Shared Decision Making Enhances Pneumococcal Vaccination Rates in Adult Patients in Outpatient Care
Abstract
1. Introduction
2. Materials and Methods
- Patient activation.
- Bi-directional exchange of information.
- Bi-directional deliberation of options.
3. Results
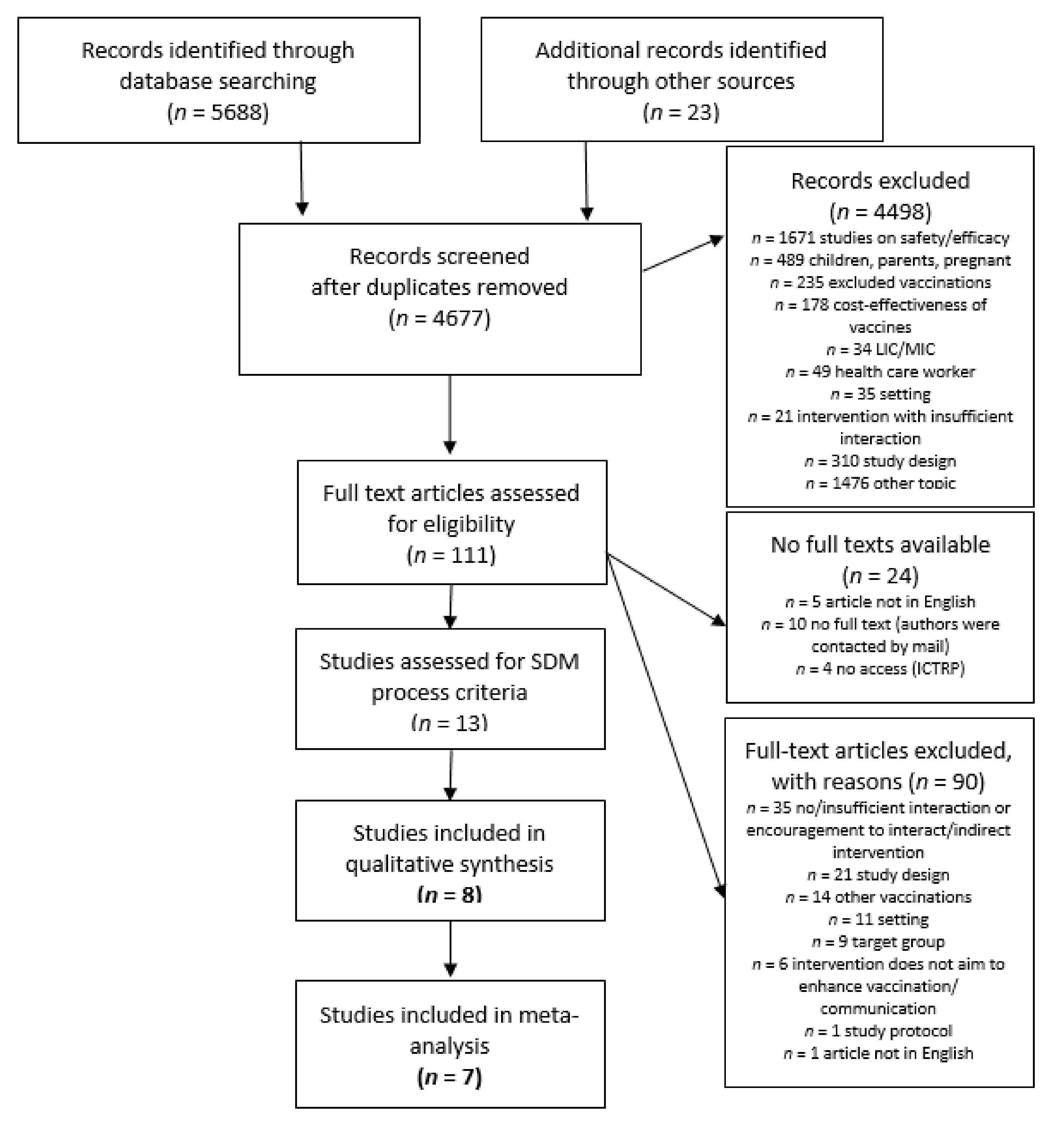
3.1. Included Studies
3.2. Characteristics of Interventions
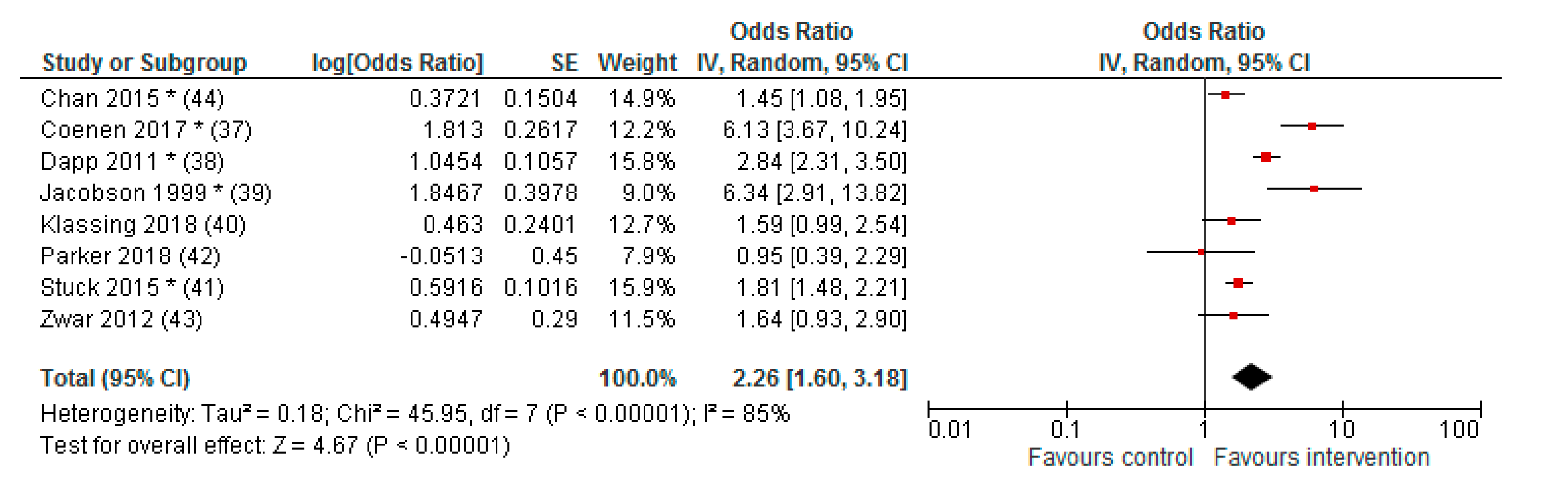
3.3. Effect of Interventions
