Comparison of Azithromycin Removal from Water Using UV Radiation, Fe (VI) Oxidation Process and ZnO Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthetic Aquatic Solution Contaminated with Azithromycin (ASCA)
2.2. ZnO Nanoparticles
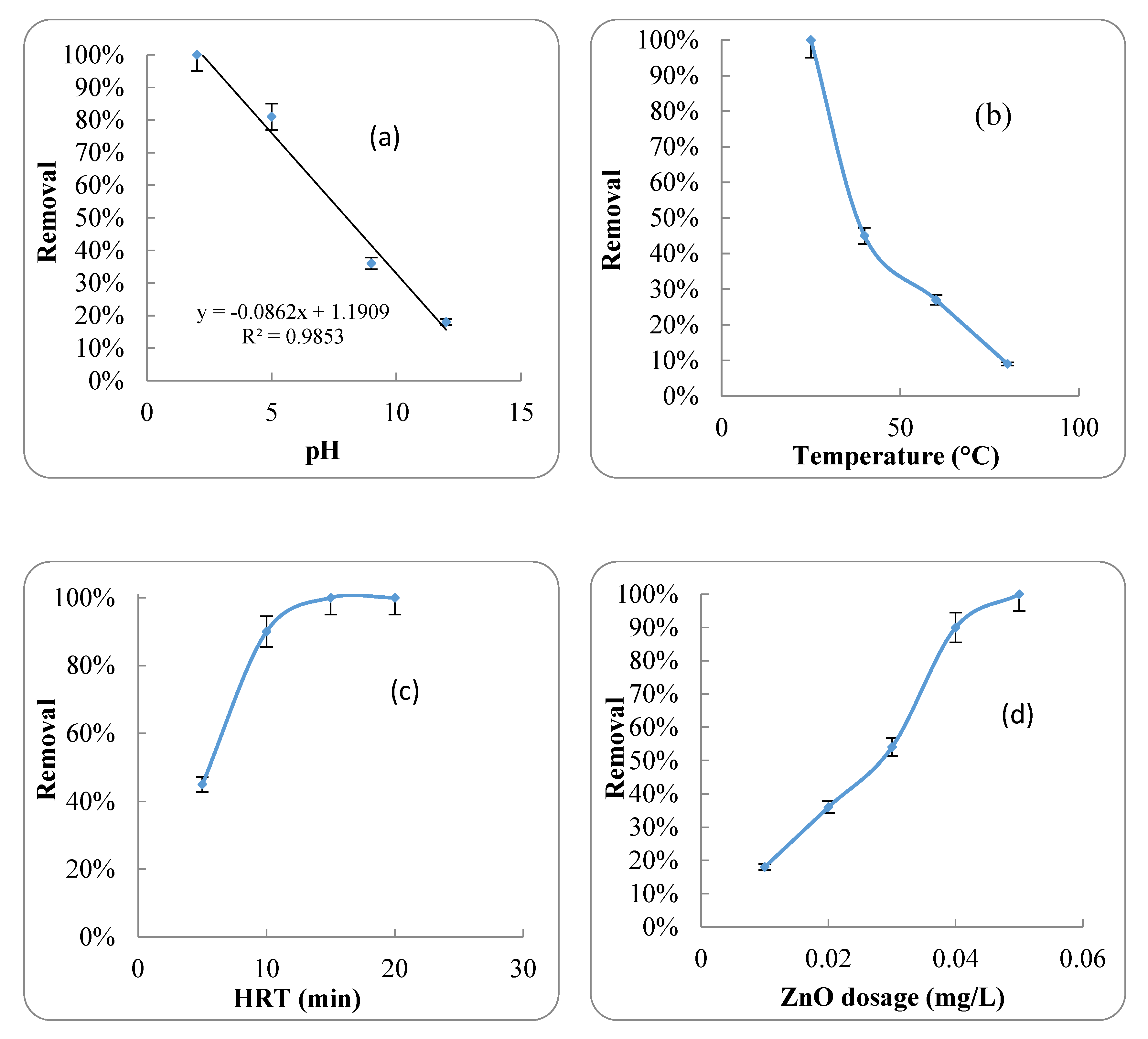
2.2.1. Effect of pH
2.2.2. Effect of ZnO Nanoparticles
2.2.3. Effect of HRT
2.2.4. Effect of Temperature
2.2.5. Determination of Isotherms
2.3. UV Radiation
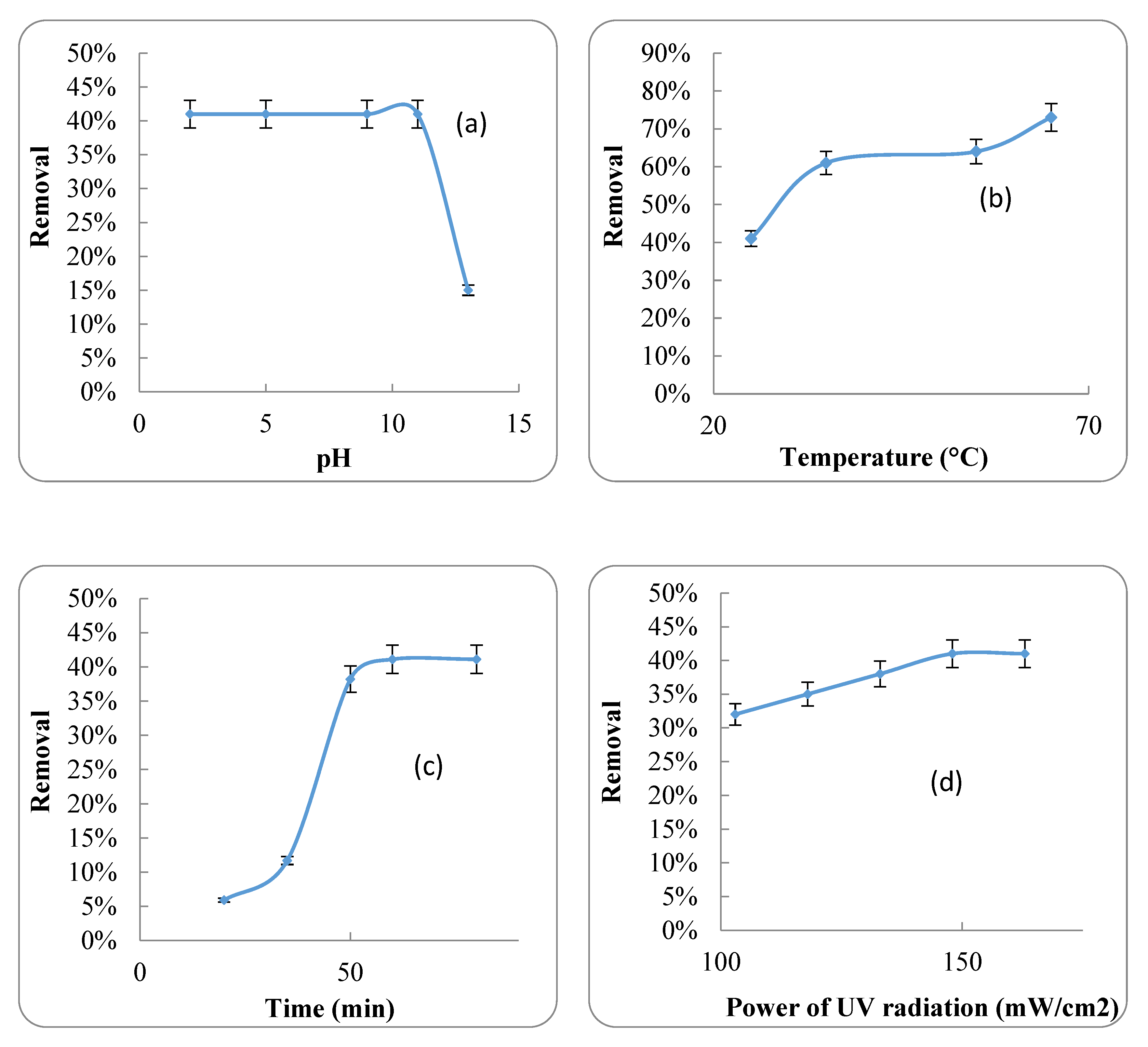
2.3.1. Effect of pH
2.3.2. Effect of Temperature
2.3.3. Effect of HRT
2.3.4. Effect of UV Radiation
2.4. Fe (VI)
2.4.1. Production of Ferrate (VI)
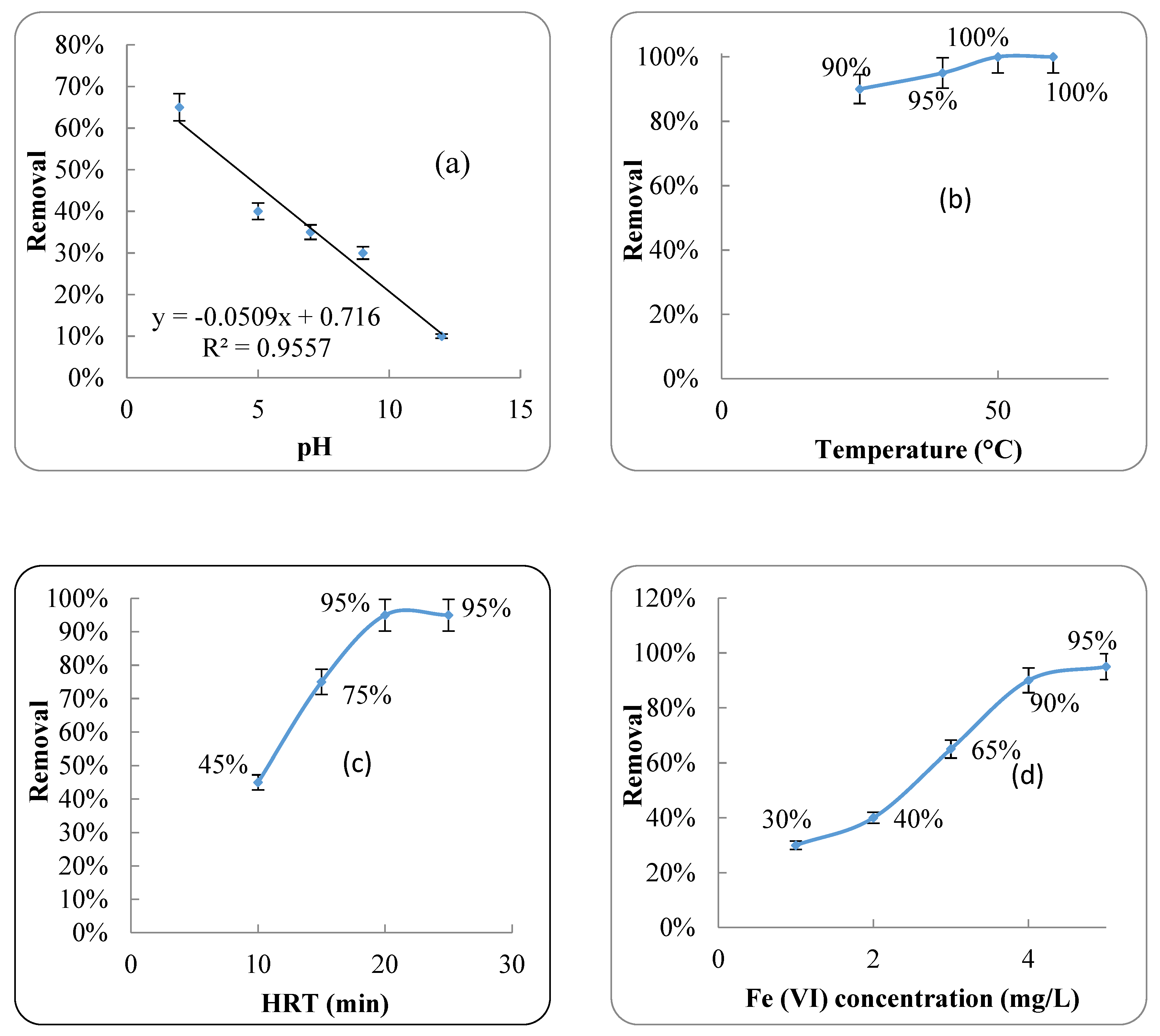
2.4.2. Effect of pH
2.4.3. Effect of Fe (VI) Concentration
2.4.4. Effect of HRT
2.4.5. Effect of Temperature
2.5. Analytical Methods
3. Results
4. Discussion
4.1. ZnO Nanoparticles
Isotherms
4.2. UV Radiation
4.3. Fe (VI) Oxidation Process
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yadav, K.K.; Kumar, S.; Pham, Q.B.; Gupta, N.; Rezania, S.; Kamyab, H.; Yadav, S.; Vymazal, J.; Kumar, V.; Tri, D.Q.; et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review. Ecotoxicol. Environ. Saf. 2019, 182, 109362. [Google Scholar] [CrossRef]
- Nateghian, Z.; Aliabadi, E. Aspects of Environmental Pollutants on Male Fertility and Sperm Parameters. J. Environ. Treat. Tech. 2020, 8, 299–309. [Google Scholar]
- Aigberua, A.; Tarawou, T. Water Quality Index (WQI) Assessment along Inland Fresh Waters of Taylor Creek in Bayelsa State, Nigeria. J. Environ. Treat. Tech. 2019, 7, 260–269. [Google Scholar]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical industry wastewater: Review of the technologies for water treatment and reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Mellah, A.; Harik, D. Removal Pharmaceutical Pollutants by Adsorption Competitive using Powdered Activated Carbon CAP (F400). J. Environ. Treat. Tech. 2020, 8, 336–345. [Google Scholar]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Acar, J.F. Consequences of bacterial resistance to antibiotics in medical practice. Clin. Infect. Dis. 1997, 24 (Suppl. 1), S17–S18. [Google Scholar] [CrossRef]
