Value of Active Warming Devices for Intraoperative Hypothermia Prevention—A Meta-Analysis and Cost-Benefit Analysis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Incidences of Adverse Events with versus without IH
2.3. Treatments for Adverse Events and IH-Related Costs
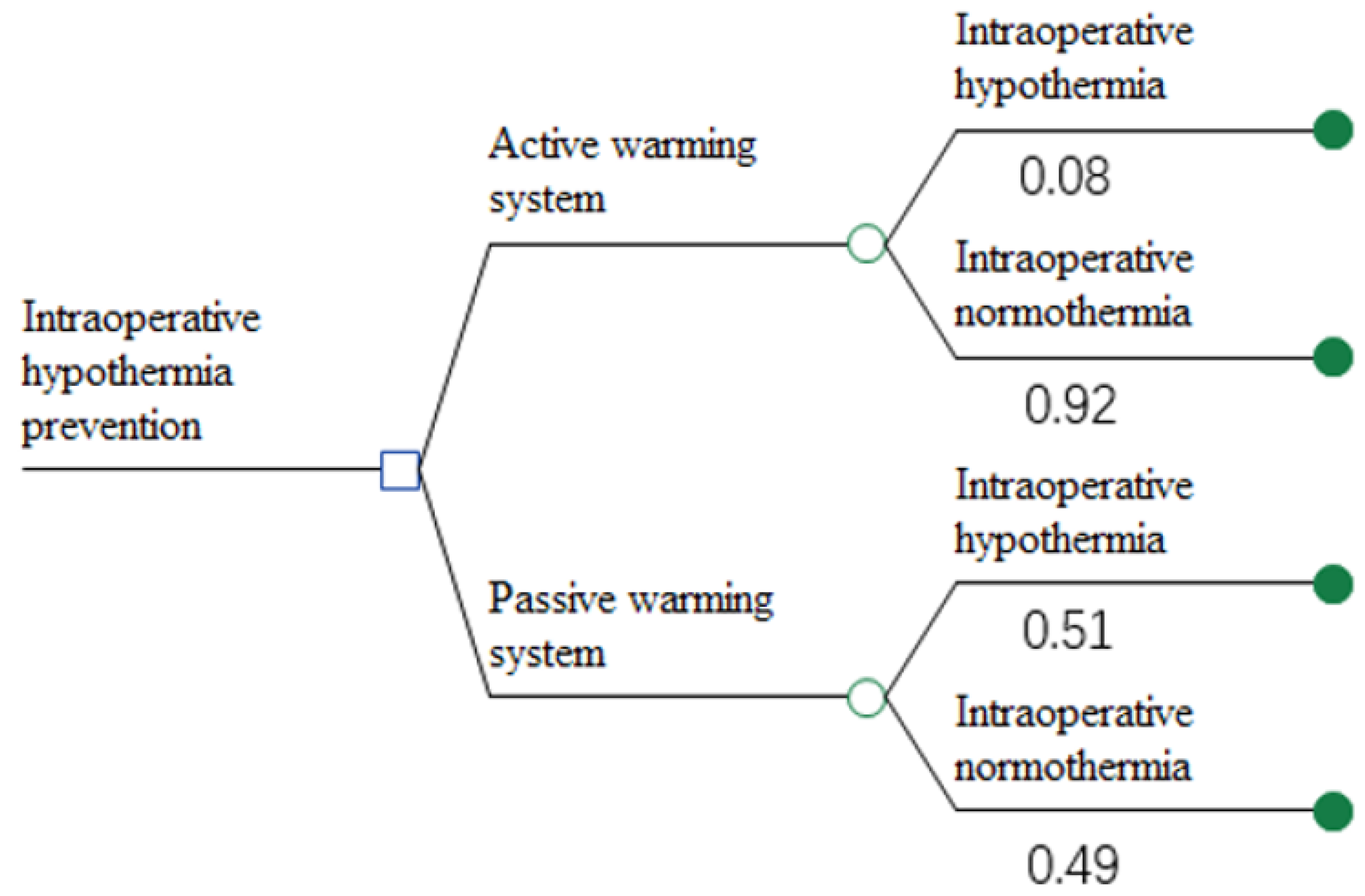
2.4. Cost-Benefit Analysis
2.5. Uncertainty Analyses
2.5.1. Deterministic Sensitivity Analysis
2.5.2. Probability Sensitivity Analysis
2.5.3. Scenario Analysis
3. Results
3.1. Treatments Costs of Individual Adverse Events
3.2. Total Cost for the Treatment of Intraoperative Hypothermia
3.3. Cost-Benefit Analysis of Active Warming Devices versus Passive Warming Devices for Intraoperative Hypothermia Prevention
3.3.1. Base-Case Analysis Result
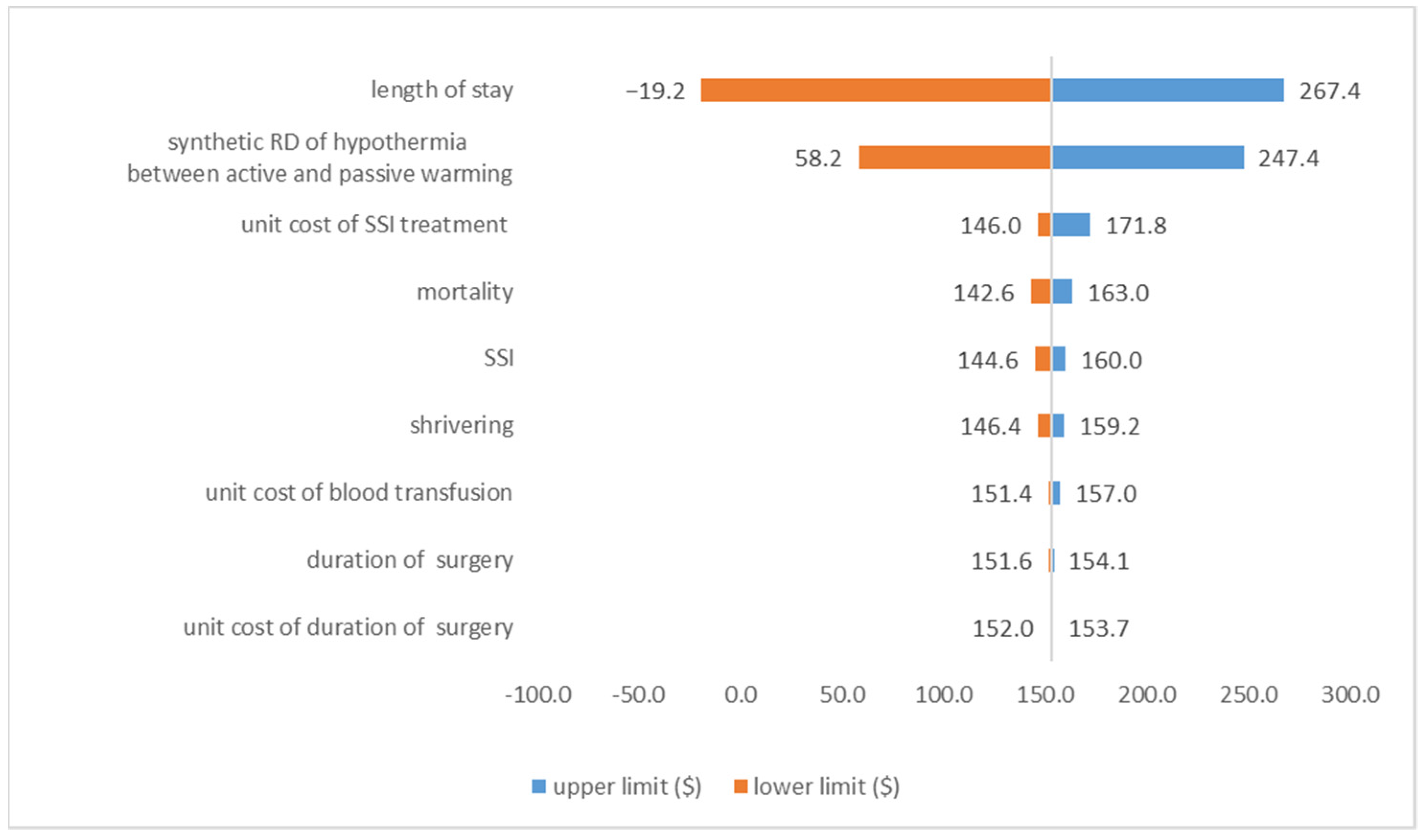
3.3.2. Deterministic Sensitivity Analysis Result
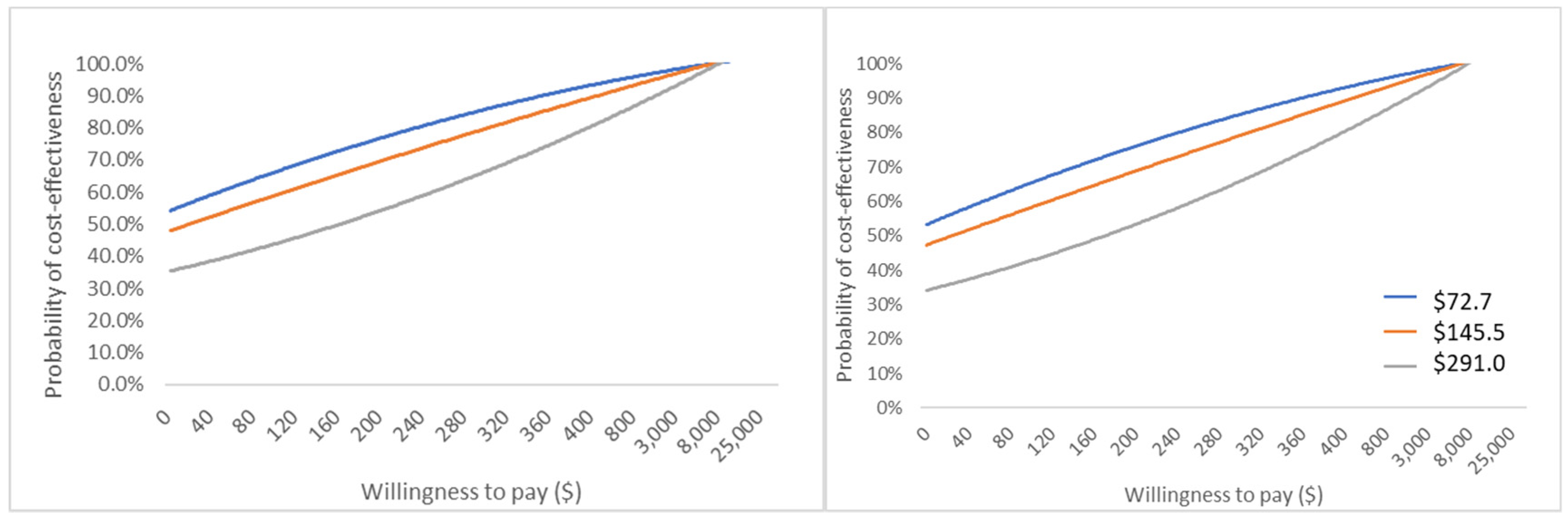
3.3.3. Probability Sensitivity Analysis Result
3.3.4. Scenario Analysis Result
4. Discussion
4.1. Lower Costs of Intraoperative Hypothermia than Expected
4.2. The Advantage of Performing CBA
4.3. The Complexity of Deciding the Value of Active Warming Devices for IH Prevention
4.4. Research Novelty and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBA | cost-benefit analysis |
| DSA | deterministic sensitivity analysis |
| FAW | forced-air warming |
| IH | intraoperative hypothermia |
| MD | mean difference |
| PSA | Probability sensitivity analysis |
| RD | risk difference |
| WTP | willingness-to-pay |
References
- Burns, S.M.; Piotrowski, K.; Caraffa, G.; Wojnakowski, M. Incidence of Postoperative Hypothermia and the Relationship to Clinical Variables. J. PeriAnesthesia Nurs. 2010, 25, 286–289. [Google Scholar] [CrossRef]
- Kurz, A.; Sessler, D.I.; Rainer, L. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 1996, 334, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Nathan, H.J.; Parlea, L.; Dupuis, J.; Hendry, P.; Williams, K.A.; Rubens, F.D.; Wells, A.G. Safety of deliberate intraoperative and postoperative hypothermia for patients undergoing coronary artery surgery: A randomized trial. J. Thorac. Cardiovasc. Surg. 2004, 127, 1270–1275. [Google Scholar] [CrossRef][Green Version]
