Abstract
Background: Titanium dioxide (TiO2) is used as a food additive in pastries, sweets, and sauces. It is recognized as safe by food safety authorities, but in recent years, governments and scientists have raised concerns about its genotoxicity. This systematic review aims to assess the potential associations between food TiO2 exposure and microbiota composition and functions. Methods: A systematic literature search was performed up to December 2020 in PubMed, Web of Science, and Scopus databases. The PRISMA guidelines followed. The risk of bias was assessed from ARRIVE and SYRCLE tools. Results: A total of 18 animal studies were included (n = 10 mice, n = 5 rats, n = 2 fruit flies, n = 1 silkworm). Studies varied significantly in protocols and outcomes assessment. TiO2 exposure might cause variations in abundance in specific bacterial species and lead to gut dysfunctions such as a reduction in SCFAs levels, goblet cells and crypts, mucus production, and increased biomarkers of intestinal inflammation. Conclusions: Although the extrapolation of these results from animals to humans remains difficult, this review highlights the key role of gut microbiota in gut nanotoxicology and stimulates discussions on the safe TiO2 use in food and dietary supplements. This systematic review was registered at PROSPERO as CRD42020223968.
Keywords:
dioxide titanium; TiO2; E171; CI 77891; food additive; gut microbiota; gut barrier; immunity; toxicity; diet 1. Introduction
Titanium dioxide (TiO2) is one of the main food additives used for its coloring and opacifying properties to improve the appearance and taste of processed foods. Food-grade TiO2 is found in over 900 food products such as pastries, sauces, ice-creams, candies, chocolates, and chewing gum. In foods, TiO2 is commonly reported as E171. It is also referred to as CI 77891 when used in cosmetics and toothpaste as a white colorant [1]. E171 consists of a wide range of particle TiO2 sizes and can contain up to 36% nanosized TiO2 particles, i.e., less than 100 nm in diameter [2,3]. Compared with their macroscopic counterparts, nanoparticles (NPs) can easier pass through the body’s cells and then into the bloodstream and internal organs such as liver, kidney, and lung tissues. Daily, the human dietary exposure dose of TiO2 NPs can reach one to four micrograms per kilogram body weight per day (µg per kg bw per day) [3]. In 1966, the Food and Drug Administration (FDA) approved the use of food-grade TiO2 referred to as INS171, specifying that the quantity of TiO2 must not exceed one percent by weight of the food [4]. In Europe, in 2006, the European Food Safety Authority (EFSA) authorized the use of E171 in food concluding that E171 is safe for consumers, having margins of safety (MoS) of 2.25 mg per kg bw per day [5,6]. However, TiO2 NPs raise health concerns among the scientific community and governments given their potential to cross the gut barrier and distribute to other organs eliciting immunological response. In June 2018, the EFSA evaluated four new in vivo and in vitro studies [7,8,9,10] assessing potential toxicities and reaffirmed the safety of E171 [11]. In April 2019, the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) published a review suggesting a genotoxic and carcinogenic potential even if further in vivo mammalian studies are warranted to confirm or rule out these hypotheses [12]. As requested by the European Commission, EFSA provided urgent scientific and technical review regarding the opinion issued by ANSES [13]. The EFSA concluded that the latest ANSES opinion does not identify any major new findings that would overrule the conclusions made in the previous two scientific opinions in 2016 and 2018. The latest ANSES opinion reiterated the previously identified uncertainties and emphasized that there was still not enough data available to carry out a proper assessment of the risks associated with the food use of E171. EFSA considered this recommendation should be revisited once the ongoing work on the physicochemical characterization of E171 will be completed. In January 2020, France has adopted a decree to ban the use of E171 in foods as a precautionary measure to protect consumers’ health.
In a scientific context of “microbiota revolution”, potential health risks of TiO2 NPs and their impact on the intestinal tract and the gut microbiota are increasingly being studied. Gut microbiota is composed of millions of bacterial species that bi-directionally interact with the host in the intestinal tract, regulating the development of immune cells. Alterations in the abundance and composition of intestinal microbiota, known as dysbiosis, are associated with host health such as brain function, lipid metabolism, immune responses, and development of diseases [14]. Recent studies reported adverse effects of in vitro exposure of intestinal epithelial cells to E171 [9,15,16]. Indeed, TiO2 NPs could damage microvilli structure and alter epithelial integrity [17,18]. TiO2 NPs can be internalized and can cross the epithelial barrier to enter the bloodstream and potentially affect the function of distant organs, such as the liver [19]. Moreover, in vitro, NPs have the potential to negatively affect intestinal functions and gut homeostasis associated with gut microbiota [20]. New evidence from numerous recent animal studies has emerged highlighting the effects of various physiological doses of TiO2 NPs on gut microbiota composition and gut homeostasis. Such evidence has not yet been systematically reviewed. Hence, we sought to systematically review current evidence from in vivo animal models to disentangle the TiO2 effects on the gut microbiome composition and functions.
2. Methods
This systematic review is structured following the general principles published in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21]. The PRISMA checklist was detailed in Table S1. Full details of the search strategies were specified and documented in a protocol that was registered at PROSPERO (https://www.crd.york.ac.uk/PROSPERO; accessed on 24 December 2020) as CRD42020223968.
2.1. Eligibility Criteria
The eligibility criteria are outlined using the PICOS format (Table 1).

Table 1.
PICOS criteria for inclusion of studies.