- Tolls, J. Sorption of Veterinary Pharmaceuticals in Soils: A Review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef]
- Wei, R.; Ge, F.; Huang, S.; Chen, M.; Wang, R. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere 2011, 82, 1408–1414. [Google Scholar] [CrossRef]
- Yang, S.; Cha, J.; Carlson, K. Simultaneous extraction and analysis of 11 tetracycline and sulfonamide antibiotics in influent and effluent domestic wastewater by solid-phase extraction and liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2005, 1097, 40–53. [Google Scholar] [CrossRef]
- Bhandari, A.; Close, L.I.; Kim, W.; Hunter, R.P.; Koch, D.E.; Surampalli, R.Y. Occurrence of ciprofloxacin, sulfamethoxazole, and azithromycin in municipal wastewater treatment plants. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2008, 12, 275–281. [Google Scholar] [CrossRef]
- Mowery, H.; Loganathan, B. Persistent organic compounds in wastewater: Azithromycin and urobilin concentrations in wastewater treatment plant samples from Murray, Kentucky, USA. Organohalogen Compd. 2007, 69, 483. [Google Scholar]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem. 2014, 6, PMC. S14459. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Jajko, M.; Sobczak, A. Removal of veterinary antibiotics from wastewater by electrocoagulation. Chemosphere 2018, 194, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, D.S.S.; Qiu, G.; Ting, Y.-P. Fate and removal of selected antibiotics in an osmotic membrane bioreactor. Chem. Eng. J. 2018, 334, 198–205. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Alexander, J.; Schwartz, T.; Fatta-Kassinos, D. Investigation of the potential of a Membrane BioReactor followed by solar Fenton oxidation to remove antibiotic-related microcontaminants. Chem. Eng. J. 2017, 310, 491–502. [Google Scholar] [CrossRef]
