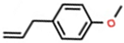
Abstract
The overuse of synthetic pesticides in plant protection strategies has resulted in numerous side effects, including environmental contamination, food staff residues, and a threat to non-target organisms. Several studies have been performed to assess the pesticidal effects of plant-derived essential oils and their components, as partially safe and effective agents, on economically important pests. The essential oils isolated from Satureja species are being used in medicinal, cosmetic, and food industries. Their great potential in pest management is promising, which is related to high amounts of terpenes presented in this genus. This review is focused on the acute and chronic acaricidal, insecticidal, and nematicidal effects of Satureja essential oil and their main components. The effects of eighteen Satureja species are documented, considering lethality, repellency, developmental inhibitory, and adverse effects on the feeding, life cycle, oviposition, and egg hatching. Further, the biochemical impairment, including impairments in esterases, acetylcholinesterase, and cytochrome P450 monooxygenases functions, are also considered. Finally, encapsulation and emulsification methods, based on controlled-release techniques, are suggested to overcome the low persistence and water solubility restrictions of these biopesticides. The present review offers Satureja essential oils and their major components as valuable alternatives to synthetic pesticides in the future of pest management.
1. Introduction
Although synthetic chemicals have been considered as the pest management strategy so far, their overuse has led to several side effects. These include soil and groundwater pollution, toxic residues on the food stuffs, pest resistance, outbreak of secondary pests, and harmful effects on non-target organisms such as fish, bees, predators, and parasites [1,2,3,4].
The plant essential oils as low-risk agents are recommended alternatives to chemical pesticides [5,6]. Essential oils are complex mixtures of aromatic and aliphatic compounds, which mainly consist of hydrocarbon monoterpenes, monoterpenoids, hydrocarbon sesquiterpenes, and sesquiterpenoids, and can be made by all plant parts, such as flowers, seeds, leaves, stems, and bark [7]. Essential oils are composed by plants as secondary metabolites with anti-herbivore activity, resulted in critical defense strategies against herbivorous pests along with other significant roles, such as allelopathic plant–plant interactions and attraction of pollinators [8]. Hence, the possibilities of pest resistance to plant-derived essential oils is very low [9]. Along with multiple modes of action and efficiency against a wide range of arthropod pests, essential oils also exhibit comparative lower toxicity on non-target organisms, such as mammals and beneficial insects compared to chemicals [10]. Additionally, with about 24–48 h half-lives, they are degraded quickly by natural degradation mechanisms and considered as biodegradable agents [9]. The pesticidal effects of essential oils isolated from several species of plant families, such as Lamiaceae, Asteraceae, Myrtaceae, Apiaceae, Cupressacae, and Rutaceae, against diverse groups of agricultural pests have been well-endorsed in recent years [11,12,13]. Along with the toxicity of plant essential oils to arthropod pests, there are promising findings against pathogenic nematodes [14,15].
The genus Satureja belongs to the Lamiaceae family, Nepetoidae subfamily, and the Mentheae tribe, that includes about 200 species of aromatic herbs and shrubs. They are broadly distributed in America, the Mediterranean area, Middle East, North Africa, and West Asia [16]. Several species from this genus, conventionally known as savory, especially summer savory (Satureja hortensis L.), are cultivated in various countries [17]. These aromatic plants possess a high content of essential oil (even about 4%) located in their leaves, stems, and flowers [18]. Numerous medicinal properties, including reduction of blood pressure, joint pains, rheumatic pains, stomachache, toothache, fever, diarrhea, dyspepsia, gastrointestinal bloating, influenza, colds, scabies and itching, eye strengthening, antioxidant, antidiabetic, and antimicrobial properties, of Satureja species, especially their extracted essential oils, are well-documented in the literature [16,19,20,21].
The present review aimed to update the current knowledge on the essential oils extracted from different Satureja species in controlling economically damaging insects, mites, ticks, and nematodes. Thus, vast amounts of individual research have been gathered from scientific databases, including Scopus, Web of Science, PubMed, and Google Scholar. Our main aim was to introduce a novel, safe, and efficient bio-rational agent(s), as alternatives to the detrimental chemicals. The search also considers the sub-lethal and biochemical changes after application of these compounds in order to obtain a thorough insight into their mode of action.
2. Pesticidal Effects of Essential Oils Extracted from Various Satureja Species
The great potential of several species from the Satureja genus, including S. aintabensis Davis, S. bachtiarica Bung, S. cilicica Davis, S. cuneifolia Ten, S. hellenica Halásky, S. hortensis L., S. intermedia C. A. Mey, S. isophylla L., S. khuzestanica Jamzad, S. montana L., S. parnassica Heldr & Sart ex Boiss, S. parvifolia (Phil) Epling, S. rechingeri Jamzad, S. sahendica Bornm, S. spicigera Boiss, S. spinosa L., S. thymbra L., and S. wiedemanniana (Avé-Lall) Velen, has been reported in the insects, mites, ticks, and nematodes’ management. As shown in Table 1, the efficiency of Satureja essential oils was assessed against a diverse group of insects from Coleoptera to Diptera, Hemiptera, Homoptera, Lepidoptera, Phthiraptera, and Thysanoptera orders, and similarly, on other arthropods, including mites and ticks, and plant pathogenic nematodes.
The pesticidal effects of Satureja essential oils can be considered from two viewpoints, i.e., lethal and sub-lethal. For example, along with acute fumigant toxicity of S. thymbra essential oil against the adults of Acanthoscelides obtectus, Ephestia. kuehniella, and Leptinotarsa decemlineata, its repellent effect on Aedes albopictus was also reported [22,23,24]. In general, there are several sub-lethal bio-efficiencies of Satureja essential oils, including repellent and antifeedant activities and adverse effects on fecundity, fertility, and life cycle. Some of these studies have also considered the biochemical mode of action in pests such as general esterase, acetylcholinesterase, and cytochrome P450 monooxygenases [25,26,27]. The studies include different developmental stages of pests, from eggs to larvae, pupae, and adults. Among the large species of Satureja studied, the essential oils of S. hortensis, S. montana, and S. thymbra are considered as the most promising in pest management (Table 1). Another prospective is the possibility of using Satureja essential oil along with other pest control agents, such as entomopathogenic fungi. For example, Hosseinzadeh et al. [28] indicated that the essential oil of S. sahendica had a significant synergistic effect with entomopathogenic fungus Beauveria bassiana against the cowpea weevil, Callosobruchus maculatus (Fabricius).

Table 1.
Reported acaricidal, insecticidal, and nematicidal effects of the essential oils isolated from different Satureja species.
Table 1.
Reported acaricidal, insecticidal, and nematicidal effects of the essential oils isolated from different Satureja species.
