Mindfulness-Based Interventions for People with Schizophrenia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Method
2.1. Protocol and Registration
2.2. Selection Criteria
2.3. Sources of Information
2.4. Search
2.5. Selection of Studies
2.6. Risk of Bias Across Studies
2.7. Summary of Results
3. Results
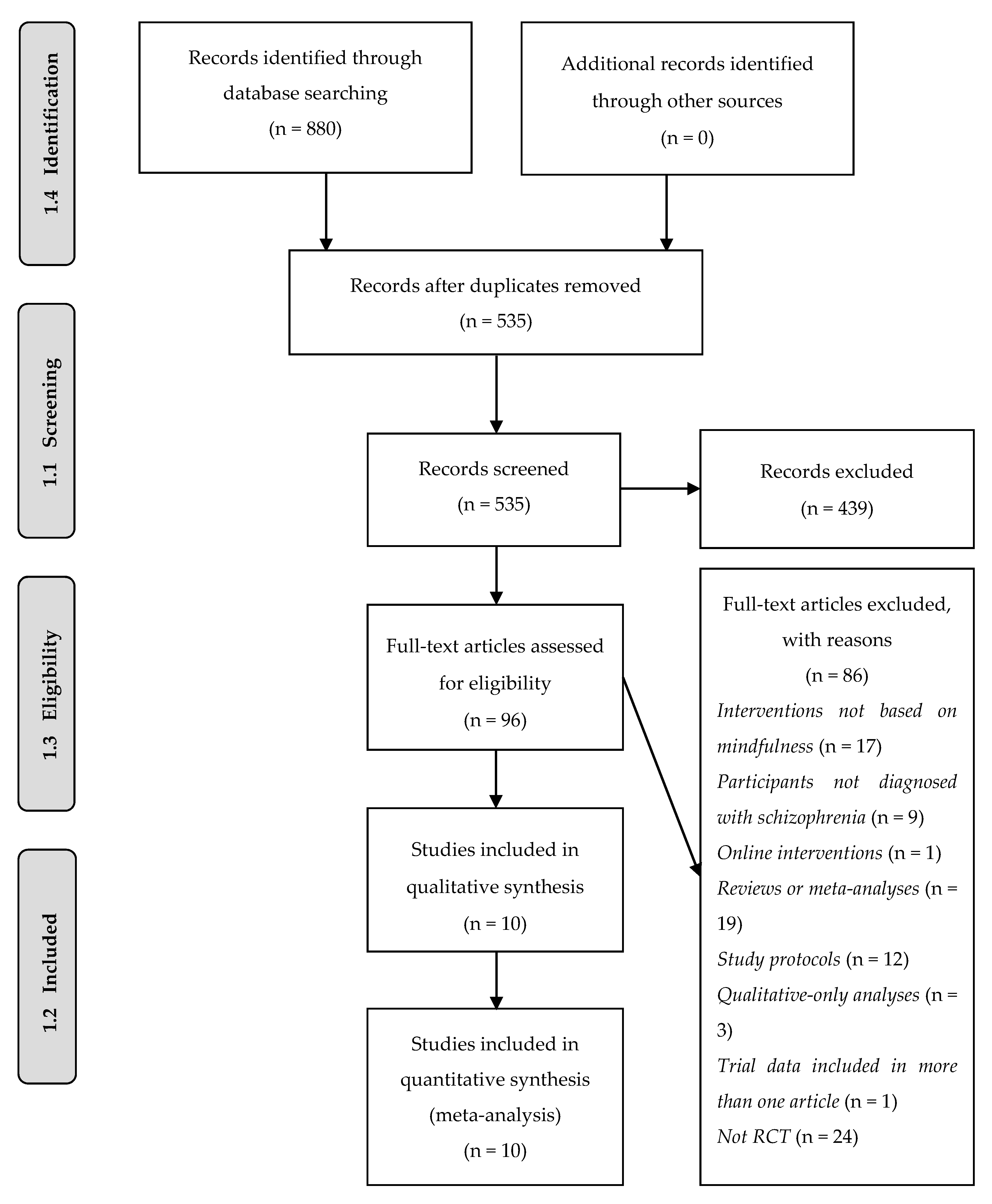
3.1. Study Selection
3.2. Study Characteristics
3.3. Design of the Studies
3.4. Participants
3.5. Interventions
3.6. Risk of Bias of the Studies
3.7. Summary of Results
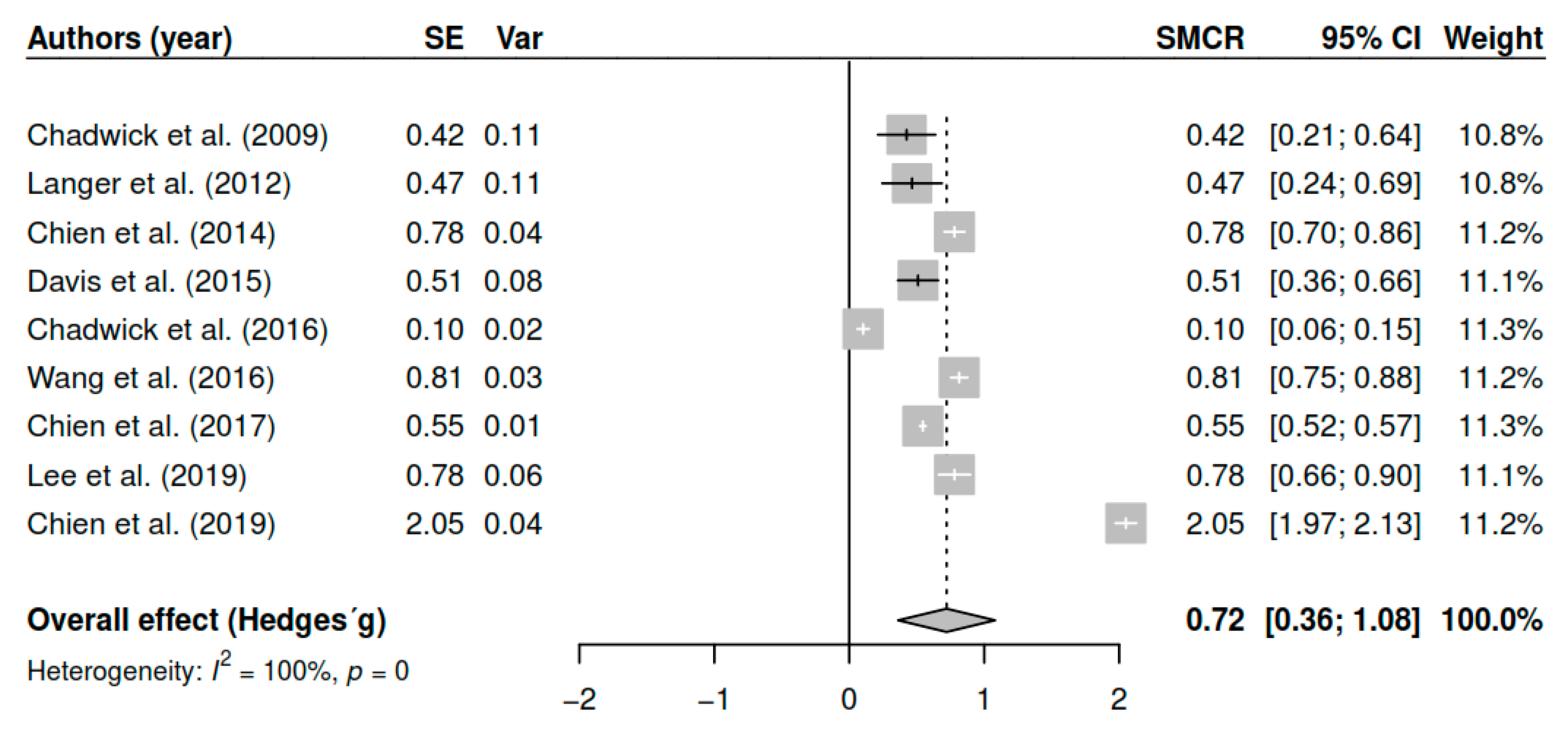
3.7.1. Effects on Overall Symptomatology (Pretest–Posttest)
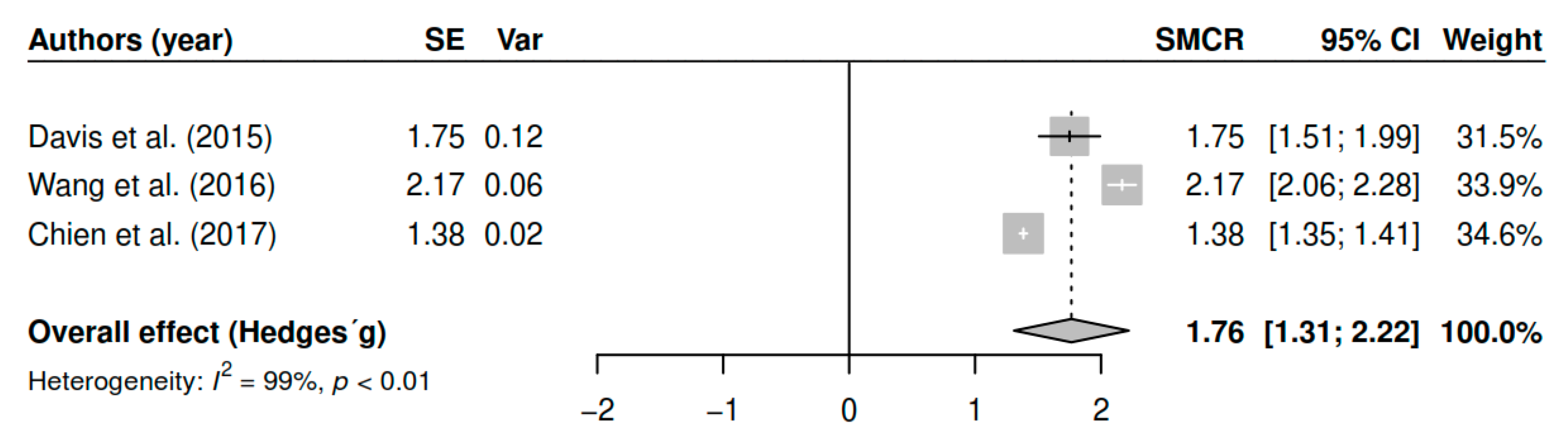
3.7.2. Effects on Overall Symptomatology (Follow-Up)
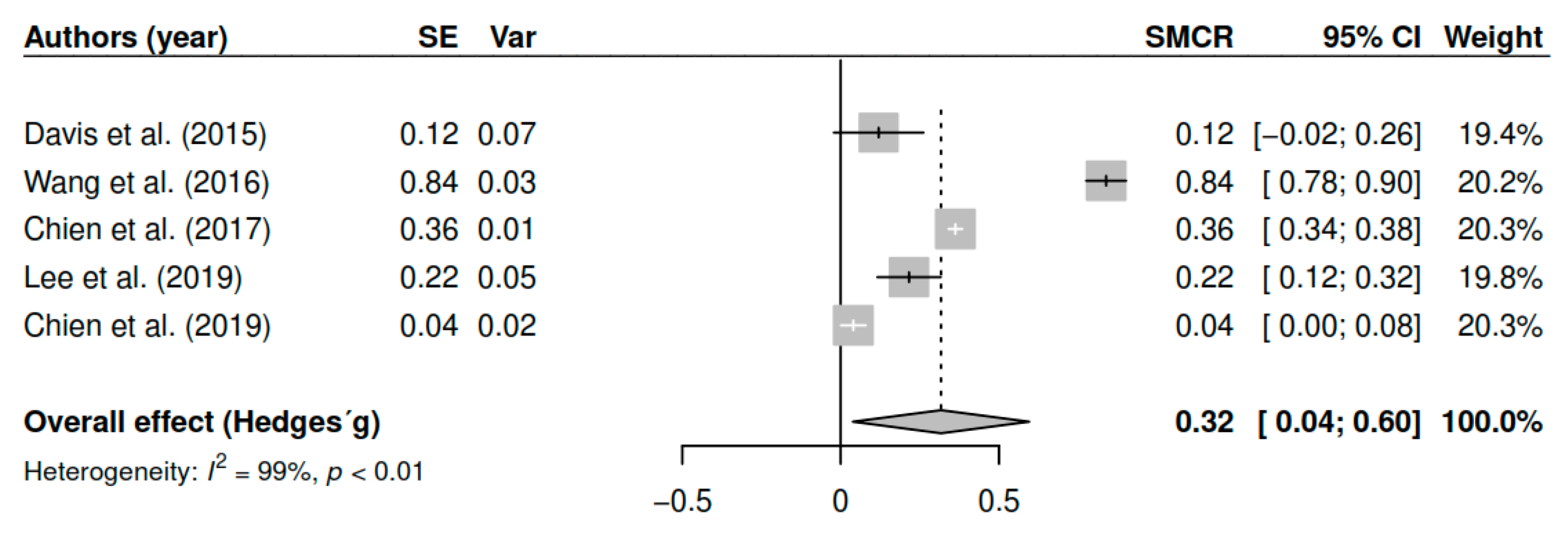
3.7.3. Effects on Positive Symptoms (Pretest–Posttest)
3.7.4. Negative Symptoms (Pretest–Posttest)
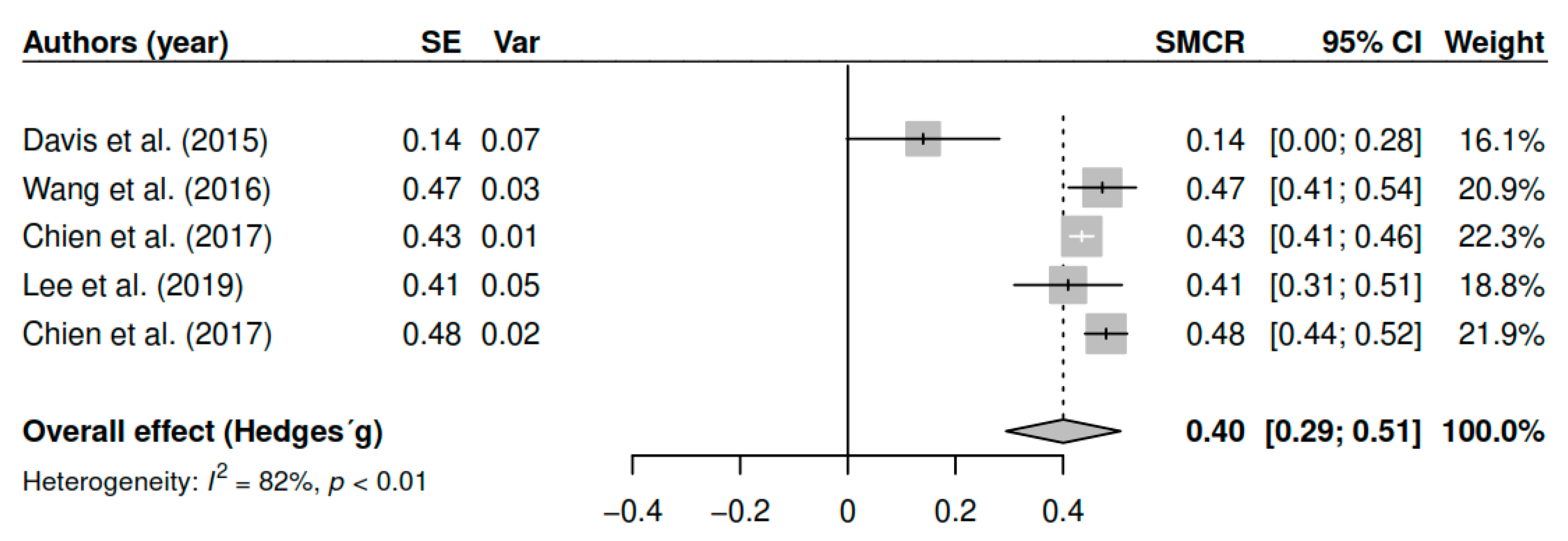
3.7.5. Effects on Mindfulness (Pretest–Posttest)
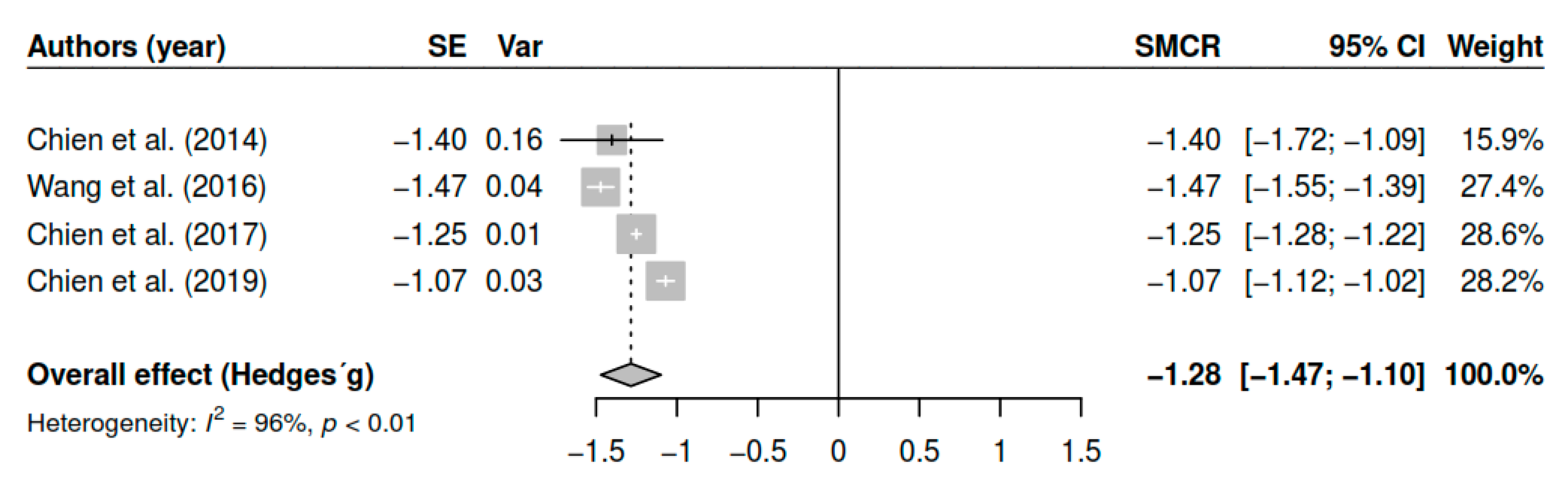
3.7.6. Effects on Functioning (Pretest–Posttest)
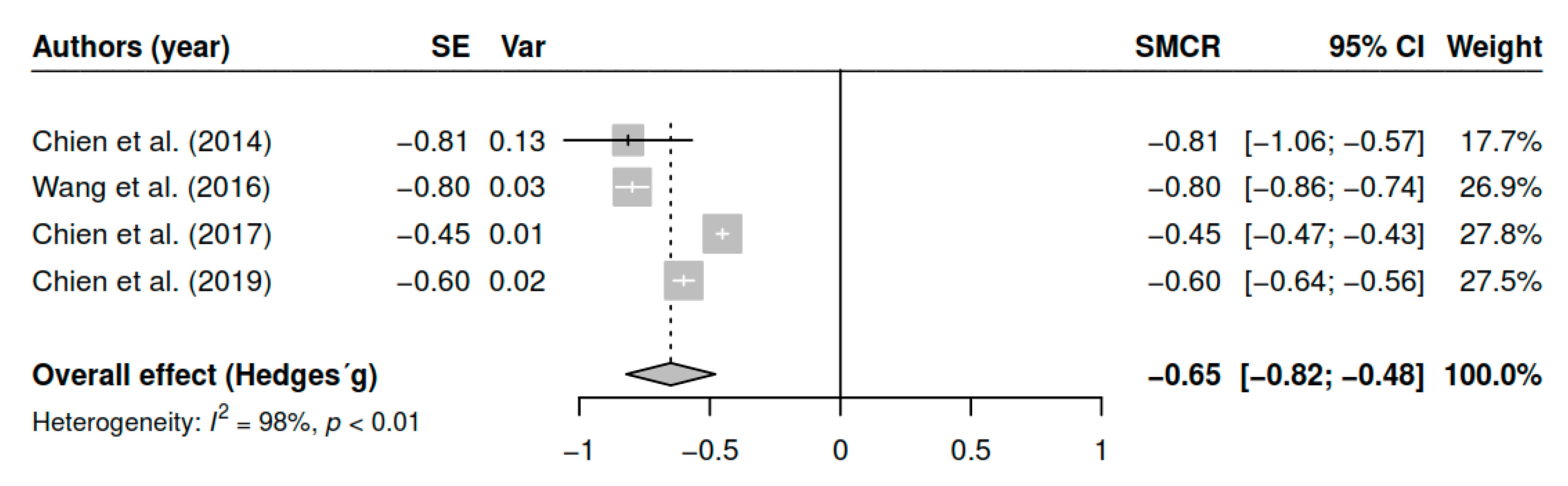
3.7.7. Effects on Awareness of Illness (Pretest–Posttest)
3.8. Additional Analyses
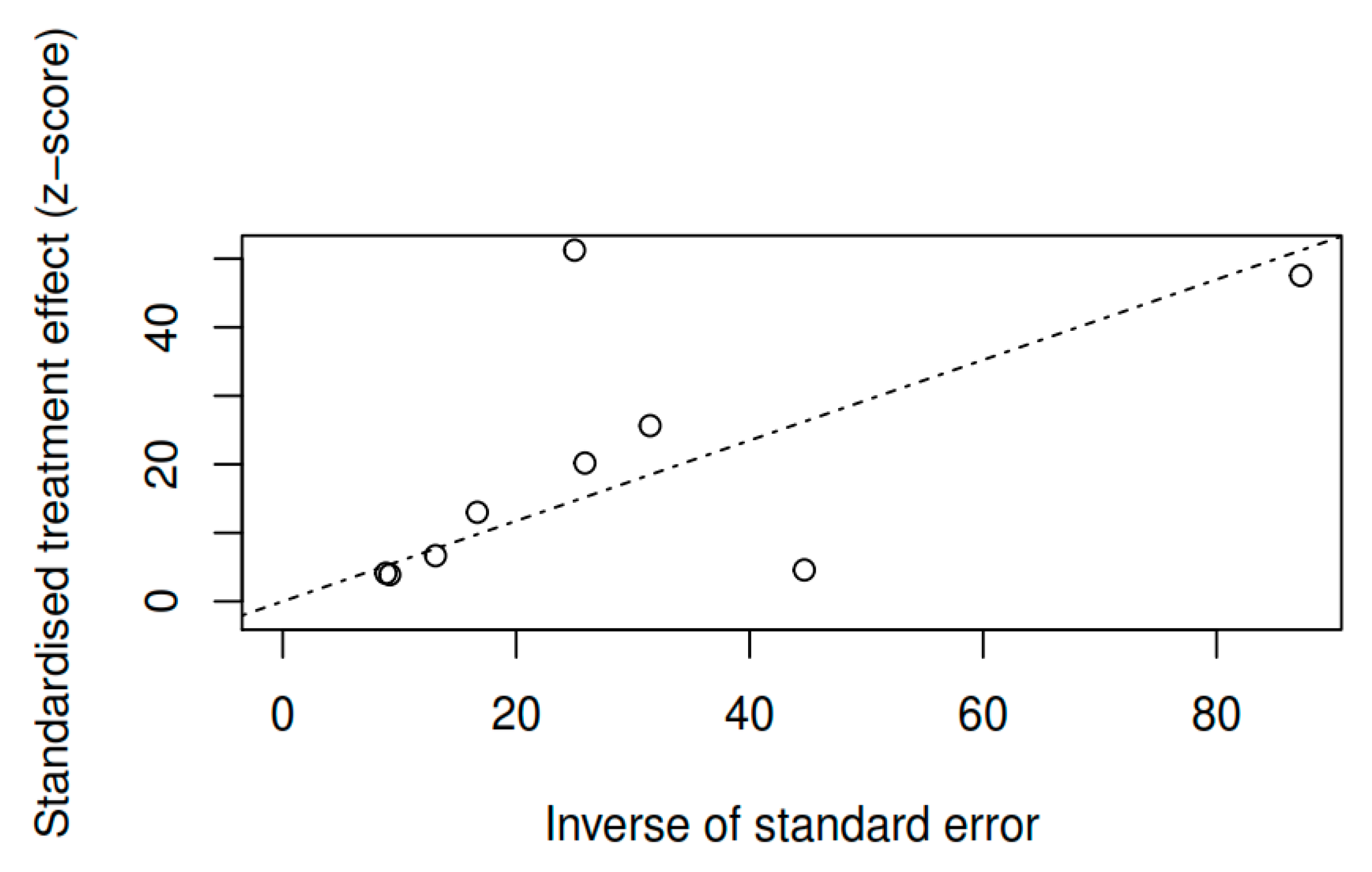
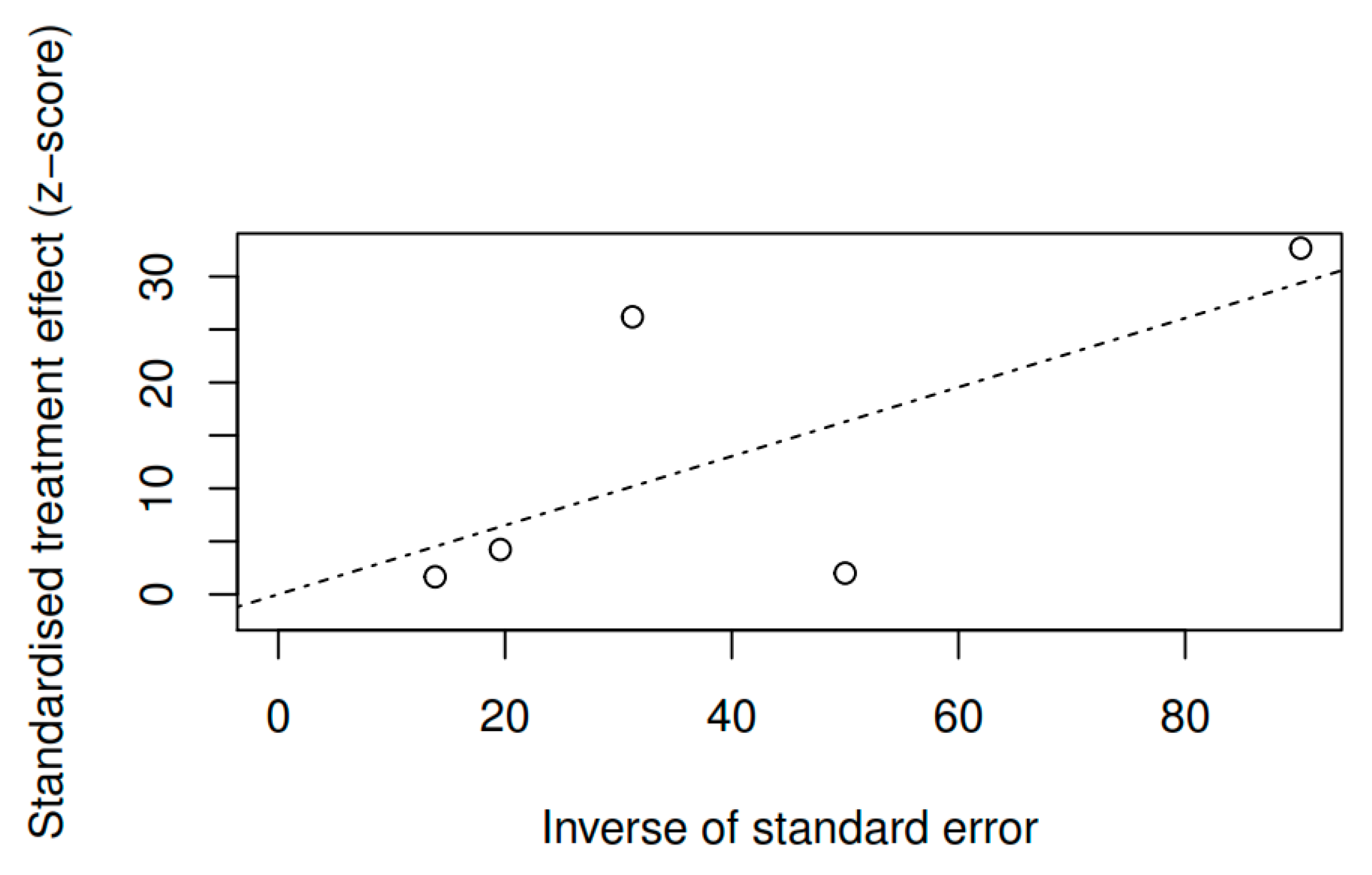
3.8.1. Results of the Meta-Regression
3.8.2. Sensitivity Analysis
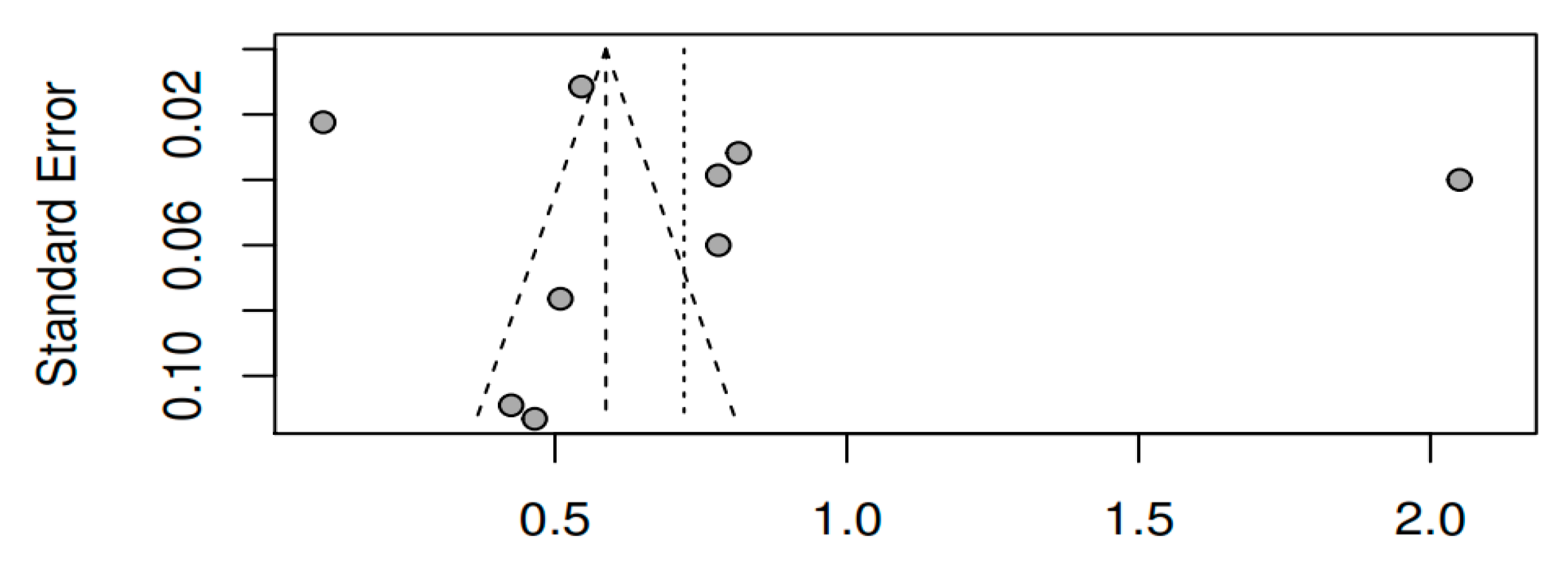
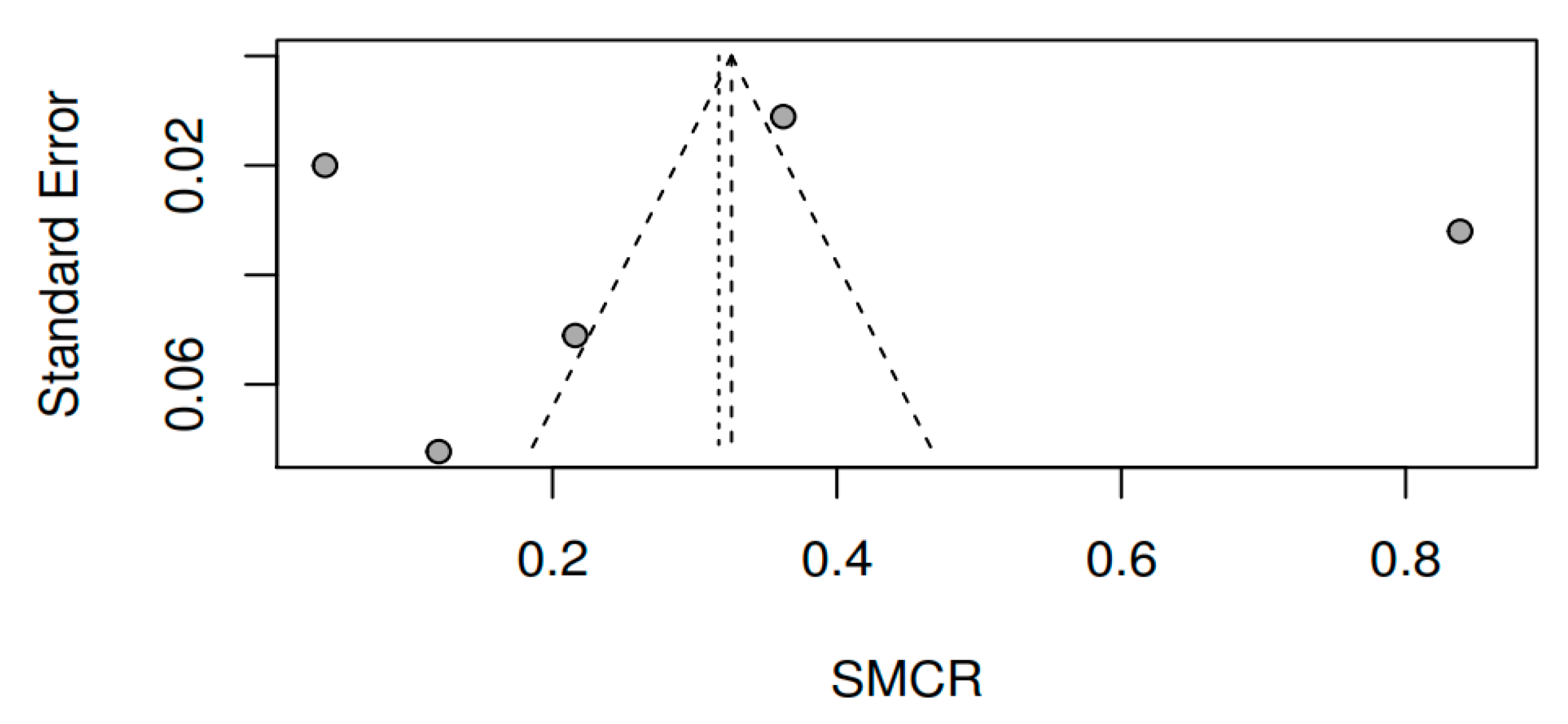
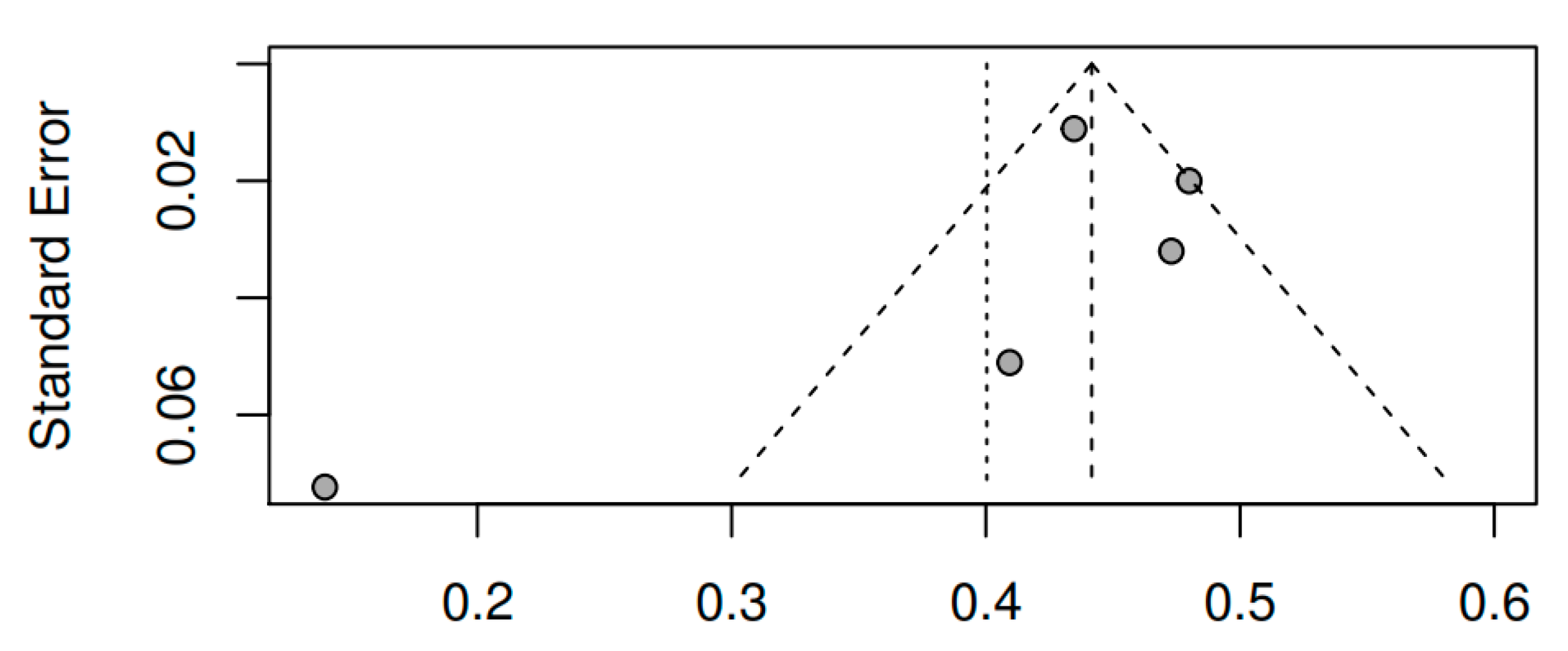
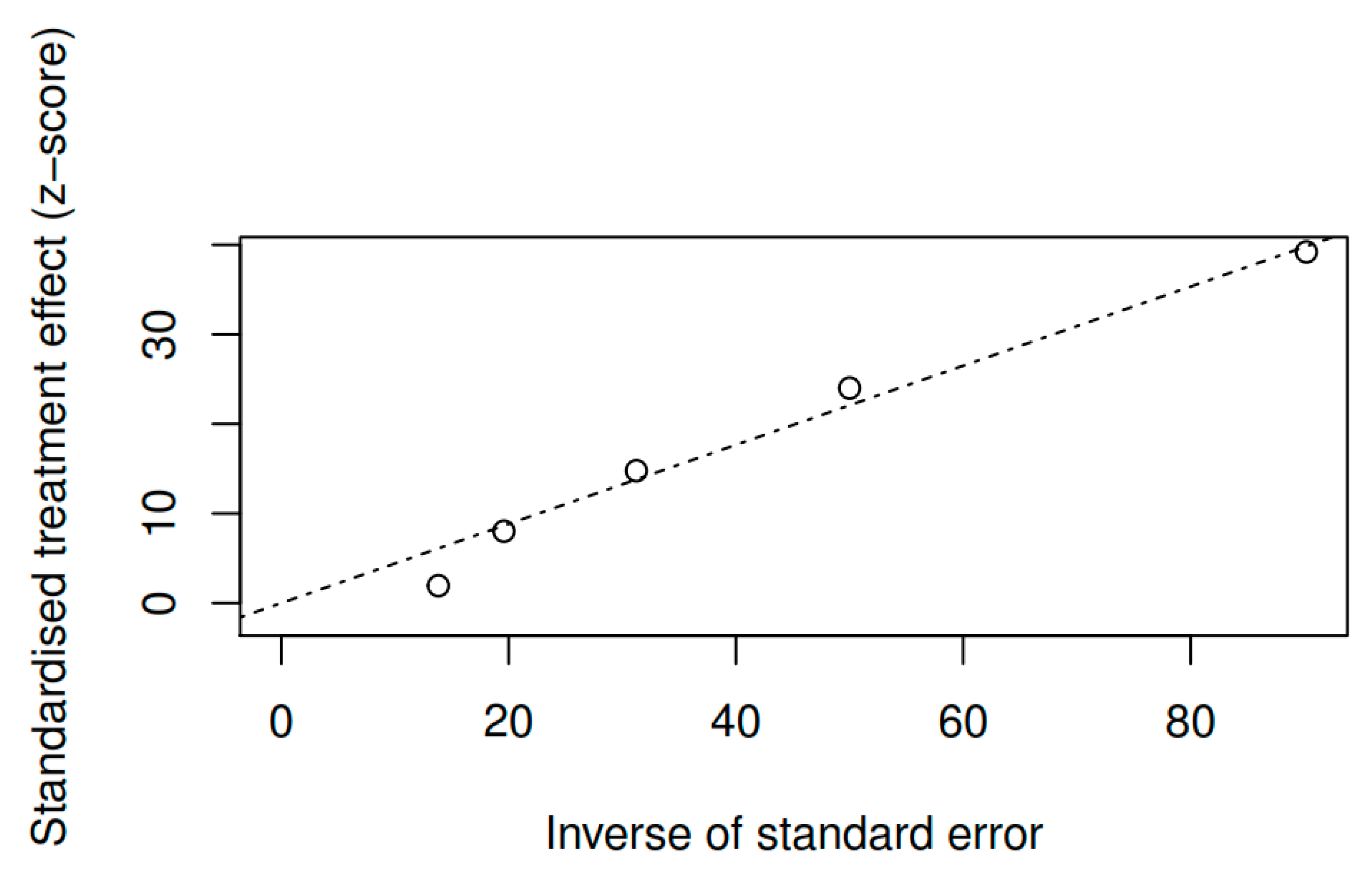
3.8.3. Publication bias and model adjustment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Santed, M.Á. Procesos psicológicos en mindfulness. In Mindfulness: Fundamentos y Aplicaciones; Santed, M.Á., Segovia, S., Eds.; Paraninfo: Madrid, Spain, 2018; pp. 21–56. [Google Scholar]
- Hanh, T.N.; Kornfield, J. Being Peace, 2nd ed.; Parallax Press: Berkeley, CA, USA, 2005; ISBN 978-1-888375-40-4. [Google Scholar]
- Kabat-Zinn, J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen. Hosp. Psychiatry 1982, 4, 33–47. [Google Scholar] [CrossRef]