- Norte, T.H.d.O.; Marcelino, R.B.P.; Medeiros, F.H.A.; Moreira, R.P.L.; Amorim, C.C.; Lago, R.M. Ozone oxidation of β-lactam antibiotic molecules and toxicity decrease in aqueous solution and industrial wastewaters heavily contaminated. Ozone Sci. Eng. 2018, 40, 385–391. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Different removal behaviours of multiple trace antibiotics in municipal wastewater chlorination. Water Res. 2013, 47, 2970–2982. [Google Scholar] [CrossRef]
- Ternes, T.A.; Stüber, J.; Herrmann, N.; McDowell, D.; Ried, A.; Kampmann, M.; Teiser, B. Ozonation: A tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res. 2003, 37, 1976–1982. [Google Scholar] [CrossRef]
- Hey, G.; Grabic, R.; Ledin, A.; La Cour Jansen, J.; Andersen, H. Oxidation of pharmaceuticals by chlorine dioxide in biologically treated wastewater. Chem. Eng. J. 2012, 185, 236–242. [Google Scholar] [CrossRef]
- Audino, F.; Toro Santamaria, J.M.; Del Valle Mendoza, L.J.; Graells, M.; Pérez-Moya, M. Removal of paracetamol using effective advanced oxidation processes. Int. J. Environ. Res. Public Health 2019, 16, 505. [Google Scholar] [CrossRef]
- Sharma, V.K.; Liu, F.; Tolan, S.; Sohn, M.; Kim, H.; Oturan, M.A. Oxidation of β-lactam antibiotics by ferrate (VI). Chem. Eng. J. 2013, 221, 446–451. [Google Scholar] [CrossRef]
- Musikavong, C.; Wattanachira, S.; Marhaba, T.F.; Pavasant, P. Reduction of organic matter and trihalomethane formation potential in reclaimed water from treated industrial estate wastewater by coagulation. J. Hazard. Mater. 2005, 127, 48–57. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Talaei, M.R.; Rezania, S. An Overview on Production and Application of Ferrate (VI) for Chemical Oxidation, Coagulation and Disinfection of Water and Wastewater. J. Environ. Chem. Eng. 2017, 5, 1828–1842. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, L.; Li, Z.; Pi, K.; Deng, Y. One-step Ferrate(VI) treatment as a core process for alternative drinking water treatment. Chemosphere 2020, 242, 125134. [Google Scholar] [CrossRef] [PubMed]
