Abstract
Volatile organic compounds (VOCs) are ubiquitous in the environment. In the United States (U.S.), tobacco smoke is the major non-occupational source of exposure to many harmful VOCs. Exposure to VOCs can be assessed by measuring their urinary metabolites (VOCMs). The Population Assessment of Tobacco and Health (PATH) Study is a U.S. national longitudinal study of tobacco use in the adult and youth civilian non-institutionalized population. We measured 20 VOCMs in urine specimens from a subsample of adults in Wave 1 (W1) (2013–2014) to characterize VOC exposures among tobacco product users and non-users. We calculated weighted geometric means (GMs) and percentiles of each VOCM for exclusive combustible product users (smokers), exclusive electronic cigarette (e-cigarette) users, exclusive smokeless product users, and tobacco product never users. We produced linear regression models for six VOCMs with sex, age, race, and tobacco user group as predictor variables. Creatinine-ratioed levels of VOCMs from exposure to acrolein, crotonaldehyde, isoprene, acrylonitrile, and 1,3-butadiene were significantly higher in smokers than in never users. Small differences of VOCM levels among exclusive e-cigarette users and smokeless users were observed when compared to never users. Smokers showed higher VOCM concentrations than e-cigarette, smokeless, and never users. Urinary VOC metabolites are useful biomarkers of exposure to harmful VOCs.
1. Introduction
Volatile organic compounds (VOCs) are ubiquitous in the environment. In the United States (U.S.), tobacco smoke is the major non-occupational source of exposure to many harmful VOCs [1]. Tobacco smoke contains over 8000 chemicals, including carcinogenic and toxic VOCs such as acrylonitrile and benzene [2]. Lower levels of VOCs have been reported in electronic cigarette (e-cigarette) refill solutions and emissions; e-cigarette use has increased significantly worldwide, especially among youth [3,4]. Regardless of exposure source, high levels of harmful VOCs are a significant public health concern. Previous epidemiologic studies have suggested respiratory effects of VOC exposure (e.g., asthma exacerbation, lung cancer) [5,6,7]. Moreover, controlled human exposure studies have suggested the inflammatory effects of VOCs (i.e., dose-related increases in lower and upper respiratory symptoms) [8,9].
Exposure to VOCs can be assessed by measuring their metabolites in urine. While measuring VOCs in blood can provide a direct assessment of VOC levels in vivo [1,10], urinary biomarkers of VOC exposure have a longer biological half-life than VOCs in blood and are more stable during storage and handling [11]. Volatile organic compound metabolites (VOCMs) have been examined in the U.S. population through the National Health and Nutrition Examination Survey (NHANES) since 2005 [12,13]. However, the NHANES is not designed to address tobacco-related exposures specifically, and thus includes relatively few users of tobacco products other than cigarettes. Population data are needed to better evaluate harmful VOC exposures resulting from use of various tobacco products.
The Population Assessment of Tobacco and Health (PATH) Study is a U.S. cohort study of 45,971 adults and youth, aged 12 years or older, that studies tobacco use and its health effects in the population [14]. In Wave 1 (W1) of the study, we measured VOCMs in urine specimens collected from adult tobacco users and non-users to characterize and compare VOC exposures among tobacco users and non-users in the U.S. The primary aim of the study was to estimate urinary concentrations of 20 VOCMs stratified by four user groups (e.g., Every day established exclusive combustible product user, Every day established exclusive e-cigarette user, Every day established exclusive smokeless product user, and never user). The measurement of urinary biomarkers of VOC exposure will help characterize the prevalence and magnitude of exposure to tobacco-related VOCs.
2. Materials and Methods
2.1. Study Design
We used biomarker and questionnaire data from W1 of the PATH Study, conducted from September 12, 2013 to December 15, 2014. Adult tobacco users, young adults aged 18 to 24, and African Americans were oversampled relative to population proportions. The weighting procedures were adjusted for oversampling and nonresponse. Combined with the use of a probability sample, the weighted data allowed the estimates produced by the PATH Study to be representative of the non-institutionalized, civilian U.S. population. Further details regarding the PATH Study design and methods are published elsewhere [14]. Details on survey interview procedures, questionnaires, sampling, weighting, and information on accessing the data are available at https://doi.org/10.3886/Series606. The Westat Institutional Review Board approved the study design and data collection protocol.