- Santed, M.Á.; Segovia, S. Mindfulness: Fundamentos y Aplicaciones; Paraninfo: Madrid, Spain, 2018. [Google Scholar]
- Segal, Z.V.; Mark, G.W.; John, D.T. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse, 2nd ed.; Guilford Press: New York, NY, USA, 2012; ISBN 978-1-4625-0750-4. [Google Scholar]
- De Vibe, M.; Bjørndal, A.; Fattah, S.; Dyrdal, G.M.; Halland, E.; Tanner-Smith, E.E. Mindfulness-based stress reduction (MBSR) for improving health, quality of life and social functioning in adults: A systematic review and meta-analysis. Campbell Syst. Rev. 2017, 13, 1–264. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Tucker, R.P.; Greene, P.A.; Davidson, R.J.; Kearney, D.J.; Simpson, T.L. Mindfulness-based cognitive therapy for the treatment of current depressive symptoms: A meta-analysis. Cogn. Behav. Ther. 2019, 48, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. Mindfulness-based interventions in context: Past, present, and future. Clin. Psychol. Sci. Pract. 2003, 10, 144–156. [Google Scholar] [CrossRef]
- Crane, R.S.; Brewer, J.; Feldman, C.; Kabat-Zinn, J.; Santorelli, S.; Williams, J.M.G.; Kuyken, W. What defines mindfulness-based programs? The warp and the weft. Psychol. Med. 2017, 47, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Carmody, J. Eastern and Western Approaches to Mindfulness: Similarities, Differences, and Clinical Implications. In The Wiley Blackwell Handbook of Mindfulness; Ie, A., Ngnoumen, C.T., Langer, E.J., Eds.; John Wiley & Sons: Chichester, UK, 2014; ISBN 978-1-11829-489-5. [Google Scholar]
- Hayes, S.C.; Strosahl, K.D.; Wilson, K.G. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change; Guilford Press: New York, NY, USA, 1999; ISBN 978-1-57230-481-9. [Google Scholar]
- Linehan, M. Cognitive-Behavioral Treatment of Borderline Personality Disorder, 1st ed.; The Guilford Press: New York, NY, USA, 1993; ISBN 978-0-89862-183-9. [Google Scholar]