- Machala, L.; Zajíček, P.; Kolařík, J.; Mackuľak, T.; Filip, J. Ferrates as Powerful Oxidants in Water Treatment Technologies. In Advanced Nano-Bio Technologies for Water and Soil Treatment; Filip, J., Cajthaml, T., Najmanová, P., Černík, M., Zbořil, R., Eds.; Springer International Publishing: Cham, The Netherlands, 2020; pp. 177–201. [Google Scholar]
- Gholami, M.; Mohammadi, M.; Zare-Hoseinabadi, A.; Taghizadeh, S.; Behzad Behbahani, A.; Arab Solghar, R.; Amani, A.M. Preparation of ZnXFe3-XO4@chitosan Nanoparticles As an Adsorbent for Methyl Orange and Phenol. J. Environ. Treat. Tech. 2019, 7, 245–249. [Google Scholar]
- Dayang, W.; Dongbo, P. Taurine prevents ultraviolet B induced apoptosis in retinal ganglion cells. Cutan. Ocul. Toxicol. 2018, 37, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, K. Modeling of Adsorption in a Packed Bed Tower, the Case Study of Methane Removal and Parametric Calculation. J. Environ. Treat. Tech. 2019, 7, 324–333. [Google Scholar]
- Jin, L.; Chai, L.; Yang, W.; Wang, H.; Zhang, L. Two-Dimensional Titanium Carbides (Ti3C2Tx) Functionalized by Poly (m-phenylenediamine) for Efficient Adsorption and Reduction of Hexavalent Chromium. Int. J. Environ. Res. Public Health 2020, 17, 167. [Google Scholar] [CrossRef]
- Asha, E.; Parveen Fatemeh, R.; Kar Woon, L.; Mohd Hafiz, J.; Mohd Azrul, N.; Sun, J.S.; Shao, W.; Ismail, S. Treatment of Dye Wastewater by Functionalization of Bentonite-Methylene Blue with Sodium Persulfate. J. Environ. Treat. Tech. 2019, 7, 229–233. [Google Scholar]
- Talaiekhozani, A.; Banisharif, F.; Eskandari, Z.; Talaei, M.R.; Park, J.; Rezania, S. Kinetic Investigation of 1, 9-Dimethyl-Methylene Blue Zinc Chloride Double Salt Removal from Wastewater using Ferrate (VI) and Ultraviolet Radiation. JKSUS 2020, 32, 213–222. [Google Scholar] [CrossRef]