2.2. Participants
Participants completed the W1 adult interview and provided detailed information about their tobacco product use (i.e., self-reported). Our analyses focused on the following four mutually exclusive tobacco use categories: (1) Every Day Established Exclusive Combustible Product User (Smokers); (2) Every Day Established Exclusive E-cigarette User (electronic nicotine delivery system (ENDS) Users); (3) Every Day Established Exclusive Smokeless Product User (Smokeless Users); and (4) Never Users of Tobacco (Never Users) (see Table 1). In these analyses, tobacco products are defined as follows: combustible products, which include cigarettes, cigars, cigarillos, little filtered cigars, pipe, and hookah; e-cigarettes; and smokeless tobacco products, which include loose snus, pouched snus, chewing tobacco, dip, snuff, spit tobacco, and dissolvable tobacco.

Table 1.
Tobacco user group definitions.
2.3. Biospecimen Collection Procedures
Full-void spot urine specimens from 11,501 consenting participants were self-collected in a 500 mL polypropylene container (Globe Scientific, Mahwah, NJ, USA), immediately placed in a Crēdo Cube shipper (Series 4–496, Minnesota Thermal Science, Plymouth, MN, USA) certified to hold contents 2–8 °C for at least 72 h, and shipped overnight to the PATH Study biorepository. Each specimen was divided into aliquots and stored in FluidX® (Brooks Life Sciences, Chelmsford, MA, USA) polypropylene cryovials at −80 °C. Study participants used tobacco products ad libitum during the in-home interview, without controlling the timing of last tobacco use and spot urine collection.
2.4. Chemical Analysis
2.4.1. Volatile Organic Compound Metabolites (VOCMs)
In all, 20 biomarkers of exposure to VOCs (Table 2) were measured in spot urine using isotope dilution UPLC-MS/MS as described by Alwis et al. [15] and modified by Alwis et al. [16]. Briefly, samples were assayed by analyzing a 50 µL aliquot of each specimen through an ultra-high-performance liquid chromatography system (Waters Inc., Milford, MA, USA) coupled with electrospray ionization (ESI) tandem mass spectrometry (Sciex API 5500 Triple Quad, Applied Biosystems, Foster City, CA, USA). The mass spectrometer was operated using negative-ion ESI and in scheduled multiple reaction monitoring mode. The ion source temperature was held at 650 °C, and the electrospray ion voltage was −4000 V. Urine specimens were assayed with a 1:10 dilution (50 µL urine +25 µL mixed internal standard +425 µL 15 mM ammonium acetate). The mobile phase consisted of 15 mM ammonium acetate, pH 6.8 (mobile phase A), and acetonitrile (mobile phase B). Unknown concentrations were determined using the peak area ratio of a known standard to the stable isotope-labeled internal standard. The limits of detection (LODs) ranged from 0.5 ng/mL to 15 ng/mL. The reported data meet the quality requirements of the Centers for Disease Control and Prevention (CDC) Division of Laboratory Sciences [17].

Table 2.
Urinary volatile organic compound metabolites (VOCMs) measured for the Population Assessment of Tobacco and Health (PATH) Study Wave 1 (W1).
2.4.2. Urinary Creatinine
Urinary biomarker concentrations can be influenced by urine dilution, which can vary markedly from void to void and may confound statistical inference [18]. Urine dilution can be accounted for by scaling urinary analyte concentration to the urinary concentration of creatinine, a compound formed endogenously by lean body mass and excreted at a fairly constant rate. Creatinine-corrected values were calculated for urinary biomarkers of respondents with levels of creatinine between 10 and 370 mg/dL. This correction helped to avoid the confounding effects of overly dilute or hyper-concentrated urines [19]. Creatinine in urine was measured by an enzymatic assay on a commercial automated clinical chemistry analyzer, and the LOD was 1.1 mg/dL.
3. Statistical Analysis
The PATH Study recruited participants through a multistage probability sample. Applying survey weights produced robust estimates. Balanced repeated replication (BRR) with a Fay’s adjustment factor of 0.3 was used for variance estimation. We used this estimation approach as implemented in the PROC DESCRIPT, PROC CROSSTAB, and PROC VARGEN procedures of SUDAAN version 11.0.1 (Research Triangle Institute, Research Triangle Park, NC, USA) called from the SAS statistical software application version 9.4 (SAS Institute, Cary, NC, USA). Statistical significance was set to 0.05.
