Abstract
Sarcopenia is a physiopathological process associated with aging, caused by reduction of muscle strength, muscle quality and physical performance, and associated with an increased risk of falls, physical disability and premature death. There is no effective treatment for sarcopenia, but physical exercise seems to be highly effective at counteracting the decline in muscle mass and strength associated with aging. Recently, sarcopenia has been recognized as an emerging issue in people living with HIV (PLWH). Despite adequate treatment with combination antiretroviral therapy (cART), PLWH may exhibit an early occurrence of some aging-related conditions, including sarcopenia, frailty and falls, and this is likely resulting from high rates of comorbidities, high-risk behaviours, chronic immune activation and cART-specific factors. In this review, we discuss the potential mechanisms and the clinical relevance of sarcopenia in PLWH, and present data from longitudinal studies of physical activity in this population. Despite none of these studies having specifically addressed the benefits of physical exercise on sarcopenia, there is evidence that exercise is effective to increase aerobic capacity and muscle strength, and to improve body composition and inflammatory outcomes in PLWH. Therefore, the expected benefits of physical exercise are likely to translate into a successful and specific intervention for prevention and treatment of sarcopenia in this population.
1. Introduction
Sarcopenia is defined by the progressive reduction of muscle mass, muscle strength and function occurring in the elderly and in people with chronic conditions, such as metabolic syndrome, cardiovascular disease or cancer [1]. Starting from the age of thirty, our body faces a slow natural loss of muscle tissue. However, this process is accelerated in the elderly and in people with some pathological conditions. Its prevalence in the elderly population is largely variable, ranging from 5% to 50% depending on age, gender, pathological conditions and diagnostic criteria [2]. As examples, a large study of community-dwelling older people from United Kingdom—mean age 67 years—showed a prevalence of sarcopenia of 4.6% in men and 7.9% in women [3], whereas an earlier large survey in New Mexico documented an increased overall prevalence with age, from below 24% in women and men below 70 years of age to >50% in those over 80 [4].
Sarcopenia is clinically relevant because it is associated with an increased frequency of adverse health outcomes, such as frailty, functional disabilities, falls and, in general, quality-of-life impairment [5,6,7]. There is no effective treatment for sarcopenia, but physical exercise seems to be highly effective at counteracting the decline in muscle mass and strength associated with aging. Based on the evidence that resistance exercise increases muscle mass and strength, a recent position paper from the Society of Sarcopenia Cachexia and Wasting disorders (SCWD) recommended resistance exercise to any older persons for both secondary prevention and/or treatment of sarcopenia [8].
Sarcopenia is an emerging health issue in people living with HIV (PLWH). After more than three decades since the beginning of the AIDS epidemic, and following the introduction of combination antiretroviral therapy (cART), HIV infection has now become a chronic and manageable condition [9]. Consequently, the average life span of PLWH approaches today that of the general population [10]. However, the combination of aging with chronic inflammation, lifestyle factors and cART toxicities has contributed to an increasing risk of developing chronic diseases, also including sarcopenia, frailty and falls [11]. There are only few data on the prevalence of sarcopenia in PLWH, although, similarly to other chronic diseases, this syndrome seems to occur more frequently and at an earlier age than in the general population.
The aim of this review is to discuss the mechanisms and the clinical relevance of sarcopenia in PLWH, to describe the efficacy of physical activity interventions on muscle mass and function, and to provide evidence towards an effective treatment approach for sarcopenia in this population.
2. Mechanisms of Sarcopenia in People Living with HIV
A number of factors play a role in the development of sarcopenia in the general population, including lack of physical exercise, hormonal changes, nutritional deficiencies and low protein intake, metabolic alterations and chronic inflammation [12]. Among these risk factors, the presence of metabolic alterations and increased immune activation are key features of HIV infection in cART-treated persons. Chronic immune activation is characterized by an increased release of proinflammatory mediators, presence of dysfunctional T-regulatory cells and a T-cell-senescent phenotype. These alterations are also observed in the elderly, overall defining the conditions of “immunosenescence” and “inflammaging” [13]. Immune activation in PLWH may result from the persistence of HIV infection in latently infected cells, but other factors are likely to contribute, including cART, risk behaviours, e.g., smoking or use of drugs, and other comorbidities [14]. In turn, chronic inflammation is one of the most important risk factors for the developing of non-AIDS diseases, including cardiovascular, kidney or liver disease, cancers, some neurological diseases and “geriatric syndromes”, including sarcopenia, frailty and falls [15].