- Eskandari, Z.; Talaiekhozani, A.; Talaie, M.R.; Banisharif, F. Enhancing ferrate(VI) oxidation process to remove blue 203 from wastewater utilizing MgO nanoparticles. J. Environ. Manag. 2019, 231, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Federation, W.E.; Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Moussavi, G.; Mahmoudi, M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard. Mater. 2009, 168, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Collings, P.J.; Hird, M. Introduction to Liquid Crystals: Chemistry and Physics; CRC Press: London, UK, 2017. [Google Scholar]
- Anslyn, E.V.; Dougherty, D.A. Modern Physical Organic Chemistry; University Science Books: Mill Valley, CA, USA, 2006. [Google Scholar]
- El-Kemary, M.; El-Shamy, H.; El-Mehasseb, I. Photocatalytic degradation of ciprofloxacin drug in water using ZnO nanoparticles. J. Lumin. 2010, 130, 2327–2331. [Google Scholar] [CrossRef]
- Agarwal, M.; Dave, M.; Upadhayaya, S. Adsorption of Formaldehyde on Treated Activated Carbon and Activated Alumina. Curr. World. Environ. 2011, 6, 53–59. [Google Scholar] [CrossRef]
- Mohamad Amran, M.S.; Dalia Khalid, M.; Wan Azlina, W.A.K.; Azni, I. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar]
- Hudlicky, M. Oxidations in Organic Chemistry; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- Elmolla, E.S.; Chaudhuri, M. Comparison of different advanced oxidation processes for treatment of antibiotic aqueous solution. Desalination 2010, 256, 43–47. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227. [Google Scholar] [CrossRef]
- Tan, F.; Sun, D.; Gao, J.; Zhao, Q.; Wang, X.; Teng, F.; Quan, X.; Chen, J. Preparation of molecularly imprinted polymer nanoparticles for selective removal of fluoroquinolone antibiotics in aqueous solution. J. Hazard. Mater. 2013, 244, 750–757. [Google Scholar] [CrossRef]