A total of 11,501 samples with VOCM data were retrieved from PATH Study W1 data. Of those, 4075 people were excluded because of missing demographic variables, 2049 people were excluded for dual/poly product use, and an additional 128 people were excluded for missing creatinine measurements (i.e., creatinine-ratioed VOCM values were used for all descriptive statistics). Furthermore, 28 people were excluded if they reported smoking >100 cigarettes per day. Because the distribution of measurements was strongly right-skewed, VOCM concentrations were log-transformed to calculate the weighted geometric mean (GM) of each VOCM by user group, and the weighted percentile (e.g., 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentile) numerical distribution of the data for each VOCM by user group.
3.1. Multivariate Analysis and Selected VOCM Analyses
We investigated the effect of selected predictors on VOCM levels by tobacco user group. Sample-weighted multivariate linear regression models stratified were fit to W1 data, where the dependent variables were urinary concentrations (ng/mL) of six VOCMs (2COEMA, 2CYEMA, 3HPMA, 3HMPMA, 4HMBEMA, and t4HBEMA). Sex, age, race/ethnicity, education, tobacco user group, and creatinine concentration were examined as predictors in each model. These six VOCMs were selected because their parent VOCs (acrolein, acrylonitrile, crotonaldehyde, isoprene, and 1,3-butadiene) have high cancer and non-cancer risk indices based on exposure to a single cigarette per day [20]. Moreover, they have been prioritized by the World Health Organization as part of a strategy for tobacco regulation based on product performance assessments, with the goal of reducing the mainstream smoke levels of selected constituents [21,22]. The selected VOCMs are also included in the Food and Drug Administration (FDA)’s Established List of Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke [23].
Because the distribution of measurements was strongly right-skewed, which would have adversely affected hypothesis testing, urinary VOCM concentration data were natural log-transformed for regression analysis. We report coefficients from these models along with their 95% confidence intervals and p-values. To interpret categorical predictors in the model, if the exponentiated coefficient estimate is greater or less than 1, the predictive percentage increase or decrease in VOCM concentrations is calculated as the estimate minus one then multiplied by 100.
3.2. PATH Study W1 and NHANES 2015–2016 VOCM Comparison
VOCM data from the PATH Study Wave 1 (2013–2014) for six VOCMs (2COEMA, 2CYEMA, 3HPMA, 3HMPMA, 4HMBEMA, and t4HBEMA) were compared with results from the NHANES 2015–2016 (UVOCS_I). The NHANES is a population-based survey designed to assess health and nutritional status through a cross-sectional observation of a complex, multistage probability sample representative of the civilian, non-institutionalized population of the United States [12]. Participants were classified as smokers and non-users as previously described [13,24,25]. Only smokers (serum cotinine > 10 ng/mL) and non-users (serum cotinine ≤ 10 ng/mL) were examined in this comparison since there were not enough NHANES study participants who could be apportioned into the other two user groups studied in this report. Only NHANES study participants 18 years or older were selected. In order to directly compare with NHANES participants, PATH Study Wave 1 participants were also classified as smokers and non-users based on their serum cotinine level. We performed two-sample t-tests on weight-adjusted samples to determine whether these two studies had equal GMs for the same analytes used in the multivariate analysis. Bonferroni correction was used to adjust for multiple comparisons to avoid the inflation of type I error.
4. Results
4.1. VOCM Geometric Means and Percentiles by Tobacco User Group
We examined 5221 subjects, after excluding participants who could not be assigned to any of the four abovementioned user groups. The sample size for each tobacco user group was as follows: (1) Smokers, n = 3156; (2) ENDS Users, n = 149; (3) Smokeless Users, n = 353; and (4) Never Users, n = 1563. Overall detection frequencies for 10 VOCMs were >99%; the detection frequency was lowest for 1CYHEMA (35.98%; Table S1, Supplementary Materials). We calculated creatinine- and non-creatinine-ratioed GMs by demographic and tobacco user group for 20 VOCMs (Tables S2–S21, Supplementary Materials). In addition, selected percentiles by tobacco user groups (both creatinine- and non-creatinine-ratioed) are provided in Tables S22–S41. Figure 1 presents GMs (95% confidence intervals) of six VOCMs by specific tobacco product user groups as described above. Concentrations of three biomarkers (BZMA, PHMA, and TTCA) were similar between all four tobacco user groups.
