Comparison between Gradual Reduced Nicotine Content and Usual Nicotine Content Groups on Subjective Cigarette Ratings in a Randomized Double-Blind Trial
Abstract
1. Introduction
2. Materials and Methods
Statistical Methods
3. Results
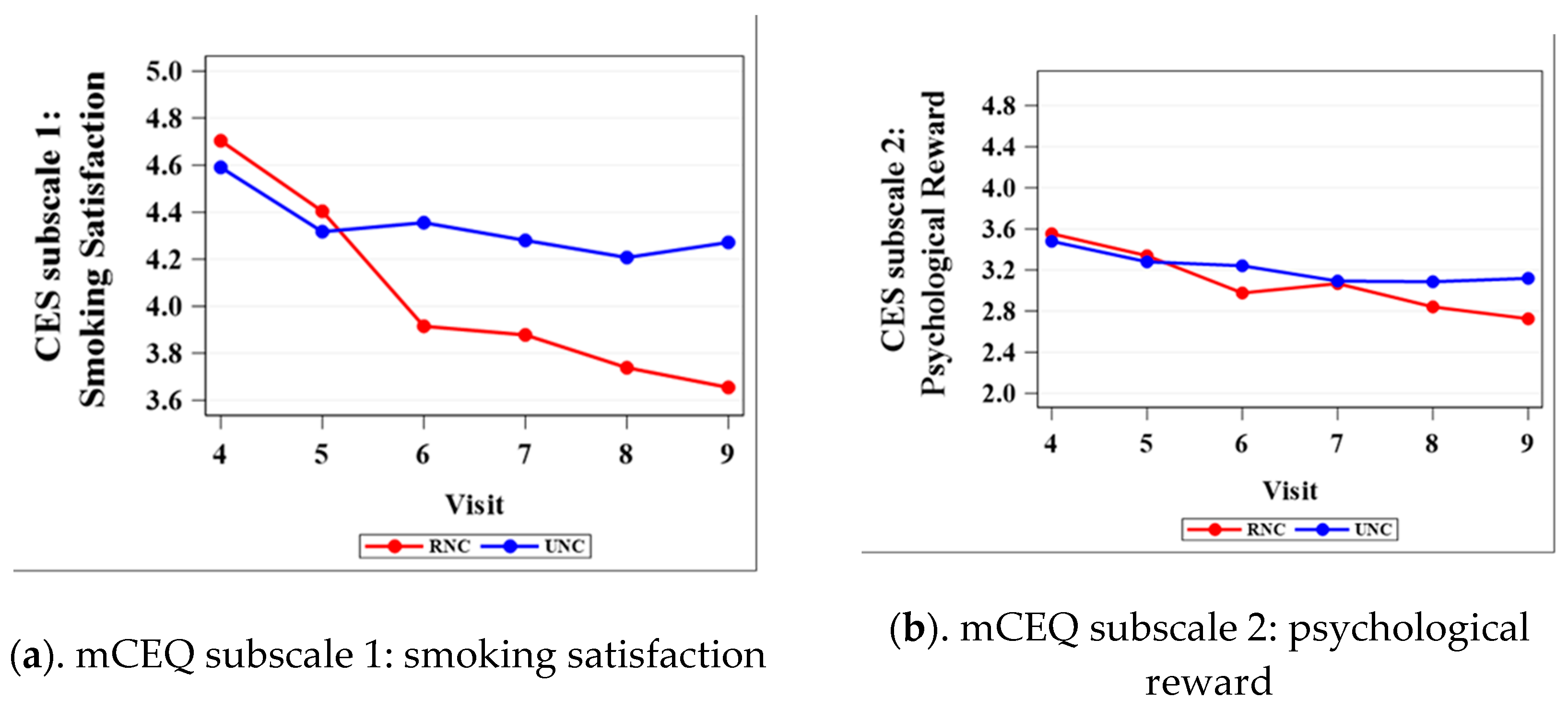
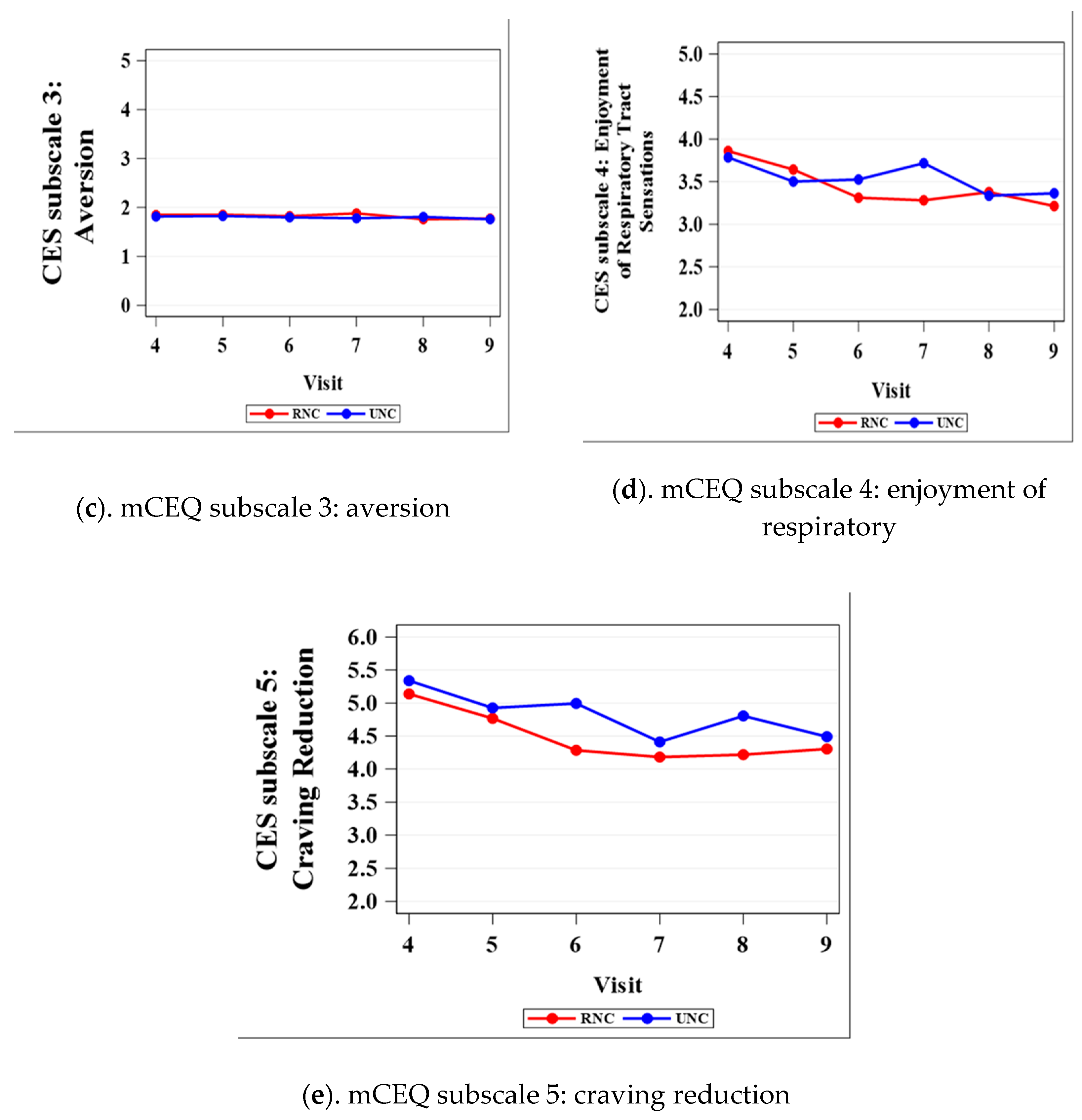
3.1. Modified Cigarette Evaluation Questionnaire (mCEQ)
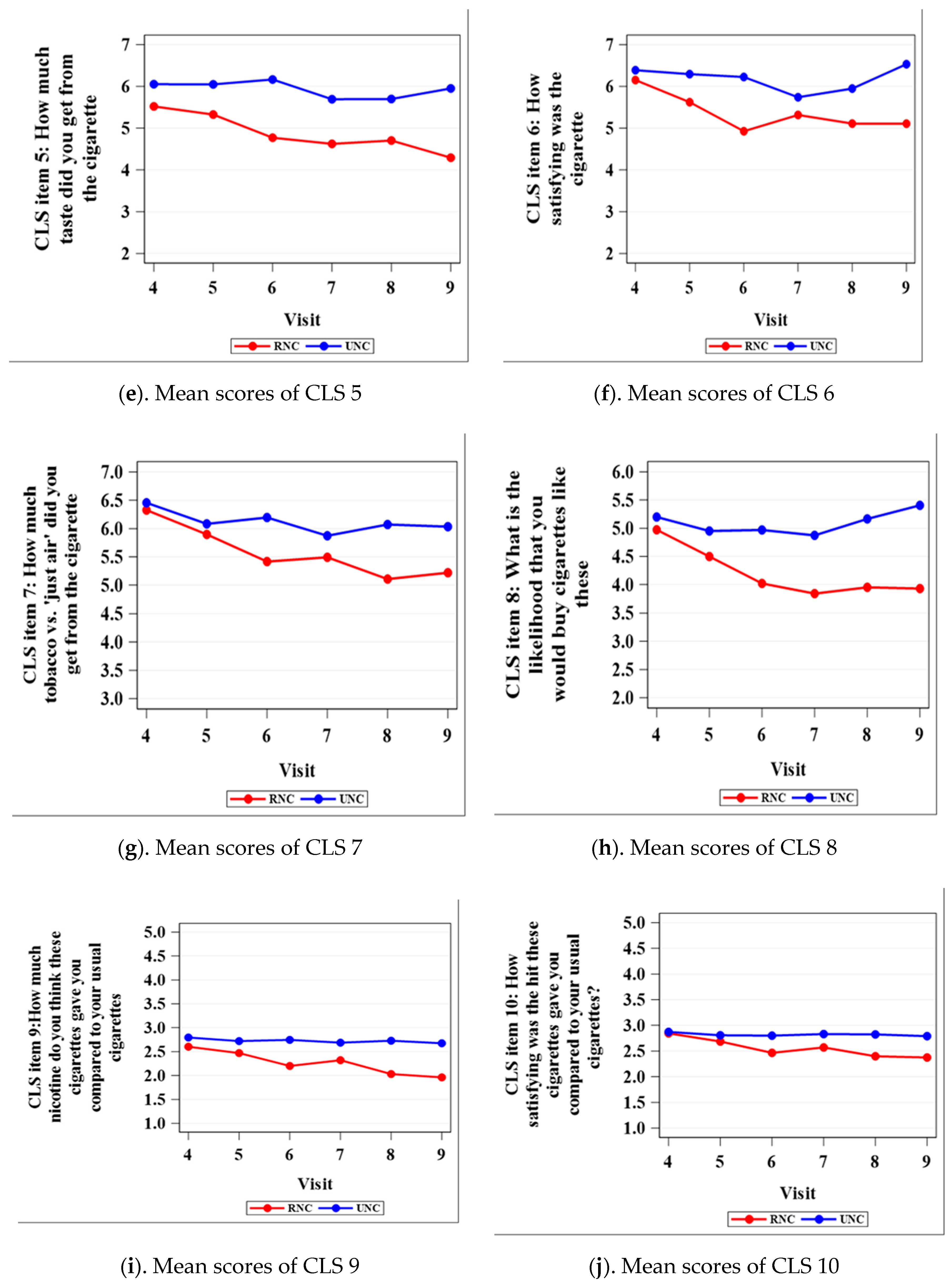
3.2. Cigarette-Liking Scale
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Creamer, M.R.; Wang, T.W.; Babb, S.; Cullen, K.A.; Day, H.; Willis, G.; Jamal, A.; Neff, L. Tobacco product use and cessation indicators among adults—United States, 2018. Morb. Mortal. Wkly. Rep. 2019, 68, 1013. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A. Current cigarette smoking among adults—United States, 2005–2015. Morb. Mortal. Wkly. Rep. 2016, 65, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Rockville, MD. 2014. Available online: https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm (accessed on 8 July 2020).
- Apelberg, B.J.; Feirman, S.P.; Salazar, E.; Corey, C.G.; Ambrose, B.K.; Paredes, A.; Richman, E.; Verzi, S.J.; Vugrin, E.D.; Brodsky, N.S.; et al. Potential public health effects of reducing nicotine levels in cigarettes in the United States. N. Engl. J. Med. 2018, 378, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes: 83 FR 11818; Federal Register: Washington, DC, USA, 2018; pp. 11818–11843.