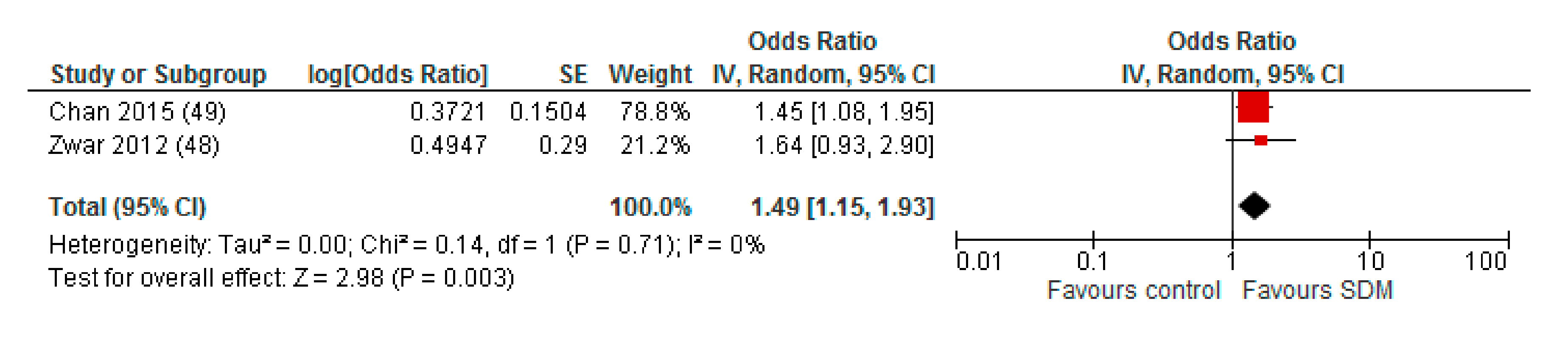
3.4. Subgroup Analyses
3.5. Sensitivity Analysis
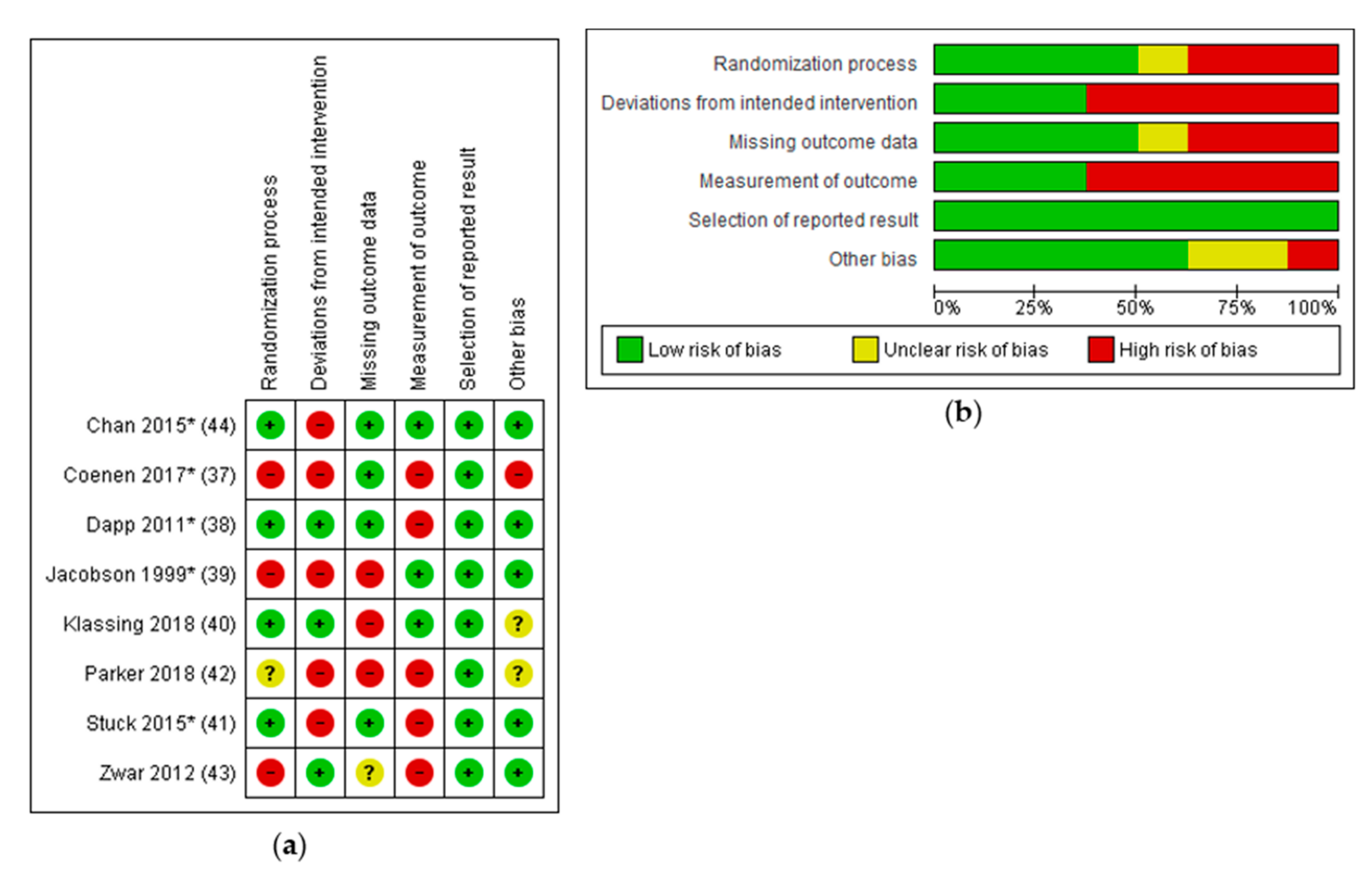
3.6. Risk of Bias in Included Studies
4. Discussion
4.1. Summary of Results
4.2. Interpretation of Results
4.3. Comparison to Previous Research
4.4. Strenghts and Limitations
4.5. Further Research
4.6. Implications for Policy and Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. Sensitivity