Abstract
Staphylococcus aureus is an important bacterial pathogen. This study utilized known staphylococcal epidemiology to track S. aureus between patients, surfaces, staff hands and air in a ten-bed intensive care unit (ICU). Methods: Patients, air and surfaces were screened for total colony counts and S. aureus using dipslides, settle plates and an MAS-100 slit-sampler once a month for 10 months. Data were modelled against proposed standards for air and surfaces, and ICU-acquired staphylococcal infection. Whole-cell genomic typing (WGS) demonstrated possible transmission pathways between reservoirs. Results: Frequently touched sites were more likely to be contaminated (>12 cfu/cm2; p = 0.08). Overall, 235 of 500 (47%) sites failed the surface standard (≤2.5 cfu/cm2); 20 of 40 (50%) passive air samples failed the “Index of Microbial Air” standard (2 cfu/9 cm plate/h), and 15/40 (37.5%) air samples failed the air standard (<10 cfu/m3). Settle plate data were closer to surface counts than automated air data; the surface count most likely to reflect pass/fail rates for air was 5 cfu/cm2. Surface counts/bed were associated with staphylococcal infection rates (p = 0.012). Of 34 pairs of indistinguishable S. aureus, 20 (59%) showed autogenous transmission, with another four (12%) occurring between patients. Four (12%) pairs linked patients with hand-touch sites and six (18%) linked airborne S. aureus, staff hands and hand-touch sites. Conclusion: Most ICU-acquired S. aureus infection is autogenous, while staff hands and air were rarely implicated in onward transmission. Settle plates could potentially be used for routine environmental screening. ICU staphylococcal infection is best served by admission screening, systematic cleaning and hand hygiene.
1. Introduction
There is a limited evidence base for everything that we do in the name of infection prevention and control. This is because infection control itself is a very young science and has not yet amassed sufficient evidence to achieve universally agreed practices. One of the most problematic deficits is the lack of knowledge on the exact mechanism by which patients acquire pathogens in the healthcare environment. Transmission to, from and between patients, staff and the environment has not been fully elucidated and this means that it is impossible to prioritise or target infection prevention interventions for patient benefit. In order to focus on transmission dynamics, it was decided to embark on a study that might have the potential to pinpoint the exact pathway leading to healthcare-acquired infection (HAI). While an experimental unit would make it easier to track pathogens between reservoirs, this would not provide real-world data. So an intensive care unit (ICU) in a district general hospital (DGH) in Scotland was chosen for the surface-air-sampling study (SASS), which took place throughout 2015.
Elucidating dynamic transmission requires an indicator pathogen. Staphylococcus aureus represents a useful marker of hospital hygiene since it colonises 1 in 3 people, including staff, patients and visitors, and is thus found in air and on hands and surfaces, including equipment [1]. This organism was the obvious choice to investigate transmission between all major reservoirs in a clinical unit. It is also amenable to whole-cell genomic typing strategies.
This commentary summarises the study; why it was performed, how it was performed, and the main results, which were published in a sequence of three papers by the authors of this article [2,3,4]. The findings suggest the main direction of travel for the study pathogen, S. aureus, as well as highlighting the most important reservoirs in an ICU. It draws together the implications from all three papers and offers a series of recommendations for healthcare workers charged with controlling infection in the critical care environment.
2. Methods
2.1. Setting
The study took place in a 10-bed adult ICU in a semirural health board in Scotland [2,3]. The ICU has nine beds in the main area of the unit and one isolation room (Figure 1). At least 600 patients are admitted each year with a daily turnover of at least 1–5 patients. The case mix includes pneumonia, major trauma, sepsis, cardiac conditions and postoperative support. The ICU is mechanically ventilated with rates maintained at 10 air changes per hour at constant temperature and humidity. Detergent cleaning of general surfaces is performed daily by domestic staff, with near-patient sites cleaned twice daily by nurses using detergent wipes. Detergent wipes are also used to clean clinical equipment, with a once weekly bleach (Actichlor Plus™) disinfection [2]. Patients colonised or infected with hospital pathogens (e.g., methicillin-resistant S. aureus (MRSA); vancomycin-resistant enterococci; or Clostridium difficile) are isolated and the room or bed space disinfected with bleach on a daily basis.
