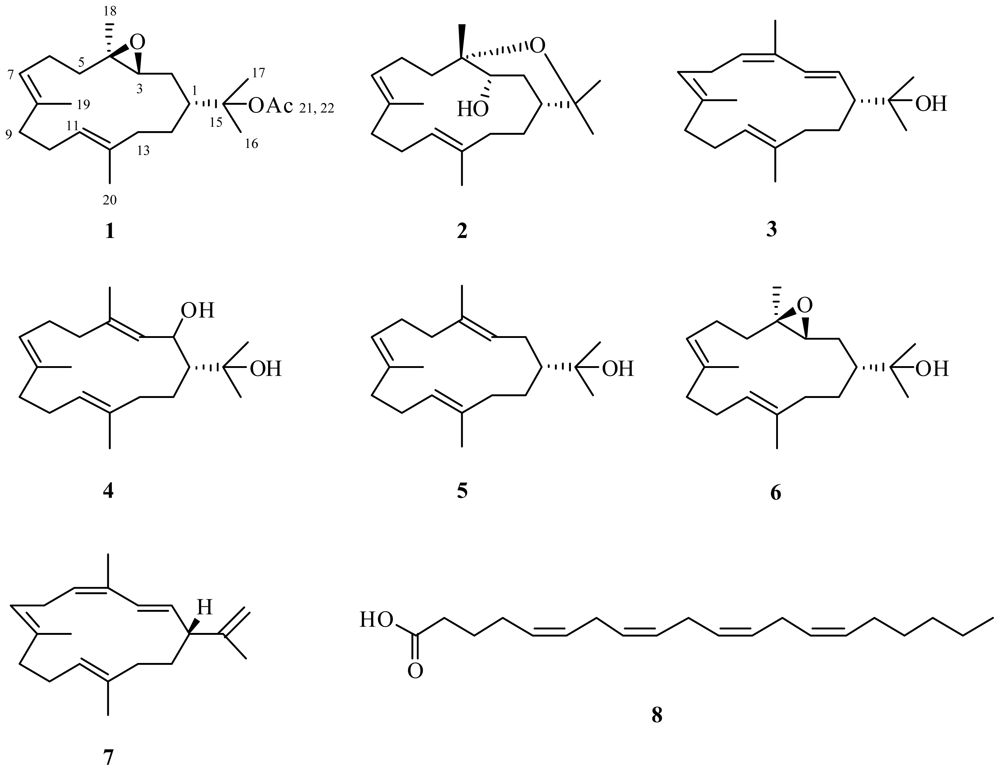
Abstract
Methanol extracts of two specimens of the soft coral Nephthea sp. collected from the Seribu Islands, Indonesia, were active in an anticancer bioassay. One new (1) and four known diterpenes (2–5) based on the cembrane carbon skeleton were isolated from these extracts, as was arachidonic acid (8). The structures of all compounds were elucidated using NMR, including 1,1-ADEQUATE and 1D gradient selective NOESY where applicable to determine the relative stereochemistry. Spectroscopic data, including 1H and 13C NMR, UV, IR and optical rotations are reported when enough material was available and where this has not been done previously. Inhibition assays employing three cancer cell lines; SF-268 (CNS), MCF-7 (breast), and H460 (lung) were used to guide the isolation of all compounds.
1. Introduction
A large diversity of marine organisms have been shown to produce secondary metabolites as a means of defense [1–4], many of these compounds also possess interesting biological activities [5–7]. Soft corals are no exception [8–12]. Investigations of soft corals from Indonesian waters have been limited with only six such reports on six unrelated soft coral species [13–18], the first of these appearing in 1997 [13]. Since 2002 [15,16], aside from the research undertaken by Fattorusso et al. [17], Wang et al. [18], and the current authors [19–22], there is little work being done with soft corals from this region of the world, even though they are likely to be rich sources of biologically active secondary metabolites. The information presented here resulted from a formal cooperation between the Indonesia Research Center for Marine and Fisheries Product Processing and Biotechnology, and the Australian Institute of Marine Science (AIMS), funded by an AusAID PSLP Indonesia grant. The investigation of two Nephthea (Alcyonacea, Nephtheidae) species, whose methanol (MeOH) extracts exhibited anticancer properties, resulted in the isolation of a new cembrane 3,4-epoxy-nephthenol acetate (1) along with five known compounds: decaryiol (2), 15-hydroxy-cembrenene (3), 2-hydroxy-nephthenol (4), nephthenol (5) and arachidonic acid (8). This report describes the structural elucidation of (1) and clearly shows that soft corals of Indonesian origin have significant potential as sources of biologically active and drug development lead compounds.