2.2. Data Sources and Search Strategy
The search was carried out on 1 December 2020 using three electronic databases, MEDLINE (via PubMed), ISI Web of Science, and Scopus. Multiple search terms are used including the microbiome, microflora, intestinal microbiota, gut microbiota, titanium dioxide, TiO2, and E171. The search string for each database is described in Table S2. Hand searching of eligible studies was done to find studies that may not have been found in the databases.
2.3. Study Selection
The study selection process was independently carried out by two reviewers (P.R.; E.R.). All articles generated from the electronic search were imported into Mendeley© (Elsevier, Amsterdam, The Netherlands), a references management software, and duplicates were removed. Titles and abstracts were screened for eligibility based on inclusion criteria. All titles assessed as ineligible were excluded. Differences in judgment during the selection process between the two reviewers were settled by discussion and consensus.
2.4. Data Extraction and Reporting
After full-text analysis, the following information was extracted from the included articles: title, author information, year of publication, type of study performed, assessed outcome/s, the animal model used, animal gender, age, and weight at baseline, administered dose, length of study, administration route, and main conclusions.
Data was reported using an Excel© (Microsoft Office, Redmond, WA, USA) spreadsheet specifically developed for this study. Each full-text article was retrieved, and any ineligible articles were excluded from the reasoning reported. Differences in judgment between two reviewers (P.R.; E.R.) were settled by discussion and consensus.
2.5. Quality Assessment
The quality of the included studies was assessed following the Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines [22]. These guidelines consist of the minimum information that animal research studies should include such as the number and specific characteristics of animals, details of housing and husbandry, experimental and statistical methods, reporting and interpretation of the results.
Moreover, SYRCLE’s risk of bias tool [23] was used to assess the risk of bias of animal studies. SYRCLE’s tool is an adapted version of the Risk of Bias tool provided by the Cochrane Collaboration. It consists of ten entries associated with selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. Quality assessment was independently performed by two reviewers (P.R. and E.R.) and a consensus should be reached for discrepancies.
3. Results
3.1. Study Selection
The flow diagram in Figure 1 displays the results of the literature search and study selection process. A total of 6254 studies were initially identified. After duplicate removal, 4915 studies remained for titles and abstracts screening. Thirteen studies were excluded for the following reasons: in vitro studies (n = 8) [9,19,24,25,26,27,28,29], no assessment outcomes of interest (n = 1) [30], microbiota of mussel hemolymph (n = 1) [31], review (n = 1) [32], Chinese language (n = 1) [33], TiO2 and bisphenol A co-exposure (n = 1) [34]. Eighteen studies [8,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] were identified for inclusion in the systematic review.
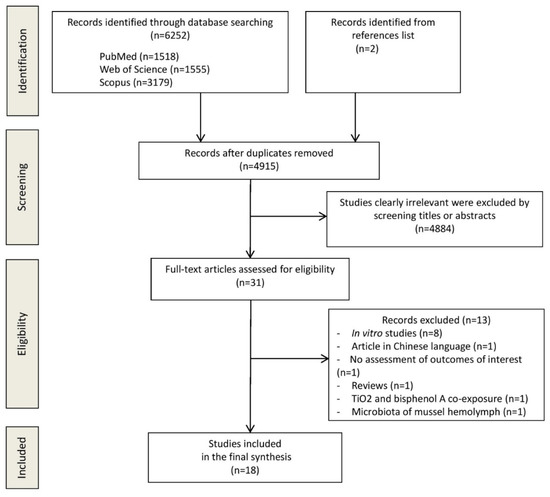
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram.
3.2. Study Characteristics
Included studies used different animal models: C57BL/6J mice (n = 5) [44,45,47,50,51], Sprague-Dawley rats (n = 3) [36,37,43], C57BL/6 (n = 3) [35,39,40], Wistar rats (n = 2) [8,48], Drosophila Melanogaster (n = 2) [42,46], CD-1 mice (n = 1) [38], ICR mice (n = 1) [49], and Bombyx mori (n = 1) [41]. Sample size ranged from 8 [43] to 80 animals [38]. Dose exposure ranged from 2 mg/day/kg body weight of TiO2 NPs [36,37,42,45] to 1 g/day/kg body weight of TiO2 NPs [39] and exposure period ranged from 24 h [39] to 100 days [8]. The characteristics of each included study are detailed in Table 2.

Table 2.
Characteristics of included animal studies (listed by animal type).
3.3. Quality Assessment
The detailed results of quality assessment are presented in Tables S3 and S4. First, the quality of the eighteen included animal studies was assessed through the ARRIVE guidelines. As a result, the included animal articles adequately provide an accurate title and abstract, a structured and thorough introduction, an ethical statement only for mammalian studies, and an adequate study design except for two studies [44,45] which are unclear. None of them justified the sample size, and consequently, the use of a too small number of animals may lead to a lack of experimental statistical significance given the use of too many animals may lead to unnecessary wastage of resources and ethical issues. Only one study did not clearly describe statistical methods [42]. Baseline characteristics (body weight, age, and gender) before treatment are reported in five of the total of studies [8,40,48,49,50]. For twelve studies [35,36,37,39,41,42,43,44,45,46,47,51], body weight was not specified, and for three studies [38,41,42], age was not reported. All studies adequately reported and interpreted their results in terms of numbers analyzed, outcomes, adverse events, interpretation, and generalizability.