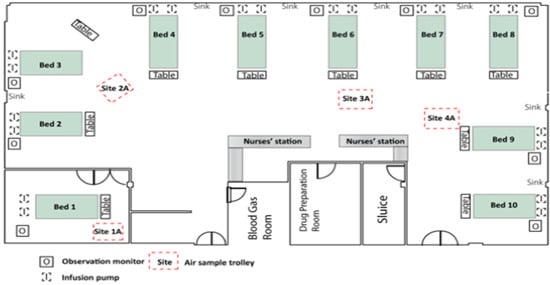
Figure 1.
Aerial view of ICU (intensive care unit).
2.2. Environmental Screening
The study ran for ten months, with a different morning chosen for sampling each month and with different intervals between study days [2,3]. Clinical staff were not told when screening was planned in order to circumvent any Hawthorne-type effect. Consideration was given to staffing levels and ICU bed occupancy (≥50%) before beginning the sampling protocol. There was a period of at least two hours after routine cleaning before systematic sampling began.
Double-sided dipslides coated with nutrient and staphylococcal-selective (Baird–Parker) agars were employed for hard surface sampling (Hygiena International, Watford, UK) [5]. Each slide was pressed firmly onto five near-patient sites for 10 s at a pressure of 25 g/cm2. Chosen sites were panels on the intravenous fluid pump and cardiac monitor; right and left bedrails and bed table [2,3]. The slides were incubated at 35 °C in CO2 for 48–72 h, depending upon colony size. Aerobic colony counts (ACC) per cm2 were categorised as follows: no growth; scanty growth <2.5 cfu/cm2; light growth 2.5–12 cfu/cm2; moderate growth 12–40 cfu/cm2; and heavy growth >40 cfu/cm2. Potential coagulase-positive staphylococci were captured from the selective agar, recultured and tested for coagulase production and antibiotic susceptibilities in accordance with routine laboratory protocol [2,3,4,5].
Settle plates (9 cm) were used for passive air sampling. These contained the same nutrient and selective agars in order to measure total counts and S. aureus (cfu/9 cm plate/h) [6]. They were placed on 1 metre high trolleys for one hour, with one in the isolation room and three in the main ICU (Figure 1). We performed automated air sampling at the same positions as the settle plate trolleys with an MAS-100 slit sampler based on the Andersen impactor principle (Merk, Germany) [7]. ICU air stream was directed onto nutrient and selective agar plates for 10 × 1 min [3]. The plates were processed using the same protocols for total bioburden and presence of S. aureus [2,3,4,5].
2.3. Patients, Visitors and Staff
Patients admitted to ICU are routinely screened for S. aureus; screening is repeated twice weekly and on discharge. Regular sampling of nose, perineum, wounds and urine enabled us to establish carrier patients (transient or permanent), staphylococcal colonization pressure, duration of staphylococcal shedding and investigate acquisition incidents occurring in ICU. Visitors were not screened, but staff voluntarily placed finger tips onto blood agar for enumeration and isolation of any possible S. aureus. Patients with confirmed S. aureus infection occurring >48 h after admission were assumed to be ICU-acquired and these were defined according to national guidelines [8].
2.4. Staphylococcal Genotyping
Staphylococcal isolates were sent to the Staphylococcal Reference Laboratory (National Infection Service, Public Health England, Colindale) for spa typing and MLST-CC assignments. Use of the spa server (http://spa.ridom.de/mlst.shtml), MLST database (http://saureus.mlst.net) and in-house PHE database helped to establish identity between strains [9]. Isolates with epidemiological links and related spa types were subjected to whole genomic sequencing (WGS) as previously described [10]. Single-nucleotide polymorphism (SNP) analysis was used to determine phylogenetic relationships between isolates at the core genome level (https://github.com/phe-bioinformatics/PHEnix). Any pairs or clusters of isolates with <50 SNPs between them suggested identity and these were explored further [11].
3. Results
Initial examination compared the surface bioburden of hand-touch sites, in order to model the level of microbial soil against the number of times the site was actually handled [2]. Staff were observed touching study sites from an average of 6/h (cardiac monitor) to 37/h (bed table) (Table 1) [2]. Just ten S. aureus (including one methicillin-resistant S. aureus: MRSA) were recovered from 500 screened sites around the patients’ beds. These comprised four from the left bedrail, two each from bed table and intravenous pump, and one isolate each from right bedrail and cardiac monitor. Seven isolates were linked with gross contamination (>12 cfu/cm2) of a specific site (p = 0.005), and six of these were recovered from the highest number of touched sites (bed table and bedrails) (Table 1).