- Goldberg, S.B.; Tucker, R.P.; Greene, P.A.; Davidson, R.J.; Wampold, B.E.; Kearney, D.J.; Simpson, T.L. Mindfulness-based interventions for psychiatric disorders: A systematic review and meta-analysis. Clin. Psychol. Rev. 2017, 59, 52–60. [Google Scholar] [CrossRef]
- Epstein, M.D.; Lieff, J.D. Psychiatric complications of meditation practice. J. Transpers. Psychol. 1981, 13, 137–147. [Google Scholar]
- Walsh, R.; Roche, L. Precipitation of acute psychotic episodes by intensive meditation in individuals with a history of schizophrenia. Am. J. Psychiatry 1979, 136, 1085–1086. [Google Scholar] [CrossRef]
- Kuijpers, H.J.; Van der Heijden, F.; Tuinier, S.; Verhoeven, W.M.A. Meditation-induced psychosis. Psychopathology 2007, 40, 461–464. [Google Scholar] [CrossRef]
- Bach, P.; Hayes, S.C. The use of acceptance and commitment therapy to prevent the rehospitalization of psychotic patients: A randomized controlled trial. J. Consult. Clin. Psychol. 2002, 70, 1129–1139. [Google Scholar] [CrossRef]
- Chadwick, P. Person-Based Cognitive Therapy for Distressing Psychosis; John Wiley & Sons: Chichester, UK, 2006; ISBN 0-470-02984-6. [Google Scholar]
- Naeem, F.; Khoury, B.; Munshi, T.; Ayub, M.; Lecomte, T.; Kingdon, D.; Farooq, S. Brief Cognitive Behavioral Therapy for Psychosis (CBTp) for Schizophrenia: Literature Review and Meta-analysis. Int. J. Cogn. Ther. 2016, 9, 73–86. [Google Scholar] [CrossRef]
- Lemos-Giráldez, S. Esquizofrenia y Otros Trastornos Psicóticos, 1st ed.; Síntesis: Madrid, Spain, 2015; ISBN 978-84-9077-205-8. [Google Scholar]
- Jauhar, S.; Laws, K.R.; McKenna, P.J. CBT for schizophrenia: A critical viewpoint. Psychol. Med. 2019, 49, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.M.; Erickson, D.H.; Brenner, C.A. Cognitive-behavioral therapy for medication-resistant psychosis: A meta-analytic review. Psychiatr. Serv. 2014, 65, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Hazell, C.M.; Hayward, M.; Cavanagh, K.; Strauss, C. A systematic review and meta-analysis of low intensity CBT for psychosis. Clin. Psychol. Rev. 2016, 45, 183–192. [Google Scholar] [CrossRef]