Secondly, the risk of bias of the included animal studies was assessed using SYRCLE’s tool. In regards to sequence generation, in twelve out of eighteen studies, the allocation sequence was randomly generated and applied. However, in eleven out of 12 studies, the investigators did not describe the sequence generation process such as the use of a random number table or a computer random number generation. Only in the study of Zhang et al. [50], mice were randomly allocated into the control group and the TiO2 NPs’ group using a web-based randomization service. For all studies, it is not clear how animals were allocated to different groups. In addition, for all studies, all groups had similar characteristics at baseline. Regarding allocation concealment, the concealment was not clear for all studies. Indeed, no studies have explicated the concealed procedure when the investigators have allocated the animals to different groups. Moreover, all included studies have a high risk of performance bias. Indeed, the animals did not randomly house during the experiment and it is not clear whether the investigators did not blind from knowledge which intervention each animal received during the experiment. Additionally, overall, it is not specified whether the investigators did not select animals at random for outcome assessment. However, the outcome assessment methods are the same in both groups for all studies. In regards to attrition and reporting bias, the risk is low for all studies since the outcome data reported in each study was completed for each outcome. All primary outcomes have been reported. Finally, the studies did not report other problems that could result in a high risk of bias. As a conclusion, according to SYRCLE’s risk of bias tool, the quality of each study is debatable due to an inadequate or unclear randomization of allocation, housing and outcome assessment, and a lack of blinding. However, the studied population has similar characteristics at baseline making the sample homogenous and avoiding confounding bias. Moreover, in regards to the reporting of outcomes (complete outcome data reporting, adequate outcome reporting), the risk of bias is low.
3.4. Results
3.4.1. TiO2 and gut Microbial Diversity
Alpha-diversity variations were measured in five studies [40,43,44,45,50]. Chao1—an estimate of species richness based on a vector or matrix of abundance data—did not significantly vary between exposed groups and controls groups in mice exposed to 100 mg/kg bw/day of TiO2 NPs for eight weeks [40], in pregnant rats exposed to 5 mg/kg bw/day of TiO2 NPs for 12 weeks [43], but decreased in mice exposed to 150 mg/kg bw/day of TiO2 NPs for 30 days (p = 0.0052) [50]. In regards to Shannon’s diversity—another index accounting for both abundance and evenness of the species with equal weighting given to abundant and rare species—no significant variations were observed between groups in mice exposed to 100 mg/kg bw/day of TiO2 NPs for eight weeks [40], in mice exposed to a diet containing 0.1% TiO2 NPs for three months [44], in mice exposed to 2, 10, 50 mg/kg bw/day of TiO2 NPs for three weeks [45], and in pregnant rats exposed to 5 mg/kg bw/day of TiO2 NPs for 13 days [43]. However, in the study of Zhang et al. [50], Shannon’s diversity decreased in mice exposed to 150 mg/kg bw/day of TiO2 NPs for 30 days (p = 0.0036) [50]. Finally, applying Simpson’s diversity index—another diversity index measuring richness and evenness in which more weighting is given to abundant species—in four out of the same studies [43,44,45,50], no significant variations were found except for the study of Zhang et al. [50] showing a significant increase after TiO2 NPs exposure (p = 0.0180).
3.4.2. TiO2 and Abundance of Individual Microbial Species
In rodents, four studies showed an increase in Firmicutes abundance after TiO2 exposure compared with controls [35,39,49,51]. Lactobacillus was the most studied genus and significantly decreased in four studies [35,36,39,44] but increased in one study [45] after TiO2 NPs exposure compared with controls. Moreover, an increase in Allobaculum abundance was reported in one study [45] while a decrease was observed in another mice model [35]. Other variations in genera and family abundance after TiO2 exposure compared with controls are observed such as an increase in Oscillospira [35,51], Turicibacter [36], and Clostridiales [43], and a decrease in Veillonella [36], Prevotella [40,51], and Dehalobacteriaceae [43].
Bacteroidetes abundance could also be influenced by TiO2 exposure in rodent models. Three studies showed a decrease in Bacteroidetes levels [35,49,51] while one study reported an increase in Bacteroidetes levels [44]. Especially, TiO2 exposure could lead to an increase in Bacteroides [40], Parabacteroides [45], and a decrease in Barnesiella [49].
Actinobacteria phylum could decrease in abundance [35] after TiO2 exposure with a decrease in Bifidobacterium spp reported in two rodent studies [35,44]. Moreover, an increase in Rhodococcus abundance [40] and a decrease in Adlercreutzia levels [45] were observed.
In regards to other phyla, Proteobacteria could increase after TiO2 exposure, as reported in three studies [40,50,51], and Desulfovibrionaceae [51] and Verrucomicrobia could decrease, in particular in the Akkermansia genus [51].
All these findings observed in rodent models showed that TiO2 exposure could impact gut microbiota composition, although the variations in specific phyla and genera abundances remain to be elucidated with large sample size animal studies using the same dose and duration of TiO2 exposure.
In regards to animal models other than rodents, a model organism Drosophila melanogaster [42] showed that the exposure of 1, 2, and 200 mg/mL dietary of three different sizes of TiO2 NPs for five days did not inhibit the growth of gut bacteria in Drosophila larva or adults. On the other hand, a silkworm model [41] showed different gut microbiota compositional variations after intake of mulberry leaves soaked in 5 mg/L TiO2 NPs and naturally dried from the third day of fifth instar larvae until morning.
3.4.3. TiO2 and SCFAs Levels
A total of six rodent studies reported between-group differences in fecal SCFA concentrations after different TiO2 NPs dose exposure and length of exposure. Three studies showed no significant variations in SCFAs levels [36,37,48] while two studies observed a decrease in SCFAs levels in mice treated with 0.1 weight percent of TiO2 NPs for eight weeks [35] and in mice treated with 50 mg TiO2/kg bw/day for three weeks [45]. Interestingly, one study [39] reported an increase of SCFAs in stools in mice exposed to 1 g/kg bw TiO2 for 14 days. This can be explained by an increase in SCFAs production or a decrease in absorption.
