Abstract
Electro-oxidation is an effective approach for the removal of 2-chlorophenol from wastewater. The modeling of the electrochemical process plays an important role in improving the efficiency of electrochemical treatment and increasing our understanding of electrochemical treatment without increasing the cost. The backpropagation artificial neural network (BP–ANN) model was applied to predict chemical oxygen demand (COD) removal efficiency and total energy consumption (TEC). Current density, pH, supporting electrolyte concentration, and oxidation–reduction potential (ORP) were used as input parameters in the 2-chlorophenol synthetic wastewater model. Prediction accuracy was increased by using particle swarm optimization coupled with BP–ANN to optimize weight and threshold values. The particle swarm optimization BP–ANN (PSO–BP–ANN) for the efficient prediction of COD removal efficiency and TEC for testing data showed high correlation coefficient of 0.99 and 0.9944 and a mean square error of 0.0015526 and 0.0023456. The weight matrix analysis indicated that the correlation of the five input parameters was a current density of 18.85%, an initial pH 21.11%, an electrolyte concentration 19.69%, an oxidation time of 21.30%, and an ORP of 19.05%. The analysis of removal kinetics indicated that oxidation–reduction potential (ORP) is closely correlated with the chemical oxygen demand (COD) and total energy consumption (TEC) of the electro-oxidation degradation of 2-chlorophenol in wastewater.
1. Introduction
Wastewater produced by various industrial processes contains large quantities of chlorophenol compounds, which are highly toxic and resistant to biological degradation [1]. The compound 2-chlorophenol is a typical chlorophenol compound that is listed as a priority pollutant by the Environmental Protection Agency, given its carcinogenic properties [2,3]. Electro-oxidation, an effective technology that does not require the use of extra reagents, is commonly used to remove chlorophenol compounds from wastewater because of its high efficiency, rapid reaction rate, and environmental friendliness [4,5]. However, the energy cost of the electro-oxidation process limits its application [6].
The establishment of appropriate models for electro-oxidation is essential given the complexity of this process. Modeling of the electrochemical process plays an important role in improving the efficiency of electrochemical treatment and a further understanding of electrochemical treatment without increasing the cost. Empirical models and semi-empirical models, such as pseudo-first-order kinetics [7], pseudo-second-order kinetics [8], a computational fluid dynamics (CFD) model, and response surface methodology (RSM) model, are usually established for the prediction of electrochemical process behaviors. Bu et al. [9] established the kinetic model of the degradation of oxcarbazepine (OXC) using electrochemically-activated persulfate (EC/PS) based on two assumptions. Conventional mathematical or mechanistic models can be used to predict the final state of the system only under given circumstances [10]. Wang et al. [11] calculated the velocity distribution and turbulence distribution of a new type of tubular plunger flow reactor by CFD. CFD can reveal the mass transfer process and mechanism of an electrochemical reactor, but it is still affected by grid mass, transfer mode, and calculation [12,13]. Song et al. [14] optimized the electrochemical simultaneous removal of the ammonia nitrogen process using RSM, which showed a good prediction. The main disadvantage of RSM is that it cannot effectively improve approximation accuracy, even with an increase in the number of sample points. Electrochemistry is a complex non-linear process, and it is difficult to explain it clearly through traditional empirical and semi-empirical modeling.
In contrast to traditional mathematical models, scholars have done some research on the non-linear prediction model of the electrochemical process. Artificial Neural Networks (ANNs) do not require the modeling of a detailed mathematical formulation of a system and have been used to determine complex relationships between input and output data [15]. Daneshvar et al. [16] established an ANN model for the decolorization process of dyeing wastewater by electroflocculation. This model can predict the color removal rate under different experimental conditions. Researchers pointed out that ANN has good prospects for the prediction of complex systems [17,18]. Belkacem et al. [19] applied a backpropagation artificial neural network (BP–ANN) prediction of oxytetracycline removal in an electro-oxidation system, which chose 14 nodes from the hidden layer, the LM (Levenberg-Marquardt)algorithm, the logsig transfer function of the hidden layer, and the purelin transfer function of the output layer. However, the researchers did not verify the reliability of the network or compare the algorithms and transfer functions on the network. Moreover, BP–ANN easily falls into the local minimum and has a poor global convergence rate [20]. The further optimization of the BP–ANN has also attracted growing attention [21]. Particle swarm optimization (PSO) is an algorithm that simulates the foraging behavior of birds [22]. Khajeh and coworkers [23] integrated PSO in a BP–ANN model for the specification of optimal initial weights and threshold values by updating generations to avoid the local minimum and achieve global convergence quickly and correctly.
Establishing an efficient and reliable ANN model for predicting the behavior of electrochemical oxidation processes can reduce energy cost and is a fundamental step toward their control. The input parameters of ANN network are one of the key factors in establishing an ANN network. Oxidation–reduction potential (ORP) has been employed as an integrated indicator in various fields to describe the redox characteristic of any given chemical reaction system [24]. ORP has a good relationship with the chemical oxygen demand (COD) of electro-oxidation [25]. Wang and coworkers [26] constructed a model of the multiparameter linear relationship between ORP and Qsp (specific electrical charge) and between a COD and Cl−1 concentration to reflect quantitatively the effect of the current density, Cl−1 concentration, pollutant load, and reaction time on the electro-oxidation system. Basha et al. [27] built a BP–ANN model to predict the effect of electro-oxidation on COD removal, but ORP was not considered in the input parameters.
In this study, PSO–BP–ANN models were constructed to predict the COD removal efficiency and total energy consumption (TEC) of electro-oxidation. ORP was used as one of the input parameters. First, BP–ANN and the selection of the number of hidden layers and training algorithm were discussed in detail. Then, the PSO algorithm was used to optimize the weight and threshold of BP–ANN and identify the optimal parameters of the PSO algorithm. Experimental values were compared with output variables predicted by PSO–BP–ANN. The importance of each input variable was determined.
2. Materials and Methods
2.1. Data Set
All electro-oxidation experiments were conducted with a 3 L-capacity laboratory-scale plate cell with a circulating tank. The used datasets were obtained from a previous study [25]. A total of 190 experimental runs (Table A1) were performed in the galvanostatic mode under a current density of 8 mA cm−2 to 25 mA cm−2, an original pH of 3 to 11, an electrolyte concentration of 0.05mol L−1 to 0.12 mol L−1, a reaction time of 0 h to 2 h, and ORP values of −68 mV to 500 mV, as shown in Table 1.

Table 1.
Experimental conditions. ORP, oxidation–reduction potential.
During the Electro-oxidation, an ORP (SX-630, Sanxin, China) and a pH (SX711, Sanxin, China) probe were installed in the electrolysis bath for online monitoring of ORP/pH. COD was determined according to Chinese standard HJ/T 399-2007 with slight modifications. The solution was measured at a wavelength of 440 nm using a UV-visible spectrophotometer (UV-2910, Hitachi, Japan).