Analysis: Studies with a Maximum of Two RoB Domains Assessed as “High Risk”
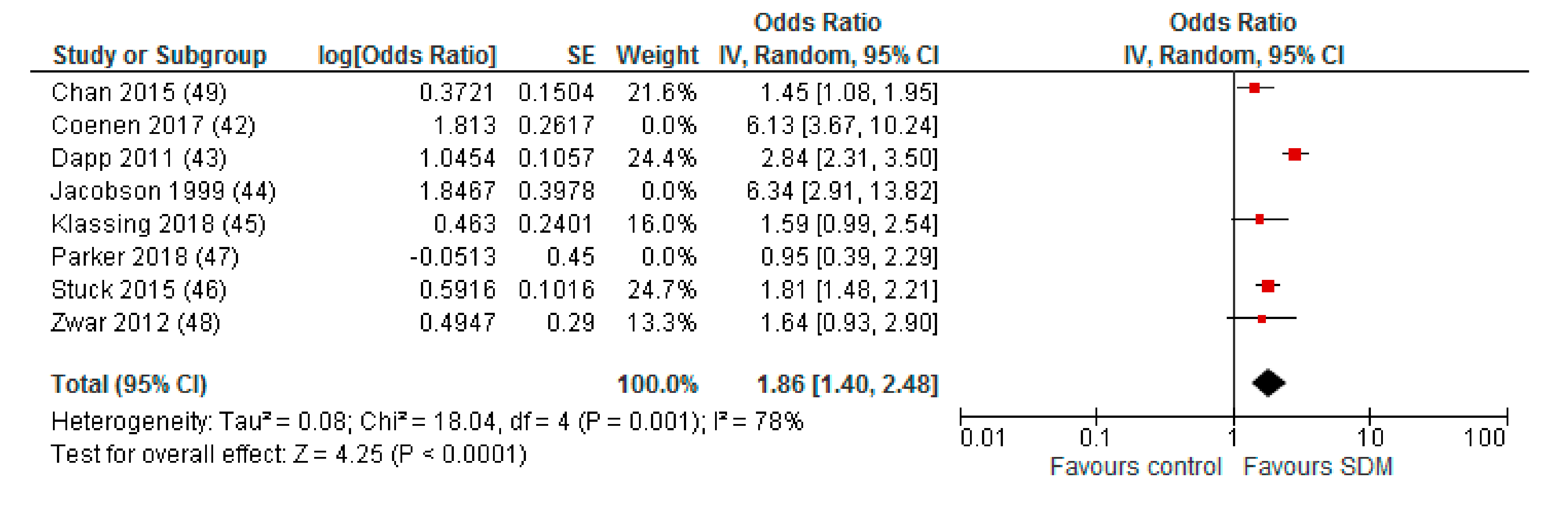
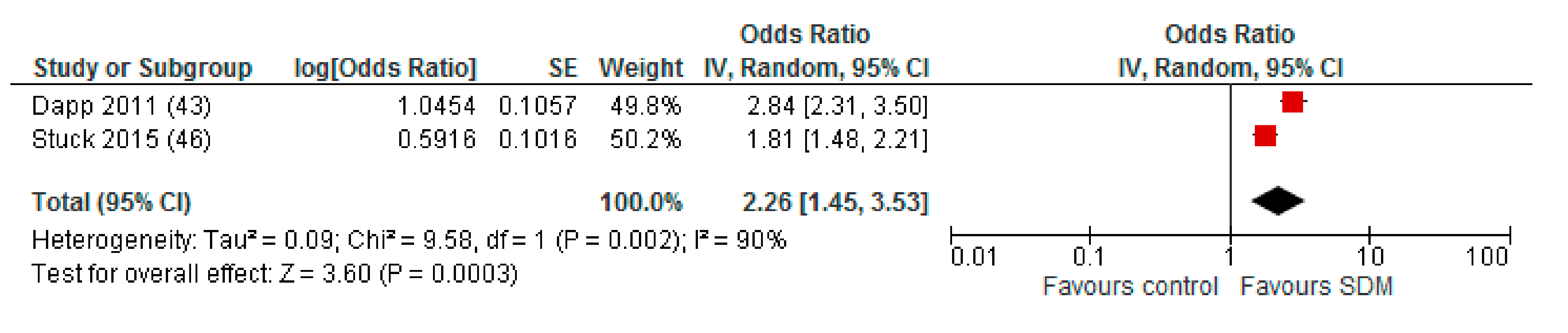
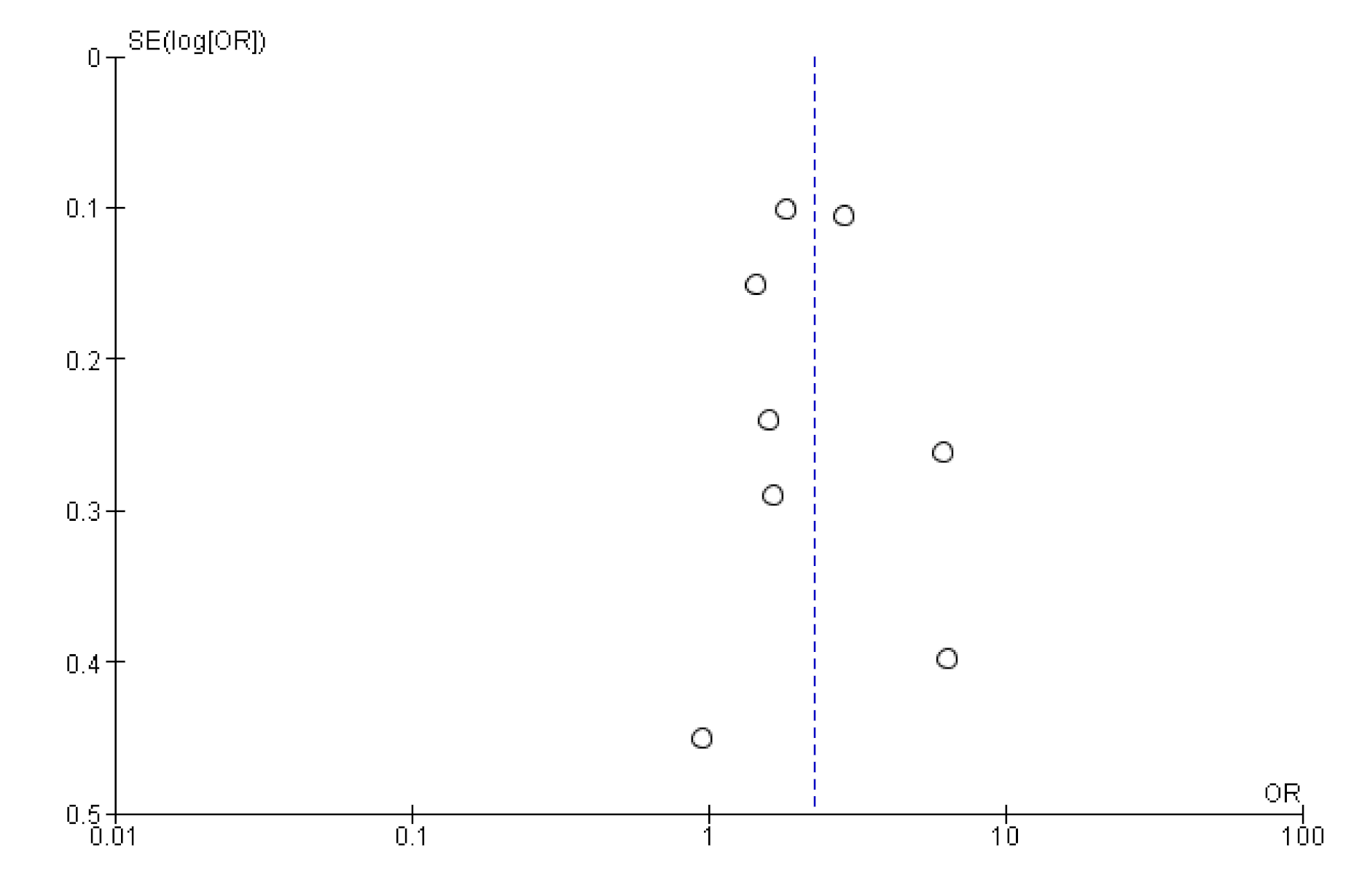
Appendix B. Funnel Plot of Effects on Pneumococcal Vaccination Rate
References
- Blasi, F.; Mantero, M.; Santus, P.; Tarsia, P. Understanding the burden of pneumococcal disease in adults. Clin. Microbiol. Infect. 2012, 18, 7–14. [Google Scholar] [CrossRef]
- Weycker, D.; Strutton, D.; Edelsberg, J.; Sato, R.; Jackson, L.A. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine 2010, 28, 4955–4960. [Google Scholar] [CrossRef]
- European Center for Disease Prevention and Control. Disease Factsheet about Pneumococcal Disease. Available online: https://www.ecdc.europa.eu/en/pneumococcal-disease/facts (accessed on 25 May 2020).