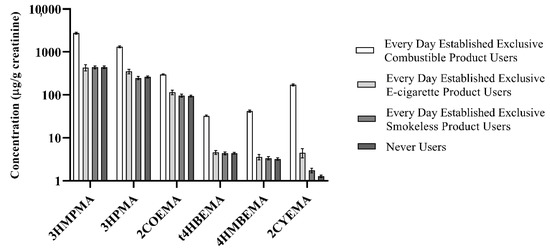
Figure 1.
Creatinine-ratioed sample-weighted geometric means (GMs) (95% confidence interval) of selected VOCMs by tobacco user group in the PATH Study Wave 1 (2013–2014).
Overall, the relative abundance of VOCM GMs by tobacco user group follows the same pattern, i.e., Smokers had the highest VOCM GMs, followed by ENDS Users. Smokeless and Never Users had the lowest GMs out of the four groups and were about the same for most VOCMs. Of note, statistically significant differences between all four tobacco user group GMs were observed only for 2CYEMA and 3HPMA levels when compared to the reference group. Creatinine-ratioed 2CYEMA GMs (standard error) were as follows (µg/g creatinine): 172 (4.77) (Smokers), 4.51 (0.560) (ENDS), 1.76 (0.114) (Smokeless), and 1.27 (0.043) (Never Users). For 3HPMA, creatinine-ratioed GMs (standard error) were as follows (µg/g creatinine): 1.32 × 103 (33.0) (Smokers), 354 (22.3) (ENDS), 249 (10.1) (Smokeless), and 262 (7.66) (Never Users).
4.2. Multivariate Analysis of Tobacco Smoke and Demographic Associations with Selected VOCMs
We found significantly higher (p < 0.0001) VOCM levels between Smokers and Never Users for 2COEMA (206%), 2CYEMA (>12,000%), 3HPMA (385%), 3HMPMA (505%), 4HMBEMA (1193%), and t4HBEMA (617%) after adjusting for age, sex, race, education level, and creatinine (Tables S42–S47). Contrastingly, race/ethnicity and age variables were associated with smaller magnitude changes in VOCM levels (Table 3). A positive number indicates significantly higher (p < 0.05) levels of VOCMs when compared to the reference group. Alternatively, a negative number indicates significantly lower levels of VOCMs when compared to the reference group. After controlling for other cofactors, females had significantly higher urinary 2COEMA, 3HMPMA, 4HMBEMA, and t4HBEMA levels compared to males. Likewise, after controlling for tobacco use and other cofactors and using participants’ age of 25–34 years as the reference, young adults (18–24 years) had significantly lower urinary concentrations of 2COEMA, 3HPMA, 3HMPMA, 4HMBEMA, and t4HBEMA (p-values <0.0009), and older adults (≥ 55 years) had statistically significantly higher urinary concentrations for the same five VOCMs (range 17.2–35.5%; p-values < 0.0008). In both cases, 2CYEMA levels showed no statistically significant changes among age groups.

Table 3.
Summary of sample-weighted multivariate regression modeling of predictor variables for six urinary VOC metabolites and selected demographics.
4.3. PATH Study W1 and NHANES 2015–2016 VOCM Comparison
We compared weighted GMs (ng/mL) of selected VOCMs (2COEMA, 2CYEMA, 4HMBEMA, 3HMPMA, 3HPMA, and t4HBEMA) in the PATH Study Wave 1 and the NHANES 2015–2016 for Never Users and Smokers (as defined in previous sections) (Table 4). After adjusting for multiple comparisons, we observed small, but statistically significant, differences in GM levels for 3HPMA, 2COEMA, and 3HMPMA in Smokers (p-values < 0.0083, PATH > NHANES). For Smokers, 2CYEMA GMs were not statistically different between PATH Study Wave 1 and NHANES 2015–2016 participants (68.4 and 58.0 ng/mL, respectively); the same pattern was observed for 4HMBEMA. Additionally, we observed small, but statistically significant, differences in GM levels for t4HBEMA, 3HPMA, and 3HMPMA in Never Users (p-values < 0.0009, PATH > NHANES). Like Smokers, 2CYEMA and 4HMBEMA GMs were not statistically different between Never Users in PATH Study Wave 1 and NHANES 2015–2016 participants (1.25 and 1.41 ng/mL, and 3.14 and 3.22 ng/mL, respectively).

Table 4.