| Pests | Satureja Species | Bioassay and Target Pest | Efficiency |
|---|---|---|---|
| Insects | S. aintabensis Davis | Contact assay (on treated filter papers) against the adult females of the turnip aphid (Lipaphis pseudobrassicae (Davis)). | Significant toxicity with LC50 (lethal concentration to kill 50% of tested insects) of 1.7 mg/mL after 1 h [29]. |
| S. bachtiarica Bung | Aqueous suspension of essential oil against the third- and fourth-instar larvae of the Asian malaria mosquito (Anopheles stephensi) and filariasis vector (Culex quinquefasciatus Say). | The larval mortality of 100% at the concentration of 160 ppm after 24 h [30]. | |
| Fumigant and repellency assays (by impregnated filter papers in glass vials and Petri dishes, respectively) against the adults of red flour beetle (Tribolium castaneum (Herbst)). | Significant fumigant toxicity (LC50 = 4.71 mg/L) and repellent action (100% at the concentration of 1% v/v after 8 h) [31]. | ||
| Fumigant assay (by impregnated filter papers) against the fourth-instar larvae of tomato leafminer (Tuta absoluta (Meyrick)) | Significant fumigant toxicity (LC50 = 25.03 µL/L) and reduction in activity of general esterases (α and β) (p < 0.05) [25]. | ||
| S. cilicica Davis | Contact assay (on treated filter papers) against the Colorado potato beetle (Leptinotarsa decemlineata Say). | High mortality of the first (97.7%), second (95.5%), third (91.1%), and fourth (97.7%) instar larvae and the adults (84.4%) at 20 µL/cm2 after 96 h [24]. | |
| S. cuneifolia Ten | Fumigant assay (by impregnated filter papers) on field-collected sand flies (Diptera: Psychodidae: Phlebotomie). | The knockdown rate of 100% at the concentration of 20.0 µL/L after 0.5 h [32]. | |
| Contact assay (on treated filter papers) against L. decemlineata. | High mortality of the first (93.3%), second (91.1%), third (95.5%), and fourth (88.8%) instar larvae and the adults (86.6%) at 20 µL/cm2 after 96 h [24]. | ||
| S. hortensis L. | Aqueous suspension of essential oil against the larvae of the C. quinquefasciatus. | Significant toxicity (LC50 = 36.0 μg/mL), the reduction in the adult emergence by a quarter of the control (p < 0.05), and 100% oviposition deterrence by the concentration of 200 ppm [33]. | |
| Fumigant assay (by impregnated filter papers) against the adults of bean weevils (Bruchus dentipes (Baudi)). | The mortality of 100% at the concentration of 20.0 µL/L after 24 h [34] | ||
| Fumigant assay (by impregnated filter papers) against the cotton whitefly (Bemisia tabaci) on the eggplant leaves. | The 100% mortality of adult females at 2.4 mL/cm3 of essential oil after 24 h [35]. | ||
| Fumigant assay (by impregnated filter papers) against the adults of B. tabaci on cucumber leaves. | The mortality of 100% at 2 µL/L of essential oil after 12 h [36]. | ||
| Contact assay (on treated filter papers) against the adults of C. maculatus. | Toxic to the adults with LC50 values of 5.36 and 6.41 µL/cm2 on the males and females, respectively [37]. | ||
| Fumigant assay (by impregnated filter papers) against the adults of C. maculatus. | The 91.2% adult mortality at 60 mL/L and the 94.5% egg mortality at 4.3 mL/L of essential oil after 24 h [38]. | ||
| Fumigant assay (by impregnated filter papers) against the adults of maize weevil (Sitophilus zeamais Motschulsky). | The 100% mortality at the concertation of 10 µL/L after 96 h exposure time [39]. | ||
| Leaf dipping method against the larvae of mulberry pyralid (Glyphodes pyloalis Walker) | Significant feeding inhibition (44.35% at the concentration of 0.025%), decrease in the amount of protein, lipid, carbohydrates, and the activity of α-amylase, esterase, and glutathione S-transferase (p < 0.05) [40]. | ||
| Antifeedant assay (by treated flour disk) on first-instar larvae of the Indian meal moth (Plodia interpunctella Hübner). | Significant reduction in the relative growth (0.01 mg/day) and consumption (0.31 mg/day) rates of larvae treated by 0.22 µL/cm2 of essential oil compared to control (0.05 and 0.10 mg/day, respectively) (p < 0.05) [41]. | ||
| In-vivo repellent assay (by counting the number of bites on the back of rabbits) against the adult females of A. stephensi. | A protection time of 4.16 h at ED50 (effective dose) of 5.63 mg/cm2 [42]. | ||
| Contact assay (by direct spraying) on the larvae of the American White Butterfly (Hypantria cunea Drury). | The 68.8% mortality of third- and fourth-instars larvae at 1.67 µL/cm2 after 96 h [43] | ||
| Spraying on black chokeberry inflorescences ingested by the larvae of grey Knot-horn (Acrobasis advenella (Zinck)). | Significant reduction in the amount of α- and β-glucosidase of treated larvae and the emergence and longevity of adults [17]. | ||
| Fumigant assay (by impregnated filter papers) on the third-instar larvae of Mediterranean flour moth (Ephestia kuehniella Zeller). | A mortality of 88.3% at 60 µL/L after 24 h (LC50 = 30.09 µL/L) [44]. | ||
| Oviposition deterrence and feeding-site assays (by choice test with treated black chokeberry infructescences) on A. advenella. | Significant reduction in laid eggs (3.89%) and feeding site of larvae (27.35%) compared to control groups (17.15% and 4.69%, respectively) [45]. | ||
| Fumigant assay (by impregnated filter papers) against the adults of lesser grain borer (Rhyzopertha dominica (Fabricius)) and T. castaneum. | Significant toxicity against both insects with LC50 values of 16.47 and 25.75 µL/L after 72 h, respectively [46]. | ||
| S. intermedia C. A. Mey | Fumigant assay (by impregnated filter papers) against the adults of saw-toothed beetle (Oryzaephilus surinamensis (L.)), R. dominica, the khapra beetle (Trogoderma granarium Everts), and T. castaneum, and contact assay (leaf dipping method) on the adult female of the oleander aphid (Aphis nerii). | High fumigant and contact toxicity against all pests with LC50 values of 8.15, 12.83, 2.49, and 35.61 µL/L, and 418.38 µg/mL, respectively [47]. | |
| S. isophylla L. | Fumigant assay (by impregnated filter papers) against cabbage aphid (Brevicoryne brassica L.) and black bean aphid (Aphis fabae Scop) on acacia leaves. | Significant fumigant toxicity against both insects with LC50 values of 7.33 and 14.29 µL/L, respectively [48]. | |
| Fumigant assay (by impregnated filter papers) against A. fabae on acacia leaf. | Significant fumigant toxicity against adult females (LC50 = 14.29 µL/L) and nymph production detergency at 8.53 µL/L (p < 0.05) [49]. | ||
| Fumigant assay (by impregnated filter papers) against the adults of R. dominica and T. castaneum. | High mortality of R. dominica (98.7%) and T. castaneum (90.0%) at 35.3 and 55.0 µL/L concentrations respectively, after 72 h [50]. | ||
| S. khuzestanica Jamzad | In vivo mosquito repellents assay for human skin (from elbow to wrist) against the adults of A. stephensi. | Significant reduction in the number of mosquito bites compared to the control group (p < 0.01) [51]. | |
| Toxicity assay (by impregnated potato leaves in Petri dishes) on the adults of L. decemlineata. | Significant mortality of the fourth-instar larvae and adults with LC50 values of 23.36 and 167.96 ppm, respectively [52]. | ||
| Fumigant and repellent assays (by impregnated filter papers in glass vials and Petri dishes, respectively) against the adults of T. castaneum. | Significant fumigant toxicity (LC50 = 2.51 mg/L) and repellent action (100% at the concentration of 1% v/v after 8 h) [31]. | ||
| Fumigant assay (by impregnated filter papers) against the fourth-instar larvae of T. absoluta. | Significant fumigant toxicity (LC50 = 17.51 µL/L) and reduction in activity of general esterases (α and β) (p < 0.05) [25]. | ||
| S. montana L. | Aqueous suspension of essential oil on the fourth-instar larvae of common house mosquito (Culex pipiens L.). | Significant larvicidal activity with LC50 value of 37.70 mg/L [53]. | |
| Repellent assay (by treated green bean leaves in Petri dishes) on the Western flower thrips (Frankliniella occidentalis). | A complete repellency (100%) at the concentration of 2.0% after 1 h [54]. | ||
| Contact assay (topical application) against the fruit fly (Drosophila suzukii (Matsumura)). | Significant toxicity with LC50 values of 2.95 and 4.59 µg/fly on the male and female adults, respectively [26]. | ||
| Aqueous suspension of essential oil against the third-instar larvae of C. quinquefasciatus | High larvicidal effectiveness with LC50 value of 25.6 μL/L [55]. | ||