- Hatsukami, D.K.; Kotlyar, M.; Hertsgaard, L.A.; Zhang, Y.; Carmella, S.G.; Jensen, J.A.; Allen, S.; Shields, P.G.; Murphy, S.E.; Stepanov, I.; et al. Reduced nicotine content cigarettes: Effects on toxicant exposure, dependence and cessation. Addiction 2010, 105, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Donny, E.C.; Denlinger, R.L.; Tidey, J.W.; Koopmeiners, J.S.; Benowitz, N.L.; Vandrey, R.G.; Al’Absi, M.; Carmella, S.G.; Cinciripini, P.M.; Dermody, S.S.; et al. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med. 2015, 373, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Hatsukami, R.K.; Luo, X.; Jensen, J.A.; Al’Absi, M.; Allen, S.; Carmella, S.G.; Chen, M.; Cinciripini, P.M.; Denlinger-Apte, R.; Drobes, D.J.; et al. Effect of immediate vs. gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: A randomized clinical trial. JAMA 2018, 320, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Dains, K.M.; Hall, S.M.; Stewart, S.L.; Wilson, M.; Dempsey, D.; Jacob, P. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol. Biomark. Prev. 2012, 21, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Mercincavage, M.; Souprountchouk, V.; Tang, K.Z.; Dumont, R.L.; Wileyto, E.P.; Carmella, S.G.; Hecht, S.S.; Strasser, A.A. A Randomized Controlled Trial of Progressively Reduced Nicotine Content Cigarettes on Smoking Behaviors, Biomarkers of Exposure, and Subjective Ratings. Cancer Epidemiol Biomark. Prev. 2016, 25, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.; O’Connor, R.J. Reduced nicotine cigarettes: Smoking behavior and biomarkers of exposure among smokers not intending to quit. Cancer Epidemiol Biomark. Prev. 2014, 23, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Shiffman, S.; Kurland, B.F.; Scholl, S.M.; Mao, J.M. Nondaily smokers’ changes in cigarette consumption with very low-nicotine-content cigarettes: A randomized double-blind clinical trial. JAMA Psychiatry 2018, 75, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.T.; Donny, E.C.; Luo, X.; Allen, A.M.; Carroll, D.M.; Denlinger-Apte, R.L.; Dermody, S.S.; Koopmeiners, J.S.; McClernon, F.J.; Pacek, L.R.; et al. The impact of gradual and immediate nicotine reduction on subjective cigarette ratings. Nicotine Tob. Res. 2019, 21, S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Lee, J.; Stitzer, M.L. Nicotine-containing versus de-nicotinized cigarettes: Effects on craving and withdrawal. Pharmacol. Biochem. Behav. 1997, 57, 159–165. [Google Scholar] [CrossRef]
- Cappelleri, J.C.; Bushmakin, A.G.; Baker, C.L.; Merikle, E.; Olufade, A.O.; Gilbert, D.G. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict. Behav. 2007, 32, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.M.; Allen, S.I.; Veldheer, S.; Martinez, D.J.; Horn, K.; Livelsberger, C.; Modesto, J.; Kuprewicz, R.; Wilhelm, A.; Hrabovsky, S.; et al. Reduced nicotine content cigarettes in smokers of low socioeconomic status: Study protocol for a randomized control trial. Trials 2017, 18, 300. [Google Scholar] [CrossRef] [PubMed]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerstrom, K.