3.4.4. TiO2 and Metabolism
A total of seven studies [36,37,39,42,43,49,50] showed significant metabolic variations in TiO2 exposed animals compared with controls. Lipopolysaccharides (LPS) proportionally increased in mice exposed to 2, 10, and 50 mg/kg bw/day of TiO2 for 30 days [36], in mice exposed to 10, 40, and 160 mg/kg bw/day of TiO2 for 28 days [49], and in mice exposed to 10 μL/g bw/day for eight weeks [51]. Interestingly, in TiO2-treated mice fed with a high-fat diet (HFD), LPS significantly increased compared with TiO2-treated mice fed with a high fiber diet (CHOW diet) [51]. Triglycerides levels (TG) levels increased in mice after exposure to 160 mg/kg bw/day of TiO2 for 28 days, while TG levels reduced in rats exposed to 10 and 50 mg/kg bw/day of TiO2 for 90 days. Moreover, glucose levels could increase after TiO2 exposure, as reported in two rodent model studies [43,49]. Interestingly, in Sprague-Dawley pregnant rats, exposure of 5 mg/kg/day of TiO2 NPs for 12 weeks could strengthen genes about type 2 diabetes mellitus related to function and lipid biosynthesis, compared with controls [43].
The two Drosophila model studies [42,46] reported contradictory results. One study showed no alterations of pupation cycle, weight, and lipid levels after 1, 2, and 200 mg/mL dietary TiO2 NPs of different sizes for five days while Richter and colleagues [46] demonstrated alterations of metabolic gut homeostasis with significant changes in pupation, time to pupation, reduction of body size, and glucose levels.
3.4.5. TiO2 and Gut Barrier Permeability
Bettini et al. observed no significant changes in epithelial paracellular permeability in the E171 group in comparison to the controls [8]. Additionally, a previous study [48] found no effect compared with controls on mucin O-glycosylation in the small intestine of the rats following 7- or 60-day TiO2 exposure, regardless of TiO2 type (E171 and NM-105) or E171 dose tested (0.1 mg/kg bw/day and 10 mg/kg bw/day). Another study [39] showed that at 24 h post-gavage, MUC2 gene expression was lower in TiO2 NP-treated mice (1 g/kg bw/day) compared with controls but this trend was reversed from 48 h post-gavage to seven days with an elevated expression of mucin-2 for the rest of the study.
On the other hand, in mice exposed to 0.1 weight percent of TiO2, goblet cells and crypts significantly decreased compared to controls. Furthermore, three studies [45,49,51] reported a decrease in MUC2 gene expression in mice treated with TiO2 NPs. Yan et al. [49] also reported a reduction of mucus thickness in all exposed mice compared with controls. Interestingly, MUC2 gene expression and crypt length significantly decreased in TiO2-treated mice fed with HFD compared with TiO2-treated mice fed with CHOW diet [51].
3.4.6. TiO2 and Inflammatory Responses
A total of ten studies have assessed levels of different gut microbiota associated biomarkers of mucosal immunity and intestinal inflammation such as interleukins (IL) levels, number of T reg cells, macrophages, and T helper cells. A reduction of T reg cells numbers was found in food-grade E171 treated mice after 100 days [8] and in mice exposed to a diet containing 0.1% TiO2 NPs for three months [44]. Inflammatory cytokines levels increased in exposed rodents compared with controls in the majority of studies: IL1 [49,51], IL2 [38], IL6 [8,36,45,49,51], IL10 [45], IL12 [35], IL17 [8,35], IL18 [8], as well as TNFα levels [45,49,51]. The production of macrophages and the expression of β defensin gene are also stimulated [45]. Interestingly, TiO2 NPs decreased the CD4+ T cells, T regs, and macrophages in the mesenteric lymph nodes and increased neutrophil gelatinase-associated lipocalin (LCN2) levels in mice aggravating the DSS-induced chronic colitis [44]. Moreover, IL-1 levels, IL-6 levels, TNFα levels increased in TiO2-treated mice fed with HFD compared with TiO2-treated mice fed CHOW diet [51]. All these results showed the potential involvement of TiO2 in the imbalances in intestinal and systemic immune responses.
4. Discussion
This systematic review of animal studies found that TiO2 dietary exposure might increase or decrease abundance in specific bacterial species, even if an overall impact on bacterial α-diversity has not been clearly demonstrated. Moreover, this review highlights that TiO2 exposure could lead to perturbations in intestinal metabolism, gut barrier integrity, and gut immunity.
The limited effect of TiO2 exposure on α-diversity of the gut microbiota was found in the majority of included studies. This could be explained by the short duration of the interventions, not exceeding three months. The lack of effects of different dietary interventions on gut microbiota diversity has been shown in previous systematic reviews investigating the effects of dietary patterns or dietary interventions—such as dietary fiber interventions or probiotics interventions—on gut microbiota [52]. Long-term studies are required to assess this hypothesis. In regards to bacterial abundances, in various included studies [35,39,44,49], significant compositional changes are reported after TiO2 exposure compared with controls. TiO2 exposure could lead to an alteration of the Firmicutes/Bacteroides ratio, a depletion of Lactobacillus, and enrichment of Proteobacteria [40,50]. Interestingly, these microbial variations are also found in studies investigating the effect of other food nanoparticles such as nano-Ag, ZnO, and SiO2 exposure [53]. Lactobacillus is a genus well-known to produce SCFAs, metabolites involved in host metabolism, while Proteobacteria might be overrepresented in inflammatory intestinal and extra-intestinal diseases. Indeed, this observed dysbiosis is also a hallmark of inflammatory bowel disease, colorectal cancer, or chronic metabolic disorders such as obesity [54].