- Turner, D.T.; van der Gaag, M.; Karyotaki, E.; Cuijpers, P. Psychological interventions for psychosis: A meta-analysis of comparative outcome studies. Am. J. Psychiatry 2014, 171, 523–538. [Google Scholar] [CrossRef]
- Abba, N.; Chadwick, P.; Stevenson, C. Responding mindfully to distressing psychosis: A grounded theory analysis. Psychother. Res. 2008, 18, 77–87. [Google Scholar] [CrossRef]
- Ashcroft, K.; Barrow, F.; Lee, R.; MacKinnon, K. Mindfulness groups for early psychosis: A qualitative study. Psychol. Psychother. 2012, 85, 327–334. [Google Scholar] [CrossRef]
- Khoury, B.; Lecomte, T.; Gaudiano, B.A.; Paquin, K. Mindfulness interventions for psychosis: A meta-analysis. Schizophr. Res. 2013, 150, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Cramer, H.; Lauche, R.; Haller, H.; Langhorst, J.; Dobos, G. Mindfulness-and acceptance-based interventions for psychosis: A systematic review and meta-analysis. Glob. Adv. Health Med. 2016, 5, 30–43. [Google Scholar] [CrossRef]
- Louise, S.; Fitzpatrick, M.; Strauss, C.; Rossell, S.L.; Thomas, N. Mindfulness- and acceptance-based interventions for psychosis: Our current understanding and a meta-analysis. Schizophr. Res. 2018, 192, 57–63. [Google Scholar] [CrossRef]
- Jansen, J.E.; Gleeson, J.; Bendall, S.; Rice, S.; Alvarez-Jimenez, M. Acceptance- and mindfulness-based interventions for persons with psychosis: A systematic review and meta-analysis. Schizophr. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Sterne, J.; Savović, J.; Page, M.; Hróbjartsson, A.; Boutron, I.; Reeves, B.; Eldridge, S. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2). Available online: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed on 25 January 2020).
- Becker, B.J. Synthesizing standardized mean-change measures. Br. J. Math. Stat. Psychol. 1988, 41, 257–278. [Google Scholar] [CrossRef]