Weighted geometric means (GMs, ng/mL) comparisons of six selected VOCMs in the PATH Study Wave 1 and the National Health and Nutrition Examination Survey (NHANES) 2015–2016 data stratified by smokers and never users.
5. Discussion
We quantified 20 VOCMs in urine samples collected as part of the PATH Study Wave 1 (2013–2014) and present herein these data as U.S. reference values for different types of tobacco product users. We detected at least one of the 20 VOCMs in over 99% of sampled participants, confirming widespread exposure to harmful VOCs in the general U.S. adult population. This finding is consistent with data from the NHANES [1,13,24].
The creatinine-ratioed geometric means of 17 VOCMs followed the same pattern: Smokers > ENDS Users > Smokeless Users > Never Users. Specifically, the use of combustible tobacco products was associated with higher levels of selected metabolites of acrolein, acrylamide, acrylonitrile, 1,3-butadiene, crotonaldehyde, cyanide, N,N-dimethylformamide/methyl isocyanate, ethylbenzene/styrene, isoprene, propylene oxide, styrene, o-xylene, and m,p-xylene. This pattern is consistent with data from the NHANES and other previous reports [26,27,28,29]. In addition, we present creatinine-ratioed percentiles for each VOCM by tobacco user group to describe VOCM concentration distributions. Females generally showed higher creatinine-ratioed VOCM GM levels compared to males regardless of the tobacco user group classification, with only a few exceptions (e.g., 2COEMA ENDS male and female users had the same GMs). Non-Hispanic Whites had the highest GMs across all tobacco user groups with some exceptions. However, those exceptions should be interpreted with caution because they have low statistical precision. They are based on a sample size of less than 50 (in each case), or the coefficient of variation of the estimate is larger than 30%.
We investigated the effect of selected predictors on VOCM levels by tobacco user group. Levels of all six modeled VOCMs were significantly higher (p < 0.0001) in Smokers when compared to Never Users; the percent increase attributable to smoking was 2COEMA (206%), 3HPMA (385%), 3HMPMA (505%), t4HBEMA (617%), 4HMBEMA (1193%), and 2CYEMA (> 12,000%). These higher exposures are understandable based on the microgram quantities of the parent VOCs in the mainstream smoke from a single cigarette [30]. The 120-fold higher levels of 2CYEMA in smokers compared with non-users underscores the value of 2CYEMA as a selective smoke exposure biomarker. Interestingly, regression models also indicate that urinary 3HPMA and 2CYEMA were marginally higher in ENDS Users than Never Users, possibly because of higher secondhand smoke exposure and/or occasionally unreported smoking in ENDS users (many of whom are former smokers). We also observed differences in some VOCM levels by sex, after controlling for confounders. These differences in VOCM levels in females may be partially explained by creatinine adjustments. While urinary creatinine excretion is relatively consistent in an individual, the amount excreted can vary significantly between individuals based on lean body mass and other physiological factors. Creatinine production tends to be higher in males compared to females and higher in non-Hispanic Blacks compared with other races [18]. Additionally, VOCM levels differed modestly by age and race/ethnicity, after controlling for confounders. The predicted effect size for these demographic variables was consistently small across all age and race/ethnicity categories (Table 3) compared with tobacco smoke. These data confirm that tobacco smoke exposure is a more impactful determinant of VOCM levels than are demographic variables.
PATH Study W1 GMs for 2COEMA, 2CYEMA, 3HPMA, 3HMPMA, 4HMBEMA, and t4HBEMA were marginally higher in magnitude compared to those for NHANES 2015–2016 participants (4 of 6 were statistically higher). The demographic characteristics for PATH Study W1 and NHANES 2015–2016 participants were very similar, notwithstanding the difference in the number of participants in each study. The higher levels of smoke-related VOCM biomarkers may result from a combination of two factors: (1) the PATH Study selects participants based on tobacco use and thus may include heavier smokers than those of the NHANES, and (2) the PATH Study’s urine collection is likely sooner after last tobacco product use compared with that of the NHANES. In both studies, the GM ratio of 2CYEMA in Smokers/Never Users exceeded 40:1 despite the fact that we did not exclude non-users who have secondhand smoke exposure. The consistency of higher urinary 2CYEMA in smokers across both studies provides further evidence for the use of 2CYEMA as a smoke exposure biomarker.
