Abstract
Aerosol generation and a wide range of pathogens originating from the oral cavity of the patient contaminate various surfaces of the dental clinic. The aim was to determine the efficacy of vaporized hydrogen peroxide fogging on pathogens related to the dental environment and its possible application in dentistry. PICOS statement (Population, Intervention, Comparison/Control, Outcome and Study design statement) was used in the review. Six electronic databases were searched for articles published from 2010 to 2020. Articles written in English reporting vaporized hydrogen peroxide on pathogens deemed to be relevant to the dental environment were assessed. The quality of the studies was assessed using the risk-of-bias assessment tool designed for the investigation of vaporized hydrogen peroxide application in dentistry. A total of 17 studies were included in the qualitative synthesis. The most commonly reported single bacterial pathogen was Methicillin-resistant Staphylococcus aureus in five studies, and the viruses Feline calicivirus, Human norovirus, and Murine norovirus were featured in three studies. The results of the studies reporting the log kill were sufficient for all authors to conclude that vaporized hydrogen peroxide generation was effective for the assessed pathogens. The studies that assessed aerosolized hydrogen peroxide found a greater log kill with the use of vaporized hydrogen peroxide generators. The overarching conclusion was that hydrogen peroxide delivered as vaporized hydrogen peroxide was an effective method to achieve large levels of log kill on the assessed pathogens. The hydrogen peroxide vapor generators can play a role in dental bio-decontamination. The parameters must be standardized and the efficacy assessed to perform bio-decontamination for the whole clinic. For vaporized hydrogen peroxide generators to be included in the dental bio-decontamination regimen, certain criteria should be met. These include the standardization and efficacy assessment of the vaporized hydrogen peroxide generators in dental clinics.
1. Introduction
Infection control has always been a core objective in dentistry; however, it has risen to greater importance given the SARS-CoV-2 pandemic. Aerosolized viruses and bacteria, such as Tuberculosis, Candida auris, and Staphylococcus aureus, once inhaled by either patient or healthcare worker, can result in far-reaching health consequences [,,].
Infected droplets can be spread by dental instruments from the mouth of the dental patient, such as high-speed rotating handpieces and ultrasonic devices. The contaminated aerosol settle on exposed surfaces, resulting in environmental contamination. Virus or bacterial pathogens may survive on inanimate surfaces for prolonged periods of time and, as a result, serve as reservoirs for cross-contamination. These aforementioned surfaces increase the risk of transferring pathogens to patients through hand contact []. The surfaces most frequently touched in the dental environment include switches/buttons on the dental chair, dental light, radiography equipment, dental material containers, dental curing lights, lasers, and computer equipment. Despite surface disinfection protocols, many of the inanimate objects are not routinely disinfected and together with the hands of staff become vectors for transmission of healthcare-associated infections [,]. Studies determined that established disinfection methods showed their inability to eliminate environmental contamination of certain pathogens that are associated with direct transmission []. In addition, an increase in the bacterial load after the use of a neutral detergent was reported. A neutral detergent is composed of surfactants, fillers, and chelating agents. They are typically not used in pathogen eradication []. Enhanced cleaning that deviates from traditional surface cleaning (traditional surface cleaning or terminal cleaning aims to reduce the number of pathogens on surfaces to reduce transmission) not only reduced the bacterial load in the environment but also reduced the number of organisms on the hands of staff. Disinfection procedures that involve physical contact with the surfaces (spray, wipe, and spray techniques) are widely used but are usually labor-intensive and not always effective, as it is impossible to reach all hidden surfaces. For this reason, it is imperative to investigate the efficacy and then adopt other infection control approaches to decontaminate the dental environment between patients and minimize the risk of transmission of diseases [,].
As a result of this need for increased efficacy of decontamination for dentistry, there is potential in the use of gaseous decontamination either on its own or after conventional cleaning. Vaporized hydrogen peroxide (VHP) can be a suitable decontaminant as it is effective for the in vitro inactivation of enveloped and non-enveloped viruses [,]. Hydrogen peroxide is an effective biocide in its gaseous (vaporized and aerosolized) form against viruses, spores, fungi, and bacteria [,]. The vaporized solution of hydrogen peroxide, which is based on water, is activated by plasma and acts as an oxidizing and disinfecting agent when it settles and contacts the surfaces of all objects in the room. In doing so, it attacks essential cell components, such as the DNA, lipids, and protein of the pathogen cell wall [].