- Berical, A.C.; Harris, D.; Dela Cruz, C.S.; Possick, J.D. Pneumococcal Vaccination Strategies. An Update and Perspective. Ann. Am. Thorac. Soc. 2016, 13, 933–944. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC). Pneumococcal Vaccination: What Everyone Should Know. Available online: https://www.cdc.gov/vaccines/vpd/pneumo/public/index.html (accessed on 25 August 2020).
- National Health Service (NHS). Pneumococcal Vaccine Overview. Available online: https://www.nhs.uk/conditions/vaccinations/pneumococcal-vaccination/ (accessed on 25 August 2020).
- World Health Organization (WHO). Pneumococcal Conjugate Vaccines. Available online: https://www.who.int/biologicals/areas/vaccines/pneumo/en/ (accessed on 25 August 2020).
- Australian Government Department of Health. National Immunisation Program Schedule. 2019. Available online: https://www.health.gov.au/resources/publications/national-immunisation-program-schedule-landscape (accessed on 25 August 2020).
- NHS. NHS Vaccinations and when to Have Them. Available online: https://www.nhs.uk/conditions/vaccinations/nhs-vaccinations-and-when-to-have-them/ (accessed on 28 May 2020).
- Robert Koch-Institut. Impfkalender (Standardimpfungen) für Säuglinge, Kinder, Jugendliche und Erwachsene. Epidemiologisches Bulletin; Robert Koch-Institut: Berlin, Germany, 2019; p. 34. [Google Scholar]
- CDC. Recommended Adult Immunization Schedule. 2020. Available online: https://www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.html (accessed on 28 May 2020).
- Dyda, A.; Karki, S.; Hayen, A.; MacIntyre, C.R.; Menzies, R.; Banks, E.; Kaldor, J.M.; Liu, B. Influenza and pneumococcal vaccination in Australian adults: A systematic review of coverage and factors associated with uptake. BMC Infect. Dis. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Theidel, U.; Kuhlmann, A.; Braem, A. Pneumocccal vaccination rates in adults in Germany—An analysis of statutory health insurance data on more than 850 000 individuals. Dtsch. Arztebl. Int. 2013, 110, 743–750. [Google Scholar] [PubMed]
- CDC. Adult VaxView. 2008 through 2018 Adult Vaccination Coverage Trend Report. Available online: https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/data-reports/general-population/trend/index.html (accessed on 28 August 2020).
- WHO. MOV Intervention Guidebook for Implementing and Monitoring Activities to Reduce Missed Opportunities for Vaccination; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Anderson, E.L. Recommended solutions to the barriers to immunization in children and adults. Mol. Med. 2014, 111, 344–348. [Google Scholar]
- Lode, H.; Ludwig, E.; Kassianos, G. Pneumococcal Infection—Low Awareness as a Potential Barrier to Vaccination: Results of a European Survey. Adv. Ther. 2013, 30, 387–405. [Google Scholar] [CrossRef]
- Rehm, S.J.; File, T.M.; Metersky, M.; Nichol, K.L.; Schaffner, W. Identifying Barriers to Adult Pneumococcal Vaccination: An NFID Task Force Meeting. Postgrad. Med. J. 2012, 124, 71–79. [Google Scholar] [CrossRef]
- Johnson, D.R.; Nichol, K.L.; Lipczynski, K. Barriers to adult immunization. Am. J. Med. 2008, 121, 28–35. [Google Scholar] [CrossRef]
- Dube, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J. Vaccine hesitancy: An overview. Hum. Vaccines Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef]
- Holt, D.; Bouder, F.; Elemuwa, C.; Gaedicke, G.; Khamesipour, A.; Kisler, B.; Kochhar, S.; Kutalek, R.; Maurer, W.; Obermeirer, P.; et al. The importance of the patient voice in vaccination and vaccine safety—Are we listening? Clin. Microbiol. Infect. 2016, 22, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Hobson-West, P. ‘Trusting blindly can be the biggest risk of all’: Organised resistance to childhood vaccination in the UK. Sociol. Health Illn. 2007, 29, 198–215. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, N.E.; Butler, R.; Dubé, E. Addressing barriers to vaccine acceptance: An overview. Hum. Vaccines Immunother. 2018, 14, 218–224. [Google Scholar] [CrossRef]
- European Observatory on Health Systems. The Organization and Delivery of Vaccination Services in the European Union; Rechel, B., Richardson, E., Eds.; WHO: London, UK, 2018. [Google Scholar]
- Stiggelbout, A.M.; Pieterse, A.H.; de Haes, J.C. Shared decision making: Concepts, evidence, and practice. Patient Educ. Couns. 2015, 98, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.; Gafni, A.; Whelan, T. Shared decision-making in the medical encounter: What does it mean? (or it takes at least two to tango). Soc. Sci. Med. 1997, 44, 681–692. [Google Scholar] [CrossRef]
- Charles, C.; Gafni, A.; Whelan, T. Decision-making in the physician-patient encounter: Revisiting the shared treatment decision-making model. Soc. Sci. Med. 1999, 49, 651–661. [Google Scholar] [CrossRef]
- Elwyn, G.; Froch, D.; Thomson, R.; Joseph-Williams, N.; Lloyd, A.; Kinnersley, P.; Cording, E.; Tomson, D.; Dodd, C.; Rollnick, S.; et al. Shared decision making: A model for clinical practice. J. Gen. Intern. Med. 2012, 27, 1361–1367. [Google Scholar] [CrossRef]
- Makoul, G.; Clayman, M.L. An integrative model of shared decision making in medical encounters. Pat. Educ. Couns. 2006, 60, 301–312. [Google Scholar] [CrossRef]
- World Bank. World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 4 November 2020).