| Contact assay (on treated filter papers) against L. decemlineata. | High mortality of the first (100%), second (97.7%), third (95.5%), and fourth (97.7%) instar larvae and the adults (88.8%) at the concentration of 20 µL/cm2 after 96 h [24]. | ||
| S. parnassica Heldr & Sart ex Boiss | Aqueous suspension of essential oil on the fourth-instar larvae C. pipiens. | Significant larvicidal activity with LC50 value of 37.70 mg/L [53]. | |
| S. parvifolia (Phil.) Epling | Fumigant assay (by impregnated filter papers) on the adult-females of the head louse (Pediculus humanus capitis De Geer). | Significantly toxic with KT50 value (time to 50% knockdown) of 36.06 min at 60 µL of essential oil concentration [56]. | |
| Repellent assay (by treated filter papers in Petri dishes) against the nymphs of kissing bug (Triatoma infestans Klug). | The repellency of 100% and 76.0% at the concentration of 0.5% (w/v) after 1 and 24 h [57]. | ||
| S. rechingeri Jamzad | Fumigant and repellency assays (by impregnated filter papers in glass vials and Petri dishes, respectively) against the adults of T. castaneum. | Significant fumigant toxicity (LC50 = 3.27 mg/L) and repellent action (100% at the concentration of 1% v/v) after 8 h [31]. | |
| Fumigant assay (by impregnated filter papers) against the fourth-instar larvae of T. absoluta. | Significant fumigant toxicity (LC50 = 34.33 µL/L) and reduction in activity of general esterases (α and β) (p < 0.05) [25]. | ||
| S. sahendica Bornm | Fumigant assay (by impregnated filter papers) against the adults of C. maculatus. | Significant toxicity with LC50 value of 22.42 µL/L [28]. | |
| S. spicigera Boiss | Fumigant assay (by impregnated filter papers) against the adults of granary weevil (Sitophilus granarius (L.)). | The 94.27% mortality at the concentration of 20.0 µL/L after 86 h [58]. | |
| Fumigant assay (by impregnated filter papers) against S. zeamais. | The mortality of 100% at concertation of 10 µL/L after 96 h exposure time [39]. | ||
| Contact assay (on treated filter papers) against L. decemlineata. | High mortality of the first (100%), second (100%), third (95.5%), and fourth (95.5%) instar larvae and the adults (80.0%) at 20 µL/cm2 after 96 h [24]. | ||
| S. spinosa L. | Aqueous suspension of essential oil on the fourth-instar larvae C. pipiens. | Significant larvicidal toxicity with LC50 value of 37.70 mg/L [53]. | |
| S. thymbra L. | Aqueous suspension of essential oil on the fourth-instar larvae C. pipiens. | Significant larvicidal toxicity with LC50 value of 37.70 mg/L [53]. | |
| Fumigant assay (by impregnated filter papers) against E. kuehniella and P. interpunctella. | The 100% egg mortality of E. kuehniella and P. interpunctella at 200 μL/L after 96 h [59]. | ||
| Fumigant assay (by impregnated filter papers) against the adults of E. kuehniella, P. interpunctella, and bean weevil (Acanthoscelides obtectus Say). | The 100% mortality of E. kuehniella, P. interpunctella (at 9 and 25 µL/L respectively, after 24 h), and A. obtectus (195 µL/L after 144 h) [22]. | ||
| Fumigant assay (by impregnated filter papers) against E. kuehniella. | Significant adulticidal toxicity (LC50 = 13.92 µL/L after 12 h) and reduction in the larval and adult emergence and egg production compared to control groups (p < 0.05) [60]. | ||
| Fumigant (by impregnated filter papers on the adults) and aqueous suspension (on the larvae) assays on African malaria mosquito (Anopheles gambiae Giles). | The 100% mortality of adults and larvae at 32.2 µg/mL and 3 mg/mL of essential oil respectively, after 24 h [61]. | ||
| Spraying on grape leaves against the nymphs and female adults of the vine mealybug (Planococcus ficus (Signoret)). | Significant mortality on nymphs (LC50 = 2.7 mg/mL) and adults (LC50 = 6.3 mg/mL) after 24 h [62]. | ||
| In vivo larvicidal assay in basins against the larvae of dengue vector (Aedes albopictus Skuse). | Significant larval mortality (96.00% at 29 mg/L of the essential oil) after 24 h [23]. | ||
| Contact assay (on treated filter papers) against L. decemlineata. | High mortality of the first (100.0%), second (95.5%), third (97.7%), and fourth (95.5%) instar larvae and the adults (97.7%) at 20 µL/cm2 after 96 h [24]. | ||
| S. wiedemanniana (Avé-Lall) Velen | Contact toxicity (on treated filter papers) against the adult females L. pseudobrassicae. | Significant toxicity with LC50 of 1.0 mg/mL after 1 h [29]. | |
| Mites and Ticks | S. bachtiarica | Fumigant (by impregnated filter papers) and repellency assays (by treated leaf discs) against the two-spotted spider mite (Tetranychus urticae Koch) in Petri dishes. | Significant fumigant toxicity (LC50 = 44.06 µL/L) and high repellent action at 44.06 µL/L after 24 h [27]. |
| S. hortensis | Fumigant assay (by impregnated filter papers) against T. urticae on fresh leaves of bean. | The 96.6% mortality of nymphs and adults of T. urticae at concentration of 3.13 µL/L after 96 h [63]. | |
| Fumigant (by impregnated filter papers) and contact (leaf dipping method) assays on the adults of T. urticae. | Significant fumigant and contact toxicity with LC50 values of 7.074 μL/L and 0.876% (v/v), respectively [64]. | ||
| Fumigant assays (by impregnated filter papers) against T. urticae on bean leaves. | Significant toxicity against the adults and eggs with 24 h LC50 values of 1.44 and 1.31 µL/L [65]. | ||
| S. khuzestanica | Fumigant (by impregnated filter papers) and repellency assays (by treated leaf discs) against T. urticae in Petri dishes. | Significant fumigant toxicity (LC50 = 31.11 µL/L) and high repellent action at 18.85 µL/L after 24 h [27]. | |
| S. sahendica | Fumigant assay (by impregnated filter papers) against T. urticae on bean leaf discs. | Significant adulticidal (24 h LC50 = 0.98 µL/L) and ovicidal (72 h LC50 = 0.54 µL/L) toxicity [66]. | |
| S. thymbra | Fumigant assay (by treated cotton wick) on the adults of the Mediterranean tick (Hyalomma marginatum). | The complete mortality (100%) at 40.0 µL/L within 3 h [67]. | |
| Nematodes | S. hellenica Halácsy | Immersion of the cotton root-knot nematode (Meloidogyne incognita (Kofold & White)) and the root-knot nematode (Meloidogyne javanica (Treub)) in aqueous suspension of essential oil. | The 100% paralysis of the second-stage juveniles (J2) of both species at the concentration of 2000 µL/L after 96 h [68]. |
| S. montana | Immersion of the mixed stages of pine wood nematode (Bursaphelenchus xylophilus Nickle) in aqueous suspension of essential oil. | The 100% mortality of nematodes exposed to a 2 mg/mL solution after 24 h [69]. | |
| Spraying of the aqueous suspension of essential oil on B. xylophilus co-cultured with Pinus pinaster shoot. | Significant decrease in the population growth of nematode compared to the control groups (p < 0.05) [70]. | ||
| Spraying of the aqueous suspension of essential oil on the Columbia root-knot nematode (Meloidogyne chitwoodi Golden) co-cultured with Solanum tuberosum hairy roots. | Significant decrease in the population growth of nematode compared to the control groups (p < 0.05) [71]. |
Furthermore, as shown in Table 1, in addition to agricultural pests, the acute toxicity and repellent action of Satureja essential oils against larvae and adults of blood-sucking mosquitos that carry pathogenic agents were also approved. For example, high susceptibility of the Asian malaria mosquito (A. stephensi) and the filariasis vector mosquito (C. quinquefasciatus) to the essential oil of S. bachtiarica was reported, in which 100% larval mortality of both insects was attained by the concentration of 160 ppm after 24 h exposure time [30].
3. Relationship between Compositions of Satureja Essential Oils with Pesticidal Properties
The major compounds of essential oils of different Satureja species’ insecticidal, acaricidal, and nematicidal activities are depicted in Table 2. Some compounds such as γ-terpinene, borneol, carvacrol, p-cymene, and thymol were identified in many species. For example, thymol with high percentage is the main compound of S. aintabensis, S. bachtiarica, S. cilicica, S. intermedia, S. isophylla, S. montana, S. parnassica, S. sahendica, S. spinosa, S. thymbra, and S. wiedemanniana essential oils. However, some compounds, such as estragole, piperitenone, piperitenone oxide, α-terpineol, β-caryophyllene, and β-myrcene, were recognized in a species: estragole in the S. hortensis, Piperitenone and piperitenone oxide in S. parvifolia essential oil, and β-myrcene in S. isophylla essential oil (Table 2).