Sarcopenia is characterized by a progressive loss of muscle fibres that are replaced by adipose tissue, increasing fibrosis and changes in muscle metabolism [16]. Several mechanisms have been suggested to explain how persistent inflammation may lead to these changes in the muscular tissue (Figure 1). A first potential mechanism involves mitochondrial dysfunction. Immune activation is known to increase reactive oxygen species (ROS) intracellular concentration and cause redox balance disturbances [17], which, in turn, may lead to mitochondrial DNA damage due to its proximity to free radical sources and the relative lack of a protein scaffold. Consequently, mitochondrial DNA mutations can impair mitochondrial protein synthesis, determining loss of oxidative phosphorylation efficiency and, ultimately, premature cell senescence [18].
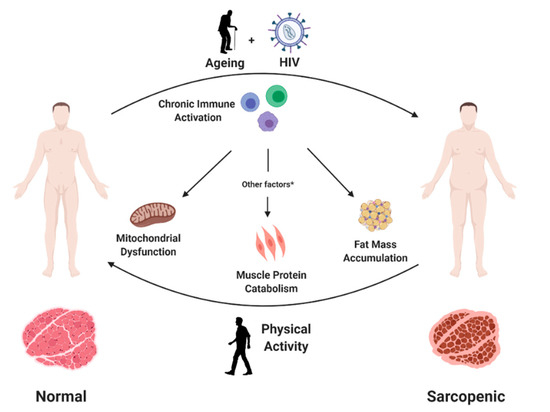
Figure 1.
Persistent HIV infection and ageing contribute to development of sarcopenia in people with HIV. Chronic immune activation, common to both conditions, may favour this process through mitochondrial dysfunction, increased muscle protein catabolism and muscle fat accumulation. Other factors include a low protein intake and vitamin D deficiency, among others. In contrast, physical exercise may prevent and partly reduce sarcopenia through increasing muscle mass and function and reducing chronic inflammation.
Another consequence of increased immune activation is the accumulation of macrophages and other immune cells in the adipose tissue, with an increased release of pro-inflammatory cytokines and adipokines [19]. These inflammatory mediators may favour lipid accumulation and trigger protein catabolism with loss of muscle mass and further increase local inflammation [20].
Some traditional risk factors may also specifically promote sarcopenia progression in PLWH, including a low vitamin D level [21] and factors associated with lifestyle [22]. The relevance of vitamin D for skeletal muscle metabolism has been highlighted in recent years, and ageing is associated with vitamin D deficiency likely resulting from decreased sun exposure and ability to synthesize Vitamin D, and reduced expression of vitamin D receptor in muscle tissue [21]. Vitamin D deficiency seems indeed to be more prevalent in PLWH compared to the general population, possibly associated with immune activation, exposure to specific antiretroviral drugs and high prevalence of metabolic diseases [23]; low-function subjects had a greater frequency of vitamin D deficiency compared to high-function subjects [24]. Among factors associated with lifestyle, cigarette smoking is also more prevalent in PLWH than in the general population [25] and it may also increase the chance of developing sarcopenia [22].
