for the years 1986–2005 [55]. The risks of outcomes related to higher temperatures will increase, but there may be a decrease in cold-related outcomes due to a decrease in the frequency and intensity of cold extremes. Future research should focus on heat impacts; however, it is still important to quantify the impacts of low temperatures due to ongoing cold spells and climate variability. Lastly, this work can be utilised, together with the growing body of research on climate change and health, to motivate for more rigorous climate mitigation and adaptation strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19159109/s1, Table S1. Search terms used in Medline; Table S2. Modified OHAT Risk of Bias Tool [25].
Author Contributions
S.K. and D.P.L. conceptualised the study with input from H.A.B. and B.N. on methodology. D.P.L. undertook the literature search with contribution from M.F.C., D.P.L., S.K., B.N. and H.A.B. prepared the original draft with all authors contributing to review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
B.N., M.F.C. and S.K. were funded by the Research Council of Norway (RCN) (grant number 312601); the Natural Environment Research Council (NERC) (grant numbers NE/T013613/1, NE/T01363X/1), the Swedish Research Council for Health, Working Life and Welfare in collaboration with the Swedish Research Council (Forte) (grant number 2019–01570); and the National Science Foundation (NSF) (grant number ICER-2028598); coordinated through a Belmont Forum partnership (CHAMNHA project). Part of this work was funded through the HE2AT Center, a grant supported by the NIH Common Fund, which is managed by the Fogarty International Center. NIH award number 1U54TW012083-01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This paper was developed from a master’s dissertation during D.P.L.’s studies at the London School of Hygiene and Tropical Medicine (LSHTM). D.P.L. would like to acknowledge the contributions of H.A.B. and S.K., the library staff at LSHTM for their assistance with designing the search strategy, and for the feedback from anonymous markers that graded the dissertation.
Conflicts of Interest
M.F.C. and D.P.L. hold investments in the fossil fuel industry through their pension funds. The University of the Witwatersrand holds investments in the fossil fuel industry through their endowments and other financial reserves.
References
- World Health Organization. Climate Change and Health. Geneva. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health (accessed on 20 February 2022).
- IPCC. Climate change 2021: The physical science basis summary for policymakers working group I contribution to the sixth assessment report of the intergovernmental panel on climate change. In Climate Change 2021: The Physical Science Basis; IPCC: Geneva, Switzerland, 2021; 3949p. [Google Scholar]
- Luber, G.; McGeehin, M. Climate change and extreme heat events. Am. J. Prev. Med. 2008, 35, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Basu, R. High ambient temperature and mortality: A review of epidemiologic studies from 2001 to 2008. Environ. Health 2009, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Remenyi, T.A.; White, C.J.; Johnston, F.H. Heatwave and health impact research: A global review. Health Place 2018, 53, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kovats, R.S.; Hajat, S. Heat stress and public health: A critical review. Annu. Rev. Public Health 2008, 29, 41–55. [Google Scholar] [CrossRef]
- Hajat, S.; Kosatky, T. Heat-related mortality: A review and exploration of heterogeneity. J. Epidemiol. Community Health 2010, 64, 753–760. [Google Scholar] [CrossRef]