Table 2.
Main components of the Satureja species essential oils documented as promising insecticidal, acaricidal, and nematicidal agents.
The identified compounds in the essential oils of Satureja species are categorized in the monoterpene hydrocarbon, monoterpenoid, sesquiterpene hydrocarbon, sesquiterpenoid, and phenylpropanoid groups (see Table 3). Indeed, the majority of recognized compounds are in the monoterpene group, with lower molecular weight than others, and only three compounds belong to other categories. There is sufficient evidence that the monoterpenes, especially monoterpenoids, have high pesticidal properties, and some novel and reliable outcomes in this field are shown in Table 3. For example, the toxicity of thymol, as one of main components in several species of the Satureja genus, was reported against the African cotton leafworm (Spodoptera littoralis Boisduval), the bed bugs (Cimex lectularius L.), the Colorado potato beetle (Leptinotarsa decemlineata Say), the granary weevil (Sitophilus granarius (L.)), the green peach aphid (Myzus persicae (Sulzer)), and the root-knot nematode (Meloidogyne javanica (Treub) Chitwood) [73,76,77]. It can be concluded from these studies that the presence of higher total monoterpenoid content of essential oils had a positive correlation with their pesticidal activity [78,79,80,81]. Thus, the acaricidal, insecticidal, and nematicidal effects of Satureja essential oils may be related to the high amounts of compounds listed in Table 3. It was also demonstrated that the phenolic monoterpenoids such as thymol with CH(CH3)2 functional group displayed significantly higher pesticidal effects compared to other terpenes, such as carvacrol and eugenol with CH3 and OCH3 functional groups, respectively [82,83]. However, the synergistic acaricidal, insecticidal, and nematicidal effects of minor components such as α- and β-pinene, camphor, menthol, sabinene, and thujene should also be considered [84,85,86,87]. For instance, the synergistic insecticidal action of terpenes that have methyl functional groups such as p-cymene and limonene with borneol is another consideration already reported by Pavela [83].

Table 3.
Characteristics and pesticidal activities of main components identified in Satureja species.
4. Modes of Action of Essential Oils and Their Components
The acetylcholinesterase (AChE) is actively involved in metabolic conversion of ‘acetylcholine’ in the synaptic cleft of arthropods and has two catalytic and peripheral target sites. The insect-specific cysteine residue positioned at the acetylcholinesterase active site is a proposed target site for developing insecticides to reduce off-target toxicity [94]. On the other hand, inhibition of pest-specific acetylcholinesterase will decrease the risk of utilized pesticides on non-target organisms, such as mammals [94]. Some essential oils and compounds are reported to bind with these target sites to inhibit the AChE action [95,96,97]. Park et al. [26] revealed that the essential oil of S. montana had significant AChE inhibitory activity against the fruit fly (Drosophila suzukii (Matsumura)), along with high toxicity. The inhibition of AChE leads to acetylcholine accumulation, hyperactivity, paralysis, and death of the pest. Along with terpenes, the well-known phenylpropane estragole has also shown AChE inhibitory effects [98,99]. It should be noted that the AChE inhibition can occur in both contact and fumigation methods of used essential oils [100,101]. Octopamine, as a neurotransmitter, neuromodulator, and hormone, is one of the important biogenic amines in invertebrates and is released at times of high energy demands [102]. Octopamine receptor alteration is considered as another mode of action of essential oils or their components [103]. The blockage of gamma-amino butyric acid (GABA) and nicotinic acetylcholine (nAChR) receptors has also been documented in some studies [97,104].
Beside the neurotoxic modes of pesticidal action of essential oils and compounds, there are several studies indicating enzymatic and non-enzymatic effects. The destructive effects of essential oils and their compounds on esterases and glutathione S-transferases (GSTs) as imperative detoxifying enzymes in arthropod pests are reported [88,105,106]. Disruption of the function of detoxifying enzymes may reduce the probability of pest resistance [107], and this has been clearly depicted by essential oils and their components. Farahani et al. [27] showed that the essential oil of S. khuzestanica had adverse effects on cytochrome P450 monooxygenases (P450, responsible for the oxidative metabolism of a variety of xenobiotics and endogenous compounds) function of two spotted spider mites (Tetranychus urticae Koch), along with toxic and repellent activities. The adverse effects of these agents on digestive enzymes such as lipases, proteases, α-amylases, α-glucosidases, and β-glucosidases were also reported [106], which can be very effective in reducing the nutritional efficiency of pests. Effects on energy reservoirs of the pest by decreasing the protein, glucose, and triglyceride contents and disrupting the action of immunological and hematological parameters are the other reasons to approve the multiple modes of action of these eco-friendly bio-pesticides [108,109].
5. Proposed New Formulations for Greenhouse and Field Applications
Although great potential for acaricidal, insecticidal, and nematicidal activity of Satureja essential oils and compounds have been reported, limitations such as susceptibility to light, moisture, oxygen, and temperature may restrict their application in the pest management strategies [5]. Indeed, the use of essential oils and their components in non-crop agriculture in the management of stored product pests, flies, and cockroaches is effective [110]. Additionally, the larvicidal activity of essential oils by treating standing water and waterways and their repellent effects on adults may be useful in mosquito management (See Table 1 and Table 3 for examples). Due to the disadvantage of low persistence in environmental conditions, the application of essential oils in crop agriculture can be limited [6]. Soft body and sucking pests (viz., aphids, thrips, and mites) are usually controlled by essential oils on crops, particularly under low pest pressure [110]. For example, Western flower thrip and green peach aphid were successfully controlled by the essential oil-based insecticide Ectrol (EcotecTM, California, USA) on lettuce and strawberry. However, partial efficiency was achieved against larger chewing insect pests, such as coleopterans and lepidopterans [110].
Nanoencapsulation based on the controlled release technique has been offered to overcome the lack of persistence restriction of bio-pesticides [111]. In the nanoencapsulation process, the active agent as a solid, liquid, or gas is surrounded by a thin layer of natural or synthesized polymer or a membrane to keep the core active agent from harmful environmental factors [112]. Generally, reducing the amount of active ingredients and minimizing evaporation and its controlled release are main advantages of nanoencapsulation [111]. However, along with above-mentioned advantages, expensive and difficult processes of the creation of nano-formulations should be considered. In the study of Ahmadi et al. [65], encapsulation of S. hortensis essential oil in chitosan-tripolyphosphate nanoparticles improved its ovicidal and adulticidal toxicity against T. urticae. Along with high toxicity, nanoencapsulation of S. hortensis essential oil in chitosan-tripolyphosphate nanoparticles enhanced its persistence so that 80% and 15% mortality was achieved for nano-encapsulated and pure essential oil formulation after 14 days. Usha Rani et al. [113] evaluated the antifeedant activity of pure and silica nanoparticles-based capsulated α-pinene and linalool against the tobacco cutworm (Spodoptera litura F.) and the castor semi-looper (Achaea janata L.). Although both terpenes had significant antifeedant effects, nano-capsule formulation augmented their effectiveness up to 10 and 25 times for A. Janata and S. litura, respectively. The same results regarding the enhancing toxicity and persistence of other essential oils by encapsulation in polymeric and non-polymeric materials, such as poly(ethylene glycol), myristic acid-chitosan, and mesoporous material, were also documented [114,115,116]. The preparation of nano-emulsions is another applicable method to solve the solubility restriction of essential oils in water and is more effective with minute quantities of toxic substances, both in medicinal and agricultural pest management prospects [117,118]. Further, the combination of essential oils with other protectants such as microbial agents may enhance their effectiveness. For example, the combination of S. sahendica essential oil with entomopathogenic fungus Beauveria bassiana augmented its toxicity against cowpea weevil, and insect pest mortality increased from 50% after a 1-day exposure time to 80% after 7 days [28].