- Morris, S.B.; DeShon, R.P. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol. Methods 2002, 7, 105–125. [Google Scholar] [CrossRef]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: Orlando, FL, USA, 1985; ISBN 978-0-12-336380-0. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Del Re, A.C.; Hoyt, W.T. Package ‘MAd’. Available online: https://cran.r-project.org/web/packages/MAd/MAd.pdf (accessed on 25 January 2020).
- RDC Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2006. [Google Scholar]
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.T.; Langan, D.; Salanti, G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 2016, 7, 55–79. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Cheung, M.W.-L.; Ho, R.C.M.; Lim, Y.; Mak, A. Conducting a meta-analysis: Basics and good practices. Int. J. Rheum. Dis. 2012, 15, 129–135. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Chichester, UK, 2009; ISBN 978-1-119-96437-7. [Google Scholar]
- Ausina, J.B.; Meca, J.S. Meta-Análisis en Ciencias Sociales y de la Salud; Síntesis: Madrid, Spain, 2015; ISBN 978-84-9077-124-2. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Schwarzer, G. Meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- Chadwick, P.; Hughes, S.; Russell, D.; Russell, I.; Dagnan, D. Mindfulness groups for distressing voices and paranoia: A replication and randomized feasibility trial. Behav. Cogn. Psychother. 2009, 37, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Langer, Á.I.; Cangas, A.J.; Salcedo, E.; Fuentes, B. Applying mindfulness therapy in a group of psychotic individuals: A controlled study. Behav. Cogn. Psychother. 2012, 40, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, E.; Kavak, F. Effects of Mindfulness-Based Psychoeducation on the Internalized Stigmatization Level of Patients With Schizophrenia. Clin. Nurs. Res. 2018. [Google Scholar] [CrossRef]
- World Health Organization. ICD 10: International Statistical Classification of Diseases and Related Health Problems, 10th ed.; World Health Organization: Geneva, Switzerland, 1992; ISBN 978-92-4-154419-1. [Google Scholar]