3. Epidemiological and Clinical Aspects of Sarcopenia in People Living with HIV
According to the original 2010 consensus of the European Working Group on Sarcopenia in Older People (EWGSOP) on the definition and diagnosis of sarcopenia [26] and the updated 2019 version [1], the operational definition of sarcopenia is based on the assessment of three specific aspects: (i) muscle strength, as measured by handgrip strength or a chair-stand test; (ii) muscle quality, as measured by total body or appendicular skeletal muscle mass by magnetic resonance imaging (MRI), computer tomography (CT) or dual-energy X-ray absorptiometry (DEXA), and further adjustable for height, weight or body mass index; and (iii) physical performance, as determined by measuring gait speed, e.g., during a six-minute walking test, or by using other composite tests, including walking, balance and chair-standing exercises. Sarcopenia is diagnosed in the presence of low values for the first two criteria, whereas it is considered severe when also the third criterion is met [1]. In PLWH, a large number of studies have assessed the prevalence and described the characteristics of one or more of these parameters taken separately, which were usually altered compared to the general population [9,27]. For instance, low muscle strength and impairment of physical performance are often found to be impaired in PLWH and these parameters are indeed used in the definition of frailty, another common syndrome in people ageing with HIV [27,28,29]. On the other hand, the assessment of muscle quality and volume by CT showed significantly lower muscle density and greater fat infiltration in PLWH compared to HIV-uninfected controls [30], whereas DEXA studies of body composition show that treated HIV-infected persons gain more fat, but lose lean mass over time compared with HIV-uninfected persons [31].
However, only few studies have reported the prevalence of sarcopenia as a syndrome resulting from the combination of the above measures in treated PLWH [27,32,33]. The Multicenter AIDS Cohort Study (MACS) showed a prevalence of 17% among 185 men with HIV, median age 60, with no differences compared to HIV-negative people of the same age [27]. Conversely, prevalence was 24% in a smaller Brazilian study [32], enrolling 33 HIV-positive persons, mean age 59 years old, with a 4.95 higher risk compared to older—mean age 70 years old—HIV-negative controls. Similarly, the prevalence of sarcopenia among 153 HIV-infected Asians was higher in those above 50 years of age compared to HIV-uninfected controls matched by age, sex and ethnicity (17% vs. 4%), but not significantly different when people below the age of 50 were included (10 vs. 6%) [33]. In a large Spanish cohort of PLWH assessed in the period 2000–2016, the overall prevalence of sarcopenia in those aged >50 years was 27.8%, with a different gender distribution, involving 43% of women and 8.8% of men [34].
Some of these studies also identified specific risk factors for sarcopenia in PLWH, in addition to traditional risk factors such as female gender, increasing age and high body mass index. These included low education level and employment status, long duration of HIV infection, low CD4+ T-cell counts at the start of cART and long-time exposure to certain nucleoside reverse transcriptase inhibitors (NRTIs), i.e., zidovudine, stavudine or didanosine [27,33,34]. Finally, specific health outcomes were also analysed in relation to the presence of sarcopenia, including mortality risk scores, quality of life, healthcare utilization, functional disability and falls. Out of these, sarcopenia was associated with a five-fold higher mortality scores and a four-fold higher risk of functional disability [27].
4. Exercise Intervention to Counteract Sarcopenia in PLWH
Sedentary lifestyle is one of the principal causes for loss of muscle mass and strength, which, in turn, determines further reduction of activity levels with further muscle weakness [35]. In contrast, regular physical exercise is highly effective at counteracting the decline in muscle mass and strength and, possibly, also in reducing the chronic inflammation associated with aging.
Indeed, physical activity represents the most effective strategy in the management of sarcopenia in the general population and in specific patient groups [36]. However, no study has specifically addressed the benefits of physical activity on sarcopenia in PLWH. There are several differences in sarcopenia between PLWH and the general elderly population, and the benefits of physical activity may also differ. PLWH may show a greater degree of immune activation and develop sarcopenia at an earlier age. In addition, the rate of sedentary lifestyle seems to be higher in PLWH than in the general population, as well as the frequency of risk behaviours such as smoking or drug use, which may limit adherence to exercise [37]. We have here reviewed the longitudinal interventional studies of physical activity in PLWH focusing on the outcomes that could be relevant for management of sarcopenia, i.e., physical fitness, body composition and inflammatory indexes (Table 1).

Table 1.
Longitudinal interventional studies that assessed the effect of physical activity on physical fitness, body composition and inflammatory markers in cART-treated people living with HIV.