There are some important limitations to our study. Notably, we did not evaluate dual/poly use in our report since other studies have reported dual/poly use analyses using PATH Study Wave 1 (2013–2014) data [29]. The analyses performed by Goniewicz et al. are focused on characterizing harmful and addictive exposures in dual users of cigarettes and e-cigarettes. Additionally, other studies also characterized tobacco exposure biomarkers (including VOCs) in other combinations of tobacco product use [31]. Of note, we measured biomarkers of exposure to 20 different VOCs. However, we did not assess exposure to formaldehyde, which is a known human carcinogen found in emissions from some ENDS products [32]. Another limitation is that the PATH Study W1 captures data on first generation e-cigarettes that differ in product characteristics and operational features compared to e-cigarette products on the market currently. Our report describes the users of first-generation e-cigarettes [33], which were not as common as they are today (n = 149 for our analysis). Efforts are currently underway to characterize exposures related to the use of nicotine salt e-cigarettes in more recent waves of both the PATH Study and the NHANES. These analyses will be able to reference our current study as a baseline measure. We believe that it is important to document VOC exposures in the U.S. population at different times, and thus better understand the impact of changes in products, behaviors, and regulations.
6. Conclusions
We present the first comprehensive VOC exposure data in users of combustible tobacco products, e-cigarettes, smokeless tobacco products, and never users during the PATH Study Wave 1 (2013–2014), based on the analysis of 20 urinary biomarkers of exposure. Users of combustible tobacco products had significantly higher concentrations of most VOCMs than users of non-combustible tobacco products and never users. Users of smokeless tobacco products and e-cigarette users have VOC exposure similar to never users for most VOCMs, but not for 2CYEMA. Our findings suggest that selected VOCMs are suitable biomarkers for monitoring tobacco smoke exposure. Specifically, the biomarker 2CYEMA (its parent compound is acrylonitrile) was shown to effectively distinguish between combustible and non-combustible tobacco product users, and never users. The data presented in this study establish a baseline of exposures to VOCs to identify exposure trends resulting from changes in tobacco products (e.g., the emergence of nicotine salt e-cigarettes) and use patterns.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/15/5408/s1.
Author Contributions
Conceptualization, V.R.D.J. and B.C.B.; Methodology, V.R.D.J., B.C.B., W.Z., and L.Z.; Formal Analysis, V.R.D.J., B.C.B., W.Z, L.Z, C.R., K.C., D.T., D.B.; Investigation, V.R.D.J., B.C.B., W.Z, and L.Z.; Resources, V.R.D.J., B.C.B., A.Y.D.V.-P., and D.v.B.; Data Curation, C.R., K.C., D.T., D.B., E.S.; Writing—Original Draft Preparation, V.R.D.J.; Writing—Review & Editing, V.R.D.J., B.C.B., W.Z, L.Z., C.R., K.C., D.T., D.B., A.Y.D.V.-P., D.v.B., G.L., J.T.C., H.L.K., E.S., M.L.G., and A.H.; Supervision, B.C.B.; Project Administration, V.R.D.J., B.C.B.; Funding Acquisition, B.C.B. and D.v.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project is supported with Federal funds from the National Institute on Drug Abuse, National Institutes of Health, and the Center for Tobacco Products, Food and Drug Administration (FDA), Department of Health and Human Services, under a contract to Westat (Contract Nos. HHSN271201100027C and HHSN271201600001C) and through an interagency agreement between the Center for Tobacco Products, the FDA, and the Centers for Disease Control and Prevention.
Acknowledgments
The authors wish to thank the Volatile Organic Compound Metabolites Team, Tobacco and Volatiles Branch, Centers for Disease Control and Prevention for the laboratory analysis of the PATH Study Wave 1 biospecimens. The views and opinions expressed in this report are those of the authors and do not necessarily represent the views, official policy, or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies. Use of trade names is for identification purposes and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the U.S. Department of Health and Human Services.
Conflicts of Interest
Maciej L. Goniewicz has received a research grant from Pfizer and served as a member of a scientific advisory board to Johnson & Johnson.