- Chersich, M.F.; Pham, M.D.; Areal, A.; Haghighi, M.M.; Manyuchi, A.; Swift, C.P.; Wernecke, B.; Robinson, M.; Hetem, R.; Boeckmann, M.; et al. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: Systematic review and meta-analysis. BMJ 2020, 371, m3811. [Google Scholar] [CrossRef]
- Gronlund, C.J. Racial and Socioeconomic Disparities in Heat-Related Health Effects and Their Mechanisms: A Review. Curr. Epidemiol. Rep. 2014, 1, 165–173. [Google Scholar] [CrossRef]
- Xu, Z.; Sheffield, P.E.; Su, H.; Wang, X.; Bi, Y.; Tong, S. The impact of heat waves on children’s health: A systematic review. Int. J. Biometeorol. 2014, 58, 239–247. [Google Scholar] [CrossRef]
- Xu, Z.; Etzel, R.A.; Su, H.; Huang, C.; Guo, Y.; Tong, S. Impact of ambient temperature on children’s health: A systematic review. Environ. Res. 2012, 117, 120–131. [Google Scholar] [CrossRef]
- Helldén, D.; Andersson, C.; Nilsson, M.; Ebi, K.L.; Friberg, P.; Alfvén, T. Climate change and child health: A scoping review and an expanded conceptual framework. Lancet Planet Health 2021, 5, e164–e175. [Google Scholar] [CrossRef]
- Sheffield, P.E.; Landrigan, P.J. Global climate change and children’s health: Threats and strategies for prevention. Environ. Health Perspect. 2011, 119, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Scovronick, N.; Sera, F.; Acquaotta, F.; Garzena, D.; Fratianni, S.; Wright, C.Y.; Gasparrini, A. The association between ambient temperature and mortality in South Africa: A time-series analysis. Environ. Res. 2018, 161, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Schinasi, L.H.; Bloch, J.R.; Melly, S.; Zhao, Y.; Moore, K.; de Roos, A.J. High ambient temperature and infant mortality in Philadelphia, Pennsylvania: A case-crossover study. Am. J. Public Health 2020, 110, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J. Pediatric thermoregulation: Considerations in the face of global climate change. Nutrients 2019, 11, 2010. [Google Scholar] [CrossRef]
- Chang, H.Y.; Sung, Y.H.; Wang, S.M.; Lung, H.L.; Chang, J.H.; Hsu, C.H.; Hung, H.F. Short- and Long-Term Outcomes in Very Low Birth Weight Infants with Admission Hypothermia. PLoS ONE 2015, 10, e0131976. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Sheffield, P.E.; Hu, W.; Su, H.; Yu, W.; Qi, X.; Tong, S. Climate change and children’s health—A call for research on what works to protect children. Int. J. Environ. Res. Public Health 2012, 9, 3298–3316. [Google Scholar] [CrossRef]
- Kakkad, K.; Barzaga, M.L.; Wallenstein, S.; Azhar, G.S.; Sheffield, P.E. Neonates in Ahmedabad, India, during the 2010 Heat Wave: A climate change adaptation study. J. Environ. Public Health 2014, 2014, 946875. [Google Scholar] [CrossRef]
- Sarofim, M.C.; Saha, S.; Hawkins, M.D.; Mills, D.D.; Hess, J.; Kinney, P.; Schwartz, J.; Juliana St., A. Temperature-Related Death and Illness: The Impacts of Climate Change on Human Health in the United States, A Scientific Assessment; U.S. Global Change Research Program: Washington, DC, USA, 2016; pp. 43–68.
- Basu, R.; Pearson, D.; Sie, L.; Broadwin, R. A Case-Crossover Study of Temperature and Infant Mortality in California. Paediatr. Perinat. Epidemiol. 2015, 29, 407–415. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.; Hajat, S.; Armstrong, B.; Wilkinson, P. Declining vulnerability to temperature-related mortality in London over the 20th century. Am. J. Epidemiol. 2006, 164, 77–84. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Zeitschrift 2006, 15, 259–263. [Google Scholar] [CrossRef]
- National Toxicology Program. OHAT Risk of Bias Rating Tool for Human and Animal Studies. 2015. Available online: https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf (accessed on 18 May 2022).