Physical activity interventions are usually followed by improvement in physical function, because of an increase in cardiorespiratory and muscular fitness [38]. Cardiorespiratory fitness (CRF) reflects the integrated ability of the human organism to transport oxygen from the atmosphere to the mitochondria to perform physical work. CRF depends on a linked chain of processes, including pulmonary ventilation and diffusion, ventricular function, ventricular–arterial coupling, ability of the vasculature to accommodate and efficiently transport blood from the heart to match oxygen requirements and ability of the muscle cells to receive and use the oxygen and nutrients delivered by the blood [38]. CRF thus quantifies the functional capacity of an individual and is considered a reflection of total body health. Physical activity contributes to improved CRF, approximately for 45%–50% [38]. The maximal oxygen uptake (O2max) is the gold standard for measuring the integrated cardiopulmonary-muscle oxidative function, and studies using the O2max as fitness outcome have shown that and adequate physical exercise intervention invariably improves CRF in PLWH (Table 1).
Muscular fitness is a general term used to describe muscular performance in relation to strength, endurance and overall health. Muscular strength is measured in (i) dynamic strength: measure of the maximum weight that can be lifted once (1 Repetition Maximum, 1RM); (ii) static strength: measure of the maximum force that one can apply to an unmoving object (e.g., handgrip strength); and (iii) muscular endurance: measured through multiple lifting repetitions using weights that are below one’s maximum capacity. Muscular strength and endurance improve when muscle fibres grow stronger and new muscles form, and when the supply of oxygen and energy to the muscles becomes more efficient. In PLWH, several studies have proven that adequate resistance training exercises is successful in improving muscular strength, as assessed by using all of the above approaches (Table 1).
In longitudinal studies of physical activity in PLWH, the combination of improved cardiovascular and muscular fitness was associated with other health outcomes with relevance on sarcopenia, including improvements in body composition and inflammatory markers. In particular, a number of studies reported a reduction in fat mass and an increase in fat-free mass, using either dual-energy X ray absorptiometry, computed tomography or bio-impedentiometry. These results were observed irrespectively of type of exercise (endurance or resistance, or a combination of both), its duration and frequency (from 20 to 60 min for 2/3 times a week) and the duration of the study (from 6 to 48 weeks). A few recent studies have also investigated the effects of physical activity on inflammatory outcomes in PLWH. Most of these showed a reduction of soluble markers of inflammation, such as high-sensitivity C-reactive protein, Interleukin-6 and Interleukin-8 (Table 1). Additionally, physical exercise—both endurance and combined exercise training—was also followed by a marked decrease of the frequency of CD8+/CD38+/HLA-DR+ activated T-cells [60].
5. Conclusions
PLWH may show a degree of musculoskeletal impairment and an increased risk of sarcopenia, likely resulting from a combination of HIV-associated chronic immune activation, metabolic complications and advancing ageing. Different physical activity interventions have shown to be effective to increase muscle mass and function in this population. However, no study has so far addressed sarcopenia as specific target of physical exercise intervention in PLWH. Nevertheless, physical activity, in its various blends, increases aerobic capacity and muscle strength and this is associated with improvement in body composition and inflammatory outcomes. The information from longitudinal trials of physical exercise is relevant in order to prescribe cost-effective tailored exercise interventions and protocols that focus specifically for prevention and treatment of sarcopenia in PLWH.
Author Contributions
M.B. and P.C. designed the present review, reviewed the literature and wrote the manuscript. G.B., L.G. and F.T. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Italian Ministry of Health (Ricerca Corrente).