- American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Publishing: Washington, DC, USA, 1994; ISBN 978-0-89042-061-4. [Google Scholar]
- American Psychiatric Association. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision; American Psychiatric Publishing: Washington, DC, USA, 2000; ISBN 978-0-89042-025-6. [Google Scholar]
- American Psychiatric Association. DSM-5: Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- Chien, W.T.; Bressington, D.; Yip, A.; Karatzias, T. An international multi-site, randomized controlled trial of a mindfulness-based psychoeducation group programme for people with schizophrenia. Psychol. Med. 2017, 47, 2081–2096. [Google Scholar] [CrossRef]
- Chien, W.T.; Cheng, H.Y.; McMaster, T.W.; Yip, A.L.K.; Wong, J.C.L. Effectiveness of a mindfulness-based psychoeducation group programme for early-stage schizophrenia: An 18-month randomised controlled trial. Schizophr. Res. 2019, 212, 140–149. [Google Scholar] [CrossRef]
- Chien, W.T.; Thompson, D.R. Effects of a mindfulness-based psychoeducation programme for Chinese patients with schizophrenia: 2-year follow-up. Br. J. Psychiatry 2014, 205, 52–59. [Google Scholar] [CrossRef]
- Lee, K.-H. A randomized controlled trial of mindfulness in patients with schizophrenia. Psychiatry Res. 2019, 275, 137–142. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Petrini, M.; Morisky, D.E. Comparison of the quality of life, perceived stigma and medication adherence of Chinese with schizophrenia: A follow-up study. Arch. Psychiatr. Nurs. 2016, 30, 41–46. [Google Scholar] [CrossRef]
- Chadwick, P.; Strauss, C.; Jones, A.-M.; Kingdon, D.; Ellett, L.; Dannahy, L.; Hayward, M. Group mindfulness-based intervention for distressing voices: A pragmatic randomised controlled trial. Schizophr. Res. 2016, 175, 168–173. [Google Scholar] [CrossRef]