Table 1.
Microbial soil categories for five hand-touch sites on ICU.
While the bed table was the most frequently touched site, it did not deliver the highest amount of bioburden as expected (Figure 2). We wondered whether staff using alcohol gel from bottles on the bed table may have transferred gel to table surfaces, or generated microaerosol that settled on sampling sites. This premise was tested by removing the bottle of alcohol gel from one bed table in the middle of the ICU and rescreening the table during ten unannounced visits. Five of ten dipslides yielded >12 cfu/cm2, which was higher than the proportion from either bedrail and allowed us to confirm the relationship between touch frequency and surface soil (Figure 2). There is clearly a quantitative association between the number of times a site is touched and the amount of aerobic soil recovered from that surface [2]. Furthermore, there is a higher chance of isolating S. aureus from a surface if it is already heavily contaminated with microbial soil.
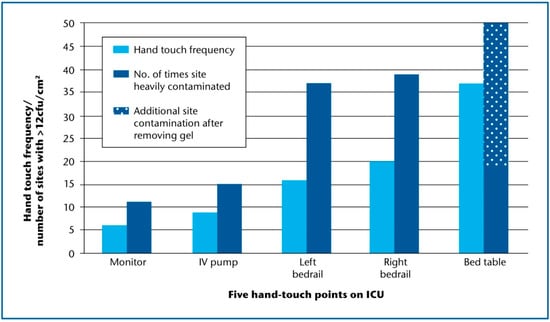
Figure 2.
Hand-touch frequency and gross microbial soil for five near-patient sites on ICU. Average hand-touch frequency/site/h following ten observational audits; each site (n = 5) in ten bed spaces was screened on ten occasions; gross microbial soil defined as no. of screens exceeding 12 cfu/cm2; ICU: intensive care unit.
The second analysis involved modelling quantitative surface bioburden against microbial counts gathered from both passive and active air sampling within the 2 h sampling period on ICU [3]. Five hundred near-patient sites yielded quantifiable bioburden, ranging from no growth to heavy growth (>40 cfu/cm2) (Table 1). The microbiological surface standard chosen for these sites was <2.5 cfu/cm2, which gave an overall 47% failure rate [12]. Comparing the data from both air sampling methods allowed a proportionate comparison of pass or fail according to the surface standard.
Passive air sampling delivered values of 0–40 cfu/plate/h, with >2 cfu/plate/h recovered from 20 of the 40 plates (Table 2). This suggested a failure rate of 50%, if using the index of microbial air contamination (IMA) [6]. The IMA proposes a standard of ≤2 cfu/plate/h, while the standard for active air sampling is <10 cfu/m3 [7]. Fifteen of forty samples produced >10 cfu/m3, thus providing a failure rate of 37.5%. Thus, proportionate fails rate from passive air sampling (50%) was closer to the surface failure rate (47%) than the active air failure rate (37.5%) for ten study days.

Table 2.
Microbial burden categories for air (active and passive sampling) and hygiene fails according to standards.
The pass/failure rates from both air sampling methods were compared against the surface bioburden pass/fail rate on a site-by-site basis for each study day. There were just 19/40 (47.5%) pairs that agreed a pass or fail status between active air sampling data and surface bioburden, although settle plate data showed a closer relationship with surface counts (26/40: 65%) using the 2.5 cfu/cm2 benchmark [12]. Given that this benchmark has yet to become universally established, it was questioned whether pass/fail proportions for active air and settle plate counts would demonstrate a similar relationship with surface data if another surface standard was used. Consequently, all surface bioburden data was assigned pass or fail against a range of different standards from 1–20 cfu/cm2. The closest pass/fail agreement between any air parameter and specific surface standard occurred at 5 cfu/cm2 for settle plate data, with 70% agreement (Figure 3) [3].
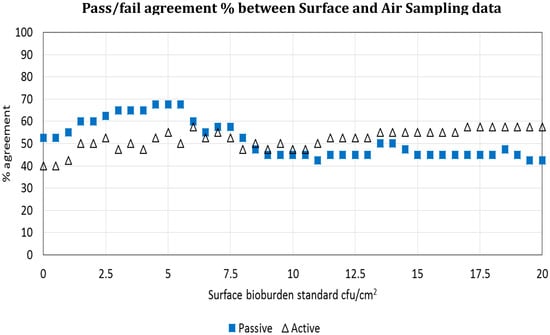
Figure 3.