6. Conclusions
Along with antibacterial, antifungal, antiviral, and general importance in medicinal, food, and cosmetic industries [119,120,121], the essential oils isolated from different species of Satureja genus could have great potential in the management of detrimental mite and tick Acari, insects, and nematodes. Pesticidal effects of Satureja species essential oils, which may be commonly related to their main terpenes [67,83,86], were reported as lethal contact and fumigant toxicity to sublethal repellent action, developmental inhibitory effects, adverse effects on the feeding, life cycle, oviposition, and egg hatching, and biochemical disturbances, such as reduction in general esterase content and inhibition of acetylcholinesterase and cytochrome P450 monooxygenases functions (see Table 1 and Table 3). Such multiple modes of action of essential oils and their compounds, in addition to reducing pest resistance, can affect a wide range of pests [5,9]. Despite all of the mentioned advantages, high volatility or lack of persistence and insolubility in water are the main restrictions in the commercialization and extensive application of these compounds [110]. Accordingly, their application is principally focused against indoor non-crop pests such as storage pests, flies, and cockroaches [96,114]. Further, the acute toxicity against larvae and repellent activity on the adults of mosquitos that carry pathogens and suck blood were also documented in Table 1 and Table 3. However, with micro- and nano-encapsulation on the basis of controlled release techniques, their persistence can be increased [122]. Although nano-emulsification is also a suitable way to dissolve essential oils in water [123,124], it is possible to increase their effectiveness by combined application with microbial control agents, such as entomopathogenic fungi [28]. These less-toxic substances may help in agriculture and environmental protection and can be proposed to countries that apply extreme amounts of synthetic pesticides. However, effects on beneficial and non-target organisms, residues on food products, and more importantly, considering a method for lower cost of Satureja essential oils and their components, should also be investigated in future research.
Author Contributions
Conceptualization, A.E.; methodology, A.E.; investigation, A.E., J.J.S., and M.Z.; resources, A.E., J.J.S., and M.Z.; writing—original draft preparation, A.E. and P.K.; writing—review and editing, A.E., J.J.S., M.Z., and P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This study received financial support from the University of Mohaghegh Ardabili, which is greatly appreciated. The publication of this review was financially supported by Chiang Mai University, Thailand.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heckel, D.G. Insecticide resistance after silent spring. Science 2012, 337, 1612–1614. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Di Bartolomeis, M.; Kegley, S.; Mineau, P.; Radford, R.; Klein, K. An assessment of acute insecticide toxicity loading (AITL) of chemical pesticides used on agricultural land in the United States. PLoS ONE 2019, 14, e0220029. [Google Scholar] [CrossRef]
- Ramadan, N. Aluminum phosphide poisoning; a case of survival. Asian Pac. J. Med. Toxicol. 2019, 8, 28–89. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnasson, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–425. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Theis, N.; Lerdau, M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003, 164, 93–102. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticidal, deterrents, and repellents in modern agriculture and increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneise, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Ikbal, C.; Pavela, R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pest. Sci. 2019, 92, 971–986. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential oils extracted from deferent species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef]
- Andrés, M.F.; González-Coloma, A.; Sanz, J.; Burillo, J.; Sainz, P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 2012, 11, 371–390. [Google Scholar] [CrossRef]
- Eloh, K.; Kpegba, K.; Sasanelli, N.; Koumaglo, H.K.; Caboni, P. Nematicidal activity of some essential plant oils from tropical West Africa. Int. J. Pest Manag. 2019, 66, 131–141. [Google Scholar] [CrossRef]
- Momtaz, S.; Abdollahi, M. An update on pharmacology of Satureja species; from antioxidant, antimicrobial, antidiabetes and antihyperlipidemic to reproductive stimulation. Int. J. Pharmacol. 2010, 6, 454–461. [Google Scholar] [CrossRef]
- Magierowicz, K.; Górska-Drabik, E.; Sempruch, C. The insecticidal activity of Satureja hortensis essential oil and its active ingredient carvacrol against Acrobasis advenella (Zinck.) (Lepidoptera, Pyralidae). Pestic. Biochem. Physiol. 2019, 153, 122–128. [Google Scholar] [CrossRef]
- Tepe, B. Inhibitory effect of Satureja on certain types of organisms. Rec. Nat. Prod. 2015, 9, 1–18. [Google Scholar]
- Macia, M.J.; Garcia, E.; Vidaurre, P.J. An ethnobotanical survey of medicinal plants commercialized in the markets of La Paz and El Alto, Bolivia. J. Ethnopharmacol. 2005, 97, 337–350. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Evergetis, E.; Mallouchos, A.; Kalpoutzakis, E.; Nychas, G.J.; Haroutounian, S.A. Characterization of the essential oil volatiles of Satureja thymbra and Satureja parnassica: Influence of harvesting time and antimicrobial activity. J. Agric. Food Chem. 2006, 54, 3139–3345. [Google Scholar] [CrossRef]
- Farzaneh, M.; Kiani, H.; Sharifi, R.; Reisi, M.; Hadian, J. Chemical composition and antifungal effects of three species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) essential oils on the main pathogens of strawberry fruit. Postharvest Biol. Technol. 2015, 109, 145–151. [Google Scholar] [CrossRef]
- Ayvaz, A.; Sagdic, O.; Karaborklu, S.; Ozturk, I. Insecticidal activity of the essential oils from different plants against three stored-product insects. J. Insect Sci. 2020, 10, 21. [Google Scholar] [CrossRef]
- Evergetis, E.; Bellini, R.; Balatsos, G.; Michaelakis, A.; Carrieri, M.; Veronesi, R.; Papachristos, D.P.; Puggioli, A.; Kapsaski-Kanelli, V.N.; Haroutounian, S.A. From bio-prospecting to field assessment: The case of carvacrol rich essential oil as a potent mosquito larvicidal and repellent agent. Front. Ecol. Evol. 2018, 6, 204. [Google Scholar] [CrossRef]
- Usanmaz-Bozhuyuk, A.; Kordali, S. Investigation of the toxicity of essential oils obtained from six Satureja species on Colorado potato beetle, Leptinotarsa decemlineata (say, 1824), (Coleoptera: Chrysomelidae). Fresenius Environ. Bull. 2018, 27, 4389–4401. [Google Scholar]
- Rahmani, S.; Azimi, S. Fumigant toxicity of three Satureja species on tomato leafminers, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Toxin Rev. 2020. [Google Scholar] [CrossRef]