There are various terms used in the literature used to describe hydrogen peroxide vapor, vaporized hydrogen peroxide (VHP), and aerosolized hydrogen peroxide (aHP). Hydrogen peroxide vapor has been used effectively to decontaminate enclosed areas, like incubators, medicine trolleys, laboratory cabinets, operating rooms, isolation room, general medical wards, and intensive care units. The air particles in the room assist with spreading the hydrogen peroxide vapor [,,,]. This process is relatively non-toxic for humans, the environment, and medical materials/devices because hydrogen peroxide vapor degrades into water and oxygen with no residue typically found [,,]. The importance of the compatibility between materials used in the dental environment with physical or liquid chemical germicides should not be overlooked.
VHP generators are no-touch decontamination and therefore circumvent problems associated with operators during manual disinfection such as incorrect application and use of cleaning agents. Due to its vapor form, it can disinfect all objects that are in contact with the air as well as hard to reach places on inanimate objects [,]. Whole-room disinfection using conventional surface cleaning followed by VHP was found to be highly effective in reducing the level of aerobic bacterial contamination below detectable counts [,]. An additional advantage is that, while the hydrogen peroxide is being dispensed by a portable vapor generator into a vaporized state for decontamination, staff do not need to remain in the room, thus, providing an opportunity to allocate staff to other tasks or continue treatment in another room. The greatest disadvantage of this form of decontamination is that the generator set-up and the preparation process have an initial learning curve and must be operated by trained personnel [].
Depending on the generator utilized it could be time-consuming and expensive to ensure the appropriate hydrogen peroxide concentration and validation are achieved [,]. Additionally, the room must be vacated and possibly pre-cleaned to remove visible contamination. VHP can irritate eyes, mucous membranes, skin, and lungs—if it is inhaled. For this reason, the room requires a venting stage to achieve a safe level of VHP before staff may enter the room [].
The efficacy of surface decontamination and techniques in dental environments has been questioned, especially in our current climate of SARS-CoV-2 hypervigilance. Contaminated hands can result in self-inoculation and act as a route for disseminating pathogens. No-touch decontamination methods can, therefore, play a pivotal role in the prevention of viral transmission in the dental environment.
2. Objective
In this review we aim to determine the efficacy of VHP fogging on pathogens related to the dental environment and its possible applications in dentistry.
3. Methods
We reported this review following the Preferred Reporting Items for Systematic Reviews and meta-analyses (PRISMA) statement []. A meta-analysis and associated plots/graphs were not completed for this systematic review.
Research questions:
Efficacy of VHP fogging against dental environment pathogens:
- −
- To what log kill are the pathogens that play a role in dentistry eliminated by VHP fogging?
- −
- What VHP fogging disinfection methods could be effective in the dental environment?
3.1. PICOS Statement
People/participants = Pathogens on surfaces exposed with VHP
Intervention/Event = VHP fogging
Comparison = Surface disinfection
Outcome = Bio-decontamination in terms of log kill
Study design = Quasi-experimental study design
3.2. Search Strategy
The following electronic databases were searched to identify articles reporting results on VHP fogging for pathogens that can contaminate the dental environment using terms with Boolean operators published between January 2010 And October 2020 (Table 1): DOAJ, Ebscohost, Pubmed/Medline, Scopus, Sceilio, and Web of Science. Studies were limited to articles written in the English language.

Table 1.
Search terms for database searches.
3.3. Eligibility Criteria
Inclusion criteria: Studies (2010–2020) published in English that investigated the efficacy of the VHP fogging on pathogens that can contaminate the dental environment were considered for inclusion. The following original research articles: Articles published in peer-reviewed, scientific journals and research conducted in dental-care or in vitro settings that implemented a no-touch disinfection method with hydrogen peroxide against any pathogen that presented with the possibility of being a contaminating pathogen from patients to the dental environment and staff were considered for inclusion.