- Martinez-Gonzalez, N.A.; Plate, A.; Senn, O.; Markun, S.; Rosemann, T.; Neuner-Jehle, S. Shared decision-making for prostate cancer screening and treatment: A systematic review of randomised controlled trials. Swiss Med. Wkly. 2018, 148. [Google Scholar] [CrossRef]
- Scholl, I.; Koelewijn-van Loon, M.; Sepucha, K.; Elwyn, G.; Légaré, F.; Härter, M.; Dirmaier, J. Measurement of shared decision making— a review of instruments. Z. Evid. Fortbild. Qual. Gesundhwes 2011, 105, 313–324. [Google Scholar] [CrossRef]
- Härter, M. Partizipative Entscheidungsfindung (Shared Decision Making)—Ein von Patienten, Ärzten und der Gesundheitspolitik geforderter Ansatz setzt sich durch. Z. Ärztl. Fortbild. Qual. Gesundhwes 2004, 98, 89–92. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions version 6.0, 2nd ed.; Higgins, J.P.T., Thomas, J., Eds.; John Wiley & Sons: Chichester, UK, 2019; Available online: https://training.cochrane.org/handbook/current (accessed on 11 November 2020).
- GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Schünemann, H., Brozek, J., Eds.; The GRADE Working Group, 2013; Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 11 November 2020).
- Coenen, S.; Weyts, E.; Jorissen, C.; de Munter, P.; Noman, M.; Ballet, V.; Vermeire, S.; van Assche, G.; Ferrante, M. Effects of Education and Information on Vaccination Behavior in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 318–324. [Google Scholar] [CrossRef]
- Dapp, U.; Anders, J.A.M.; von Renteln-Kruse, W.; Minder, C.E.; Meier-Baumgartner, H.P.; Swift, C.G. A randomized trial of effects of health risk appraisal combined with group sessions or home visits on preventive behaviors in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Thomas, D.M.; Morton, F.J.; Offutt, G.; Shevlin, J.; Ray, S. Use of a low-literacy patient education tool to enhance pneumococcal vaccination rates. A randomized controlled trial. JAMA 1999, 282, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Klassing, H.M.; Ruisinger, J.F.; Prohaska, E.S.; Melton, B.L. Evaluation of Pharmacist-Initiated Interventions on Vaccination Rates in Patients with Asthma or COPD. J. Community Health 2018, 43, 297–303. [Google Scholar] [CrossRef]
- Stuck, A.E.; Moser, A.; Morf, U.; Wirz, U.; Wyser, J.; Gillmann, G.; Born, S.; Zwahlen, M.; Iliffe, S.; Harari, D.; et al. Effect of health risk assessment and counselling on health behaviour and survival in older people: A pragmatic randomised trial. PLoS Med. 2015, 12, e1001889. [Google Scholar] [CrossRef]
- Parker, P.A.; Banerjee, S.C.; Matasar, M.J.; Bylund, C.L.; Rogers, M.; Franco, K.; Schofield, E.; Li, Y.; Levin, T.T.; Jacobsen, P.B.; et al. Efficacy of a survivorship-focused consultation versus a time-controlled rehabilitation consultation in patients with lymphoma: A cluster randomized controlled trial. Cancer 2018, 124, 4567–4576. [Google Scholar] [CrossRef]
- Zwar, N.A.; Hermiz, O.; Comino, E.; Middleton, S.; Vagholkar, S.; Xuan, W.; Wilson, S.F.; Marks, G.B. Care of patients with a diagnosis of chronic obstructive pulmonary disease: A cluster randomised controlled trial. Med. J. Aust. 2012, 197, 394–398. [Google Scholar] [CrossRef]
- Chan, S.S.; Leung, D.Y.; Leung, A.Y.; Lam, C.; Hung, I.; Chu, D.; Chan, C.K.; Johnston, J.; Liu, S.H.; Liang, R.; et al. A nurse-delivered brief health education intervention to improve pneumococcal vaccination rate among older patients with chronic diseases: A cluster randomized controlled trial. Int. J. Nurs. Stud. 2015, 52, 317–324. [Google Scholar] [CrossRef]