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Vitale, J.A.; Bonato, M.; La Torre, A. Banfi. The role of the molecular clock in promoting skeletal muscle growth and protecting against sarcopenia. Int. J. Mol. Sci. 2019, 20, 4318. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.P.; Syddall, H.E.; Jameson, K.; Robinson, S.; Denison, H.; Roberts, H.C.; Edwards, M.; Dennison, E.; Cooper, C.; Aihie Sayer, A. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: Findings from the Hertfordshire Cohort Study (HCS). Age Ageing 2013, 42, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Wayne, S.J.; Waters, D.L.; Janssen, I.; Gallagher, D.; Morley, J.E. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes. Res. 2004, 12, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. (1985) 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef]
- Vlietstra, L.; Hendrickx, W.; Waters, D.L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas. J. Ageing 2018, 37, 169–183. [Google Scholar] [CrossRef]
- Kaplan-Lewis, E.; Aberg, J.A.; Lee, M. Aging with HIV in the ART era. Semin. Diagn. Pathol. 2017, 34, 384–397. [Google Scholar] [CrossRef]
- Avila-Funes, J.A.; Aguilar-Navarro, S.; Melano-Carranza, E. Frailty, an enigmatic and controversial concept in geriatrics. The biological perspective. Gac. Med. Mex. 2008, 144, 255–262. [Google Scholar]
- Guaraldi, G.; Orlando, G.; Zona, S.; Menozzi, M.; Carli, F.; Garlassi, E.; Berti, A.; Rossi, E.; Roverato, A.; Palella, F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. 2011, 53, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.J.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Sereti, I.; Altfeld, M. Immune activation and HIV: An enduring relationship. Curr. Opin. HIV AIDS 2016, 11, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, K.L.; Brown, T.T.; Margolick, J.B.; Erlandson, K.M. Geriatric syndromes: New frontiers in HIV and sarcopenia. AIDS 2017, 31, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Nasi, M.; De Biasi, S.; Gibellini, L.; Bianchini, E.; Pecorini, S.; Bacca, V.; Guaraldi, G.; Mussini, C.; Pinti, M. Ageing and inflammation in patients with HIV infection. Clin. Exp. Immunol. 2017, 187, 44–52. [Google Scholar] [CrossRef]
- Ryall, J.G.; Schertzer, J.D.; Lynch, G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 2008, 9, 213–228. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Pinto, M.; Moraes, C.T. Mechanisms linking mtDNA damage and aging. Free Radic. Biol. Med. 2015, 85, 250–258. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Bhanji, R.A.; Narayanan, P.; Allen, A.M.; Malhi, H.; Watt, K.D. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in non-alcoholic steatohepatitis. Hepatology 2017, 66, 2055–2065. [Google Scholar] [CrossRef]
- Garcia, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H., Jr. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition 2019, 60, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Steffl, M.; Bhannon, R.W.; Petr, M.; Kohlikova, E.; Holmerova, I. Relation between cigarette smoking and sarcopenia: Meta-analysis. Physiol. Res. 2015, 64, 419–426. [Google Scholar] [PubMed]
- Hsieh, E.; Yin, M.T. Continued Interest and Controversy: Vitamin D in HIV. Curr. HIV/AIDS Rep. 2018, 15, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Erlandson, K.M.; Allshouse, A.A.; Jankowski, C.M.; MaWhinney, S.; Kohrt, W.M.; Campbell, T.B. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J. Acquir. Immune Defic. Syndr. 2013, 63, 209–215. [Google Scholar] [CrossRef]