- Frank, S.M.; Fleisher, L.A.; Breslow, M.J.; Higgins, M.S.; Olson, K.F.; Kelly, S.; Beattie, C. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 1997, 277, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Frisch, N.B.; Pepper, A.M.; Jildeh, T.R.; Shaw, J.; Guthrie, T.; Silverton, C. Intraoperative Hypothermia during Surgical Fixation of Hip Fractures. Orthopedics 2016, 39, e1170–e1177. [Google Scholar] [CrossRef] [PubMed]
- Jeyadoss, J.; Thiruvenkatarajan, V.; Watts, R.W.; Sullivan, T.; Van Wijk, R.M.A.W. Intraoperative hypothermia is associated with an increased intensive care unit length-of-stay in patients undergoing elective open abdominal aortic aneurysm surgery: A retrospective cohort study. Anaesth. Intensive Care 2013, 41, 759–764. [Google Scholar] [CrossRef]
- Billeter, A.T.; Hohmann, S.F.; Druen, D.; Cannon, R.; Polk, H.C. Unintentional perioperative hypothermia is associated with severe complications and high mortality in elective operations. Surgery 2014, 156, 1245–1252. [Google Scholar] [CrossRef]
- Mahoney, C.B.; Odom, F.J. Maintaining intraoperative normothermia: A meta-analysis of outcomes with costs. AANA J. 1999, 2, 155–164. [Google Scholar]
- Ma, Z.L.; Yi, J. Expert consensus on prevention and treatment of hypothermia in perioperative patients. Med. J. Peking Union Med Coll. Hosp. 2017, 8, 352–358. [Google Scholar]
- Oshvandi, K.; Shiri, F.H.; Fazel, M.R.; Safari, M.; Ravari, A. The effect of pre-warmed intravenous fluids on prevention of intraoperative hypothermia in cesarean section. Iran. J. Nurs. Midwifery Res. 2014, 19, 64–69. [Google Scholar] [PubMed]
- Adriani, M.B.; Moriber, N. Preoperative forced-air warming combined with intraoperative warming versus intraoperative warming alone in the prevention of hypothermia during gynecologic surgery. AANA J. 2013, 81, 446–451. [Google Scholar] [PubMed]
- Sikka, R.S.; Prielipp, R.C. Forced air warming devices in orthopaedics: A focused review of the literature. J. Bone Jt. Surg. Am. Vol. 2014, 96, e200. [Google Scholar] [CrossRef]
- Yoo, H.S.; Park, S.W.; Yi, J.W.; Kwon, M.I.; Rhee, Y.G. The effect of forced-air warming during arthroscopic shoulder surgery with general anesthesia. Arthroscopy 2009, 25, 510–514. [Google Scholar] [CrossRef]