- Jacobson Vann, J.C.; Jacobson, R.M.; Coyne-Beasley, T.; Asafu-Adjei, J.K.; Szilagyi, P.G. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst. Rev. 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Hu, J.; Majumdar, S.R.; Storie, D.A.; Rees, S.E.; Johnson, J.A. Interventions to improve influenza and pneumococcal vaccination rates among community-dwelling adults: A systematic review and meta-analysis. Ann. Fam. Med. 2012, 10, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Lorenzetti, D.L. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Friberg, F.; Granum, V.; Bergh, A.L. Nurses’ patient-education work: Conditional factors—An integrative review. J. Nurs. Manag. 2012, 20, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Bergh, A.L.; Friberg, F.; Persson, E.; Dahlborg-Lyckhage, E. Registered Nurses’ Patient Education in Everyday Primary Care Practice: Managers’ Discourses. Glob. Qual. Nurs. Res. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Bergh, A.L.; Karlsson, J.; Persson, E.; Friberg, F. Registered nurses’ perceptions of conditions for patient education—focusing on organisational, environmental and professional cooperation aspects. J. Nurs. Manag. 2012, 20, 758–770. [Google Scholar] [CrossRef]
- Gensichen, J.; Jaeger, C.; Peitz, M.; Torge, M.; Güthlin, C.; Mergenthal, K.; Kleppel, V.; Gerlach, F.M.; Petersen, J.J. Health care assistants in primary care depression management: Role perception, burdening factors, and disease conception. Ann. Fam. Med. 2009, 7, 513–519. [Google Scholar] [CrossRef][Green Version]
- OECD. Realising the Potential of Primary Health Care, OECD Health Policy Studies; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Chen, X.; Hay, J.L.; Waters, E.A.; Kiviniemi, M.T.; Biddle, C.; Schofield, E.; Li, Y.; Kaphingst, K.; Orom, H. Health Literacy and Use and Trust in Health Information. J. Health Commun. 2018, 23, 724–734. [Google Scholar] [CrossRef]
- Donohue, J.M.; Huskamp, H.A.; Wilson, I.B.; Weissman, J. Whom do older adults trust most to provide information about prescription drugs? Am. J. Geriatr. Pharmacother. 2009, 7, 105–116. [Google Scholar] [CrossRef]
- Poínhos, R.; Oliveira, B.M.P.M.; van der Lans, I.A.; Fischer, A.R.H.; Berezowska, A.; Rankin, A.; Kuznesof, S.; Stewart-Knox, B.; Frewer, L.J.; de Almeida, M.D.V. Providing Personalised Nutrition: Consumers’ Trust and Preferences Regarding Sources of Information, Service Providers and Regulators, and Communication Channels. Public Health Genom. 2017, 20, 218–228. [Google Scholar] [CrossRef]
- Paterson, P.; Meurice, F.; Stanberry, L.R.; Glismann, S.; Rosenthal, S.L.; Larson, H.J. Vaccine hesitancy and healthcare providers. Vaccine 2016, 34, 6700–6706. [Google Scholar] [CrossRef] [PubMed]
- Winston, C.A.; Mims, A.D.; Leatherwood, K.A. Increasing pneumococcal vaccination in managed care through telephone outreach. Am. J. Manag. Care 2007, 13, 581–588. [Google Scholar] [PubMed]
- Siebers, M.J.; Hunt, V.B. Increasing the Pneumococcal Vaccination Rate of Elderly Patients in a General Internal Medicine Clinic. J. Am. Geriatr. Soc. 1985, 33, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Kriston, L.; Scholl, I.; Hölzel, L.; Simon, D.; Loh, A.; Härter, M. The 9-item Shared Decision Making Questionnaire (SDM-Q-9)e). Development and psychometric properties in a primary care sample. Patient Educ. Couns. 2010, 80, 94–99. [Google Scholar]
- Sepucha, K.R.; Breslin, M.; Graffeo, C.; Carpenter, C.R.; Hess, E.P. State of the Science: Tools and Measurement for Shared Decision Making. Acad. Emerg. Med. 2016, 23, 1325–1331. [Google Scholar] [CrossRef]
- Paguio, J.A.; Yao, J.S.; Dee, E.C. Silver lining of COVID-19: Heightened global interest in pneumococcal and influenza vaccines, an infodemiology study. Vaccine 2020, 38, 5430–5435. [Google Scholar] [CrossRef]
- UNICEF Supply Division. Seasonal Influenza Vaccine: Supply Update. 2020. Available online: https://www.unicef.org/supply/documents/seasonal-influenza-vaccine-supply-note (accessed on 24 October 2020).
- Paul-Ehrlich-Institut. Federal Institut for Vaccines and Biomedicines. Pneumococcal Vaccine Pneumovax 23 Imported from Japan. Available online: https://www.pei.de/EN/newsroom/hp-news/2020/200401-pneumococcal-vaccines-from-japan.html (accessed on 14 October 2020).