A specific electrical charge (Qsp, Ah L−1) was calculated by using the following equation [26]:
where j is current density (A cm−2), A is the effective area of the electrode (cm2), V is the effective volume of the plate cell (L), and t is the reaction time during the electro-oxidation process (h).
TEC (kWh m−3) was calculated in a previous study as follows [28]:
where Qsp is a specific electrical charge, and U (V) is the cell voltage.
2.2. BP–ANN Coupled with PSO
ANNs have different architectures. The ANN used in this study has three layers: an input layer that receives electro-oxidation information, a hidden layer that processes information, and an output layer that calculates COD removal and TEC results [29]. During BP learning, the actual outputs are compared with the target values to derive error signals, which are propagated backward by layers to adjust the weights in all lower layers [30]. The architecture of a neural network and the BP algorithm is presented in Figure 1.
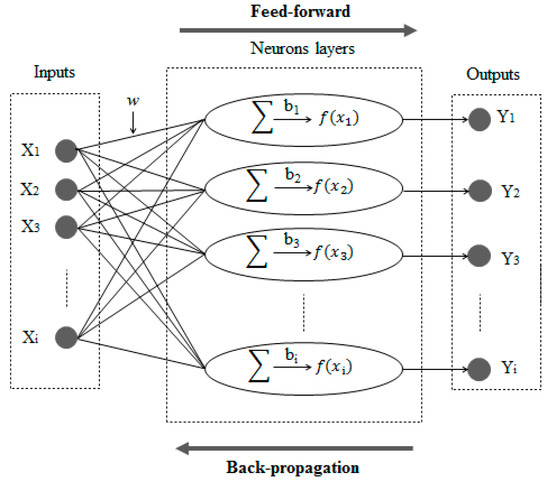
Figure 1.
Architecture of an artificial neural network (ANN) and feed-forward back-propagation algorithm.
The flowchart of BP–ANN coupled with PSO is shown in Figure 2. The ANN model was developed using MATLAB R2016a software. A total of 190 runs of the electro-oxidation process data were applied to develop the models for the prediction of COD removal efficiency and TEC. The available data were divided into training, validation, and testing subsets, of which 80% (152) were randomly selected for network training, 10% (19) were used for validation, and 10% (19) were applied to test network accuracy. Current density, original pH, electrolyte concentration, oxidation time, and ORP were used as five input parameters, and COD removal efficiency and TEC were considered as the two output.
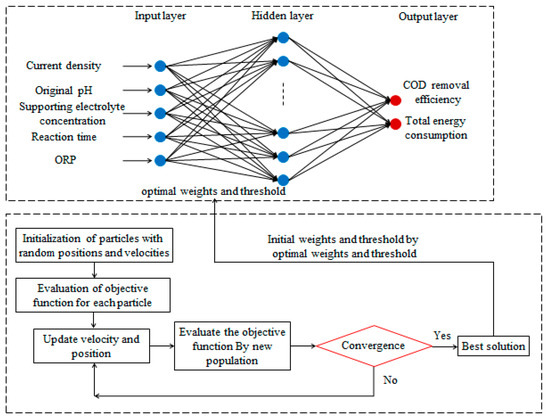
Figure 2.
Flowchart of a backpropagation artificial neural network (BP–ANN) combined with particle swarm optimization (PSO).
Two prediction score metrics, the coefficient of correlation (R2), and mean square error (MSE), were computed using the following equations to evaluate the fitting and prediction accuracy of the constructed models [31]:
where , , n is the number of samples used for modeling, fexp is the experimental value, and fANN is the network-predicted value.
3. Results and Discussion
3.1. Removal Kinetics
The apparent reaction rate constants for COD removal were calculated in accordance with Equation (5) [32]:
where COD0 and CODt are the COD values of the initial and final pollutant concentrations (mg L−1), respectively; t is the electrolysis time (min); and K is the apparent reaction rate constant (min−1). The apparent reaction rate constants calculated in accordance with Equation (3) for the current densities of 8, 10, 12, 14, 15, 18, 20, and 25 mA cm−2 were 0.0072, 0.0107, 0.0118, 0.0160, 0.0202, 0.0212, 0.0224, and 0.0232 min−1, respectively. The linear relationship between the logarithmic values of COD and electrolysis time is depicted in Figure 3. Table 2 shows that the correlation coefficient R2 of linear fitting was greater than 0.9989. This result indicates that COD removal satisfies the first-order reaction kinetics equation.
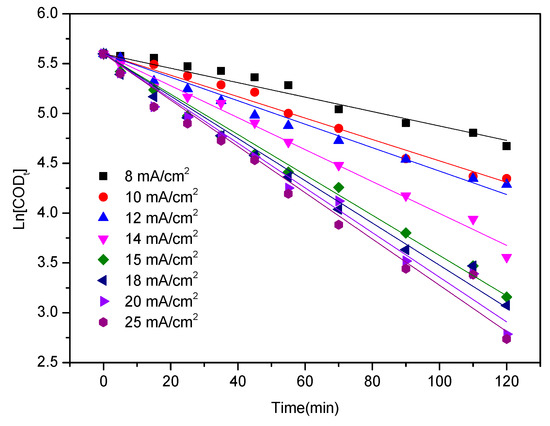
Figure 3.
Linear relationship between the logarithmic values of chemical oxygen demand (COD) and electrolysis time.

Table 2.
K and correlation coefficient values under various current densities.
Other parameters, such as temperature (T), pH value, and electricity can be obtained when the influent quality and flow rate are held constant in the electrolytic cell. The kinetic constant K is only related to current density (j) under the conditions of the original pH of 3 and Na2SO4 concentration of 0.10 mol L−1 [11].
From Equation (5), Equation (7) can be expressed as
which describes the relationship among COD, current density, and oxidation time.
The optimal electro-oxidation conditions were initially determined by considering the effective factors of current density, original pH value, and electrolyte concentration. A COD removal efficiency of 100% was obtained with the optimal operating parameters of a current density of 15 mA cm−2, an original pH of 3, and a Na2SO4 concentration of 0.10 mol L−1 at 120 min. The dependencies of the values of COD, ORP, TEC, and Qsp under a current density of 15 mA cm−2, an original pH of 3, and a Na2SO4 concentration of 0.10 mol L−1 during electrochemical oxidation are shown in Figure 4. COD removal efficiency, TEC, and Qsp increased with electro-oxidation time. COD removal efficiency, TEC, Qsp, and ORP were 77.9%, 24.2 kWh m−3, 1.375 Ah L−1, and 383 mV, respectively, when oxidation time was 1 h. The ORP value decreased from 494 mV to 190 mV within 5 min of electrolysis and then increased gradually to 500 mV during degradation.