Exclusion criteria: Articles, such as editorials, commentaries, non-peer-reviewed articles, systematic reviews, scoping reviews, pathogen outbreaks, conference papers, and surveillance reports, were excluded. Studies in which the pathogen assessed against hydrogen peroxide was not able to contaminate the dental environment were ignored including studies that evaluated VHP as an adjunct to service cleaning.
3.4. Study Selection
Two reviewers independently assessed the eligibility of the searched studies. The titles and abstracts were primarily screened to identify whether the criteria were met. The full texts of selected studies during primary screening were reviewed for the final study selection. Any discrepancies were resolved by sharing opinions and consultation with the other author, if necessary.
3.5. Data Extraction
After data extraction, two reviewers independently extracted data, such as information on the pathogen used and on what type of surface, outcome level (log kill), the methodology of use including manufacturer instructions. Included also was the control measures in terms of study standardization, the parameters of the hydrogen peroxide in parts per million, monitoring of the concentration, and whether the study was transferable to the dental environment due to the results and pathogen used.
3.6. Control Measures
The general control measures considered were the use of a biological indicator (BI) or electronic forms to assess the efficacy and consistency of the VHP fogging, the concentration of the VHP reached in the area, and the dwell time. Furthermore, environmental control of the fogged area to receive the vapor and the efficacy of not contaminating the assessed samples at any stage of the research to achieve accurate data collection.
3.7. Quality Assessment
The quality of studies was assessed using a critical appraisal tool that was developed by two reviewers (R.A. and R.M.). The decision to formulate an independent tool for this study was based on the specific aspects of hydrogen peroxide that were being investigated. The key domains that were evaluated were decontamination by hydrogen peroxide and whether this can be translated into clinical practice, including dentistry. Studies that were unclear with their objectives and did not meet the aims of the study, and were excluded.
If the studies did not use standard bacterial strains and control pathogens they were eliminated. Studies that used surface cleaning as an adjunct to VHP decontamination were excluded as well. If articles were not clear on where they fall within the context of the criteria, they were discussed between the two reviewers, and, if necessary, an agreement was reached with the corresponding author. Differing opinions were, therefore, discussed between the two reviewers and a consensus was reached.
The specific information assessed under each domain was as follows:
(1) selection of participants included the pathogen exposed on the surface with VHP; (2) confounding variables included the correct percentage of VHP used for the machine and the equipment used per the manufacturer instructions with appropriate monitoring of the VHP parts per million and dwell time; (3) VHP exposure measurement included how the study was standardized to ensure the validity of all the specimens being treated the same and preventing contamination; (4) blinding for outcome assessment included if the authors were blinded to what sample is the control or the test specimen. This determined if the study was a quasi-experimental study design; (5) incomplete outcomes included if there were any failed samples or where the experiment could not be completed or outlier results detected, and (6) selective outcome reporting where not all the data were presented or data presented not in terms of the log kill. The results for the assessment of quality were displayed using Review Manager (RevMan) version 5.3 software (The Cochrane Collaboration, Oxford, UK).
4. Results
4.1. Search Results
A total of 493 studies were retrieved from six databases. The duplicate records were removed (n = 479), and the eligibility criteria were applied for the selection process. After reviewing the full text, 434 articles were excluded for the following reasons: irrelevant for the research topic (n = 345), unavailable full-text (n = 3), review articles (n = 24), pathogens were not transferable to dentistry (n = 11), and VHP as an adjunct to surface cleaning (n = 40). Finally, 17 articles were included in this review (Figure 1).
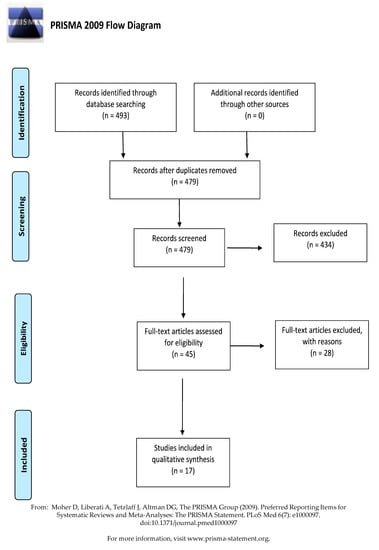
Figure 1.
PRISMA flow diagram of the study selection.