-O. The Fagerström test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Veldheer, S.; Midya, V.; Lester, C.; Liao, J.; Yingst, J.M.; Hrabovsky, S.; Allen, S.I.; Krebs, N.M.; Reinhart, L.; Evins, A.E.; et al. Acceptability of SPECTRUM research cigarettes among participants in trials of reduced nicotine content cigarettes. Tob. Regul. Sci. 2018, 4, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.A.; Kunkle, N.; Karelitz, J.L.; Michael, V.C.; Donny, E.C. Threshold dose for discrimination of nicotine via cigarette smoking. Psychopharmacology 2016, 233, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Denlinger-Apte, R.L.; Cassidy, R.N.; Colby, S.M.; Sokolovsky, A.W.; Tidey, J.W. Effects of cigarette nicotine content and menthol preference on perceived health risks, subjective ratings, and carbon monoxide exposure among adolescent smokers. Nicotine Tob. Res. 2019, 21, S56–S62. [Google Scholar] [CrossRef] [PubMed]

| No. of Visit | Visit 4 | Visit 5 | Visit 6 | Visit 7 | Visit 8 | Visit 9 |
|---|---|---|---|---|---|---|
| Nicotine Content | 7.4 mg | 3.3 mg | 1.4 mg | 0.7 mg | 0.2 mg | 0.2 mg |
| p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | |
| Satisfaction 1 | 0.5214 | 0.6304 | 0.0172 | 0.0356 | 0.0181 | 0.0026 |
| Psychological reward 2 | 0.6113 | 0.6874 | 0.0809 | 0.8768 | 0.1326 | 0.0192 |
| Aversion 3 | 0.7556 | 0.7991 | 0.8210 | 0.3690 | 0.6835 | 0.9083 |
| Enjoyment of Respiratory Tract Sensations 4 | 0.7142 | 0.4995 | 0.3161 | 0.0486 | 0.8528 | 0.5261 |
| Craving reduction 5 | 0.4241 | 0.5367 | 0.0072 | 0.4021 | 0.0388 | 0.5260 |
| Visit 4 | Visit 5 | Visit 6 | Visit 7 | Visit 8 | Visit 9 | |
|---|---|---|---|---|---|---|
| p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | |
| CLS 1 | 0.0386 | 0.0114 | <0.0001 | 0.0055 | 0.0003 | 0.0002 |
| CLS 2 | 0.4658 | 0.0235 | 0.0935 | 0.4080 | 0.0863 | 0.0516 |
| CLS 3 | 0.9012 | 0.1868 | 0.5481 | 0.5340 | 0.3355 | 0.2267 |
| CLS 4 | 0.5187 | 0.0343 | 0.0777 | 0.6848 | 0.0371 | 0.0119 |
| CLS 5 | 0.0593 | 0.0125 | <0.0001 | 0.0005 | 0.0018 | <0.0001 |
| CLS 6 | 0.4326 | 0.0290 | <0.0001 | 0.1938 | 0.0135 | <0.0001 |
| CLS 7 | 0.6193 | 0.4824 | 0.0042 | 0.1763 | 0.0010 | 0.0069 |
| CLS 8 | 0.5096 | 0.1962 | 0.0082 | 0.0054 | 0.0016 | 0.0002 |
| CLS 9 | 0.1305 | 0.0545 | <0.0001 | 0.0080 | <0.0001 | <0.0001 |
| CLS 10 | 0.8300 | 0.3574 | 0.0130 | 0.0621 | 0.0033 | 0.0054 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.; Krebs, N.M.; Zhu, J.; Foulds, J.; Horn, K.; Muscat, J.E. Comparison between Gradual Reduced Nicotine Content and Usual Nicotine Content Groups on Subjective Cigarette Ratings in a Randomized Double-Blind Trial. Int. J. Environ. Res. Public Health 2020, 17, 7047. https://doi.org/10.3390/ijerph17197047
Lin W, Krebs NM, Zhu J, Foulds J, Horn K, Muscat JE. Comparison between Gradual Reduced Nicotine Content and Usual Nicotine Content Groups on Subjective Cigarette Ratings in a Randomized Double-Blind Trial. International Journal of Environmental Research and Public Health. 2020; 17(19):7047. https://doi.org/10.3390/ijerph17197047
Chicago/Turabian StyleLin, Wenxue, Nicolle M. Krebs, Junjia Zhu, Jonathan Foulds, Kimberly Horn, and Joshua E. Muscat. 2020. "Comparison between Gradual Reduced Nicotine Content and Usual Nicotine Content Groups on Subjective Cigarette Ratings in a Randomized Double-Blind Trial" International Journal of Environmental Research and Public Health 17, no. 19: 7047. https://doi.org/10.3390/ijerph17197047
APA StyleLin, W., Krebs, N. M., Zhu, J., Foulds, J., Horn, K., & Muscat, J. E. (2020). Comparison between Gradual Reduced Nicotine Content and Usual Nicotine Content Groups on Subjective Cigarette Ratings in a Randomized Double-Blind Trial. International Journal of Environmental Research and Public Health, 17(19), 7047. https://doi.org/10.3390/ijerph17197047