References
- Chambers, D.M.; Ocariz, J.M.; McGuirk, M.F.; Blount, B.C. Impact of cigarette smoking on volatile organic compound (VOC) blood levels in the U.S. population: NHANES 2003–2004. Environ. Int. 2011, 37, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Polzin, G.M.; Kosa-Maines, R.E.; Ashley, D.L.; Watson, C.H. Analysis of volatile organic compounds in mainstream cigarette smoke. Environ. Sci. Technol. 2007, 41, 1297–1302. [Google Scholar] [CrossRef]
- Han, S.; Chen, H.; Zhang, X.; Liu, T.; Fu, Y. Levels of Selected Groups of Compounds in Refill Solutions for Electronic Cigarettes. Nicotine Tob. Res. 2016, 18, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, W.; Liao, J.; Matsuo, T.; Ito, K.; Fowles, J.; Shusterman, D.; Mendell, M.; Kumagai, K. A Device-Independent Evaluation of Carbonyl Emissions from Heated Electronic Cigarette Solvents. PLoS ONE 2017, 12, e0169811. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Delfino, R.J. Epidemiologic evidence for asthma and exposure to air toxics: Linkages between occupational, indoor, and community air pollution research. Environ. Health Perspect. 2002, 110 (Suppl. 4), 573–589. [Google Scholar] [CrossRef]
- Tagiyeva, N.; Sheikh, A. Domestic exposure to volatile organic compounds in relation to asthma and allergy in children and adults. Expert Rev. Clin. Immunol. 2014, 10, 1611–1639. [Google Scholar] [CrossRef]
- Pappas, G.P.; Herbert, R.J.; Henderson, W.; Koenig, J.; Stover, B.; Barnhart, S. The respiratory effects of volatile organic compounds. Int. J. Occup. Environ. Health 2000, 6, 1–8. [Google Scholar] [CrossRef]
- Koren, H.S.; Graham, D.E.; Devlin, R.B. Exposure of humans to a volatile organic mixture. III. Inflammatory response. Arch. Environ. Health 1992, 47, 39–44. [Google Scholar] [CrossRef]
- Chambers, D.M.; McElprang, D.O.; Waterhouse, M.G.; Blount, B.C. An improved approach for accurate quantitation of benzene, toluene, ethylbenzene, xylene, and styrene in blood. Anal. Chem. 2006, 78, 5375–5383. [Google Scholar] [CrossRef]
- Boyle, E.B.; Viet, S.M.; Wright, D.J.; Merrill, L.S.; Alwis, K.U.; Blount, B.C.; Mortensen, M.E.; Moye, J., Jr.; Dellarco, M. Assessment of Exposure to VOCs among Pregnant Women in the National Children’s Study. Int. J. Environ. Res. Public Health 2016, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- NHANES U.S. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/ (accessed on 21 July 2020).
- Bagchi, P.; Geldner, N.; de Castro, B.R.; De Jesus, V.R.; Park, S.K.; Blount, B.C. Crotonaldehyde exposure in U.S. tobacco smokers and nonsmokers: NHANES 2005–2006 and 2011–2012. Environ. Res. 2018, 163, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hyland, A.; Ambrose, B.K.; Conway, K.P.; Borek, N.; Lambert, E.; Carusi, C.; Taylor, K.; Crosse, S.; Fong, G.T.; Cummings, K.M.; et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob. Control 2017, 26, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Alwis, K.U.; Blount, B.C.; Britt, A.S.; Patel, D.; Ashley, D.L. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal. Chim. Acta 2012, 750, 152–160. [Google Scholar] [CrossRef]
- Alwis, K.U.; Bailey, T.L.; Patel, D.; Wang, L.; Blount, B.C. Measuring urinary N-acetyl-S-(4-hydroxy-2-methyl-2-buten-1-yl)-L-cysteine (IPMA3) as a potential biomarker of isoprene exposure. Anal. Chim. Acta 2016, 941, 61–66. [Google Scholar] [CrossRef]
- Caudill, S.P.; Schleicher, R.L.; Pirkle, J.L. Multi-rule quality control for the age-related eye disease study. Stat. Med. 2008, 27, 4094–4106. [Google Scholar] [CrossRef]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef]
- Boeniger, M.F.; Lowry, L.K.; Rosenberg, J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. Am. Ind. Hyg. Assoc. J. 1993, 54, 615–627. [Google Scholar] [CrossRef]
- Fowles, J.; Dybing, E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. Control 2003, 12, 424–430. [Google Scholar] [CrossRef]
- Piade, J.J.; Wajrock, S.; Jaccard, G.; Janeke, G. Formation of mainstream cigarette smoke constituents prioritized by the World Health Organization—Yield patterns observed in market surveys, clustering and inverse correlations. Food Chem. Toxicol. 2013, 55, 329–347. [Google Scholar] [CrossRef]
- Burns, D.M.; Dybing, E.; Gray, N.; Hecht, S.; Anderson, C.; Sanner, T.; O’Connor, R.; Djordjevic, M.; Dresler, C.; Hainaut, P.; et al. Mandated lowering of toxicants in cigarette smoke: A description of the World Health Organization TobReg proposal. Tob. Control 2008, 17, 132–141. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke: Established List. Available online: https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/harmful-and-potentially-harmful-constituents-tobacco-products-and-tobacco-smoke-established-list (accessed on 22 April 2020).