4.2. Characteristics of the Included Studies
The characteristics of the eligible studies are presented in (Table 2). The publication distribution was between 2010 and 2020. The majority of the articles were completed in the United Kingdom (n = 8), Sweden (n = 3), and the USA (n = 2), followed by Brazil (n = 1), France (n = 1), Germany (n = 1), and The Netherlands (n = 1).

Table 2.
The characteristics of the included studies for VHP decontamination (n = 17).
4.3. Risk of Bias in the Included Studies
The quality of the 17 selected studies was assessed, and the results are summarized in Figure 2. All the studies were of high risk for detection bias concerning blinding toward the outcome of pathogen assessment as the samples were not anonymized when sent for pathogen assessment. One study presented with an unclear risk for incomplete data representation or outlier results being present []. Confounding variables for selection bias was of unknown risk for eight of the articles [,,,,,,,]. Incomplete data presentation leading to attrition bias or at least the risk of misinterpretation of the log loss of viruses were not determined due to the various steps of virus remuneration after VHP exposure [,,,,,,,,,,].
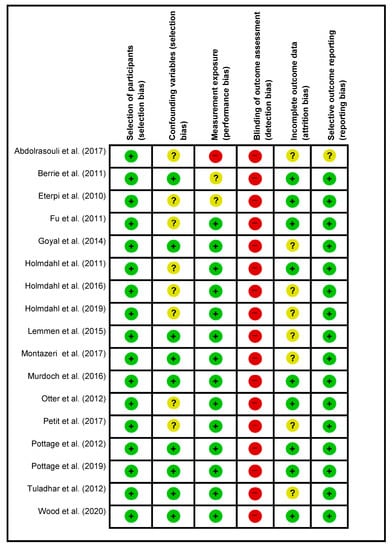
Figure 2.
Risk of bias summary.
4.4. Characteristics of VHP Decontamination on Pathogens
The characteristics of VHP decontamination are presented in Table 2 and Table 3. Bioquell was the manufacturer of 13 of the 19 machines assessed in the selected studies [,,,,,,,,,,,,]; however, irrespective of the VHP unit used, all the studies reported favorable outcomes (towards the VHP generators rather than the aerosolized hydrogen peroxide (aHP) generators) for log reduction of the assessed pathogens. Methicillin-resistant Staphylococcus aureus (MRSA) was the most used bacterial pathogen in five studies and with the viruses Feline calicivirus, Human norovirus, and Murine norovirus featured in three studies.

Table 3.
Summary of items regarded in the risk assessment of the chosen article.
Most of the VHP generators are equipped with monitoring systems, but part per million (ppm) monitoring is essential to ensure the desired concentration of hydrogen peroxide is reached for the desired dwell time. Additionally, the use of standardized validated Geobacillus stearothermophilus biological indicators [,,,,,,,,], are important to set the benchmark for the efficacy of the VHP with the manufacturer’s instructions. The surface that received the pathogen was predominantly stainless steel in the form of discs, coupons, or tape with the exceptions of cell culture well plates [,,] and cryogenic tube caps []. Two authors used stainless steel as well as some additional materials [,]. The log kill was sufficient for all the authors to conclude that VHP generation was effective for the assessed pathogens. The studies that assessed aHP found a greater log kill with VHP generators [,].
5. Discussion
Stainless steel in its nature is resilient and is a common material found in the clinical areas of medicine and dentistry. In this review, stainless steel discs were the most commonly used and preferred carrier or medium for inoculation with a bacterium, virus, or fungi. A 10 mm diameter disc was used by 5 of the 17 included studies and four studies used unspecified stainless-steel disc. This evidence from the aforementioned five studies is therefore translatable to dentistry due to the high contact surfaces in a dental clinic containing some kind of stainless-steel surface.
Some studies did not report on the hydrogen peroxide liquid and/or the percentage used in the machines [,,,,] nor on the model of the machine []. The hydrogen peroxide liquid and percentage cannot be assumed, as some liquids are optimized with additives for certain machines. The concentration of the hydrogen peroxide liquid plays a role in the time period that the machine needs to be on in order to generate the appropriate parts per million in the room. Some authors additionally did not indicate in their articles if the manufacturer’s instructions were followed. The combination of the unknown run time of the machine and unknown liquid concentration makes certain studies difficult to reproduce. Although the exposure time, dwell time, and the grams of hydrogen peroxide used per cubic meter are essential information, the ability to replicate a study requires more information [,,,,].