Is surface bioburden associated with air bioburden? Agreement between active and passive air sampling and surface bioburden using a range of surface standards from 0–20 cfu/cm2. The X axis shows the percentage pass or fail agreement between active and passive air data for each bioburden standard; the Y axis shows the surface bioburden value in cfu/cm2.
We identified eleven patients with ICU-acquired staphylococcal infections occurring within the 72 h period encompassing each study day. Taking % bed occupancy into account, the number of these infections was plotted against total surface cfus per bed (all five bed sites) for Beds 2–10 for each individual study day. We ignored data from the isolation room because all patients with ICU-acquired infections were identified in the main ICU. Bed occupancy rate-adjusted ICU-acquired staphylococcal infection was associated with average surface count for patients in the main body of the ICU (p = 0.012) (Figure 4) [3].
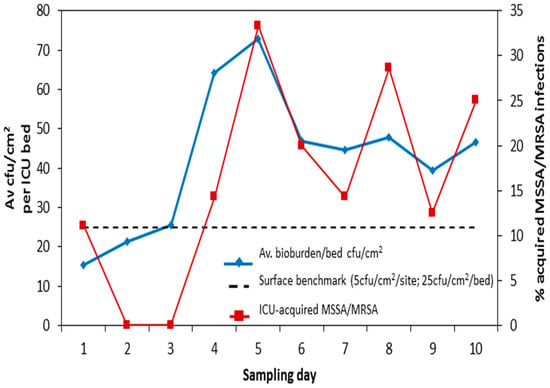
Figure 4.
Is surface bioburden associated with clinical risk? Total bioburden (5 sites)/bed (cfu/cm2) plotted against % ICU-acquired S. aureus infection (adjusted for bed occupancy) for Beds 2–10 on 10 sampling days. ICU: intensive care unit; MSSA: methicillin-susceptible S. aureus; MRSA: methicillin-resistant S. aureus.
The third analysis of data concerned results from whole genomic sequencing (WGS). WGS established 34 S. aureus clusters between reservoirs and patients, with another four pairs showing convincing phenotypical and epidemiological relationships (Table 3) [4]. There were 20 of 34 (59%) pairs that were highly related (<25 SNPs); these pairs linked a carriage strain with a strain causing acquired infection in the same patient, i.e., so-called autogenous or endogenous transmission [13]. Most of these infections were ventilator-associated (13 of 20), but there were also two central-line infections, four wound infections; one intra-abdominal infection and one abscess. The period of time between confirming colonisation and recovering the S. aureus causing infection was an average of 2.7 days (range 0–8 days). There were four other transmissions between four patient pairs with time intervals from 2–3 days to several months. Two of these pairs were highly related, but the relationship between the other two could not be verified because these isolates were EMRSA-15 and this strain was present elsewhere in the hospital [11]. Two patients had been on the same ward at the same time, but the isolation of their MRSA strains occurred over a 5 month period. The second MRSA pair involved two patients on ICU with just 4 days separating the isolation of their strains. At this time, these two patients were the only ones with MRSA in the ICU.

Table 3.
Whole-genome sequencing (WGS) categories and pathways, lineage, sites, intervals (days) and SNP (single-nucleotide polymorphism) differences of S. aureus clusters in a ten-bed ICU during a ten month study.
Four pairs of S. aureus linked hand-touch sites and patients, and all of these involved sites within the patients’ own, or adjoining, bed spaces (Table 3). One pair was classified as “uncertain” according to genomic identity definitions [14]. There were two linked isolates from bed table and cardiac monitor in adjoining bed spaces and another pair recovered from a bed table and bedrail on the same day, three bed spaces apart. There were five transmission episodes involving bedrails, with four of these implicating the left bedrail. Staff usually touch the bedrail on the patient’s right, and visitors more usually touch the bedrail on the left [2]. There were two pairs of strains involving the bed table, and the intravenous pump and cardiac monitor were also linked in two separate transmission episodes.
Despite 10 air changes/hour, there were four airborne S. aureus recovered and three of these were implicated in transmission links between a near-patient site and staff hands. There were no transmission episodes involving patients and staff hands, which was surprising because we always assume that HAI is associated with contaminated staff hands.
4. Discussion
This article describes a detailed study in one ICU in an attempt to identify transmission pathways between patients, staff and the environment using S. aureus (Figure 5) [2,3,4]. We found a relationship between the amount of times a site was touched and the total burden of microbial soil at that site. There was also a relationship between the amount of microbial soil on surfaces and the number of microbes in surrounding air, although the best relationship came from passive, rather than active, air sampling.