- Insler, S.R.; Bakri, M.H.; Nageeb, F.; Mascha, E.; Mihaljevic, T.; Sessler, D.I. An evaluation of a full-access underbody forced-air warming system during near-normothermic, on-pump cardiac surgery. Anesth. Analg. 2008, 106, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Egan, C.; Bernstein, E.; Reddy, D.; Ali, M.; Paul, J.; Yang, D.; Sessler, D.I. A randomized comparison of intraoperative PerfecTemp and forced-air warming during open abdominal surgery. Anesth. Analg. 2011, 113, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.K.; Lai, A.; Wu, A. A randomised controlled trial of the electric heating pad vs forced-air warming for preventing hypothermia during laparotomy. Anaesthesia 2007, 62, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Luo, A.L.; Xu, L.; Huang, Y.G. Forced-air warming and fluid warming minimize core hypothermia during abdominal surgery. Chin. Med. Sci. J. 2005, 20, 261–264. [Google Scholar]
- Wong, A.; Walker, S.; Bradley, M. Comparison of a radiant patient warming device with forced air warming during laparoscopic cholecystectomy. Anaesth. Intensive Care 2004, 32, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Torrie, J.J.; Yip, P.; Robinson, E. Comparison of forced-air warming and radiant heating during transurethral prostatic resection under spinal anaesthesia. Anaesth. Intensive Care 2005, 33, 733–738. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Guan, X.; Lu, Y.; Malone, D.C.; Salmon, J.W.; Ma, A.X.; Tang, W.X. Safety of intraoperative hypothermia for patients: Meta-analyses of randomized controlled trials and observational studies. BMC Anesthesiol. 2020, 20, 202. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.H. Clinical and Basic Researches of Intraoperative Hypothermia: Dalian Medical University. BMC Anesthesiol. 2010, 20, 202. [Google Scholar]
- Zhang, J.; Jing, C. Outcomes of two temperature maintenance strategies during radical resection for carcinoma of oesophagus and their effects on postoperative shivering. J. Shanghai Jiaotong Univ. 2009, 6, 712–715. [Google Scholar]
- Todd, M.M.; Hindman, B.J.; Clarke, W.R.; Torner, J.C.; Weeks, J.B.; Bayman, E.O.; Shi, Q.; Spofford, C.M.; IHAST Investigator. Perioperative fever and outcome in surgical patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2009, 64, 897–908. [Google Scholar] [CrossRef]