Attrition bias is an important component of microbiological studies and although the studies utilized positive controls that were not exposed to hydrogen peroxide vapor, the loss of pathogens during the methodology influences the results and leads to increased attrition bias. The bias within the study as a result of surviving enumerated pathogens after VHP is purely concerning the control pathogens not exposed to VHP but exposed to similar periods before enumeration. On the other hand, the determination of the possible enumerated pathogens at every step ensures that the authors can explain certain results. This was evident, e.g., as H2O2 vapor unexposed C. auris was able to survive in a desiccated state in vitro after 4 weeks, whereas non-C. auris had reduced viability (data was not shown by the authors) [].
Certain studies recognized the loss of the pathogens during the methodology and reported on the log reduction determined in the studies for viruses [,,] and bacteria [,]. The loss of pathogens has been specifically noted with viruses [] but has also occurred with bacteria [] on dry surfaces indicated at each step of the methodology with reconstitution and neutralization. The assessment of the lost pathogens provides transparency to the research. For review purposes, a greater opportunity for result comparison with other studies and duplication of the methodology can be achieved. Other positive, negative and additional control measures were employed by authors [,]. These measures provided greater validity to the methodology and less possible bias.
The appropriate sample size to receive hydrogen peroxide vapor (H2O2 vapor) in conjunction with the biological indicator next to the sample assessed confirms the H2O2 vapor efficacy to eliminate the pathogens on a biological indicator. The importance of such a measure is evident when the selective outcome reporting (reporting bias) is noted. In a study by [], one Indian (the United Kingdom outbreak unrelated) C. auris isolate grew repeatedly in two out of the triplicate wells exposed to H2O2 vapor. These authors concluded that some isolates may be more resilient to this form of disinfection []. Geobacillus stearothermophilus is the verified bacterial pathogen used on the stainless-steel discs as a biological indicator for the efficacy of bio-decontamination of hydrogen peroxide vapor generators by the machine manufacturers. The biological indicator pathogen elimination from the stainless-steel discs after hydrogen peroxide vapor is easier to achieve with high numbers of log kill, compared to hydrogen peroxide vapor systems on Methicillin-resistant Staphylococcus aureus (MRSA) [,]. The elimination of MRSA by 35% hydrogen peroxide vapor is the requirement for VHP, rather than 5% and 10% concentrations []. MRSA is quite resilient compared to the biological indicators and is a suitable parameter to assess the efficacy of VHP for dental purposes. Additionally, the studies that presented the growth medium containing additional serums added to the simulation of surface contamination.
These additional growth mediums present a greater challenge to the VHP bio-decontamination process [,,,,,,]. This applies to dentistry, as the pathogens will be present in saliva, blood, and water as organic and inorganic matter. Therefore, surface contamination from the serum poses a real-life challenge to the hydrogen peroxide vapor articles assessed in this systematic review. The result was that VHP generators with a 35% H2O2 are the most appropriate machine for dentistry; however, the parameters should be defined in more detail than is presented in the 17 reviewed articles to align the methodologies toward consistent and predictable bio-decontamination.
6. Conclusions
The overarching conclusion is that H2O2 delivered as VHP was an effective method to achieve large levels of log kill on the assessed pathogens. Within the limitations of all the studies’ parameters, including the presentation of no blinding as well as pathogen log loss during the methodology, VHP was found to be a suitable form of bio-decontamination. Head-to-head direct comparison of results between articles was not possible due to the heterogeneity in the methodologies. The essential methodology benchmarks should include the presentation of the dwell time, parts per million, and the initial concentration of the H2O2. Due to the heterogeneity of the methodologies, evaluating each article concerning the bias selection criteria was the only effective way to determine the relevance to dentistry.
All the articles have applications to dentistry bio-decontamination. They showed the efficacy of VHP in spaces and surfaces similar to a dental clinic. Further investigation of VHP in dental clinics is required with certain variables that must be known and standardized to assure the validity and reproducibility regarding the H2O2 concentration, dwell time, and a constant ppm or defined ppm range during the dwell time. The enumerated pathogens at every step of the methodology, from inoculation on the test surface to the enumeration of the exposed and unexposed samples, should be completed. This safeguard will ensure the correct determination of the log loss of pathogens. From the results of the reviewed articles, a statistically calculated sample size performed in triplicate should be standardized.