- Park, C.G.; Jang, M.; Yoon, K.A.; Kim, J. Insecticidal and acetylcholinesterase inhibitory activities of Lamiaceae plant essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Ind. Crop. Prod. 2016, 89, 507–513. [Google Scholar] [CrossRef]
- Farahani, S.; Bandani, A.; Amiri, A. Toxicity and repellency effects of three essential oils on two populations of Tetranychus urticae (Acari: Tetranychidae). Persian J. Acarol. 2020, 9, 67–82. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Mehrvar, A.; Eivazian Kary, N.; Valizadeh, H. Compatibility of some plant essential oils in combination with the entomopathogenic fungus, Beauveria bassiana against Callosobruchus maculatus (Col.: Bruchidae). Plant Pest Res. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Sampson, B.J.; Tabanca, N.; Kirimer, N.; Demirci, B.; Baser, K.H.C.; Khan, I.A.; Spiers, J.M.; Wedge, D.E. Insecticidal activity of 23 essential oils and their major compounds against adult Lipaphis pseudobrassicae (Davis) (Aphididae: Homoptera). Pest Manag. Sci. 2005, 61, 1122–1128. [Google Scholar] [CrossRef]
- Soleimani-Ahmadi, M.; Abtahi, S.M.; Madani, A.; Paksa, A.; Abadi, Y.S.; Gorouhi, M.A.; Sanei-Dehkordi, A. Phytochemical profile and mosquito larvicidal activity of the essential oil from aerial parts of Satureja bachtiarica Bunge against malaria and lymphatic filariasis vectors. J. Essent. Oil Bear. Plants 2017, 20, 328–336. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Hooshmandi, M. Insecticidal and repellent activity of three Satureja species against adult red flour beetles, Tribolium castaneum (Coleoptera: Tenebrionidae). Acta Ecol. Sin. 2017, 37, 201–206. [Google Scholar] [CrossRef]
- Cetin, H.; Ser, O.; Arserim, S.K.; Polat, Y.; Ozabek, T.; Civril, M.; Cinbilgel, I.; Ozbel, Y. Fumigant toxicity of Satureja cuneifolia and Ziziphora clinopodioides essential oils on field collected sand flies (Diptera: Psychodidae: Phlebotomie). Fresenius Environ. Bull. 2018, 27, 4258–4262. [Google Scholar]
- Pavela, R. Larvicidal property of essential oils against Culex quinquefasciatus Say (Diptera: Culicidae). Ind. Crop. Prod. 2009, 30, 311–315. [Google Scholar] [CrossRef]
- Tozlu, E.; Cakir, A.; Kordali, S.; Tozlu, G.; Ozer, H.; Akcin, T.A. Chemical compositions and insecticidal effects of essential oils isolated from Achillea gypsicola, Satureja hortensis, Origanum acutidens and Hypericum scabrum against broadbean weevil (Bruchus dentipes). Sci. Hortic. 2011, 130, 9–17. [Google Scholar] [CrossRef]
- Kim, S.I.; Chae, S.H.; Youn, H.S.; Yeon, S.H.; Ahn, Y.J. Contact and fumigant toxicity of plant essential oils and efficacy of spray formulations containing the oils against B- and Q-biotypes of Bemisia tabaci. Pest Manag. Sci. 2011, 67, 1093–1099. [Google Scholar] [CrossRef]
- Zandi-Sohani, N. Efficiency of Labiateae plants essential oils against adults of cotton whitefly (Bemisia tabaci). Indian J. Agric. Sci. 2011, 81, 1164–1167. [Google Scholar]
- Heydarzade, A.; Moravvej, G.H. Contact toxicity and persistence of essential oils from Foeniculum vulgare, Teucrium polium and Satureja hortensis against Callosobruchus maculatus (Fabricius) (Coleoptera: Bruchidae) adults. Turk. J. Entomol. 2012, 36, 507–518. [Google Scholar]
- Zandi-Sohani, N. A comparative study on fumigant toxicity of Zataria multiflora and Satureja hortensis (Lamiaceae) to Callosobruchus maculatus (Coleoptera: Chrysomelidae). Int. J. Trop. Insect Sci. 2012, 32, 142–147. [Google Scholar] [CrossRef]
- Kordali, S.; Emsen, B.; Yildirim, E. Fumigant toxicity of essential oils from fifteen plant species against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Egypt. J. Biol. Pest. Control 2013, 23, 241–246. [Google Scholar]
- Yazdani, E.; Jalali Sendi, J.; Khosravi, R.; Hajizadeh, J.; Ghadamyari, M. Effect of Satureja hortensis L. essential oil on feeding efficiency and biochemical properties of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Arch. Phytopathol. Plant Protect. 2013, 46, 328–339. [Google Scholar] [CrossRef]
- Shahab-Ghayoor, H.; Saeidi, K. Antifeedant activities of essential oils of Satureja hortensis and Fumaria parviflora against Indian meal moth Plodia interpunctella Hubner (Lepidoptera: Pyralidae). Entomol. Ornithol. Herpetol. 2015, 4, 154. [Google Scholar] [CrossRef]
- Pirmohammadi, M.; Shayeghi, M.; Vatan Doost, H.; Abaei, M.R.; Mohammadi, A.; Bagheri, A.; Khoobdel, M.; Hasan Bakhshi, H.; Pirmohammadi, M.; Tavassoli, M. Chemical composition and repellent activity of Achillea vermiculata and Satureja hortensis against Anopheles stephensi. J. Arthropod-Borne Dis. 2016, 10, 201–210. [Google Scholar] [PubMed]
- Gokturk, T.; Kordali, S.; Usanmaz Bozhuyuk, A. Insecticidal effect of essential oils against fall webworm (Hypantria cunea Drury (Lepidoptera: Arctiidae)). Nat. Prod. Commun. 2017, 12, 1659–1662. [Google Scholar] [CrossRef]
- Najafzadeh, R.; Ghasemzadeh, S.; Mirfakhraie, S. Effect of essential oils from Nepeta crispa, Anethum graveolens and Satureja hortensis against the stored-product insect “Ephestia kuehniella (Zeller)”. J. Med. Plants By-Prod. 2019, 2, 163–169. [Google Scholar] [CrossRef]
- Magierowicz, K.; Górska-Drabik, E.; Golan, K. Effects of plant extracts and essential oils on the behavior of Acrobasis advenella (Zinck.) caterpillars and females. J. Plant Dis. Prot. 2020, 127, 63–71. [Google Scholar] [CrossRef]
- Ebadollahi, A. Estragole-rich essential oil of summer savory (Satureja hortensis L.) as an eco-friendly alternative to the synthetic insecticides in management of two stored-products insect pests. Acta Agric. Slov. 2020, 115, 307–314. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Setzer, W.N. Evaluation of the toxicity of Satureja intermedia C. A. Mey essential oil to storage and greenhouse insect pests and a predator ladybird. Foods 2020, 9, 712. [Google Scholar] [CrossRef]
- Hasanshahi, G.; Abbasipour, H.; Jahan, F.; Askarianzadeh, A.; Karimi, J.; Rastegar, F. Fumigant toxicity and nymph production deterrence effect of three essential oils against two aphid species in the laboratory condition. J. Essent. Oil Bear. Plants 2016, 19, 706–711. [Google Scholar] [CrossRef]
- Jahan, F.; Abbasipour, H.; Hasanshahi, G. Fumigant toxicity and nymph production deterrence effect of five essential oils on adults of the black bean aphid, Aphis fabae Scop. (Hemiptera: Aphididae). Adv. Food Sci. 2019, 41, 48–53. [Google Scholar]
- Ebadollahi, A. Chemical profile and insecticidal activity of an Iranian endemic savory Satureja isophylla Rech. Revue Agric. 2020, 11, 20–27. [Google Scholar]
- Kayedi, M.H.; Haghdoost, A.A.; Salehnia, A.; Khamisabadi, K. Evaluation of repellency effect of essential oils of Satureja khuzestanica (Carvacrol), Myrtus communis (Myrtle), Lavendula officinalis and Salvia sclarea using standard WHO repellency tests. J. Arthropod-Borne Dis. 2014, 8, 60–68. Available online: https://jad.tums.ac.ir/index.php/jad/article/view/244 (accessed on 2 June 2021).