- Lenhardt, R.; Marker, E.; Goll, V.; Tschernich, H.; Kurz, A.; Sessler, D.I.; Narzt, E.; Lackner, F. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology 1997, 87, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Schmied, H.; Kurz, A.; Sessler, D.I.; Kozek, S.; Reiter, A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet 1996, 347, 289–292. [Google Scholar] [CrossRef]
- Kurz, A.; Sessler, D.I.; Narzt, E.; Bekar, A.; Lenhardt, R.; Huemer, G.; Lackner, F. Postoperative hemodynamic and thermoregulatory consequences of intraoperative core hypothermia. J. Clin. Anesth. 1995, 7, 359–366. [Google Scholar] [CrossRef]
- Yi, J.; Lei, Y.; Xu, S.; Si, Y.; Li, S.; Xia, Z.; Shi, Y.; Gu, X.; Yu, J.; Xu, G.; et al. Intraoperative hypothermia and its clinical outcomes in patients undergoing general anesthesia: National study in China. PLoS ONE 2017, 12, e177221. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Y.J.; Guan, X.; Ma, Y.; Ma, A.X.; Tang, W.X. Efficacy and Safety of Forced-Air Warming System versus Passive Warming Measures in Major Surgeries: A Systematic Review. J. Anesth. Clin. Care 2021, 8, 65. [Google Scholar]
- Lv, C.X.; Zou, S.H.; Wang, Y.; Zhou, L.X. Study on the effect of nursing process reengineering in emergency room. J. Tradit. Chin. Med. Manag. 2017, 25, 116–117. [Google Scholar]
- Commission, N.H. Guiding Principles for Clinical Application of Antimicrobial Agents (2015 Edition). Available online: http://www.nhc.gov.cn/ewebeditor/uploadfile/2015/09/20150928170007470.pdf:2015. (accessed on 6 October 2021).
- Leticia, M.; Forns, J.R.; Espin, J. Cost transferability problems in economic evaluation as a framework for an European health care and social costs database. Cost Eff. Resour. Alloc. 2021, 19, 1478–7547. [Google Scholar]
- Yamada, K.; Nakajima, K.; Nakamoto, H.; Kohata, K.; Shinozaki, T.; Oka, H.; Yamakawa, K.; Matsumoto, T.; Tokimura, F.; Kanai, H.; et al. Association between Normothermia at the End of Surgery and Postoperative Complications following Orthopaedic Surgery. Clin. Infect. Dis. 2020, 70, 474–482. [Google Scholar] [PubMed]