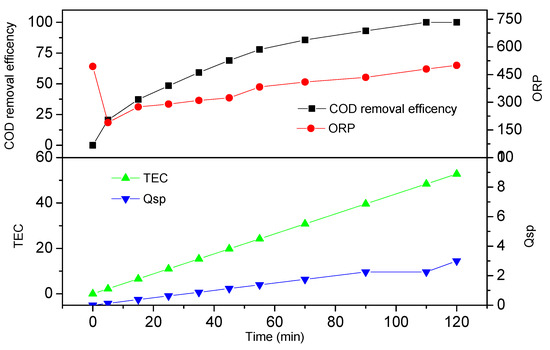
Figure 4.
COD removal efficiency, ORP, total energy consumption (TEC), and Qsp under a current density of 15 mA cm−2, original pH of 3, and an Na2SO4 concentration of 0.10 mol L−1.
The typical multiple regression equation showing the relationship among ORP, current density, original pH, Na2SO4 concentration, reaction time, and COD removal efficiency was obtained as follows:
The typical multiple regression equation representing the relationship among influential parameters and TEC was obtained and is shown below:
The R2 values for COD removal efficiency and TEC were 0.8878 and 0.93223, respectively. These values reflect a good correlation among COD, TEC, j, pH, t, Na2SO4 concentration, and ORP. ORP values provide a complete indicator of the effect of current density, electrolyte concentration, pH, and reaction time on the performance of the electro-oxidation system. Therefore, the ORP value can be used as an effective controlling factor for the prediction of COD removal efficiency and the TEC of electro-oxidation.
3.2. BP–ANN Prediction of 2-Chlorophenol Removal
The tangent sigmoid was selected as the transfer function for the input layer nodes to the hidden layer, and the purelin was selected as the transfer function for the hidden layer nodes to the output layer. All data were normalized within a range of −1 and 1 before being fed to the networks to increase training speed and facilitate modeling and prediction.
In this study, the numbers of input and output nodes were 5 and 2, respectively, and were equal to the numbers of input and output data. The number of neurons has a considerable effect on network performance. For example, the network cannot achieve the desired error if the number of neurons is too small, or overfitting may occur if the number of neurons is too large. Thus, determining the appropriate number of neurons in the hidden layer is necessary. This number can usually be determined by using the following empirical formula in accordance with Hecht–Nielsen’s theorem [33]:
where NH is the number of hidden neurons, and Ni is the number of input variables, which is 5 in the present work. Equation (11) shows that the node number in the hidden layer was approximately 11. Then, BP networks with different hidden neurons from 6–16 were compared on the basis of the maximization of R2 and the minimization of MSE for the testing dataset. Table 3 shows that the BP–ANN that contains 6–16 hidden neurons in the prediction of the electro-oxidation process. The optimal BP–ANN model provided an R2 and MSE of 0.9344 and 0.0137232 for COD removal efficiency, respectively, and an R2 and MSE of 0.9355 and 0.013127 for TEC, respectively when the hidden neurons were 10. Under the optimal network, BP–ANN in the prediction of COD removal efficiency and TEC and the correlations between the experimental and predicted sets are illustrated in Figure 5. The error range of COD was (−0.058, 0.249) and TEC (−0.079, 0.391). The network performance is good, but the error range shows that the deviation of individual points is large.

Table 3.
Evaluation of the prediction performance of the BP–ANN model for the testing dataset.
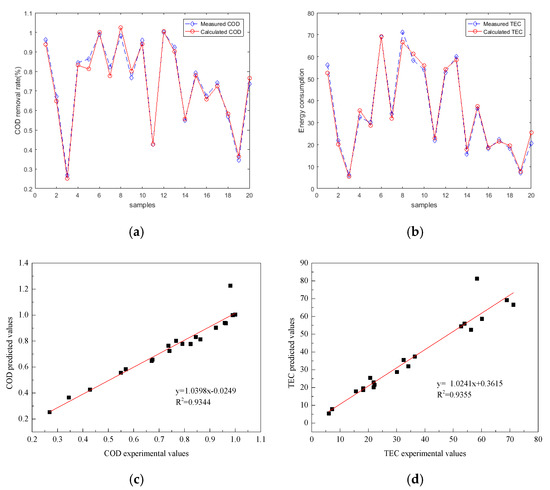
Figure 5.
Performance of the BP–ANN predicting COD removal efficiency and TEC between experimental and predicted data sets (COD removal efficiency testing set (a), TEC testing set (b)); correlations between experimental and predicted set (COD removal efficiency testing set (c), TEC testing set (d)).
The training algorithm also affects the performance of BP networks. A wide variety of training functions with 10 neurons used in the hidden layer was studied to select a good BP network. Table 4 presents the data for R2 and MSE under different training functions of BP networks. The Levenberg–Marquardt back propagation (trainlm) training algorithm, which maximized the R2 and minimized the MSE of COD removal efficiency and TEC, was identified as the best training function.

Table 4.
Predictions of backpropagation (BP) models with different training algorithms for the testing dataset.
3.3. Optimization of the Weight and Threshold Value of BP–ANN
The PSO–BP–ANN can be optimized for selection purposes by optimizing (1) swarm size, (2) maximum iteration, (3) cognition coefficient C1, and (4) social coefficient C2 (Table A2). Table 5 displayed PSO control parameters, R2, and training MSE for the testing dataset. The PSO–ANN containing a swarm size of 50, a maximum iteration of 200, C1 of 1.5, and C2 of 1.5 was selected as the best model for the electrochemical process of interest. The optimal PSO–BP–ANN models provided R2 of 0.99 and 0.9944 for COD removal efficiency and TEC, and MSE values of 0.0015526 and 0.0023456, respectively, for the testing dataset. The performance of the optimal PSO–BP–ANN in the prediction of COD removal efficiency and TEC and the correlations between the experimental and predicted sets are illustrated in Figure 6. The PSO–BP–ANN selected for the efficient prediction of 2-Chlorophenol removal in an electro-oxidation system was containing 10 hidden neurons, trainlm training algorithm, swarm size of 50, maximum iteration of 200, C1 of 1.5, and C2 of 1.5.

Table 5.
PSO–ANN with different parameters of the PSO algorithm.
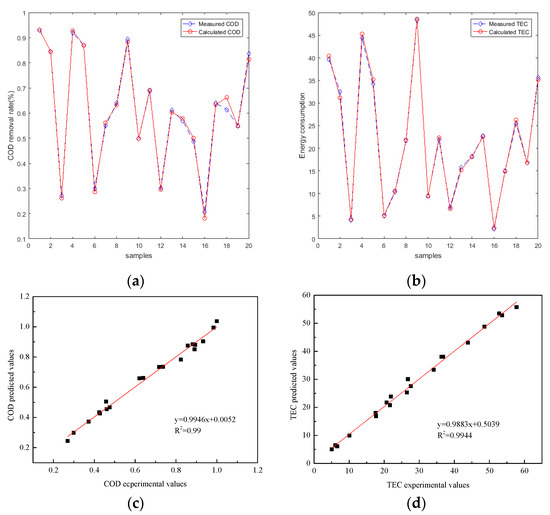
Figure 6.