- Wang, Z.; Taylor, K.; Allman-Farinelli, M.; Armstrong, B.; Askie, L.; Ghersi, D.; Bero, L.A. A systematic review: Tools for assessing methodological quality of human observational studies. NHMRC 2019. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef]
- Mannan, I.; Choi, Y.; Coutinho, A.J.; Chowdhury, A.I.; Rahman, S.M.; Seraji, H.R.; Baqui, A.H. Vulnerability of newborns to environmental factors: Findings from community based surveillance data in Bangladesh. Int. J. Environ. Res. Public Health 2011, 8, 3437–3452. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wei, L.; Duan, Y.; Li, H.; Liao, Y.; Lv, Q.; Jiang, H. Short-term effects of meteorological factors and air pollutants on hand, foot and mouth disease among children in Shenzhen, China, 2009–2017. Int. J. Environ. Res. Public Health 2019, 16, 3639. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Zhang, T.; Liu, L.; Lv, Q.; Li, X. The Association between Ambient Temperature and Childhood Hand, Foot, and Mouth Disease in Chengdu, China: A Distributed Lag Non-linear Analysis. Sci. Rep. 2016, 6, 27305. [Google Scholar] [CrossRef][Green Version]
- Scrafford, C.G.; Mullany, L.C.; Katz, J.; Khatry, S.K.; Leclerq, S.C.; Darmstadt, G.L.; Tielsch, J.M. Incidence of and risk factors for neonatal jaundice among newborns in southern Nepal. Trop Med. Int. Health 2013, 18, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.; Lundevaller, E.H.; Schumann, B. Neonatal mortality and temperature in two Northern Swedish rural parishes, 1860–1899—The significance of ethnicity and gender. Int. J. Environ. Res. Public Health 2020, 17, 1216. [Google Scholar] [CrossRef]
- Scalone, F.; Samoggia, A. Neonatal mortality, cold weather, and socioeconomic status in two northern Italian rural parishes, 1820–1900. Demogr. Res. 2018, 39, 525–560. [Google Scholar] [CrossRef]
- Fouillet, A.; Rey, G.; Laurent, F.; Pavillon, G.; Bellec, S.; Guihenneuc-Jouyaux, C.; Hémon, D. Excess mortality related to the August 2003 heat wave in France. Int. Arch. Occup. Environ. Health 2006, 80, 16–24. [Google Scholar] [CrossRef]
- Basu, R.; Ostro, B.D. A multicounty analysis identifying the populations vulnerable to mortality associated with high ambient temperature in California. Am. J. Epidemiol. 2008, 168, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Son, J.Y.; Lee, J.T.; Bell, M.L. Is ambient temperature associated with risk of infant mortality? A multi-city study in Korea. Environ. Res. 2017, 158, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Basagaña, X.; Sartini, C.; Barrera-Gómez, J.; Dadvand, P.; Cunillera, J.; Ostro, B.; Medina-Ramón, M. Heat waves and cause-specific mortality at all ages. Epidemiology 2011, 22, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.; Linares, C.; García-Herrera, R.; López, C.; Trigo, R. Impact of temperature and air pollution on the mortality of children in Madrid. J. Occup. Environ. Med. 2004, 46, 768–774. [Google Scholar] [CrossRef]
- Jhun, I.; Mata, D.A.; Nordio, F.; Lee, M.; Schwartz, J.; Zanobetti, A. Ambient Temperature and Sudden Infant Death Syndrome in the United States. Epidemiology 2017, 28, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Auger, N.; Fraser, W.D.; Smargiassi, A.; Kosatsky, T. Ambient heat and sudden infant death: A case-crossover study spanning 30 years in Montreal, Canada. Environ. Health Perspect. 2015, 123, 712–716. [Google Scholar] [CrossRef]
- Waldhoer, T.; Heinzl, H. Exploring the possible relationship between ambient heat and sudden infant death with data from Vienna, Austria. PLoS ONE 2017, 12, e0184312. [Google Scholar] [CrossRef]
- Scheers-Masters, J.R.; Schootman, M.; Thach, B.T. Heat stress and sudden infant death syndrome incidence: A United States population epidemiologic study. Pediatrics 2004, 113, e586-92. [Google Scholar] [CrossRef]