- Frazier, E.L.; Sutton, M.Y.; Brooks, J.T.; Shouse, R.L.; Weiser, J. Trends in cigarette smoking among adults with HIV compared with the general adult population, United States—2009–2014. Prev. Med. 2018, 111, 231–234. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Hawkins, K.L.; Zhang, L.; Ng, D.K.; Althoff, K.N.; Palella, F.J.J.; Kingsley, L.A.; Jacobson, L.P.; Margolick, J.B.; Lake, J.E.; Brown, T.T.; et al. Abdominal obesity, sarcopenia, and osteoporosis are associated with frailty in men living with and without HIV. AIDS 2018, 32, 1257–1266. [Google Scholar] [CrossRef]
- Erlandson, K.M.; Schrack, J.A.; Jankowski, C.M.; Brown, T.T.; Campbell, T.B. Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr. HIV/AIDS Rep. 2014, 11, 279–290. [Google Scholar] [CrossRef]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I.; Gerontopole Brussels Study Group. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef]
- Natsag, J.; Erlandson, K.M.; Sellmeyer, D.E.; Haberlen, S.A.; Margolick, J.; Jacobson, L.P.; Palella, F.J., Jr.; Koletar, S.L.; Lake, J.E.; Post, W.S.; et al. HIV infection is associated with increased fatty infiltration of the thigh muscle with aging independent of fat distribution. PloS ONE 2017, 12, e0169184. [Google Scholar] [CrossRef]
- Grant, P.M.; Kitch, D.; McComsey, G.A.; Collier, A.C.; Bartali, B.; Koletar, S.L.; Erlandson, K.M.; Lake, J.E.; Yin, M.T.; Melbourne, K.; et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS. 2016, 30, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Pinto Neto, L.F.; Sales, M.C.; Scaramussa, E.S.; da Paz, C.J.; Morelato, R.L. Human immunodeficiency virus infection and its association with sarcopenia. Braz. J. Infect. Dis. 2016, 20, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.A.A.; Megan, M.; Bashah, N.S.A.; Chong, M.L.; Sasheelaa, P.; Omar, S.F.S.; Sulaiman, H.; Azwa, I.; Tan, P.T.; Kamarulzaman, A.; et al. Assessment of sarcopenia in virally suppressed HIV-infected Asians receiving treatment. AIDS 2019, 33, 769. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, P.; Bonjoch, A.; Puig, J.; Estany, C.; Ornelas, A.; Clotet, B.; Negredo, E. High Prevalence of Sarcopenia in HIV-Infected Individuals. Biomed. Res. Int. 2018, 12, 5074923. [Google Scholar] [CrossRef]
- Landi, F.; Marzetti, E.; Martone, A.M.; Bernabei, R.; Onder, G. Exercise as a remedy for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care. 2014, 17, 25–31. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Broccatelli, M.; Savera, G.; D’Elia, M.; Pahor, M.; et al. SPRINTT Consortium. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef]
- Onen, N.F.; Overton, E.T.; Seyfried, W.; Stumm, E.R.; Snell, M.; Mondy, K.; Tebas, P. Aging and HIV infection: A comparison between older HIV-infected persons and the general population. HIV Clin. Trials 2010, 11, 100–109. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation 2016, 13, e653–e699. [Google Scholar] [CrossRef]
- Roubenoff, R.; Weiss, L.; McDermott, A.; Heflin, T.; Cloutier, G.J.; Wood, M.; Gorbach, S. A pilot study of exercise training to reduce trunk fat in adults with HIV-associated fat redistribution. AIDS 1999, 13, 1373–1375. [Google Scholar] [CrossRef]
- Agin, D.; Gallagher, D.; Wang, J.; Heymsfield, S.B.; Pierson, R.N., Jr.; Kotler, D.P. Effects of whey protein and resistance exercise on body cell mass, muscle strength, and quality of life in women with HIV. AIDS 2001, 15, 2431–2440. [Google Scholar] [CrossRef]
- Yarasheski, K.E.; Tebas, P.; Stanerson, B.; Claxton, S.; Marin, D.; Bae, K.; Kennedy, M.; Tantisiriwat, W.; Powderly, W.G. Resistance exercise training reduces hypertriglyceridemia in HIV-infected men treated with antiviral therapy. J. Appl. Physiol. (1985) 2001, 90, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R.; Abad, L.W.; Lundgren, N. Effect of acquired immune deficiency syndrome wasting on the protein metabolic response to acute exercise. Metabolism 2001, 50, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Thöni, G.J.; Fedou, C.; Brun, J.F.; Fabre, J.; Renard, E.; Reynes, J.; Varray, A.; Mercier, J. Reduction of fat accumulation and lipid disorders by individualized light aerobic training in human immunodeficiency virus infected patients with lipodystrophy and/or dyslipidemia. Diabetes Metab. 2002, 28, 397–404. [Google Scholar] [PubMed]