Performance of the particle swarm optimization BP–ANN (PSO–BP–ANN) predicting COD removal efficiency and TEC between experimental and predicted data sets (COD removal efficiency testing set (a), TEC testing set (b)); correlations between experimental and predicted set (COD removal efficiency testing set (c), TEC testing set (d)).
3.4. Assessment of the Importance of Variables
The weight matrix of the neural net can be used to assess the relative importance of various input variables for output variables [31]. The relative importance of input variables on the value of COD removal efficiency and TEC as calculated by particle swarm optimization BP–ANN (PSO–BP–ANN) is shown in Table 6. Sensitivity analysis indicated order of relative importance the operational parameters on the electro-oxidation as: electrolysis time > pH > electrolyte concentration > ORP > current density. The table indicates that all of the variables have strong effects on COD removal efficiency and TEC. Therefore, none of the variables studied in this work should be neglected in the analysis.

Table 6.
Relative importance of input variables on the value of COD removal efficiency and TEC.
4. Conclusions
In this study, the main object is development and construction of novel model that could make efficient prediction of electro-oxidation removal of 2-Chlorophenol on the basis of batch electro-oxidation experiments. The analysis of removal kinetics indicated that ORP was closely correlated with COD removal efficiency and TEC and was one of the important input parameters of PSO–BP–ANN. PSO–BP–ANN was developed through the optimization of the weights and thresholds of BP–ANN. The PSO–BP–ANN that contained 10 hidden neurons, trainlm training algorithm and possessed a swarm size of 50, maximum iteration of 200, C1 of 1.5, and C2 of 1.5 was identified as the best model for predicting 2-chlorophenol degradation through electro-oxidation. The PSO–BP–ANN model provided accurate predictions and R2 of 0.99 and 0.9944 for COD removal efficiency and TEC, and MSE values of 0.0015526 and 0.0023456 respectively for the testing dataset. The weight matrix revealed that the order of relative importance for the operational parameters of the electro-oxidation is: electrolysis time > pH > electrolyte concentration > ORP > current density. For comparative purposes, performance data for the ANN methodology in various electrochemical processes are summarized in Table A3.
Author Contributions
J.D.W. designed the research; Y.M. wrote and revised the paper; Y.M and J.Q.Y used the software analyzed, Y.L, D.M.X, F.L.H and H.P provided advice on the research.
Funding
This research has been funded by the National Natural Science Foundation of China (No.51878614) and (No.31600257). This project was supported by the “Talent Cultivation Project” (No.2018YCGC011) of Zhejiang Science and Technology Association and “Qingchuang Project”(No.2019QC20) of Zhejiang shuren university. And it also supported by the Public welfare project of Zhejiang provincial Science and Technology (No.2016C32022).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
The results of the electro-oxidation experiment.
Table A1.
The results of the electro-oxidation experiment.
| Number | Current Density | pH | Na2SO4 Concentration | Time | ORP | COD Removal Efficiency | TEC |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 6.5 | 0.1 | 5 | 12 | 0.092 | 0.940 |
| 2 | 10 | 6.5 | 0.1 | 5 | 14 | 0.112 | 1.354 |
| 3 | 14 | 6.5 | 0.1 | 5 | 71 | 0.117 | 2.018 |
| 4 | 15 | 6.5 | 0.1 | 5 | 80 | 0.123 | 2.250 |
| 5 | 16 | 6.5 | 0.1 | 5 | 125 | 0.120 | 2.443 |
| 6 | 18 | 6.5 | 0.1 | 5 | 130 | 0.136 | 3.128 |
| 7 | 20 | 6.5 | 0.1 | 5 | 144 | 0.143 | 3.133 |
| 8 | 25 | 6.5 | 0.1 | 5 | 150 | 0.160 | 3.958 |
| 9 | 15 | 3 | 0.1 | 5 | 190 | 0.206 | 2.200 |
| 10 | 15 | 4 | 0.1 | 5 | 180 | 0.183 | 2.406 |
| 11 | 15 | 5 | 0.1 | 5 | 173 | 0.163 | 2.313 |
| 12 | 15 | 7 | 0.1 | 5 | 60 | 0.151 | 2.506 |
| 13 | 15 | 9 | 0.1 | 5 | -38 | 0.119 | 2.525 |
| 14 | 15 | 11 | 0.1 | 5 | -68 | 0.088 | 2.434 |
| 15 | 15 | 3 | 0.05 | 5 | 162 | 0.105 | 2.025 |
| 16 | 15 | 3 | 0.08 | 5 | 183 | 0.153 | 2.438 |
| 17 | 15 | 3 | 0.1 | 5 | 190 | 0.206 | 2.200 |
| 18 | 15 | 3 | 0.12 | 5 | 180 | 0.183 | 2.688 |
| 19 | 8 | 6.5 | 0.1 | 15 | 50 | 0.231 | 2.820 |
| 20 | 10 | 6.5 | 0.1 | 15 | 61 | 0.271 | 4.063 |