- Eslami, A.; Nasseri, S.; Yadollahi, B.; Mesdaghinia, A.; Vaezi, F.; Nabizadeh, R.; Nazmara, S. Photocatalytic degradation of methyl tert-butyl ether (MTBE) in contaminated water by ZnO nanoparticles. J. Chem. Technol. Biotechnol. 2008, 83, 1447–1453. [Google Scholar] [CrossRef]
- Hariharan, C. Photocatalytic degradation of organic contaminants in water by ZnO nanoparticles: Revisited. Appl. Catal. A Gen. 2006, 304, 55–61. [Google Scholar] [CrossRef]
- Kaur, S.; Rani, S.; Mahajan, R.K. Adsorption kinetics for the removal of hazardous dye congo red by biowaste materials as adsorbents. J. Chem. 2012, 2013. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Eskandari, Z.; Talaei, M.R.; Salari, M. Hydrogen sulfide and organic compounds removal in municipal wastewater using ferrate (VI) and ultraviolet radiation. Environ. Health Eng. Manag. J. 2017, 4, 7–14. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Zhou, Y.; Fang, L.; Shao, Y. Degradation of atenolol by UV/peroxymonosulfate: Kinetics, effect of operational parameters and mechanism. Chemosphere 2013, 93, 2717–2724. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Eskandari, Z.; Bagheri, M.; Talaie, M.R. Removal of H2S and COD Using UV, Ferrate and UV/Ferrate from Municipal Wastewater. J. Hum. Environ. Health Promot. 2016, 2, 1–8. [Google Scholar] [CrossRef][Green Version]
- Kamarehie, B.; Ahmadi, F.; Hafezi, F.; Abbariki, A.; Heydari, R.; Karami, M.A. Experimental data of electric coagulation and photo-electro-phenton process efficiency in the removal of metronidazole antibiotic from aqueous solution. Data Brief 2018, 18, 96–101. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Salari, M.; Talaei, M.R.; Bagheri, M.; Eskandari, Z. Formaldehyde removal from wastewater and air by using UV, ferrate (VI) and UV/ferrate (VI). J. Environ. Manag. 2016, 184, 204–209. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Eskandari, Z.; Rodpeyma, S.; Bagheri, M. Design and Development of Municipal Wastewater Treatment Systems by Fe (VI) and Computation of System’s Economic Navigation. J. Adv. Med Sci. Appl. Technol. 2017, 3, 169–174. [Google Scholar] [CrossRef]