- Taghizadeh-Saroukolai, A.; Nouri-Ganbalani, G.; Rafiee-Dastjerdi, H.; Hadian, J. Antifeedant activity and toxicity of some plant essential oils to Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Plant Protect. Sci. 2014, 50, 207–216. [Google Scholar] [CrossRef]
- Michaelakis, A.; Theotokatos, S.A.; Koliopoulos, G.; Chorianopoulos, N.G. Essential oils of Satureja species: Insecticidal effect on Culex pipiens larvae (Diptera: Culicidae). Molecules 2007, 12, 2567–2578. [Google Scholar] [CrossRef] [PubMed]
- Picard, I.; Hollingsworth, R.G.; Salmieri, S.; Lacroix, M. Repellency of essential oils to Frankliniella occidentalis (Thysanoptera: Thripidae) as affected by type of oil and polymer release. J. Econ. Entomol. 2012, 105, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nathan, S.; Mehlhorn, H.; Maggi, F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef]
- Toloza, A.C.; Zygadlo, J.; Biurrun, F.; Rotman, A.; Picollo, M. Bioactivity of Argentinean essential oils against permethrin-resistant head lice, Pediculus humanus capitis. J. Insect Sci. 2010, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.; Lopez, S.; Luna, L.; Agüero, M.B.; Aragón, L.; Tapia, A.; Zacchino, S.; López, M.L.; Zygadlo, J.; Feresin, G.E. Essential oils of medicinal plants from the Central Andes of Argentina: Chemical composition, and antifungal, antibacterial, and insect-repellent activities. Chem. Biodivers. 2011, 8, 924–936. [Google Scholar] [CrossRef]
- Yildirim, E.; Kordali, S.; Yazici, G. Insecticidal effects of essential oils of eleven plant species from Lamiaceae on Sitophilus granarius (L.) (Coleoptera: Curculionidae). Rom. Biotechnol. Lett. 2011, 16, 6702–6709. [Google Scholar]
- Ayvaz, A.; Karaborklu, S.; Sagdic, O. Fumigant toxicity of five essential oils against the eggs of Ephestia kuehniella Zeller and Plodia interpunctella (Hübner) (Lepidoptera: Pyralidae). Asian J. Chem. 2009, 21, 596–604. [Google Scholar]
- Karaborklu, S.; Ayvaz, A.; Yilmaz, S.; Akbulut, M. Chemical composition and fumigant toxicity of some essential oils against Ephestia kuehniella. J. Econ. Entomol. 2011, 104, 1212–1219. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Sanna, C.; Rubiolo, P.; Basilico, N.; Colombo, E.; Scaltrito, M.M.; Ndiath, M.; Maccarone, L.; Taramelli, D.; Bicchi, C.; et al. Anti-plasmodial and insecticidal activities of the essential oils of aromatic plants growing in the Mediterranean area. Malar. J. 2012, 11, 2019. [Google Scholar] [CrossRef]
- Karamaouna, F.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.; Polissiou, M.; Papatsakona, P.; Tsora, E. Insecticidal activity of plant essential oils against the vine mealybug, Planococcus ficus. J. Insect Sci. 2013, 13, 142. [Google Scholar] [CrossRef]
- Aslan, I.; Ozbek, H.; Calmasur, O.; Sahin, F. Toxicity of essential oil vapours to two greenhouse pests, Tetranychus urticae Koch and Bremisia tabaci Genn. Ind. Crop. Prod. 2004, 19, 167–173. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Jalali Sendi, J.; Aliakbar, A.; Razmjou, J. Acaricidal activities of essential oils of Satureja hortensis (L.) and Teucrium polium (L.) against two spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). Egypt. J. Biol. Pest. Control 2015, 25, 171–176. [Google Scholar]
- Ahmadi, A.; Saber, M.; Akbari, A.; Mahdavinia, G.R. Encapsulation of Satureja hortensis L. (Lamiaceae) in chitosan/TPP nanoparticles with enhanced acaricide activity against Tetranychus urticae Koch (Acari: Tetranychidae). Ecotoxicol. Environ. Saf. 2018, 161, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Amizadeh, M.; Hejazi, M.J.; Askari-Saryazdi, G. Fumigant toxicity of some essential oils to Tetranychus urticae (Acari: Tetranychidae). Int. J. Acarol. 2013, 39, 285–289. [Google Scholar] [CrossRef]
- Cetin, H.; Cilek, J.E.; Oz, E.; Aydin, L.; Deveci, O.; Yanikoglu, A. Acaricidal activity of Satureja thymbra L. essential oil and its major components, carvacrol and gamma-terpinene against adult Hyalomma marginatum (Acari: Ixodidae). Vet. Parasitol. 2010, 170, 287–290. [Google Scholar] [CrossRef]
- Pardavella, I.; Nasiou, E.; Daferera, D.; Trigas, P.; Giannakou, I. The use of essential oil and hydrosol extracted from Satureja hellenica for the control of Meloidogyne incognita and M. javanica. Plants 2020, 9, 856. [Google Scholar] [CrossRef]
- Barbosa, P.; Faria, J.M.S.; Mendes, M.D.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Bioassays against pinewood nematode: Assessment of a suitable dilution agent and screening for bioactive essential oils. Molecules 2012, 17, 12312–12329. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Sena, I.; Moiteiro, C.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Nematotoxic and phytotoxic activity of Satureja montana and Ruta graveolens essential oils on Pinus pinaster shoot cultures and P. pinaster with Bursaphelenchus xylophilus in vitro co-cultures. Ind. Crop. Prod. 2015, 77, 59–65. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Rodrigues, A.M.; Sena, I.; Moiteiro, C.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity of Ruta graveolens and Satureja montana essential oils on Solanum tuberosum hairy roots and Solanum tuberosum hairy roots with Meloidogyne chitwoodi co-cultures. J. Agric. Food Chem. 2016, 64, 7452–7458. [Google Scholar] [CrossRef]
- Azaz, A.D.; Kürkcüoglu, M.; Satil, F.; Can Baser, K.H.; Tümen, G. In vitro antimicrobial activity and chemical composition of some Satureja essential oils. Flavour Fragr. J. 2005, 20, 587–591. [Google Scholar] [CrossRef]
- Navarro-Rocha, J.; Andrés, M.F.; Díaz, C.E.; Burillo, J.; González-Coloma, A. Composition and biocidal properties of essential oil from pre-domesticated Spanish Satureja Montana. Ind. Crop. Prod. 2020, 145, 111958. [Google Scholar] [CrossRef]
- Cabana, R.; Silva, L.R.; Valentão, P.; Viturro, C.I.; Andrade, P.B. Effect of different extraction methodologies on the recovery of bioactive metabolites from Satureja parvifolia (Phil.) Epling (Lamiaceae). Ind. Crop. Prod. 2013, 48, 49–56. [Google Scholar] [CrossRef]
- Sefidkon, F.; Akbari-Nia, A. Essential oil content and composition of Satureja sahendica Bornm. at different stages of plant growth. J. Essent. Oil Res. 2009, 21, 112–114. [Google Scholar] [CrossRef]
- Kordali, Ş.; Usanmaz, A.; Bayrak, N.; Çakır, A. Fumigation of volatile monoterpenes and aromatic compounds against adults of Sitophilus granarius (L.) (Coleoptera: Curculionidae). Rec. Nat. Prod. 2017, 11, 362–373. [Google Scholar]
- Gaire, S.; Scharf, M.E.; Gondhalekar, A.D. Toxicity and neurophysiological impacts of plant essential oil components on bed bugs (Cimicidae: Hemiptera). Sci. Rep. 2019, 9, 3961. [Google Scholar] [CrossRef]
- Badawy, M.E.; El-Arami, S.A.; Abdelgaleil, S.A. Acaricidal and quantitative structure activity relationship of monoterpenes against the two-spotted spider mite, Tetranychus urticae. Exp. Appl. Acarol. 2010, 52, 261–274. [Google Scholar] [CrossRef]
- Chiu, C.C.; Keeling, C.I.; Bohlmann, J. Toxicity of pine monoterpenes to mountain pine beetle. Sci. Rep. 2017, 7, 8858. [Google Scholar] [CrossRef]
- Kanda, D.; Kaur, S.; Kou, O. A comparative study of monoterpenoids and phenylpropanoids from essential oils against stored grain insects: Acute toxins or feeding deterrents. J. Pest. Sci. 2017, 90, 531–545. [Google Scholar] [CrossRef]
- Ramadan, G.R.M.; Abdelgaleil, S.A.M.; Shawir, M.S.; El-bakary, A.S.; Zhu, K.Y.; Phillips, T.W. Terpenoids, DEET and short chain fatty acids as toxicants and repellents for Rhyzopertha dominica (Coleoptera: Bostrichidae) and Lasioderma serricorne (Coleoptera: Ptinidae). J. Stored Prod. Res. 2020, 87, 101610. [Google Scholar] [CrossRef]
- Pavela, R. Insecticidal properties of phenols on Culex quinquefasciatus Say and Musca domestica L. Parasitol. Res. 2011, 190, 1547–1553. [Google Scholar] [CrossRef]
- Pavela, R. Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind. Crop. Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- Attia, S.; Grissa, K.L.; Lognay, G.; Heuskin, S.; Mailleux, A.C.; Hance, T. Chemical composition and acaricidal properties of Deverra scoparia essential oil (Araliales: Apiaceae) and blends of its major constituents against Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol. 2011, 104, 1220–1228. [Google Scholar] [CrossRef]