| 21 | 12 | 6.5 | 0.1 | 15 | 73 | 0.299 | 5.019 |
| 22 | 14 | 6.5 | 0.1 | 15 | 100 | 0.305 | 6.055 |
| 23 | 15 | 6.5 | 0.1 | 15 | 140 | 0.322 | 6.750 |
| 24 | 16 | 6.5 | 0.1 | 15 | 190 | 0.332 | 7.328 |
| 25 | 20 | 6.5 | 0.1 | 15 | 220 | 0.372 | 9.400 |
| 26 | 25 | 6.5 | 0.1 | 15 | 230 | 0.423 | 11.875 |
| 27 | 15 | 3 | 0.1 | 15 | 275 | 0.372 | 6.600 |
| 28 | 15 | 4 | 0.1 | 15 | 210 | 0.345 | 7.219 |
| 29 | 15 | 5 | 0.1 | 15 | 187 | 0.302 | 6.938 |
| 30 | 15 | 7 | 0.1 | 15 | 120 | 0.287 | 7.519 |
| 31 | 15 | 9 | 0.1 | 15 | 56 | 0.248 | 7.575 |
| 32 | 15 | 11 | 0.1 | 15 | 13 | 0.195 | 7.301 |
| 33 | 15 | 3 | 0.05 | 15 | 250 | 0.269 | 6.075 |
| 34 | 15 | 3 | 0.08 | 15 | 265 | 0.324 | 7.313 |
| 35 | 15 | 3 | 0.1 | 15 | 275 | 0.372 | 6.600 |
| 36 | 15 | 3 | 0.12 | 15 | 230 | 0.360 | 8.063 |
| 37 | 8 | 6.5 | 0.1 | 25 | 60 | 0.338 | 4.700 |
| 38 | 10 | 6.5 | 0.1 | 25 | 70 | 0.394 | 6.771 |
| 39 | 12 | 6.5 | 0.1 | 25 | 80 | 0.438 | 8.365 |
| 40 | 14 | 6.5 | 0.1 | 25 | 112 | 0.461 | 10.092 |
| 41 | 15 | 6.5 | 0.1 | 25 | 156 | 0.484 | 11.250 |
| 42 | 16 | 6.5 | 0.1 | 25 | 224 | 0.499 | 12.213 |
| 43 | 18 | 6.5 | 0.1 | 25 | 256 | 0.544 | 15.638 |
| 44 | 20 | 6.5 | 0.1 | 25 | 259 | 0.550 | 15.667 |
| 45 | 25 | 6.5 | 0.1 | 25 | 270 | 0.623 | 19.792 |
| 46 | 15 | 3 | 0.1 | 25 | 290 | 0.484 | 11.000 |
| 47 | 15 | 4 | 0.1 | 25 | 231 | 0.450 | 12.031 |
| 48 | 15 | 5 | 0.1 | 25 | 201 | 0.396 | 11.563 |
| 49 | 15 | 7 | 0.1 | 25 | 145 | 0.377 | 12.531 |
| 50 | 15 | 9 | 0.1 | 25 | 85 | 0.349 | 12.625 |
| 51 | 15 | 11 | 0.1 | 25 | 44 | 0.291 | 12.169 |
| 52 | 15 | 3 | 0.05 | 25 | 278 | 0.384 | 10.125 |
| 53 | 15 | 3 | 0.08 | 25 | 280 | 0.456 | 12.188 |
| 54 | 15 | 3 | 0.1 | 25 | 290 | 0.484 | 11.000 |
| 55 | 15 | 3 | 0.12 | 25 | 245 | 0.493 | 13.438 |
| 56 | 8 | 6.5 | 0.1 | 35 | 73 | 0.428 | 6.580 |
| 57 | 10 | 6.5 | 0.1 | 35 | 80 | 0.498 | 9.479 |
| 58 | 12 | 6.5 | 0.1 | 35 | 91 | 0.556 | 11.711 |
| 59 | 14 | 6.5 | 0.1 | 35 | 125 | 0.584 | 14.128 |
| 60 | 15 | 6.5 | 0.1 | 35 | 170 | 0.612 | 15.750 |
| 61 | 16 | 6.5 | 0.1 | 35 | 240 | 0.637 | 17.099 |
| 62 | 18 | 6.5 | 0.1 | 35 | 260 | 0.671 | 21.893 |
| 63 | 20 | 6.5 | 0.1 | 35 | 273 | 0.688 | 21.933 |
| 64 | 25 | 6.5 | 0.1 | 35 | 283 | 0.752 | 27.708 |
| 65 | 15 | 3 | 0.1 | 35 | 310 | 0.592 | 15.400 |
| 66 | 15 | 4 | 0.1 | 35 | 240 | 0.550 | 16.844 |
| 67 | 15 | 5 | 0.1 | 35 | 210 | 0.472 | 16.188 |
| 68 | 15 | 7 | 0.1 | 35 | 180 | 0.458 | 17.544 |
| 69 | 15 | 9 | 0.1 | 35 | 100 | 0.424 | 17.675 |
| 70 | 15 | 11 | 0.1 | 35 | 53 | 0.363 | 17.036 |
| 71 | 15 | 3 | 0.05 | 35 | 292 | 0.477 | 14.175 |
| 72 | 15 | 3 | 0.08 | 35 | 305 | 0.566 | 17.063 |
| 73 | 15 | 3 | 0.1 | 35 | 310 | 0.592 | 15.400 |
| 74 | 15 | 3 | 0.12 | 35 | 303 | 0.588 | 18.813 |
| 75 | 8 | 6.5 | 0.1 | 45 | 85 | 0.497 | 8.460 |
| 76 | 10 | 6.5 | 0.1 | 45 | 110 | 0.574 | 12.188 |
| 77 | 12 | 6.5 | 0.1 | 45 | 115 | 0.640 | 15.057 |
| 78 | 14 | 6.5 | 0.1 | 45 | 153 | 0.674 | 18.165 |
| 79 | 15 | 6.5 | 0.1 | 45 | 172 | 0.706 | 20.250 |
| 80 | 16 | 6.5 | 0.1 | 45 | 248 | 0.736 | 21.984 |
| 81 | 18 | 6.5 | 0.1 | 45 | 270 | 0.762 | 28.148 |
| 82 | 20 | 6.5 | 0.1 | 45 | 287 | 0.786 | 28.200 |
| 83 | 25 | 6.5 | 0.1 | 45 | 292 | 0.836 | 35.625 |
| 84 | 15 | 3 | 0.1 | 45 | 324 | 0.690 | 19.800 |
| 85 | 15 | 4 | 0.1 | 45 | 260 | 0.640 | 21.656 |
| 86 | 15 | 5 | 0.1 | 45 | 218 | 0.546 | 20.813 |
| 87 | 15 | 7 | 0.1 | 45 | 190 | 0.543 | 22.556 |
| 88 | 15 | 9 | 0.1 | 45 | 101 | 0.487 | 22.725 |
| 89 | 15 | 11 | 0.1 | 45 | 66 | 0.428 | 21.904 |
| 90 | 15 | 3 | 0.05 | 45 | 301 | 0.569 | 18.225 |
| 91 | 15 | 3 | 0.08 | 45 | 318 | 0.656 | 21.938 |
| 92 | 15 | 3 | 0.1 | 45 | 324 | 0.690 | 19.800 |
| 93 | 15 | 3 | 0.12 | 45 | 310 | 0.700 | 24.188 |
| 94 | 8 | 6.5 | 0.1 | 55 | 90 | 0.550 | 10.340 |
| 95 | 10 | 6.5 | 0.1 | 55 | 120 | 0.640 | 14.896 |
| 96 | 12 | 6.5 | 0.1 | 55 | 130 | 0.704 | 18.403 |
| 97 | 14 | 6.5 | 0.1 | 55 | 170 | 0.741 | 22.202 |
| 98 | 15 | 6.5 | 0.1 | 55 | 179 | 0.774 | 24.750 |
| 99 | 16 | 6.5 | 0.1 | 55 | 250 | 0.804 | 26.869 |
| 100 | 18 | 6.5 | 0.1 | 55 | 276 | 0.819 | 30.525 |
| 101 | 20 | 6.5 | 0.1 | 55 | 286 | 0.840 | 34.467 |
| 102 | 25 | 6.5 | 0.1 | 55 | 293 | 0.884 | 43.542 |
| 103 | 15 | 3 | 0.1 | 55 | 383 | 0.779 | 24.200 |
| 104 | 15 | 4 | 0.1 | 55 | 288 | 0.718 | 26.469 |
| 105 | 15 | 5 | 0.1 | 55 | 256 | 0.614 | 25.438 |
| 106 | 15 | 7 | 0.1 | 55 | 205 | 0.621 | 27.569 |
| 107 | 15 | 9 | 0.1 | 55 | 106 | 0.545 | 27.775 |