The intestinal microbiota plays a key role in gastrointestinal functions such as the digestion and fermentation of indigestible polysaccharides, differentiation of the intestinal epithelium, and the maintenance of mucosal barrier integrity, including mucus characteristics. Mucus is a viscoelastic gel that separates the intestinal epithelium from the gut lumen. It consists of water and mucins, lipids, as well as epithelial and globets cells. Goblet cells are localized in the intestinal crypts and secrete proteins such as muc-2 (encoded by MUC2 gene). Intestinal bacteria influence the shaping of the mucus regulating LPS and SCFAs. Indeed, SCFAs—mainly butyrate—stimulate muc-2 protein production and influence mucus quality. Numerous studies [55,56,57] demonstrated that germ-free mice, comparing with conventionalized mice, were provided with an underdeveloped intestinal epithelium with decreased mucus production, intestinal epithelial cell differentiation, and villus thickness. These alterations could lead to an increased permeability allowing the passage of harmful intraluminal microorganisms and microbial toxins. These bidirectional interactions between gut microbiota composition and gut barrier functions could be impaired with TiO2 exposure. Indeed, in some included studies [35,45,49], TiO2 exposure could be associated with a reduction of SCFAs, a decrease of goblet cells and crypts, a reduction of mucus production with a lower MUC2 expression. These in vivo findings confirmed the results of in vitro studies demonstrating that TiO2 NPs could alter microvilli structure and epithelial integrity [19,24]. Particularly, in vivo and ex vivo, TiO2 NPs can cross the regular ileum and follicle-associated epithelium and alter the paracellular permeability of the ileum and colon epithelia, which is a sign of integrity alteration [58]. However, three studies [8,37,48] did not show significant changes in terms of epithelium permeability, SCFAs levels, and mucus barrier impairment. Considering the TiO2 dose exposure of the studies, we can hypothesize that these discrepancies could be due to dose exposure and healthy conditions of the animals at baseline.
TiO2 NPs also could interact with gut immunity. Indeed, a majority of included studies have assessed associations between TiO2 exposure and increased biomarkers of intestinal inflammation such as increased interleukins levels. Recent in vitro studies [19,27] found that TiO2 NPs could stimulate the production of pro-inflammatory cytokines. Moreover, in vivo, the number of T reg cells decreased after 100 days of TiO2 exposure [8]. T reg cells are well-known to limit gut inflammatory responses and prevent food allergy development [59]. Thus, long-term TiO2 exposure could have an immunosuppressive role by limiting the production of T reg cells. Interestingly, there are significant changes in terms of IL production, significantly aggravated in obese mice treated with TiO2 compared with non-obese mice [35,51]. This shows that TiO2 could exacerbate intestinal inflammation in mice affected by metabolic diseases such as obesity. Mu et al. [44] analyzed the effect of TiO2 NPs on DSS-induced chronic colitis in mice showing that DSS-induced chronic colitis worsened by chronic TiO2 NPs exposure with a reduction of immune cells such as CD4 + T cells and Tregs. Further studies are required to deepen the effects of TiO2 NPs on immunity responses, and specifically on the gut microbiota immune axis.
Overall, TiO2 exposure can raise concerns if we consider the cocktail effects of daily consumption of the different food additives. Indeed, other NPs present in food, emulsifiers, and artificial sweeteners have also dysbiotic effects on gut microbiota [60]. This cocktail effect raises particular concerns since the quantity of food additives is not detailed in the ingredient list, making impossible the calculation of the daily quantity of food additives. For example, chewing one piece of chewing gum can result in an intake of 1.5–5.1 mg of TiO2 NPs [61]. These concerns are even more important in children. Indeed, candies, gums, desserts, and beverages—products containing the highest levels of TiO2 NPs—are consumed two to four times higher for children than for adults [3]. A Dutch survey estimated a mean TiO2 NPs intake of 2.16 (2.13–2.26) mg/kg bw per day among children aged two to six years old, and a mean of 0.55 (0.52–0.58) among people aged 7–69 years old, with toothpaste, candy, coffee creamer, fine bakery wares, and sauces mostly contributing to the TiO2 daily intake [62]. Childhood is a key development time for the shape of the microbiota that can have considerable consequences in later life [63]. Although TiO2 consumption has considerably increased in the last few decades in Western countries and despite dietary composition having an impact on gut and overall health [64], the possible impacts of long term effects of TiO2 are still poorly understood.
This systematic review has some limitations. Although the majority of included studies have used rodent models, the methods of administration (gastric gavage, addition to drinking water, addition to food), TiO2 doses, and exposure durations differ between studies and do not allow pooling results. Thus, since some studies detect only a limited impact on the microbiota, others reporting various significant changes, it remains difficult to reach firm conclusions. Another limitation are the very high doses used in animal studies compared to the estimated daily intake in humans. Indeed, the amount of TiO2 consumed is estimated to 1 mg of TiO2/kg bw/day in adults in the United Kingdom and Germany, while the ingested quantity can exceed 3 mg of TiO2/kg bw/day in children [3,65]. Thus, the results from animal studies cannot be directly extrapolated to humans. Furthermore, only 15% of the 16S rRNA sequence dataset for the mouse microbiota are shared with humans [66]. Since randomized controlled studies are unethical, the use of germ-free mice inoculated with the human microbiota could be feasible to elucidate the impact of TiO2 NPs on gut bacteria that colonize the human intestine. Moreover, different dietary patterns such as HFD or high fiber diet should be evaluated to compare the impact on TiO2 NPs in healthy individuals with those in poor health.