Author Contributions
Conceptualization, R.A. and R.M.; methodology, R.A. and R.M.; software, R.M.; validation, R.A. and R.M.; formal analysis, R.A. and R.M.; investigation, R.A. and R.M.; resources, R.A. and R.M.; data curation, R.A. and R.M.; writing- original, R.A and R.M.; draft preparations, R.A. and R.M.; writing review & editing R.A. and R.M.; visualization, R.A. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The language editing was funded by the author fund of R.M. The APC was funded by The University of the Western Cape and the author fund of R.M.
Institutional Review Board Statement
Not applicable for systematic reviews.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ali, S.; Muzslay, M.; Bruce, M.; Jeanes, A.; Moore, G.; Wilson, A.P.R. Efficacy of two hydrogen peroxide vapour aerial decontamination systems for enhanced disinfection of meticillin-resistant Staphylococcus aureus, Klebsiella pneumoniae and Clostridium difficile in single isolation rooms. J. Hosp. Infect. 2016, 93, 70–77. [Google Scholar] [CrossRef]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackelton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European Hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Inchingolo, F.; Lorusso, F. Environmental Disinfection of a Dental Clinic during the Covid-19 Pandemic: A Narrative Insight. BioMed Res. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Mepham, S.; Athan, B.; Mack, D.; Smith, R.; Jacobs, M.; Hobkins, S. Terminal decontamination of the Royal Free London’s high-level isolation unit after a case of Ebola virus disease using hydrogen peroxide vapour. Am. J. Infect. Control 2015, 44, 233–235. [Google Scholar] [CrossRef]
- Falag, M.E.; Thomaidis, P.C.; Kotsantis, I.K.; Sgouros, K.; Samonis, G.; Karageorgopoulos, D.E. Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: A systematic review. J. Hosp. Infect. 2011, 78, 171–177. [Google Scholar] [CrossRef]
- Chan, H.T.; White, P.; Sheorey, H.; Cocks, J.; Waters, M.-J. Evaluation of the biological efficacy of hydrogen peroxide vapour decontamination in wards of an Australian hospital. J. Hosp. 2011, 79, 125–128. [Google Scholar] [CrossRef]
- Tysiąc-Miśta, M.; Dubiel, A.; Brzoza, K.; Burek, M.; Pałkiewicz, K. Air disinfection procedures in the dental office during the covid-19 pandemic. Med. Pracy 2021, 72. [Google Scholar] [CrossRef]
- French, G.L.; Otter, J.A.; Shannon, K.P.; Adams, N.M.T.; Watling, D.; Parks, M.J. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): A comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J. Hosp. Infect. 2004, 57, 31–37. [Google Scholar] [CrossRef]
- Rogers, J.V.; Richter, W.R.; Shaw, M.Q.; Choi, Y.W. Vapour-phase hydrogen peroxide inactivates Yersinia pestis dried on polymers, steel, and glass surfaces. Lett. Appl. Microbiol. 2008, 47, 279–285. [Google Scholar] [CrossRef]
- Blazejewski, C.; Wallet, F.; Rouzé, A.; Le Guern, R.; Ponthieux, S.; Salleron, J.; Nseir, S. Efficiency of hydrogen peroxide in improving disinfection of ICU rooms. Crit. Care 2015, 19, 30. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Abdolrasouli, A.; Armstrong-James, D.; Ryan, L.; Schelenz, S. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with Candida auris. Mycoses 2017, 60, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Eterpi, M.; McDonnell, G.; Thomas, V. Decontamination efficacy against Mycoplasma. Lett. Appl. Microbiol. 2010, 52, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.Y.; Gent, P.; Kumar, V. Efficacy, efficiency and safety aspects of hydrogen peroxide vapour and aerosolized hydrogen peroxide room disinfection systems. J. Hosp. Infect. 2012, 80, 199–205. [Google Scholar] [CrossRef]