| 108 | 15 | 11 | 0.1 | 55 | 83 | 0.475 | 26.771 |
| 109 | 15 | 3 | 0.05 | 55 | 312 | 0.651 | 22.275 |
| 110 | 15 | 3 | 0.08 | 55 | 353 | 0.734 | 26.813 |
| 111 | 15 | 3 | 0.1 | 55 | 383 | 0.779 | 24.200 |
| 112 | 15 | 3 | 0.12 | 55 | 363 | 0.765 | 29.563 |
| 113 | 8 | 6.5 | 0.1 | 70 | 97 | 0.617 | 13.160 |
| 114 | 10 | 6.5 | 0.1 | 70 | 124 | 0.717 | 18.958 |
| 115 | 12 | 6.5 | 0.1 | 70 | 160 | 0.782 | 23.422 |
| 116 | 14 | 6.5 | 0.1 | 70 | 186 | 0.819 | 28.257 |
| 117 | 15 | 6.5 | 0.1 | 70 | 193 | 0.849 | 31.500 |
| 118 | 16 | 6.5 | 0.1 | 70 | 251 | 0.869 | 34.197 |
| 119 | 18 | 6.5 | 0.1 | 70 | 282 | 0.891 | 43.785 |
| 120 | 20 | 6.5 | 0.1 | 70 | 288 | 0.902 | 43.867 |
| 121 | 25 | 6.5 | 0.1 | 70 | 292 | 0.925 | 55.417 |
| 122 | 15 | 3 | 0.1 | 70 | 410 | 0.857 | 30.800 |
| 123 | 15 | 4 | 0.1 | 70 | 305 | 0.804 | 33.688 |
| 124 | 15 | 5 | 0.1 | 70 | 280 | 0.712 | 32.375 |
| 125 | 15 | 7 | 0.1 | 70 | 215 | 0.728 | 35.088 |
| 126 | 15 | 9 | 0.1 | 70 | 127 | 0.635 | 35.350 |
| 127 | 15 | 11 | 0.1 | 70 | 87 | 0.557 | 34.073 |
| 128 | 15 | 3 | 0.05 | 70 | 321 | 0.721 | 28.350 |
| 129 | 15 | 3 | 0.08 | 70 | 383 | 0.824 | 34.125 |
| 130 | 15 | 3 | 0.1 | 70 | 410 | 0.857 | 30.800 |
| 131 | 15 | 3 | 0.12 | 70 | 380 | 0.826 | 37.625 |
| 132 | 8 | 6.5 | 0.1 | 90 | 105 | 0.691 | 16.920 |
| 133 | 10 | 6.5 | 0.1 | 90 | 139 | 0.791 | 24.375 |
| 134 | 12 | 6.5 | 0.1 | 90 | 160 | 0.863 | 30.114 |
| 135 | 14 | 6.5 | 0.1 | 90 | 198 | 0.882 | 36.330 |
| 136 | 15 | 6.5 | 0.1 | 90 | 205 | 0.914 | 40.500 |
| 137 | 16 | 6.5 | 0.1 | 90 | 252 | 0.924 | 43.968 |
| 138 | 18 | 6.5 | 0.1 | 90 | 282 | 0.963 | 56.295 |
| 139 | 20 | 6.5 | 0.1 | 90 | 291 | 0.975 | 56.400 |
| 140 | 25 | 6.5 | 0.1 | 90 | 296 | 0.981 | 71.250 |
| 141 | 15 | 3 | 0.1 | 90 | 435 | 0.931 | 39.600 |
| 142 | 15 | 4 | 0.1 | 90 | 356 | 0.89 | 43.313 |
| 143 | 15 | 5 | 0.1 | 90 | 313 | 0.834 | 41.625 |
| 144 | 15 | 7 | 0.1 | 90 | 230 | 0.813 | 45.113 |
| 145 | 15 | 9 | 0.1 | 90 | 146 | 0.729 | 45.450 |
| 146 | 15 | 11 | 0.1 | 90 | 98 | 0.663 | 43.808 |
| 147 | 15 | 3 | 0.05 | 90 | 335 | 0.792 | 36.450 |
| 148 | 15 | 3 | 0.1 | 90 | 435 | 0.931 | 39.600 |
| 149 | 15 | 3 | 0.12 | 90 | 423 | 0.893 | 48.375 |
| 150 | 8 | 6.5 | 0.1 | 110 | 121 | 0.737 | 20.680 |
| 151 | 10 | 6.5 | 0.1 | 110 | 140 | 0.827 | 29.792 |
| 152 | 12 | 6.5 | 0.1 | 110 | 173 | 0.894 | 36.806 |
| 153 | 14 | 6.5 | 0.1 | 110 | 193 | 0.920 | 44.403 |
| 154 | 15 | 6.5 | 0.1 | 110 | 210 | 0.947 | 49.500 |
| 155 | 16 | 6.5 | 0.1 | 110 | 254 | 0.951 | 53.739 |
| 156 | 18 | 6.5 | 0.1 | 110 | 285 | 0.981 | 68.805 |
| 157 | 20 | 6.5 | 0.1 | 110 | 291 | 0.990 | 68.933 |
| 158 | 25 | 6.5 | 0.1 | 110 | 293 | 1.000 | 87.083 |
| 159 | 15 | 3 | 0.1 | 110 | 480 | 1.000 | 48.400 |
| 160 | 15 | 5 | 0.1 | 110 | 330 | 0.931 | 50.875 |
| 161 | 15 | 7 | 0.1 | 110 | 235 | 0.900 | 55.138 |
| 162 | 15 | 9 | 0.1 | 110 | 150 | 0.809 | 55.550 |
| 163 | 15 | 11 | 0.1 | 110 | 108 | 0.740 | 53.543 |
| 164 | 15 | 3 | 0.05 | 110 | 367 | 0.846 | 44.550 |
| 165 | 15 | 3 | 0.08 | 110 | 412 | 0.933 | 53.625 |
| 166 | 15 | 3 | 0.1 | 110 | 480 | 1 | 48.400 |
| 167 | 15 | 3 | 0.12 | 110 | 430 | 0.927 | 59.125 |
| 168 | 8 | 6.5 | 0.1 | 120 | 125 | 0.76 | 22.560 |
| 169 | 10 | 6.5 | 0.1 | 120 | 143 | 0.845 | 32.500 |
| 170 | 12 | 6.5 | 0.1 | 120 | 182 | 0.911 | 40.152 |
| 171 | 14 | 6.5 | 0.1 | 120 | 189 | 0.933 | 48.440 |
| 172 | 15 | 6.5 | 0.1 | 120 | 215 | 0.959 | 54.000 |
| 173 | 16 | 6.5 | 0.1 | 120 | 256 | 0.96 | 58.624 |
| 174 | 18 | 6.5 | 0.1 | 120 | 283 | 0.99 | 75.060 |
| 175 | 20 | 6.5 | 0.1 | 120 | 291 | 1 | 75.200 |
| 176 | 25 | 6.5 | 0.1 | 120 | 292 | 1 | 95.000 |
| 177 | 15 | 3 | 0.1 | 120 | 500 | 1 | 52.800 |
| 178 | 15 | 4 | 0.1 | 120 | 420 | 0.984 | 57.750 |
| 179 | 15 | 5 | 0.1 | 120 | 346 | 0.953 | 55.500 |
| 180 | 15 | 7 | 0.1 | 120 | 240 | 0.924 | 60.150 |
| 181 | 15 | 9 | 0.1 | 120 | 152 | 0.832 | 60.600 |
| 182 | 15 | 11 | 0.1 | 120 | 115 | 0.767 | 58.410 |
| 183 | 15 | 3 | 0.05 | 120 | 370 | 0.858 | 48.600 |
| 184 | 15 | 3 | 0.08 | 120 | 435 | 0.936 | 58.500 |
| 185 | 15 | 3 | 0.1 | 120 | 500 | 1.000 | 52.800 |
| 186 | 15 | 3 | 0.12 | 120 | 435 | 0.940 | 64.500 |
| 187 | 12 | 6.5 | 0.1 | 5 | 43 | 0.118 | 1.673 |
| 188 | 18 | 6.5 | 0.1 | 15 | 200 | 0.37 | 9.383 |
| 189 | 15 | 4 | 0.1 | 110 | 380 | 0.957 | 52.938 |
| 190 | 15 | 3 | 0.08 | 90 | 400 | 0.891 | 43.875 |

Table A2.