5. Conclusions
In conclusion, in vivo consumption of TiO2 could alter the composition and the activity of intestinal bacteria, promoting an inflammatory environment in the gut and aggravating gut barrier impairment and immune responses in animals already affected by diseases such as colitis or obesity. Therefore, although these findings did not allow us to reach firm conclusions in humans, this systematic review highlights the key role of gut microbiota in nanotoxicology in the gut and stimulates discussions on the safe TiO2 use in food and dietary supplements.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/18/4/2008/s1, Table S1. PRISMA checklist; Table S2. Search strategy; Table S3. Reporting of in vivo experiments (ARRIVE) guideline assessment for the included animal studies; Table S4. SCYRCLE’s tool for assessing the risk of bias in animal studies.
Author Contributions
Conceptualization, E.R. and P.R.; methodology, P.R.; validation, M.C.M. and A.G.; investigation, M.C.; resources, V.M.; writing—original draft preparation, P.R.; writing—review and editing, E.R.; visualization, A.G.; supervision, M.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martirosyan, A.; Schneider, Y.-J. Engineered nanomaterials in food: Implications for food safety and consumer health. Int. J. Environ. Res. Public Health 2014, 11, 5720–5750. [Google Scholar] [CrossRef]
- Yang, Y.; Doudrick, K.; Bi, X.; Hristovski, K.; Herckes, P.; Westerhoff, P.; Kaegi, R. Characterization of food-grade titanium dioxide: The presence of nanosized particles. Environ. Sci. Technol. 2014, 48, 6391–6400. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=73.575 (accessed on 15 December 2020).
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources Added to Food). Scientific opinion on the re-evaluation of titanium dioxide (E 171) as a food additive. EFSA J. 2016, 14, 4545. [Google Scholar]
- NTP (National Toxicology Program). Bioassay of Titanium Dioxide for Possible Carcinogenicity; U.S. Department of Health, Education, and Welfare: Rockville, MD, USA, 1979.
- Heringa, M.B.; Geraets, L.; van Eijkeren, J.C.H.; Vandebriel, R.J.; deJong, W.; Oomen, A.G. Risk assessment of titanium dioxide nanoparticles via oral exposure, including toxicokinetic considerations. Nanotoxicology 2016, 10, 1515–1525. [Google Scholar] [CrossRef]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, srep40373. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Martucci, N.J.; Moreno-Olivas, F.; Tako, E.; Mahler, G.J. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact 2017, 5, 70–82. [Google Scholar] [CrossRef]
- Proquin, H.; Rodríguez-Ibarra, C.; Moonen, C.G.J.; Urrutia Ortega, I.M.; Briedé, J.J.; De Kok, T.M.; Van Loveren, H.; Chirino, Y.I. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: Contribution of micro and nano-sized fractions. Mutagenesis 2017, 32, 139–149. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Evaluation of four new studies on the potential toxicity of titanium dioxide used as a food additive (E 171). EFSA J. 2018, 16, e05366. [Google Scholar] [CrossRef]
- ANSES. Avis de l’Agence Nationale de Securit e Sanitaire de l’Alimentation, de l’environnement et du Travail Relatif au Risques Lies a la Ingestion de l’Additif Alimentaire E 171. Available online: https://www.anses.fr/fr/system/files/ERCA2019SA0036.pdf (accessed on 20 December 2020).
- EFSA (European Food Safety Authority). EFSA statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA J. 2019, 17, e05714. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Dorier, M.; Béal, D.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Carriere, M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology 2017, 11, 751–761. [Google Scholar] [CrossRef]
- Dorier, M.; Brun, E.; Veronesi, G.; Barreau, F.; Pernet-Gallay, K.; Desvergne, C.; Rabilloud, T.; Carapito, C.; Herlin-Boime, N.; Carrière, M. Impact of anatase and rutile titanium dioxide nanoparticles on uptake carriers and efflux pumps in Caco-2 gut epithelial cells. Nanoscale 2015, 7, 7352–7360. [Google Scholar] [CrossRef] [PubMed]
- Faust, J.J.; Masserano, B.M.; Mielke, A.H.; Abraham, A.; Capco, D.G. Engineered nanoparticles induced brush border disruption in a human model of the intestinal epithelium. Adv. Exp. Med. Biol. 2014, 811, 55–72. [Google Scholar] [PubMed]
- Song, B.; Liu, J.; Feng, X.; Wei, L.; Shao, L. A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res. Lett. 2015, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Pedata, P.; Ricci, G.; Malorni, L.; Venezia, A.; Cammarota, M.; Volpe, M.; Iannaccone, N.; Guida, V.; Schiraldi, C.; Romano, M.; et al. In vitro intestinal epithelium responses to titanium dioxide nanoparticles. Food Res. Int. 2019, 119, 634–642. [Google Scholar] [CrossRef]