- Capella, K.M.; Roland, K.; Geldner, N.; Rey deCastro, B.; De Jesus, V.R.; van Bemmel, D.; Blount, B.C. Ethylbenzene and styrene exposure in the United States based on urinary mandelic acid and phenylglyoxylic acid: NHANES 2005–2006 and 2011–2012. Environ. Res. 2019, 171, 101–110. [Google Scholar] [CrossRef]
- Espenship, M.F.; Silva, L.K.; Smith, M.M.; Capella, K.M.; Reese, C.M.; Rasio, J.P.; Woodford, A.M.; Geldner, N.B.; Rey deCastro, B.; De Jesus, V.R.; et al. Nitromethane Exposure from Tobacco Smoke and Diet in the U.S. Population: NHANES, 2007–2012. Environ. Sci. Technol. 2019, 53, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Lorkiewicz, P.; Riggs, D.W.; Keith, R.J.; Conklin, D.J.; Xie, Z.; Sutaria, S.; Lynch, B.; Srivastava, S.; Bhatnagar, A. Comparison of Urinary Biomarkers of Exposure in Humans Using Electronic Cigarettes, Combustible Cigarettes, and Smokeless Tobacco. Nicotine Tob. Res. 2019, 21, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Shahab, L.; Goniewicz, M.L.; Blount, B.C.; Brown, J.; McNeill, A.; Alwis, K.U.; Feng, J.; Wang, L.; West, R. Nicotine, Carcinogen, and Toxin Exposure in Long-Term E-Cigarette and Nicotine Replacement Therapy Users: A Cross-sectional Study. Ann. Intern. Med. 2017, 166, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.J.; Fetterman, J.L.; Orimoloye, O.A.; Dardari, Z.; Lorkiewicz, P.; Hamburg, N.M.; DeFilippis, A.P.; Blaha, M.J.; Bhatnagar, A. Characterization of Volatile Organic Compound (VOC) metabolites in Cigarette smokers, Electronic Nicotine Device Users, Dual Users and Non- users of tobacco. Nicotine Tob. Res. 2020, 22, 264–272. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Smith, D.M.; Edwards, K.C.; Blount, B.C.; Caldwell, K.L.; Feng, J.; Wang, L.; Christensen, C.; Ambrose, B.; Borek, N.; et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open 2018, 1, e185937. [Google Scholar] [CrossRef] [PubMed]
- Pazo, D.Y.; Moliere, F.; Sampson, M.M.; Reese, C.M.; Agnew-Heard, K.A.; Walters, M.J.; Holman, M.R.; Blount, B.C.; Watson, C.H.; Chambers, D.M. Mainstream Smoke Levels of Volatile Organic Compounds in 50 U.S. Domestic Cigarette Brands Smoked With the ISO and Canadian Intense Protocols. Nicotine Tob. Res. 2016, 18, 1886–1894. [Google Scholar] [CrossRef]
- Chang, C.M.; Edwards, S.H.; Arab, A.; Del Valle-Pinero, A.Y.; Yang, L.; Hatsukami, D.K. Biomarkers of tobacco exposure: Summary of an FDA-sponsored public workshop. Cancer Epidemiol. Prev. Biomarkers 2017, 26, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.P.; Luo, W.; Pankow, J.F.; Strongin, R.M.; Peyton, D.H. Hidden formaldehyde in e-cigarette aerosols. N. Engl. J. Med. 2015, 372, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.; Chang, J.T.; Rostron, B.L.; Johnson, S.E.; Das, B.; Del Valle-Pinero, A.Y. An Examination of Device Types and Features Used by Adult Electronic Nicotine Delivery System (ENDS) Users in the PATH Study, 2015–2016. Int. J. Environ. Res. Public Health 2019, 16, 2329. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).