- Holmdahl, T.; Lanbeck, P.; Wullt, M.; Walder, M.H. A head-to-head comparison of hydrogen peroxide vapor and aerosol room decontamination systems. Infect. Control Hosp. Epidemiol. 2011, 32, 831–836. [Google Scholar] [CrossRef]
- Holmdahl, T.; Walder, M.; Uzcátegui, N.; Odenholt, I.; Lanbeck, P.; Medstrand, P.; Widell, A. Hydrogen peroxide vapor decontamination in a patient room using feline calicivirus and murine norovirus as surrogate markers for human norovirus. Infect. Control Hosp. Epidemiol. 2016, 37, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Holmdahl, T.; Odenholt, I.; Riesbeck, K.; Medstrand, P.; Widell, A. Hydrogen peroxide vapour treatment inactivates norovirus but has limited effect on post-treatment viral RNA levels. Infect. Dis. 2019, 51, 197–205. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezlia, S.; French, G.L. Impact of the suspending medium on susceptibility of meticillin-resistant Staphylococcus aureus to hydrogen peroxide vapour decontamination. J. Hosp. Infect. 2012, 82, 213–215. [Google Scholar] [CrossRef]
- Petit, B.M.; Almeida, F.C.; Uchiyama, T.R.; Lopes, F.O.C.; Tino, K.H.; Chewins, J. Evaluating the efficacy of hydrogen peroxide vapour against foot-and-mouth disease virus within a BSL4 biosafety facility. Lett. Appl. Microbiol. 2017, 65, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Tuladhara, E.; Terpstrac, P.; Koopmansa, M.; Duizera, E. Virucidal efficacy of hydrogen peroxide vapour disinfection. J. Hosp. Infect. 2012, 80, 110–115. [Google Scholar] [CrossRef]
- Goyala, S.M.; Chandera, Y.; Yezlib, S.; Otter, J.A. Evaluating the virucidal efficacy of hydrogen peroxide vapour. J. Hosp. Infect. 2014, 86, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Lemmen, S.; Scheithauer, S.; Häfner, H.; Yezli, S.; Mohr, M.; Otter, J.A. Evaluation of hydrogen peroxide vapor for the inactivation of nosocomial pathogens on porous and nonporous surfaces. Am. J. Infect. Control 2015, 43, 82–85. [Google Scholar] [CrossRef]
- Murdoch, L.E.; Bailey, L.; Banham, E.; Watson, F.; Adams, N.M.T.; Chewins, J. Evaluating different concentrations of hydrogen peroxidein an automated room disinfection system. Lett. Appl. Microbiol. 2016, 63, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, N.; Manuel, C.; Moorman, E.; Khatiwada, J.R.; Williams, L.L.; Jaykus, L.A. Virucidal Activity of Fogged Chlorine Dioxide- and Hydrogen Peroxide-Based Disinfectants against Human Norovirus and Its Surrogate, Feline Calicivirus, on Hard-to-Reach Surfaces. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Berrie, E.; Andrews, L.; Yezli, S.; Otter, J.A. Hydrogen peroxide vapour (HPV) inactivation of adenovirus. Lett. Appl. Microbiol. 2011, 52, 555–558. [Google Scholar] [CrossRef]
- Pottage, T.; Lewis, S.; Lansley, A.; Fraser, S.; Hendon-Dunn, C.; Bacon, J.; Ngabo, D.; Parks, S.R.; Bennett, A.M. Hazard Group 3 agent decontamination using hydrogen peroxide vapour in a class III microbiological safety cabinet. J. Appl. Microbiol. 2019, 128, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.P.; Richter, W.; Sunderman, M.; Worth-Calfee, M.; Serre, S.; Mickelsen, L. Evaluating the Environmental Persistence and Inactivation of MS2 Bacteriophage and the Presumed Ebola Virus Surrogate Phi6 Using Low Concentration Hydrogen Peroxide Vapor. Environ. Sci. Technol. 2020, 54, 3581–3590. [Google Scholar] [CrossRef]
- Pottage, T.; Macken, S.; Walker, J.T.; Bennett, A.M. Meticillin-resistant Staphylococcus aureus is more resistant to vaporized hydrogen peroxide than commercial Geobacillus stearothermophilus biological indicators. J. Hosp. Infect. 2012, 80, 41–45. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).