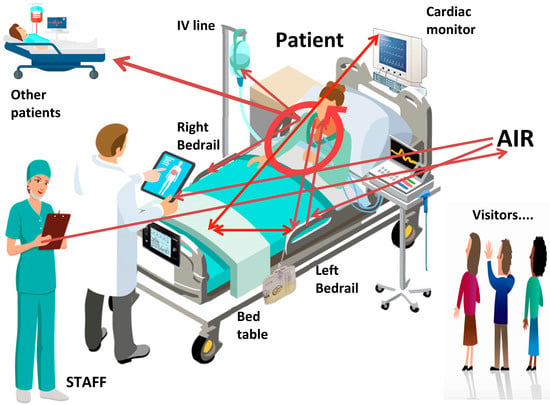
Figure 5.
Transmission pathways of S. aureus on one ICU. ICU: intensive care unit; MSSA: methicillin-susceptible S. aureus; MRSA: methicillin-resistant S. aureus.
Air samples denote only a proportion of total surface bioburden, because microbial soil found on surfaces represents a combination of air deposition and direct contact. Thus, settle plates might be more useful as a routine monitoring strategy rather than a method for investigating outbreaks. Air sampling in isolation cannot detect surface contamination from other sources, such as handling, indirect contact and spillages [3].
WGS revealed that autogenous transmission was the most important direction of transmission, i.e., between patients’ colonised and infected sites. This is, perhaps, predictable, but it is reassuring to know that exogenous infections might be prevented by cleaning and disinfection, given that the next most common pathway occurred between patients and hand-touch sites around the bed [1]. We could not find any evidence for direct transmission between patients and the air, or between staff hands and patients, although S. aureus was recovered from both air and hands during the study.
Air samples were collected in the morning, which illustrates a major limitation of the study because airborne bioburden fluctuates significantly throughout the day and could yield values that are higher than found in this study. There are additional limitations, such as the fact that the study was performed in a single ICU only; there were just 10 sampling days in 10 months; patient demographics were not reported; there were no tangible data on the effectiveness of environmental cleaning; or whether patients were appropriately isolated, including compliance with contact precautions. It is also the case that staff and visitors were not screened.
We would, however, like to suggest a number of recommendations for preventing staphylococcal infection in ICU patients:
Firstly, frequently touched near-patient sites would benefit from targeted cleaning in the ICU, although the definitions of “cleaning” and frequency are not yet established; furthermore, there is contention over choice of cleaning fluids, i.e., whether we should use disinfectant or detergent or both [15]. There is increasing awareness that routine removal of microbial soil using the “one wipe; one site; one direction” principle is sufficient for infection prevention, rather than an attempt to kill all surface flora with disinfectants.
Secondly, visitor hand hygiene should be considered in an overall infection prevention strategy for ICU [16]. This is because visitors might have played a role in transmitting S. aureus in ICU since WGS identified numerous strains without any matches from sampled reservoirs. The risk from visitors’ hands remains an unexplored issue throughout hospitals in general.
Thirdly, given that patients are more at risk from their own staphylococcal strains, they should be screened routinely for S. aureus carriage on admission to ICU and regularly thereafter [13]. There are several effective decontamination packages for S. aureus carriage which can be employed following risk assessment.
Fourthly, the relationship between bioburden on hand-touch surfaces and in the air lends itself to further research; if routine settle plates can predict increased risk for infection, then this might be a valuable asset for infection prevention practitioners and much easier to perform than surface sampling, culture and interpretation [3].
5. Conclusions
This study collected S. aureus from staff hands, patients and environmental sites in one ICU and demonstrated potential transmission pathways between all reservoirs using genomic typing strategies. New strains are constantly introduced into critical care from colonised patients, which not only pose a major risk for the carrier but also to other patients through contamination of bed space sites. We could not demonstrate transmission involving staff hands or airborne staphylococci despite obvious presence. While one study cannot comprehensively define transmission hierarchies, the data clearly supports regular cleaning of near-patient hand-touch sites, patient screening and continued emphasis toward hand hygiene for everyone.