- Driscoll, S.D.; Meininger, G.E.; Lareau, M.T.; Dolan, S.E.; Killilea, K.M.; Hadigan, C.M.; Lloyd-Jones, D.M.; Klibanski, A.; Frontera, W.R.; Grinspoon, S.K. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV-infected patients. AIDS 2004, 18, 465–473. [Google Scholar] [CrossRef]
- Driscoll, S.D.; Meininger, G.E.; Ljungquist, K.; Hadigan, C.; Torriani, M.; Klibanski, A.; Frontera, W.R.; Grinspoon, S. Differential effects of metformin and exercise on muscle adiposity and metabolic indices in human immunodeficiency virus-infected patients. J. Clin. Endocrinol. Metab. 2004, 89, 2171–2178. [Google Scholar] [CrossRef]
- Engelson, E.S.; Agin, D.; Kenya, S.; Werber-Zion, G.; Luty, B.; Albu, J.B.; Kotler, D.P. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism 2006, 2006 55, 1327–13236. [Google Scholar] [CrossRef]
- Terry, L.; Sprinz, E.; Stein, R.; Medeiros, N.B.; Oliveira, J.; Ribeiro, J.P. Exercise training in HIV-1-infected individuals with dyslipidemia and lipodystrophy. Med. Sci. Sports. Exerc. 2006, 38, 411–417. [Google Scholar] [CrossRef]
- Dolan, S.E.; Frontera, W.; Librizzi, J.; Ljungquist, K.; Juan, S.; Dorman, R.; Cole, M.E.; Kanter, J.R.; Grinspoon, S. Effects of a supervised home-based aerobic and progressive resistance training regimen in women infected with human immunodeficiency virus: A randomized trial. Arch. Intern. Med. 2006, 166, 1225–1231. [Google Scholar] [CrossRef]
- Robinson, F.P.; Quinn, L.T.; Rimmer, J.H. Effects of high-intensity endurance and resistance exercise on HIV metabolic abnormalities: A pilot study. Biol. Res. Nurs. 2007, 8, 177–185. [Google Scholar] [CrossRef]
- Hand, G.A.; Phillips, K.D.; Dudgeon, W.D.; William Lyerly, G.; Larry Durstine, J.; Burgess, S.E. Moderate intensity exercise training reverses functional aerobic impairment in HIV-infected individuals. AIDS Care 2008, 20, 1066–1074. [Google Scholar] [CrossRef]
- Lindegaard, B.; Hansen, T.; Hvid, T.; van Hall, G.; Plomgaard, P.; Ditlevsen, S.; Gerstoft, J.; Pedersen, B.K. The effect of strength and endurance training on insulin sensitivity and fat distribution in human immunodeficiency virus-infected patients with lipodystrophy. J. Clin. Endocrinol. Metab. 2008, 93, 3860–3869. [Google Scholar] [CrossRef] [PubMed]
- Farinatti, P.T.; Borges, J.P.; Gomes, R.D.; Lima, D.; Fleck, S.J. Effects of a supervised exercise program on the physical fitness and immunological function of HIV-infected patients. J. Sports Med. Phys. Fit. 2010, 50, 511–518. [Google Scholar]
- Souza, P.M.; Jacob-Filho, W.; Santarém, J.M.; Zomignan, A.A.; Burattini, M.N. Effect of progressive resistance exercise on strength evolution of elderly patients living with HIV compared to healthy controls. Clinics 2011, 66, 261–266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dudgeon, W.D.; Jaggers, J.R.; Phillips, K.D.; Durstine, J.L.; Burgess, S.E.; Lyerly, G.W.; Davis, J.M.; Hand, G.A. Moderate-Intensity Exercise Improves Body Composition and Improves Physiological Markers of Stress in HIV-Infected Men. ISRN AIDS 2012, 11, 145127. [Google Scholar] [CrossRef] [PubMed]
- Broholm, C.; Mathur, N.; Hvid, T.; Grøndahl, T.S.; Frøsig, C.; Pedersen, B.K.; Lindegaard, B. Insulin signaling in skeletal muscle of HIV-infected patients in response to endurance and strength training. Physiol. Rep. 2013, 1, e00060. [Google Scholar] [CrossRef] [PubMed]