- Davis, L.W.; Lysaker, P.H.; Kristeller, J.L.; Salyers, M.P.; Kovach, A.C.; Woller, S. Effect of mindfulness on vocational rehabilitation outcomes in stable phase schizophrenia. Psychol. Serv. 2015, 12, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.; Franck, N. Rehabilitation interventions to promote recovery from schizophrenia: A systematic review. Front. Psychiatry 2017, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.C.; Gilleen, J.; David, A.S. The relationship between cognitive insight and depression in psychosis and schizophrenia: A review and meta-analysis. Schizophr. Res. 2015, 166, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lustyk, M.K.; Chawla, N.; Nolan, R.; Marlatt, G.A. Mindfulness meditation research: Issues of participant screening, safety procedures, and researcher training. Adv. Mind Body Med. 2009, 24, 20–30. [Google Scholar] [PubMed]
- Cebolla, A.; Demarzo, M.; Martins, P.; Soler, J.; Garcia-Campayo, J. Unwanted effects: Is there a negative side of meditation? A multicentre survey. PLoS ONE 2017, 12, e0183137. [Google Scholar] [CrossRef] [PubMed]
- Jauhar, S.; McKenna, P.J.; Radua, J.; Fung, E.; Salvador, R.; Laws, K.R. Cognitive–behavioural therapy for the symptoms of schizophrenia: Systematic review and meta-analysis with examination of potential bias. Br. J. Psychiatry 2014, 204, 20–29. [Google Scholar] [CrossRef]
- Lutgens, D.; Gariepy, G.; Malla, A. Psychological and psychosocial interventions for negative symptoms in psychosis: Systematic review and meta-analysis. Br. J. Psychiatry 2017, 210, 324–332. [Google Scholar] [CrossRef]
- Birchwood, M.; Spencer, E. Psychotherapies for schizophrenia: A review. Schizophrenia 1999, 2, 147–241. [Google Scholar] [CrossRef]
- Morrison, A.P.; Barratt, S. What Are the Components of CBT for Psychosis? A Delphi Study. Schizophr. Bull. 2010, 36, 136–142. [Google Scholar] [CrossRef]
- Hodann-Caudevilla, R.M.; Serrano-Pintado, I. Revisión sistemática de la eficacia de los tratamientos basados en mindfulness para los trastornos de ansiedad. Ansiedad Y Estrés 2016, 22, 39–45. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Psychosis and Schizophrenia in Adults: Treatment and Management; National Institute for Health and Care Excellence: London, UK, 2014.