- Ntalli, G.N.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef]
- Liu, T.T.; Chao, L.K.P.; Hong, K.S.; Huang, Y.J.; Yang, T.S. Composition and insecticidal activity of essential oil of Bacopa caroliniana and interactive effects of individual compounds on the activity. Insects 2020, 11, 23. [Google Scholar] [CrossRef]
- Scalerandi, E.; Flores, G.A.; Palacio, M.; Defagó, M.T.; Carpinella, M.C.; Valladares, G.; Bertoni, A.; Palacios, S.M. Understanding synergistic toxicity of terpenes as insecticides: Contribution of metabolic detoxification in Musca domestica. Front. Plant Sci. 2018, 9, 1579. [Google Scholar] [CrossRef]
- Yeom, H.J.; Jung, C.S.; Kang, J.S.; Kim, J.; Lee, J.H.; Kim, D.S.; Kim, H.S.; Park, P.S.; Kang, K.S.; Park, I.K. Insecticidal and acetylcholine esterase inhibition activity of Asteraceae plant essential oils and their constituents against adults of the German cockroach (Blattella germanica). J. Agric. Food Chem. 2015, 63, 2241–2248. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, Y.; Wang, Y.; Lin, Z.; Wang, L.; Li, G. Toxicities of monoterpenes against housefly, Musca domestica L. (Diptera: Muscidae). Environ. Sci. Pollut. Res. Int. 2017, 24, 24708–24713. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Correa-Basurto, J.; Rodriguez-Valdez, L.M.; Sanchez-Torres, L.E.; Nogueda-Torres, B.; Nevarez-Moorillon, G.V. In vitro and in silico studies of terpenes, terpenoids and related compounds with larvicidal and pupicidal activity against Culex quinquefasciatus Say (Diptera: Culicidae). Chem. Cent. J. 2018, 12, 53. [Google Scholar] [CrossRef]
- Koliopoulos, G.; Pitarokili, D.; Kioulos, E.; Michaelakis, A.; Tzakou, O. Chemical composition and larvicidal evaluation of Mentha, Salvia, and Melissa essential oils against the West Nile virus mosquito Culex pipiens. Parasitol. Res. 2010, 107, 327–335. [Google Scholar] [CrossRef]
- Cárdenas-Ortega, N.C.; González-Chávez, M.M.; Figueroa-Brito, R.; Flores-Macías, A.; Romo-Asunción, D.; Martínez-González, D.E.; Pérez-Moreno, V.; Ramos-López, M.A. Composition of the essential oil of Salvia ballotiflora (Lamiaceae) and its insecticidal activity. Molecules 2015, 20, 8048–8059. [Google Scholar] [CrossRef]
- Pang, Y.P.; Brimijoin, S.; Ragsdale, D.W.; Zhu, K.Y.; Suranyi, R. Novel and viable acetylcholinesterase target site for developing effective and environmentally safe insecticides. Curr. Drug Targets 2012, 13, 471. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crop. Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Are monoterpenoids and phenylpropanoids efficient inhibitors of acetylcholinesterase from stored product insect strains? Flavour Fragr. J. 2015, 30, 108–112. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system—a review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef]
- Kim, S.W.; Kang, J.; Park, I.K. Fumigant toxicity of Apiaceae essential oils and their constituents against Sitophilus oryzae and their acetylcholinesterase inhibitory activity. J. Asia Pac. Entomol. 2013, 16, 443–448. [Google Scholar] [CrossRef]
- Olmedo, R.; Herrera, J.M.; Lucini, E.I.; Zunino, M.P.; Pizzolitto, R.P.; Dambolena, J.S.; Zygadlo, J.A. Essential oil of Tagetes filifolia against the flour beetle Tribolium castaneum and its relation to acetylcholinesterase activity and lipid peroxidation. Agriscientia 2015, 32, 113–121. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.; Mohamed, M.I.; Badawy, M.E.; El-arami, S.A. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 2019, 35, 518–525. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Chandu, A.G.S.; Devi, S.S. Ocimum tenuiflorum oil, a potential insecticide against rice weevil with anti-acetylcholinesterase activity. Ind. Crop. Prod. 2015, 126, 434–439. [Google Scholar] [CrossRef]
- Rand, D.; Knebel, D.; Ayali, A. The effect of octopamine on the locust stomatogastric nervous system. Front. Physiol. 2012, 3, 288. [Google Scholar] [CrossRef] [PubMed]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Coats, J.R. Quantitative structure-activity relationships of monoterpenoid binding activities to the housefly GABA receptor. Pest Manag. Sci. 2012, 68, 1122–1129. [Google Scholar] [CrossRef]
- Mojarab-Mahboubkar, M.; Sendi, J.J.; Aliakbar, A. Effect of Artemisia annua L. essential oil on toxicity, enzyme activities, and energy reserves of cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J. Plant. Prot. Res. 2015, 55, 371–377. [Google Scholar] [CrossRef]
- Oftadeh, M.; Sendi, J.J.; Ebadollahi, A. Toxicity and deleterious effects of Artemisia annua essential oil extracts on mulberry pyralid (Glyphodes pyloalis). Pestic. Biochem. Physiol. 2020, 170, 104702. [Google Scholar] [CrossRef]
- Gunderson, M.P.; Nguyen, B.T.; Cervantes Reyes, J.C.; Holden, L.L.; French, J.; Smith, B.D.; Lineberger, C. Response of phase I and II detoxification enzymes, glutathione, metallothionein and acetylcholine esterase to mercury and dimethoate in signal crayfish (Pacifastacus leniusculus). Chemosphere 2018, 208, 749–756. [Google Scholar] [CrossRef]
- Ghoneim, K. Disturbed hematological and immunological parameters of insects by botanicals as an effective approach of pest control: A review of recent progress. South. Asian. J. Exp. Biol. 2018, 1, 112–144. [Google Scholar]
- Oftadeh, M.; Sendi, J.J.; Ebadollahi, A. Biologically active toxin identified from Artemisia annua against lesser mulberry pyralid, Glyphodes pyloalis. Toxin Rev. 2020. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Ibrahim, S.S. Essential oil nanoformulations as a novel method for insect pest control in horticulture. In Horticultural Crops, 2nd ed.; Baimey, H.K., Hamamouch, N., Kolombia, Y.A., Eds.; IntechOpen: London, UK, 2019; pp. 1–14. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Usha Rani, P.; Madhusudhanamurthy, J.; Sreedhar, B. Dynamic adsorption of α-pinene and linalool on silica nanoparticles for enhanced antifeedant activity against agricultural pests. J. Pest Sci. 2014, 87, 191–200. [Google Scholar] [CrossRef]
- Gonzalez, J.O.; Gutierrez, M.M.; Ferrero, A.A.; Band, B.F. Essential oils nanoformulations for stored-product pest control-Characterization and biological properties. Chemosphere 2015, 100, 130–138. [Google Scholar] [CrossRef]
- Ziaee, M.; Moharramipour, S.; Mohsenifar, A. Toxicity of Carum copticum essential oil-loaded nanogel against Sitophilus granarius and Tribolium confusum. J. Appl. Entomol. 2015, 138, 763–771. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Jalali Sendi, J.; Aliakbar, A. Efficacy of nanoencapsulated Thymus eriocalyx and Thymus kotschyanus essential oils by a mesoporous material MCM-41 against Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol. 2017, 110, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, S.; Clarke, S.K.; Nirmala, M.J.; Tyagi, B.K.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsion of eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull. Entomol. Res. 2014, 104, 393–402. [Google Scholar] [CrossRef]
- Adak, T.; Barik, N.; Patil, N.B.; Govindharaj, G.P.P.; Gadratagi, B.G.; Annamalai, M.; Mukherjee, A.K.; Rath, P.C. Nanoemulsion of eucalyptus oil: An alternative to synthetic pesticides against two major storage insects (Sitophilus oryzae (L.) and Tribolium castaneum (Herbst)) of rice. Ind. Crop. Prod. 2020, 143, 111849. [Google Scholar] [CrossRef]
- Tepe, B.; Cilkiz, M. A pharmacological and phytochemical overview on Satureja. Pharm. Biol. 2016, 54, 375–412. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, I.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Velescu, B.S.; Anuta, V.; Ortan, A.; Jinga, V. Phytochemical profile and biological activities of Satureja hortensis L.: A review of the last decade. Molecules 2018, 23, 2458. [Google Scholar] [CrossRef]
- Sefidkon, F.; Emami Bistgani, Z. Integrative review on ethnobotany, essential oil, phytochemical, agronomy, molecular and pharmacological properties of Satureja species. J. Essent. Oil Res. 2021, 33, 114–132. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Review nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and technology of nanoemulsion-based pesticide formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green micro- and nanoemulsions for managing parasites, vectors and pests. Nanomaterials 2019, 9, 1285. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).