Author Contributions
Conceptualization, S.J.D.; methodology, C.E.A., S.J.D. and collaborators (see Acknowledgments); formal analysis, S.J.D.; data curation, C.E.A. and collaborators (see Acknowledgments); writing—original draft preparation, S.J.D. and C.E.A.; writing—review and editing, S.J.D., C.E.A. and collaborators (please see Acknowledgments); project administration, S.J.D.; funding acquisition, S.J.D. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by NHS Lanarkshire Research & Development, with consumables funded by UNISON, the people’s healthcare union (UK).
Acknowledgments
We wish to acknowledge all the ICU staff at Hairmyres hospital, particularly Veronica Watson, and all the microbiology laboratory staff. Genotyping was performed at the Staphylococcal reference laboratory at HPA, Colindale and we thank Donald Morrison, Angela Kearns and Bruno Pichon for expert help. Statistical modelling was done by Marco-Felipe King, and ventilation data analysis by Professor Cath Noakes (University of Leeds, UK). This article is based on three publications in the Journal of Hospital Infection, the editor of which has kindly given permission for replication of tables and figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dancer, S.J. Importance of the environment in MRSA acquisition: The case for hospital cleaning. Lancet Infect. Dis. 2008, 8, 101–113. [Google Scholar] [CrossRef]
- Adams, C.E.; Smith, J.; Robertson, C.; Watson, V.; Dancer, S.J. Examining the relationship between surface bioburden and frequently touched sites in Intensive Care. J. Hosp. Infect. 2017, 95, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Adams, C.E.; King, M.-F.; Robertson, C.; Noakes, C.; Dancer, S.J. Is there a relationship between airborne and surface microbes in the critical care environment? J. Hosp. Infect. 2018, 100, e123–e129. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J.; Adams, C.E.; Smith, J.; Pichon, B.; Kearns, A.; Morrison, D. Tracking Staphylococcus aureus in ICU using Whole-Genome Sequencing. J. Hosp. Infect. 2019, 103, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, A.; Stewart, M.; Hunter, J.; Yip, B.; Reid, D.; Robertson, C. How quickly do hospital surfaces become contaminated after detergent cleaning? Healthc. Infect. 2013, 18, 3–9. [Google Scholar] [CrossRef]
- Pasquarella, C.; Pitzurra, O.; Svino, A. The index of microbial air contamination. J. Hosp. Infect. 2000, 46, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Pasquarella, C.; Vitali, P.; Saccani, E.; Manotti, P.; Boccuni, C.; Ugolotti, M. Microbial air monitoring in operating theatres: Experience at the University Hospital of Parma. J. Hosp. Infect. 2012, 81, 50–57. [Google Scholar] [CrossRef] [PubMed]
- NHS Scotland National Infection Prevention and Control Manual (NIPCM). 2012. Available online: http://www.nipcm.scot.nhs.uk (accessed on 18 March 2020).
- Harmsen, D.; Claus, H.; Witte, W.; Rothganger, J.; Claus, H.; Turnwald, D. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef] [PubMed]
- Lahuerta-Marin, A.; Guelbenzu-Gonzalo, M.; Pichon, B.; Allen, A.; Doumith, M.; Lavery, J.F. First report of lukM-positive livestock-associated methicillin-resistant Staphylococcus aureus CC30 from fattening pigs in Northern Ireland. Vet. Microbiol. 2016, 182, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Coll, F.; Harrison, E.M.; Toleman, M.S.; Reuter, S.; Raven, K.E.; Blane, B. Longitudinal genomic surveillance of MRSA in the UK reveals transmission patterns in hospitals and the community. Sci. Transl. Med. 2017, 9, 413. [Google Scholar] [CrossRef]
- White, L.; Dancer, S.J.; Robertson, C.; MacDonald, J. Are hygiene standards useful in assessing infection risk? Am. J. Infect. Control 2008, 36, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.A.; van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.M.; Ludden, C.; Brodrick, H.J.; Blane, B.; Brennan, G.; Morris, D. Transmission of methicillin-resistant Staphylococcus aureus in long-term care facilities and their related healthcare networks. Genome Med. 2016, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J.; Kramer, A. Four steps to clean hospitals: Look; Plan; Clean; and Dry. J. Hosp. Infect. 2019, 103, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Birnbach, D.J.; Rosen, L.F.; Fitzpatrick, M.; Arheart, K.L.; Munoz-Price, L.S. An evaluation of hand hygiene in an intensive care unit: Are visitors a potential vector for pathogens? J. Infect. Public Health 2015, 8, 570–574. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).