- Scottish Intercollegiate Guidelines Network. Management of Schizophrenia: A National Clinical Guideline; Scottish Intercollegiate Guidelines Network: Edinburgh, Scotland, 2013; ISBN 978-1-905813-96-4.

| Variable | β | z1 | se | 95% CI | p |
|---|---|---|---|---|---|
| Overall symptomatology | |||||
| Age | <−0.01 | −0.74 | 0.32 | [−0.02; 0.01] | 0.46 |
| Gender | <0.01 | −0.17 | 0.01 | [−0.01; 0.01] | 0.86 |
| Duration | <0.01 | 1.29 | 0.01 | [−0.01; 0.03] | 0.20 |
| Treatment | 0.14 | 1.79 | 0.08 | [−0.01; 0.29] | 0.07 |
| Quality | 0.05 | 0.47 | 0.11 | [−0.16; 0.26] | 0.64 |
| Adherence | −0.01 | −0.92 | 0.01 | [−0.04; 0.02] | 0.36 |
| Control group | 0.23 | 1.37 | 0.17 | [−0.10; 0.55] | 0.17 |
| Positive symptoms | |||||
| Age | −0.01 | −0.63 | 0.01 | [−0.04; 0.02] | 0.53 |
| Gender | 0.01 | 0.86 | 0.01 | [−0.01; 0.02] | 0.39 |
| Duration | 0.03 | 2.31 | 0.01 | [0.01; 0.06] | 0.02 * |
| Treatment | 0.05 | 0.18 | 0.26 | [−0.46; 0.55] | 0.86 |
| Quality | −0.12 | −0.80 | 0.15 | [−0.42; 0.18] | 0.42 |
| Adherence | −0.04 | −1.63 | 0.03 | [−0.09; 0.01] | 0.10 |
| Control group | 0.12 | 0.31 | 0.40 | [−0.67; 0.92] | 0.76 |
| Negative symptoms | |||||
| Age | −0.01 | −2.01 | 0.01 | [−0.01; <−0.01] | 0.04 * |
| Gender | 0.01 | 2.47 | 0.01 | [<0.01; 0.01] | 0.01 * |
| Duration | 0.01 | 1.08 | 0.01 | [−0.01; 0.02] | 0.28 |
| Treatment | 0.12 | 1.46 | 0.08 | [−0.04; 0.29] | 0.14 |
| Quality | −0.08 | −2.05 | 0.04 | [−0.16; −0.01] | 0.04 * |
| Adherence | −0.01 | −0.85 | 0.01 | [−0.03; 0.01] | 0.40 |
| Control group | −0.02 | −0.09 | 0.17 | [−0.34; −0.31] | 0.92 |
| Variable | Estimator | Hedges’ g | 95% CI | p |
|---|---|---|---|---|
| Overall symptomatology | ||||
| (Pretest–posttest) | DL | 0.72 | [0.38; 1.05] | <0.01 * |
| ML | 0.72 | [0.39; 1.06] | <0.01 * | |
| EB | 0.72 | [0.36; 1.08] | <0.01 * | |
| Overall symptomatology | ||||
| (Follow-up) | DL | 1.76 | [1.18; 2.35] | <0.01 * |
| ML | 1.76 | [1.39; 2.14] | <0.01 * | |
| EB | 1.76 | [1.31; 2.21] | <0.01 * | |
| Positive symptoms | ||||
| DL | 0.32 | [0.07; 0.56] | 0.01 * | |
| ML | 0.32 | [0.07; 0.57] | 0.01 * | |
| EB | 0.32 | [0.04; 0.59] | 0.02 * | |
| Negative symptoms | ||||
| DL | 0.42 | [0.36; 0.48] | <0.01 * | |
| ML | 0.40 | [0.36; 0.48] | <0.01 * | |
| EB | 0.40 | [0.28; 0.51] | <0.01 * | |
| Mindfulness | ||||
| DL | 1.41 | [0.66; 2.15] | <0.01 * | |
| ML | 1.45 | [0.55; 2.35] | <0.01 * | |
| EB | 1.49 | [0.36; 2.61] | 0.01 * | |
| Functioning | ||||
| DL | −1.28 | [−1.44; −1.12] | <0.01 * | |
| ML | −1.28 | [−1.44; −1.12] | <0.01 * | |
| EB | −1.28 | [−1.46; −1.10] | <0.01 * | |
| Awareness of illness | ||||
| DL | −0.65 | [−0.81; −0.50] | <0.01 * | |
| ML | −0.65 | [−0.79; −0.50] | <0.01 * | |
| EB | −0.65 | [−0.82; −0.48] | <0.01 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodann-Caudevilla, R.M.; Díaz-Silveira, C.; Burgos-Julián, F.A.; Santed, M.A. Mindfulness-Based Interventions for People with Schizophrenia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 4690. https://doi.org/10.3390/ijerph17134690
Hodann-Caudevilla RM, Díaz-Silveira C, Burgos-Julián FA, Santed MA. Mindfulness-Based Interventions for People with Schizophrenia: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2020; 17(13):4690. https://doi.org/10.3390/ijerph17134690
Chicago/Turabian StyleHodann-Caudevilla, Ricardo M., Cintia Díaz-Silveira, Francisco A. Burgos-Julián, and Miguel A. Santed. 2020. "Mindfulness-Based Interventions for People with Schizophrenia: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 17, no. 13: 4690. https://doi.org/10.3390/ijerph17134690
APA StyleHodann-Caudevilla, R. M., Díaz-Silveira, C., Burgos-Julián, F. A., & Santed, M. A. (2020). Mindfulness-Based Interventions for People with Schizophrenia: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 17(13), 4690. https://doi.org/10.3390/ijerph17134690