- Wang, G.M.; Zhang, K.G.; Li, X.J.; Jiang, M.J.; Zhang, L. Evaluation of direct economic losses due to surgical site infections in patients of neurosurgery department. Chin. J. Nosocomiol. 2015, 25, 2542–2544. [Google Scholar]
- Yi, J.; Xiang, Z.; Deng, X.; Fan, T.; Fu, R.; Geng, W.; Guo, R.; He, N.; Li, C.; Li, L.; et al. Incidence of Inadvertent Intraoperative Hypothermia and Its Risk Factors in Patients Undergoing General Anesthesia in Beijing: A Prospective Regional Survey. PLoS ONE 2015, 10, e136136. [Google Scholar] [CrossRef] [PubMed]
- Sari, S.; Aksoy, S.M.; But, A. The incidence of inadvertent perioperative hypothermia in patients undergoing general anesthesia and an examination of risk factors. Int. J. Clin. Pract. 2021, 2, e14103. [Google Scholar]
- Ahmet, Y.; Gamze, T.; Cigdem, U.K.; Cevdet, Y. Perioperative temperature monitoring in general and neuraxial anesthesia: A survey study. Ain-Shams J. Anesthesiol. 2020, 12, 12. [Google Scholar]
| Treatment | Unit Cost Median (min, max) | Aggregate Unit Cost Per Case (min, max) |
|---|---|---|
| Surgical site infection | 243.9 (128.0, 566.9) | |
| Debridement and suturing | 15.6 (9.3, 34.4) | |
| Daily dressing change | 3.1 (1.9, 6.1) | |
| Antibiotic administration (sulbactam and ampicillin, four times daily for 7 days [30]) | 19.8 (8.3, 184.2) | |
| intravenous fluid administration | 1.1 (0.5, 2.2) | |
| Drug susceptibility test | 7.4 *,a | |
| Blood culture& pathogen identification | 44.6 *,b | |
| Interleukin-6 analysis (three times) | 6.5 (3.2, 11.8) | |
| Procalcitonin analysis (three times) | 24.1 (6.4, 45.6) | |
| Complete blood count test (three times) | 3.3 (0.2, 4.5) | |
| Erythrocyte sedimentation rate analysis (three times) | 1.2 (0.6, 1.9) | |
| Blood transfusion | 40.4 (37.1, 50.4) | |
| Cross-matching | 0.8 (0.5, 1.9) | |
| Blood (/200 mL) (whole blood transfusions) | 38.0 (35.3, 45.0) | |
| Blood storage(/bag) | 1.6 (1.3, 3.5) | |
| Chill/Shivering | 58.8 (58.8, 58.8) | |
| Tramadol 50 mg | 14.3 *,c | |
| Blood culture | 44.6 *,d | |
| Hospital stay | 136.5 (136.5, 136.5) | |
| Bed use(/day) | 136.5 * | |
| Surgical duration | 16.0 (2.5, 27.3) | |
| Monitoring during anesthesia(/h) | 16.0 (2.5, 27.3) | |
| Death | 1209.1 (997.6, 2192.4) | |
| Resuscitative care [29] | 1209.1 (997.6, 2192.4) |