Partly of PSO–BP–ANN training function code.
Table A2.
Partly of PSO–BP–ANN training function code.
| Training Function Code |
|---|
| net = newff(inputn,outputn,hiddennum,{‘logsig’,‘purelin’},‘traingdx’); c1 = 1.5; c2 = 1.5; maxgen = 200; sizepop = 50; Vmax = 1; Vmin = −1; popmax = 5; popmin = −5; for i = 1:sizepop pop(i,:) = 5 * rands(1,numsum); V(i,:) = 1 * rands(1,numsum); fitness(i) = fun(pop(i,:),inputnum,hiddennum,outputnum,net,inputn,outputn); [bestfitness bestindex] = min(fitness); zbest = pop(bestindex,:); gbest = pop; fitnessgbest = fitness; fitnesszbest = bestfitness; for i = 1:maxgen V(j,:) = w * V(j,:) + c1 * rand * (gbest(j,:) − pop(j,:)) + c2 * rand * (zbest − pop(j,:)); V(j,find(V(j,:) > Vmax)) = Vmax; V(j,find(V(j,:) < Vmin)) = Vmin; pop(j,:) = pop(j,:) + 0.2 * V(j,:); pop(j,find(pop(j,:) > popmax)) = popmax; pop(j,find(pop(j,:) < popmin)) = popmin; pos = unidrnd(numsum); if rand > 0.95 pop(j,pos) = 5 * rands(1,1); fitness(j) = fun(pop(j,:),inputnum,hiddennum,outputnum,net,inputn,outputn); for j = 1:sizepop if fitness(j) < fitnessgbest(j) gbest(j,:) = pop(j,:); fitnessgbest(j) = fitness(j); if fitness(j) < fitnesszbest zbest = pop(j,:); fitnesszbest = fitness(j); |

Table A3.
ANN models for applications in various electrochemical processes.
Table A3.
ANN models for applications in various electrochemical processes.
| Type of Process | Input Variable | Output Variable | Types of the ANN Model | R2 | References |
|---|---|---|---|---|---|
| electrocoagulation | Current density, electrolysis time, initial pH and dye concentration, conductivity, retention time of sludge and distance between electrodes | Color removal efficiency | BP–ANN | 0.974 | Daneshvar et al. [16] |
| electro-oxidation | Intensity of current, reaction time, pH, nature of electrolyte, concentration of electrolyte | Degradation rate of oxytetracycline | BP–ANN | 0.99 | Belkacem et al. [19] |
| electrochemically activated persulfate | Electrolysis time, applied current, persulfate, pH | Sulfamethoxazoleremoval efficicency | BP–ANN | 0.9398 | Zhang et al. [10] |
| electrocoagulation-flotation | Initial HA concentration, initial pH, electrical conductivity, current density, number of pulses | Humica acid | BP–ANN | 0.966 | Hasani et al. [34] |
References
- Ahlborg, U.G.; Thunberg, T.M.; Spencer, H.C. Chlorinated phenols: Occurrence, toxicity, metabolism, and environmental impact. CRC Crit. Rev. Toxicol. 1980, 7, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Vlastors, D.; Antonopoulou, M.; Konstantinou, L. Evaluation of toxicity and genotoxicity of 2-chlorophenol on bacteria, fish and human cells. Sci. Total Environ. 2016, 551, 649–655. [Google Scholar] [CrossRef]
- Leblanc, G.A. Acute Toxicity of Priority Pollutants to Water Flea (Daphnia magna). Bull. Environ. Contam. Toxicol. 1980, 24, 684–691. [Google Scholar] [CrossRef]
- Ajeel, M.A.; Aroua, M.K.; Daud, W.M.A.W. Anodic degradation of 2-chlorophenol by carbon black diamond and activated carbon composite electrodes. Electrochim. Acta 2015, 180, 22–28. [Google Scholar] [CrossRef]
- Duan, X.; Zhao, C.; Liu, W.; Zhao, X.; Chang, L. Fabrication of a novel PbO2 electrode with a graphene nanosheet interlayer for electrochemical oxidation of 2-chlorophenol. Electrochim. Acta 2017, 240, 424–436. [Google Scholar] [CrossRef]
- Arellano-Gonzalez, M.A.; Gonzalez, I.; Texier, A.C. Mineralization of 2-chlorophenol by sequential electrochemical reductive dechlorination and biological processes. J. Hazard. Mater. 2016, 314, 181–187. [Google Scholar] [CrossRef]
- Mascia, M.; Vacca, A.; Polcaro, A.M.; Palmas, S.; Ruiz, J.R.; Da Pozzo, A. Electrochemical treatment of phenolic waters in presence of chloride with boron-doped diamond (BDD) anodes: Experimental study and mathematical model. J. Hazard. Mater. 2010, 174, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.A.; Abu-Zurayk, R.A.; Hamadneh, I.; Al-Dujaili, A.H. Phenol adsorption on biochar prepared from the pine fruit shells: Equilibrium, kinetic and thermodynamics studies. J. Environ. Manag. 2018, 226, 377–385. [Google Scholar] [CrossRef]
- Bu, L.J.; Zhou, S.Q.; Zhou, S.; Bi, C.; Zhu, S.M.; Gao, N.Y. Iron electrode as efficient persulfate activator for oxcarbazepine degradation: Performance, mechanism, and kinetic modeling. Sep. Purif. Technol. 2017, 178, 66–74. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, W.; Qiu, J.; Jin, H.; Ma, H.; Li, Z.; Cang, D. Modeling and optimization study on sulfamethoxazole degradation by electrochemically activated persulfate process. J. Clean. Prod. 2018, 197, 297–305. [Google Scholar] [CrossRef]
- Wang, J.D.; Li, T.T.; Zhou, M.M.; Li, X.P.; Yu, J.M. Characterization of hydrodynamics and mass transfer in two types of tubular electrochemical reactors. Electrochim. Acta 2015, 173, 698–704. [Google Scholar] [CrossRef]
- Shi, X.; Xiang, Y.; Wen, L.X.; Chen, J.F. CFD analysis of liquid phase flow in a rotating packed bed reactor. Chem. Eng. J. 2013, 228, 1040–1049. [Google Scholar] [CrossRef]
- Guo, T.Y.; Shi, X.; Chu, G.W.; Xiang, Y.; Wen, L.X.; Chen, J.F. Computational fluid dynamics analysis of the micromixing effciency in a rotating-packed-bed reactor. Ind. Eng. Chem. Res. 2016, 55, 4856–4866. [Google Scholar] [CrossRef]