- Kolba, N.; Guo, Z.; Olivas, F.M.; Mahler, G.J.; Tako, E. Intra-amniotic administration (Gallus gallus) of TiO2, SiO2, and ZnO nanoparticles affect brush border membrane functionality and alters gut microflora populations. Food Chem. Toxicol. 2020, 135, 110896. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and me-ta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Limage, R.; Tako, E.; Kolba, N.; Guo, Z.; García-Rodríguez, A.; Marques, C.N.H.; Mahler, G.J. TiO2 nanoparticles and com-mensal bacteria alter mucus layer thickness and composition in a gastrointestinal tract model. Small 2020, 16, e2000601. [Google Scholar] [CrossRef]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.H.; Walker, V.K. Impact of food-grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef]
- Agans, R.T.; Gordon, A.; Hussain, S.; Paliy, O. Titanium dioxide nanoparticles elicit lower direct inhibitory effect on human gut microbiota than silver nanoparticles. Toxicol. Sci. 2019, 172, 411–416. [Google Scholar] [CrossRef]
- Dorier, M.; Béal, D.; Tisseyre, C.; Marie-Desvergne, C.; Dubosson, M.; Barreau, F.; Houdeau, E.; Herlin-Boime, N.; Rabilloud, T.; Carriere, M. The food additive E171 and titanium dioxide nanoparticles indirectly alter the homeostasis of human intestinal epithelial cells in vitro. Environ. Sci. Nano 2019, 6, 1549–1561. [Google Scholar] [CrossRef]
- Taylor, A.A.; Marcus, I.M.; Guysi, R.L.; Walker, S.L. metal oxide nanoparticles induce minimal phenotypic changes in a model colon gut microbiota. Environ. Eng. Sci. 2015, 32, 602–612. [Google Scholar] [CrossRef]
- Waller, T.; Chen, C.; Walker, S.L. Food and industrial grade titanium dioxide impacts gut microbiota. Environ. Eng. Sci. 2017, 34, 537–550. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, Y.; Zhao, X.; Sheng, L.; Wang, L. Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int. J. Nanomed. 2017, 12, 6197–6204. [Google Scholar] [CrossRef] [PubMed]
- Auguste, M.; Lasa, A.; Pallavicini, A.; Gualdi, S.; Vezzulli, L.; Canesi, L. Exposure to TiO2 nanoparticles induces shifts in the microbiota composition of Mytilus galloprovincialis hemolymph. Sci. Total. Environ. 2019, 670, 129–137. [Google Scholar] [CrossRef]
- Mercier-Bonin, M.; Despax, B.; Raynaud, P.; Houdeau, E.; Thomas, M. Mucus and microbiota as emerging players in gut nanotoxicology: The example of dietary silver and titanium dioxide nanoparticles. Crit. Rev. Food Sci. Nutr. 2018, 58, 1023–1032. [Google Scholar] [CrossRef]
- Han, S.; Chen, Z.; Zhou, D.; Zheng, P.; Zhang, J.; Jia, G. Effects of titanium dioxide nanoparticles on fecal metabolome in rats after oral administration for 90 days. J. Peking Univ. 2020, 52, 457–463. [Google Scholar]
- Chen, L.; Guo, Y.; Hu, C.; Lam, P.K.; Lam, J.C.; Zhou, B. Dysbiosis of gut microbiota by chronic coexposure to titanium dioxide nanoparticles and bisphenol A: Implications for host health in zebrafish. Environ. Pollut. 2018, 234, 307–317. [Google Scholar] [CrossRef]
- Cao, X.; Han, Y.; Gu, M.; Du, H.; Song, M.; Zhu, X.; Ma, G.; Pan, C.; Wang, W.; Zhao, E.; et al. Foodborne titanium dioxide nanoparticles induce stronger adverse effects in obese mice than non-obese mice: Gut microbiota dysbiosis, colonic inflammation, and proteome alterations. Small 2020, 16, e2001858. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zheng, P.; Zhou, D.; Zhou, S.; Jia, G. Effect of oral exposure to titanium dioxide nanoparticles on lipid me-tabolism in Sprague-Dawley rats. Nanoscale 2020, 12, 5973–5986. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, J.; Ma, L.; Li, N.; Liu, H.; Wang, J.; Zheng, L.; Liu, C.; Wang, X.; Zhao, X.; et al. Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials 2010, 31, 894–899. [Google Scholar] [CrossRef]
- Kurtz, C.C.; Mitchell, S.; Nielsen, K.; Crawford, K.D.; Mueller-Spitz, S.R. Acute high-dose titanium dioxide nanoparticle ex-posure alters gastrointestinal homeostasis in mice. J. Appl. Toxicol. 2020, 40, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, S.; Lei, R.; Gu, W.; Qin, Y.; Ma, S.; Chen, K.; Chang, Y.; Bai, X.; Xia, S.; et al. Oral administration of rutile and anatase TiO2 nanoparticles shifts mouse gut microbiota structure. Nanoscale 2018, 10, 7736–7745. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, F.; Lu, Z.; Fang, Y.; Qu, J.; Mao, T.; Wang, H.; Chen, J.; Li, B. Effects of TiO2 nanoparticles on intestinal microbial composition of silkworm, Bombyx mori. Sci. Total. Environ. 2020, 704, 135273. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Sun, L.; Zhong, Z.-T.; Zhu, J.; Song, H.-Y. Effects of titanium dioxide nanoparticles on intestinal commensal bacteria. Nucl. Sci. Tech. 2016, 27, 1–5. [Google Scholar] [CrossRef]
- Mao, Z.; Li, Y.; Dong, T.; Zhang, L.; Zhang, Y.; Li, S.; Hu, H.; Sun, C.; Xia, Y. Exposure to titanium dioxide nanoparticles during pregnancy changed maternal gut microbiota and increased blood glucose of rat. Nanoscale Res. Lett. 2019, 14, 26. [Google Scholar] [CrossRef]