| Adverse Event | Surgical Site Infection | Intraoperative Blood Loss | Intra/Postoperative Chill | Duration of Surgery | Hospital Stay | Mortality |
|---|---|---|---|---|---|---|
| Difference of adverse events | RD = 0.14 95% CI (0.06, 0.21) | MD = 131.90 95% CI (117.42, 146.38) | RD = 0.32 95% CI (0.06, 0.58) | MD = −0.16 95% CI (−0.34, 0.03) | MD = 1.40 95% CI (−0.35, 3.14) | RD = 0.00 95% CI (−0.02, 0.02) |
| Cost ($) | 243.9 (128.0, 566.9) | 40.4 (37.1, 50.4) | 58.8 (58.8, 58.8) | 16.0 (2.5, 27.3) | 136.5 (136.5, 136.5) | 1209.1 (997.6, 2192.4) |
| △Cost active-passive | WTP | ||||||
|---|---|---|---|---|---|---|---|
| $0.0 | $20.0 | $90.0 | $150.0 | $230.0 | $900.0 | $2000.0 | |
| $72.2 (¥500) | 56.2% | 57.6% | 64.7% | 71.9% | 80.0% | 99.9% | 100.0% |
| $145.5 (¥1000) | 49.8% | 51.6% | 57.5% | 62.9% | 72.5% | 99.5% | 100.0% |
| $290.1 (¥2000) | 34.3% | 36.7% | 44.2% | 50.0% | 57.2% | 98.4% | 100.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Wang, Z.; Lu, Y.; Guan, X.; Ma, Y.; Malone, D.C.; Salmon, J.W.; Ma, A.; Tang, W. Value of Active Warming Devices for Intraoperative Hypothermia Prevention—A Meta-Analysis and Cost-Benefit Analysis. Int. J. Environ. Res. Public Health 2021, 18, 11360. https://doi.org/10.3390/ijerph182111360
Xu H, Wang Z, Lu Y, Guan X, Ma Y, Malone DC, Salmon JW, Ma A, Tang W. Value of Active Warming Devices for Intraoperative Hypothermia Prevention—A Meta-Analysis and Cost-Benefit Analysis. International Journal of Environmental Research and Public Health. 2021; 18(21):11360. https://doi.org/10.3390/ijerph182111360
Chicago/Turabian StyleXu, He, Zijing Wang, Yijuan Lu, Xin Guan, Yue Ma, Daniel C. Malone, Jack Warren Salmon, Aixia Ma, and Wenxi Tang. 2021. "Value of Active Warming Devices for Intraoperative Hypothermia Prevention—A Meta-Analysis and Cost-Benefit Analysis" International Journal of Environmental Research and Public Health 18, no. 21: 11360. https://doi.org/10.3390/ijerph182111360
APA StyleXu, H., Wang, Z., Lu, Y., Guan, X., Ma, Y., Malone, D. C., Salmon, J. W., Ma, A., & Tang, W. (2021). Value of Active Warming Devices for Intraoperative Hypothermia Prevention—A Meta-Analysis and Cost-Benefit Analysis. International Journal of Environmental Research and Public Health, 18(21), 11360. https://doi.org/10.3390/ijerph182111360