| Jovian | Genalized | D-R | Temkin | Freundlich | Langmuir | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax | Kj | R2 | N | KG | R2 | qmax | β | R2 | KT | B1 | R2 | kf | n | R2 | qm | Ke | R2 |
| 116.33 | 0.002 | 0.6074 | 0.2008 | 0.10856 | 0.6199 | 4.3 × 1043 | 3 × 10−8 | 0.15 | 3.11 × 10−87 | 0.5242 | 0.0191 | 0.99 | −263.2 | 0.012 | 0.098 | −20242.91 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talaiekhozani, A.; Joudaki, S.; Banisharif, F.; Eskandari, Z.; Cho, J.; Moghadam, G.; Rezania, S. Comparison of Azithromycin Removal from Water Using UV Radiation, Fe (VI) Oxidation Process and ZnO Nanoparticles. Int. J. Environ. Res. Public Health 2020, 17, 1758. https://doi.org/10.3390/ijerph17051758
Talaiekhozani A, Joudaki S, Banisharif F, Eskandari Z, Cho J, Moghadam G, Rezania S. Comparison of Azithromycin Removal from Water Using UV Radiation, Fe (VI) Oxidation Process and ZnO Nanoparticles. International Journal of Environmental Research and Public Health. 2020; 17(5):1758. https://doi.org/10.3390/ijerph17051758
Chicago/Turabian StyleTalaiekhozani, Amirreza, Sahar Joudaki, Farhad Banisharif, Zeinab Eskandari, Jinwoo Cho, Ghasem Moghadam, and Shahabaldin Rezania. 2020. "Comparison of Azithromycin Removal from Water Using UV Radiation, Fe (VI) Oxidation Process and ZnO Nanoparticles" International Journal of Environmental Research and Public Health 17, no. 5: 1758. https://doi.org/10.3390/ijerph17051758
APA StyleTalaiekhozani, A., Joudaki, S., Banisharif, F., Eskandari, Z., Cho, J., Moghadam, G., & Rezania, S. (2020). Comparison of Azithromycin Removal from Water Using UV Radiation, Fe (VI) Oxidation Process and ZnO Nanoparticles. International Journal of Environmental Research and Public Health, 17(5), 1758. https://doi.org/10.3390/ijerph17051758