- Mu, W.; Wang, Y.; Huang, C.; Fu, Y.; Li, J.; Wang, H.; Jia, X.; Ba, Q. Effect of Long-Term Intake of Dietary Titanium Dioxide Nanoparticles on Intestine Inflammation in Mice. J. Agric. Food Chem. 2019, 67, 9382–9389. [Google Scholar] [CrossRef] [PubMed]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the food additive titanium dioxide (E171) on gut microbiota-host interaction. Front. Nutr. 2019, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.W.; Shull, G.M.; Fountain, J.H.; Guo, Z.; Musselman, L.P.; Fiumera, A.C.; Mahler, G.J. Titanium dioxide nanoparticle exposure alters metabolic homeostasis in a cell culture model of the intestinal epithelium and Drosophila melanogaster. Nanotoxicology 2018, 12, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.A.; Morón, B.; Becker, H.M.; Lang, S.; Atrott, K.; Spalinger, M.R.; Scharl, M.; Wojtal, K.A.; Fischbeck-Terhalle, A.; Frey-Wagner, I.; et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: Role of the NLRP3 inflammasome. Gut 2017, 66, 1216–1224. [Google Scholar] [CrossRef]
- Talbot, P.; Radziwill-Bienkowska, J.M.; Kamphuis, J.B.J.; Steenkeste, K.; Bettini, S.; Robert, V.; Noordine, M.-L.; Mayeur, C.; Gaultier, E.; Langella, P.; et al. Food-grade TiO2 is trapped by intestinal mucus in vitro but does not impair mucin O-glycosylation and short-chain fatty acid synthesis in vivo: Implications for gut barrier protection. J. Nanobiotechnol. 2018, 16, 1–14. [Google Scholar] [CrossRef]
- Yan, J.; Wang, D.; Li, K.; Chen, Q.; Lai, W.; Tian, L.; Lin, B.; Tan, Y.; Liu, X.; Xi, Z. Toxic effects of the food additives titanium dioxide and silica on the murine intestinal tract: Mechanisms related to intestinal barrier dysfunction involved by gut micro-biota. Environ. Toxicol. Pharmacol. 2020, 80, 103485. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, X.; Cheng, S.; Fan, J.; Qin, X.; Wang, T.; Zhang, Y.; Zhang, J.; Qiu, Y.; Qiu, J.; et al. Titanium dioxide nanoparticles via oral exposure leads to adverse disturbance of gut microecology and locomotor activity in adult mice. Arch. Toxicol. 2020, 94, 1173–1190. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, L.; Liu, Z.; Zhou, Q.; Zhu, Y.; Zhao, Y.; Yang, X. Long-term exposure to titanium dioxide nanoparticles promotes diet-induced obesity through exacerbating intestinal mucus layer damage and microbiota dysbiosis. Nano Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of diet on the gut microbiota: Rethinking intervention duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef]
- Lamas, B.; Martins Breyner, N.; Houdeau, E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: Potential consequences for host health. Part. Fibre Toxicol. 2020, 17, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Rodríguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E.V. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015, 16, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Jakobsson, H.E.; Holmén-Larsson, J.; Schütte, A.; Ermund, A.; Rodríguez-Piñeiro, A.M.; Arike, L.; Wising, C.; Svensson, F.; Bäckhed, F.; et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 2015, 18, 582–592. [Google Scholar] [CrossRef]
- Becker, S.; Oelschlaeger, T.A.; Wullaert, A.; Vlantis, K.; Pasparakis, M.; Wehkamp, J.; Stange, E.F.; Gersemann, M. Bacteria regulate intestinal epithelial cell differentiation factors both in vitro and in vivo. PLoS ONE 2013, 8, e55620. [Google Scholar]
- Brun, E.; Barreau, F.; Veronesi, G.; Fayard, B.; Sorieul, S.; Chanéac, C.; Carapito, C.; Rabilloud, T.; Mabondzo, A.; Herlin-Boime, N.; et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part. Fibre Toxicol. 2014, 11, 13. [Google Scholar] [CrossRef]
- Coombes, J.L.; Maloy, K.J. Control of intestinal homeostasis by regulatory T cells and dendritic cells. Semin. Immunol. 2007, 19, 116–126. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Gasbarrini, A.; Mele, M.C. Food additives, gut microbiota, and irritable Bowel syndrome: A hidden track. Int. J. Environ. Res. Public Heal. 2020, 17, 8816. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-X.; Cheng, B.; Yang, Y.-X.; Cao, A.; Liu, J.-H.; Du, L.-J.; Liu, Y.; Zhao, Y.; Wang, H. Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum. Small 2013, 9, 1765–1774. [Google Scholar] [CrossRef]
- Rompelberg, C.; Heringa, M.B.; Van Donkersgoed, G.; Drijvers, J.; Roos, A.; Westenbrink, S.; Peters, R.; Van Bemmel, G.; Brand, W.; Oomen, A.G. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology 2016, 10, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Alvarez, A.-S.; De Vos, W.M. The gut microbiota in the first decade of life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Bachler, G.; Von Goetz, N.; Hungerbuhler, K. Using physiologically based pharmacokinetic (PBPK) modeling for dietary risk assessment of titanium dioxide (TiO2) nanoparticles. Nanotoxicology 2014, 9, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).