- Chang, H.P.; Li, C.Y.; Chang, Y.H.; Hwang, S.L.; Su, Y.H.; Chen, C.W. Sociodemographic and meteorological correlates of sudden infant death in Taiwan. Pediatr. Int. 2013, 55, 11–16. [Google Scholar] [CrossRef]
- Xu, Z.; Crooks, J.L.; Black, D.; Hu, W.; Tong, S. Heatwave and infants’ hospital admissions under different heatwave definitions. Environ. Pollut. 2017, 229, 525–530. [Google Scholar] [CrossRef]
- Gosai, A.; Salinger, J.; Dirks, K. Climate and respiratory disease in Auckland, New Zealand. Aust. N. Z. J. Public Health 2009, 33, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Nenna, R.; Evangelisti, M.; Frassanito, A.; Scagnolari, C.; Pierangeli, A.; Antonelli, G.; Midulla, F. Respiratory syncytial virus bronchiolitis, weather conditions and air pollution in an Italian urban area: An observational study. Environ. Res. 2017, 158, 188–193. [Google Scholar] [CrossRef]
- Kim, J.M.; Jeon, J.S.; Kim, J.K. Weather and its effects on RSV A and B infections in infants and children in Korea. Australas. Med. J. 2017, 10, 997–1002. [Google Scholar]
- Hoeppner, T.; Borland, M.; Babl, F.E.; Neutze, J.; Phillips, N.; Krieser, D.; Paediatric Research in Emergency Departments International Collaborative (PREDICT). Influence of weather on incidence of bronchiolitis in Australia and New Zealand. J. Paediatr. Child Health 2017, 53, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Sekii, K.; Baba, T.; Ueno, D.; Ohishi, A. Seasonal variation in the international normalized ratio of neonates and its relationship with ambient temperature. BMC Pediatr. 2016, 16, 97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheffield, P.E.; Herrera, M.T.; Kinnee, E.J.; Clougherty, J.E. Not so little differences: Variation in hot weather risk to young children in New York City. Public Health 2018, 161, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.A. Hand-Foot-and-Mouth Disease (HFMD): Practice Essentials, Background, Pathophysiology. 2021. Available online: https://emedicine.medscape.com/article/218402-overview (accessed on 18 May 2022).
- Xu, Z.; Hu, W.; Jiao, K.; Ren, C.; Jiang, B.; Ma, W. The effect of temperature on childhood hand, foot and mouth disease in Guangdong Province, China, 2010–2013: A multicity study. BMC Infect. Dis. 2019, 19, 969. [Google Scholar] [CrossRef] [PubMed]
- Kambarami, R.; Chidede, O. Neonatal hypothermia levels and risk factors for mortality in a tropical country. Cent. Afr. J. Med. 2003, 49, 103–106. [Google Scholar] [PubMed]
- Jones, M.E.; Ponsonby, A.-L.; Dwyer, T.; Gilbert, N. The Relation between Climatic Temperature and Sudden Infant Death Syndrome Differs among Communities: Results from an Ecologic Analysis. Epidemiology 1994, 5, 332–336. [Google Scholar] [CrossRef]
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Belesova, K.; Boykoff, M.; Montgomery, H. The 2019 report of The Lancet Countdown on health and climate change: Ensuring that the health of a child born today is not defined by a changing climate. Lancet 2019, 394, 1836–1878. [Google Scholar] [CrossRef]
- Colston, J.M.; Zaitchik, B.; Kang, G.; Peñataro Yori, P.; Ahmed, T.; Lima, A.; Kosek, M.N. Use of earth observation-derived hydrometeorological variables to model and predict rotavirus infection (MAL-ED): A multisite cohort study. Lancet Planet Health 2019, 3, e248–e258. [Google Scholar] [CrossRef]
- Carlton, E.J.; Woster, A.P.; DeWitt, P.; Goldstein, R.S.; Levy, K. A systematic review and meta-analysis of ambient temperature and diarrhoeal diseases. Int. J. Epidemiol. 2016, 45, 117. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.; Birch, C.E.; Marsham, J.H.; Part, C.; Hajat, S.; Chersich, M.F.; Ebi, K.L.; Luchters, S.; Nakstad, B.; Kovats, S. Past and projected climate change impacts on heat-related child mortality in Africa. Environ. Res. Lett. 2022, 17, 074028. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).