- Ezema, C.I.; Onwunali, A.A.; Lamina, S.; Ezugwu, U.A.; Amaeze, A.A.; Nwankwo, M.J. Effect of aerobic exercise training on cardiovascular parameters and CD4 cell count of people living with human immunodeficiency virus/acquired immune deficiency syndrome: A randomized controlled trial. Niger. J. Clin. Pract. 2014, 7, 543–548. [Google Scholar]
- Ahmad, B.; Glufke, K.; Grau, M.; Sandig, D.; Rockstroh, J.; Vogel, M.; Wasmuth, J.C.; Bloch, W.; Brixius, K. Influence of endurance training and marathon running on red cell deformability in HIV patients. Clin. Hemorheol. Microcirc. 2014, 57, 355–366. [Google Scholar] [CrossRef]
- Garcia, A.; Fraga, G.A.; Vieira, R.C., Jr.; Silva, C.M.; Trombeta, J.C.; Navalta, J.W.; Prestes, J.; Voltarelli, F.A. Effects of combined exercise training on immunological, physical and biochemical parameters in individuals with HIV/AIDS. J. Sports. Sci. 2014, 32, 785–792. [Google Scholar] [CrossRef]
- Zanetti, H.R.; da Cruz, L.G.; Lourenço, C.L.; Neves, F.F.; Silva-Vergara, M.L.; Mendes, E.L. Does nonlinear resistance training reduce metabolic syndrome in people living with HIV? A randomized clinical trial. J. Sports Med. Phys. Fit. 2017, 57, 678–684. [Google Scholar]
- Bonato, M.; Galli, L.; Passeri, L.; Longo, V.; Pavei, G.; Bossolasco, S.; Bertocchi, C.; Cernuschi, M.; Balconi, G.; Merati, G.; et al. A pilot study of brisk walking in sedentary combination antiretroviral treatement (cART)- treated patients: Benefit on soluble and cell inflammatory markers. BMC Infect. Dis. 2017, 17, 61. [Google Scholar] [CrossRef]
- Pedro, R.E.; Candido, N.; Guariglia, D.A.; Melo, B.P.; Bertolini, D.A.; Peres, S.B.; Franzói de Moraes, S.M. Exercise improves cytokine profile in HIV-infected people: A randomized clinical trial. Cytokine 2017, 99, 18–23. [Google Scholar] [CrossRef]
- Zanetti, H.R.; Cruz, L.G.; Lourenço, C.L.; Neves Fde, F.; Silva-Vergara, M.L.; Mendes, E.L. Non-linear resistance training reduces inflammatory biomarkers in persons living with HIV: A randomized controlled trial. Eur. J. Sport. Sci. 2016, 16, 1232–1239. [Google Scholar] [CrossRef]
- Oursler, K.K.; Sorkin, J.D.; Ryan, A.S.; Katzel, L.I. A pilot randomized aerobic exercise trial in older HIV-infected men: Insights into strategies for successful aging with HIV. PLoS ONE 2018, 13, e0198855. [Google Scholar] [CrossRef] [PubMed]
- Vingren, J.L.; Curtis, J.H.; Levitt, D.E.; Duplanty, A.A.; Lee, E.C.; McFarlin, B.K.; Hill, D.W. Adding Resistance Training to the Standard of Care for Inpatient Substance Abuse Treatment in Men with Human Immunodeficiency Virus Improves Skeletal Muscle Health Without Altering Cytokine Concentrations. J. Strength Cond. Res. 2018, 32, 76–82. [Google Scholar] [CrossRef] [PubMed]
- De Brito-Neto, J.G.; de Andrade, M.F.; de Almeida, V.D.; Paiva, D.C.C.; de Morais, N.M.; Bezerra, C.M.; Fernandes, J.V.; do Nascimento, E.G.C.; Fonseca, I.A.T.; de Medeiros Fernandes, T.A.A. Strength training improves body composition, muscle strength and increases CD4+ T lymphocyte levels in people living with HIV/AIDS. Infect. Dis. Rep. 2019, 11, 7925. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, H.R.; Gonçalves, A.; Paranhos Lopes, L.T.; Mendes, E.L.; Roever, L.; Silva-Vergara, M.L.; Neves, F.F.; Resende, E.S. Effects of exercise training and statin use in people living with HIV with dyslipidemia. Med. Sci. Sports Exerc. 2019. [Google Scholar] [CrossRef]
- Bonato, M.; Turrini, F.; De Zan, V.; Meloni, A.; Plebani, M.; Brambilla, E.; Giordani, A.; Vitobello, C.; Caccia, R.; Piacentini, M.F.; et al. A Mobile Application for Exercise Intervention in People Living with HIV. Med. Sci. Sports Exerc. 2019, 52, 425–433. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).