- WHO; UNICEF. Immunization in the Context of COVID-19 Pandemic. Frequently Asked Questions (FAQ) 2020. Available online: WHO/2019-nCoV/immunization_services/FAQ/2020.1 (accessed on 14 October 2020).
- Ledig, T.; Egidi, G.; Schneider-Rathert, W.; Uebel, T. Impfen um jeden Preis? Impfmüdigkeit in Deutschland? Ein Positionspapier der Deutschen Gesellschaft für Allgemeinmedizin und Familienmedizin (DEGAM). Z. Allg. Med. 2009, 85. [Google Scholar] [CrossRef]
| Study | Country | Patients/Age | Intervention | Vaccination Rate (IG) | Vaccination Rate (CG) | n (IG) | n (CG) | Effect Size/p-Value for Vaccination Rate | Follow up |
|---|---|---|---|---|---|---|---|---|---|
| Chan 2015 * (44) | Hong Kong | 65+ with chronic disease | Telephone outreach and face-to-face session | 57.2% | 48.1% | 1251 | 1266 | ARR (95% CI): 1.20 (1.06–1.37) | 3 months |
| Coenen 2017 * (37) | Belgium | Inflammatory bowel disease patients | Face-to-face session | 62% | 23% | 86 (PP) 104 (ITT) | 107 (PP) 206 (ITT) | p < 0.001 (PP) OR (95% CI): 6.13 (3.67–10.24) (ITT) | 8 months |
| Dapp 2011 * (38) | Germany | 60+ | Computer generated feedback for patient and HCP, group session or home visit for patient and training for HCP, discussion empowering educational material | 47% | 23.8% | 568 | 1342 | OR (95% CI): 2.8 (2.3–3.5), p < 0.001 | 1 year |
| Jacobson 1999 * (39) | USA | 65+ or chronic disease | Discussion empowering educational material (to be used in consultation) | 19.9% | 3.8% | 221 | 212 | RR (95% CI): 5.28 (2.80–9.93), p < 0.001 | 1 day |
| Klassing 2018 (40) | USA | 18+ with Asthma/COPD | Telephone outreach | 59.7% | 55.7% | 77 (PP) 216 (ITT) | 70 (PP) 269 (ITT) | p = 0.76 (PP) OR (95% CI): 1.59 (0.99–2.44) | 5 months |
| Parker 2018 (42) | USA | 18+ lymphoma survivors | New face-to-face consultation and communication skills training for HCP | 14% | 14% | 117 | 81 | logistic HLM: OR (95% CI): 0.95 (0.39–2.32) PH model, HR (95% CI): 0.91 (0.51–1.63) | 12 months |
| Stuck 2015 * (41) | Switzerland | 65+ | Computer generated feedback for patient and HCP, telephone outreach and face-to-face session (home visit) for patients and training for HCP, discussion empowering educational material | 31.3% | 20.2% | 827 | 1320 | OR (95% CI): 1.90 (1.52–2.37), p < 0.001 | 2 years |
| Zwar 2012 (43) | Australia | 40–80 years with COPD | Face-to-face session (home visit) | 72.7% | 61.7% | 161 | 169 | OR 1.64 (0.93–2.89), p = 0.09 | 12 months |
| Subgroup | Number of Studies | OR (95% CI) | I2 |
|---|---|---|---|
| Activation | |||
| impersonal | 3 [38,39,41] | 2.79 (1.73–4.50) * | 88% |
| by nurse | 2 [43,44] | 1.49 (1.15–1.93) * | 0% |
| by physician | 2 [37,42] | 2.50 (0.40–15.52) | 92% |
| Information | |||
| by nurse | 5 [37,38,41,43,44] | 2.32 (1.57–3.43) * | 88% |
| by physician | 2 [39,42] | 2.48 (0.39–15.95) | 90% |
| Deliberation | |||
| by nurse | 3 [37,43,44] | 2.42 (0.99–5.89) | 91% |
| by physician | 4 [38,39,41,42] | 2.38 (1.50–3.77) * | 85% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuehne, F.; Sanftenberg, L.; Dreischulte, T.; Gensichen, J. Shared Decision Making Enhances Pneumococcal Vaccination Rates in Adult Patients in Outpatient Care. Int. J. Environ. Res. Public Health 2020, 17, 9146. https://doi.org/10.3390/ijerph17239146
Kuehne F, Sanftenberg L, Dreischulte T, Gensichen J. Shared Decision Making Enhances Pneumococcal Vaccination Rates in Adult Patients in Outpatient Care. International Journal of Environmental Research and Public Health. 2020; 17(23):9146. https://doi.org/10.3390/ijerph17239146
Chicago/Turabian StyleKuehne, Flora, Linda Sanftenberg, Tobias Dreischulte, and Jochen Gensichen. 2020. "Shared Decision Making Enhances Pneumococcal Vaccination Rates in Adult Patients in Outpatient Care" International Journal of Environmental Research and Public Health 17, no. 23: 9146. https://doi.org/10.3390/ijerph17239146
APA StyleKuehne, F., Sanftenberg, L., Dreischulte, T., & Gensichen, J. (2020). Shared Decision Making Enhances Pneumococcal Vaccination Rates in Adult Patients in Outpatient Care. International Journal of Environmental Research and Public Health, 17(23), 9146. https://doi.org/10.3390/ijerph17239146