- Song, Q.A.; Li, M.; Wang, L.; Ma, X.J.; Liu, F.; Liu, X. Mechanism and Optimization of Electrochemical System for Simultaneous Removal of Nitrate and Ammonia. J. Hazard. Mater. 2019, 363, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Lesnik, K.L.; Liu, H. Predicting microbial fuel cell biofilm communities and bioreactor performance using artificial neural networks. Environ. Sci. Technol. 2017, 51, 10881–10892. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Khataee, A.R.; Djafarzadeh, N. The use of artificial neural networks (ANN) for modeling of decolorization of textile dye solution containing C. I. Basic Yellow 28 by electrocoagulation process. J. Hazard. Mater. 2006, 137, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, A.R.; Moradi, M. Performance and modeling of UVpersulfate Ce(IV) process as a dual oxidant photochemical treatment system: Kinetic study and operating cost estimation. Chem. Eng. J. 2018, 347, 243–251. [Google Scholar] [CrossRef]
- Lin, C.H.; Yu, R.F.; Cheng, W.P.; Liu, C.R. Monitoring and control of UV and UV-TiO2 disinfections for municipal wastewater reclamation using artificial neural networks. J. Hazard. Mater. 2012, 209, 348–354. [Google Scholar] [CrossRef]
- Belkacem, S.; Bouafia, S.; Chabani, M. Study of oxytetracycline degradation by means of anodic oxidation process using platinized titanium (Ti/Pt) anode and modeling by artificial neural networks. Process Saf. Environ. Prot. 2017, 111, 170–179. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Mazlina, M.K.S.; Radiah, A.B.D.; Syafiie, S. Development of a hybrid PSO–ANN model for estimating glucose and xylose yields for microwave-assisted pretreatment and the enzymatic hydrolysis of lignocellulosic biomass. Neural Comput. Appl. 2016, 30, 1111–1121. [Google Scholar] [CrossRef]
- Fan, M.; Hu, J.; Cao, R.; Xiong, K.; Wei, X. Modeling and prediction of copper removal from aqueous solutions by nZVI/rGO magnetic nanocomposites using ANN-GA and ANN-PSO. Sci. Rep. 2017, 7, 18040. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Tyagi, I.; Gupta, V.K.; Ghaedi, M.; Masoomzade, M.; Ghaedi, A.M.; Mirtamizdoust, B. Kinetics and thermodynamics of methyl orange adsorption from aqueous solutions-artificial neural network-particle swarm optimization modeling. J. Mol. Liq. 2016, 218, 354–362. [Google Scholar] [CrossRef]
- Khajeh, M.; Sarafraz-Yazdi, A.; Moghadam, A.F. Modeling of solid-phase tea waste extraction for the removal of manganese and cobalt from water samples by using PSO-artificial neural network and response surface methodology. Arab. J. Chem. 2017, 10, S1663–S1673. [Google Scholar] [CrossRef]
- Kishimoto, N.; Hatta, M.; Kato, M.; Otsu, H. Effects of oxidation–reduction potential control and sequential use of biological treatment on the electrochemical Fenton-type process. Process Saf. Environ. 2017, 105, 134–142. [Google Scholar] [CrossRef]
- Mei, Y.; Lu, Y.; Ye, Z.P.; Xu, D.M.; Pan, H.; Wang, J.D. Impacts of operating parameters on oxidation-reduction potential and COD removal during the electrochemical removal of 2-chlorophenol. Desalin. Water Treat. 2019, 140, 199–206. [Google Scholar] [CrossRef]
- Wang, J.D.; Zhang, T.; Mei, Y.; Pan, B.J. Treatment of reverse-osmosis concentrate of printing and dyeing wastewater by electro-oxidation process with controlled oxidation-reduction potential (ORP). Chemosphere 2018, 201, 621–626. [Google Scholar] [CrossRef]
- Basha, C.A.; Soloman, P.A.; Velan, M. Electrochemical degradation of specialty chemical industry effluent. J. Hazard. Mater. 2010, 176, 154–164. [Google Scholar] [CrossRef]
- Chong, W.; Yin, L.F.; Niu, J.F.; Hou, L.A. Insights of ibuprofen electro-oxidation on metal-oxide-coated Ti anodes: Kinetics, energy consumption and reaction mechanisms. Chemosphere 2018, 163, 584–591. [Google Scholar]
- Thankachan, T.; Prakash, K.S.; David Pleass, C.; Rammasamy, D.; Prabakaran, B.; Jothi, S. Artificial neural network to predict the degraded mechanical properties of metallic materials due to the presence of hydrogen. Int. J. Hydrog. Energy 2017, 42, 28612–28621. [Google Scholar] [CrossRef]
- Ketabchi, N.; Naghibzadeh, M.; Adabi, M.; Esnaashari, S.S.; Faridi-Majidi, R. Preparation and optimization of chitosan/polyethylene oxide nanofiber diameter using artificial neural networks. Neural Comput. Appl. 2016, 8, 3131–3143. [Google Scholar] [CrossRef]
- Ghaedi, A.M.; Vafaei, A. Applications of artificial neural networks for adsorption removal of dyes from aqueous solution: A review. Adv. Colloid Interface Sci. 2017, 45, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Y.; Yao, J.C.; Pan, W.L.; Wang, J.D. Industrial-scale application of the plunger flow electro-oxidation reactor in wastewater depth treatment. Environ. Sci. Pollut. 2016, 23, 18288–18295. [Google Scholar] [CrossRef] [PubMed]
- Guliyev, N.J.; Ismailov, V.E. On the approximation by single hidden layer feedforward neural networks with fixed weights. Neural Netw. 2018, 98, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Hasani, G.; Daraei, H.; Shahmoradi, B.; Gharibi, F.; Maleki, A.; Yetilmezsoy, K.; McKay, G. A novel ANN approach for modeling of alternating pulse current electrocoagulation-flotation (APC-ECF) process: Humic acid removal from aqueous media. Process Saf. Environ. Prot. 2018, 117, 111–124. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).