2. Results and Discussion
Compound 1 was isolated as a yellow oil from Nephthea sp. specimen A. Mass spectrometric analysis of the compound showed it to have the molecular formula C22H36O3 and therefore have five double bond equivalents of unsaturation. From the 1H and 13C NMR data of 1, it was evident that the molecule contained two C=C double bonds (δC 123.6 d, 126.2 d, 132.4 s, 134.9 s) and one C=O (δC 170.2 s) double bond as the only multiple bonds establishing it as bicyclic. The 1D and 2D NMR data of 1 also revealed the presence of an acetate (δC 170.2 s, 85.6 s, 22.8 q, 1.98 s [C-21, 22 and 15, respectively]) and an ether (δC 63.4 d and 61.5 s, δH 2.84 [dd, 9.4, 3.4 Hz], [C-3 and C-4, respectively]). From the 1H-1H COSY spectrum of 1, three continuous chains of coupling were discerned; from H2-13 to H-3 via H2-14, H-1 and H2-2, respectively; from H2-5 to H3-19, via H2-6 and H-7; and from H2-9 to H3-20, via H2-10, and H-11, respectively. From long-range 1H-13C couplings observed between H3-18 and C-3, C-4 and C-5; between H3-19 and C-7, C-8 and C-9; and between H3-20 and C-11, C-12 and C-13, and from 1,1-ADEQUATE cross-peaks [23] (See Table 1), it was possible to link together the three proton spin systems into a continuous carbon chain to form the first ring within 1. The chemical shifts associated with C-3, C-4 and H-3 indicated the ether functionality was in fact an epoxide, and hence formed the second ring within 1, fulfilling the requirement for five double bond equivalents of unsaturation. The protons associated with two of the three unassigned methyl groups demonstrated long-range 1H-13C couplings between each others carbon and, C-1 and C-15, giving rise to a gem-dimethyl constellation attached to C-1, leaving the acetate function to reside at C-15, leading to the planar structure of 1. The geometry of the two C=C double bonds within 1 were both deduced to be E based on the 13C NMR chemical shifts of C-19 and C-20 (δC 16.7 and 15.3, respectively). Based on the observation that the 13C NMR signals for C-2 and C-18 (δC 30.8 and 16.6, respectively) occurred significantly upfield of that for C-5 (δC 39.3), resulting from steric compression due to their cis orientation, the relative stereochemistry of the epoxide is as shown in 1 [24]. This deduction was further supported by comparison of the 13C NMR chemical shift data for C-3, C-4 and C-18 in 1 with those for 6, as well as the 1H NMR data associated with H-3 in both compounds. Selective 1D NOESY excitation of the epoxide proton H-3 (δ 2.84) gave rise to signals corresponding to H-1 (δ 2.22), H-2 (δ 1.56), H-5 (δ 1.13), H-7 (δ 5.23) and H-11 (δ 5.13); this information, and comparison of both 1H and 13C NMR data with those for 6 confirmed the relative configuration at C-1, 3 and 4 to be deduced as shown in 1. Unfortunately, the absolute stereochemistry of 1 could not be determined as the compound was unstable and degraded before an optical rotation could be obtained. Literature searches for this molecule revealed it to be a new compound and the acetylated derivative of 3,4-epoxy-nephthenol, 6 [28].

Table 1.
1H and 13C NMR data (600 MHz basic frequency, CDCl3) for 3,4-epoxy-nephthenol acetate (1) and 13C NMR data for 3,4-epoxy-nephthenol (6) (22.63 MHz, CDCl3)
HRESIMS of 2, also isolated from Nephthea sp. specimen A, resulted in an [M + Na]+ ion corresponding to a molecular formula of C20H34O2 and hence four degrees of double bond unsaturation. The 1H and 13C NMR data of 2 revealed the presence of two C=C double bonds (δC 132.6 s, 132.1 s, 128.0 d and 127.7 d) as the only multiple bonds within the molecule indicating a bicyclic structure. Carbon chemical shifts indicated the presence of three oxygenated carbons (δC 76.8 s, 75.1 s and 70.3 d), two of which formed an ether linkage accounting for one of the rings within 2, and the third an alcohol (δH 4.20 [dd, 11.7, 5.6 Hz, axial proton]). Database and literature searches using this information and comparison of spectroscopic data with literature values confirmed 2 to be the known compound decaryiol [28]. Selective 1D NOESY experiments enabled the relative configuration of 2 to be determined as shown; NOESY correlations were observed from H-3 to H-1, H-2, H-5, H-6, H-7 and H-11; from H-7 to H-3, H-5, H-6 (δH 2.62 br and 1.88 s) and H-9 (δH 2.20 br and 2.17 s); from H-11 to H-1, H-2, H-3, H-9, H-10 and H-13; and from H-18 to H-2.
Accurate mass measurement of 3 showed it to have the molecular formula C20H32O and to contain five double bond equivalents of unsaturation. From the 1H and 13C NMR data of 3 it was evident that the molecule contained four C=C double bonds (δC 134.6 s, 133.4 d, 132.1 s, 131.5 s, 127.9 d, 127.3 d, 126.3 d and 126.2 d), revealing it to be monocyclic. It was also evident from this data that 3 had one carbon attached to an oxygen, therefore an hydroxyl, functionality (δC 72.3 s). As for 1, three chains of 1H-1H coupling were discerned from the COSY spectrum of 3; from H2-13 to H-3 via H2-14, H-1 and H2-2, respectively; from H3-18 to H3-19, via H-5, H2-6 and H-7; and from H2-9 to H3-20, via H2-10, and H-11, respectively. From long-range 1H-13C couplings observed between H3-18 (δC 19.9, Δ4 Z configuration) and C-4 and C-5; between H3-19 (δC 14.5, Δ7 E configuration) and C-7, C-8 and C-9; and between H3-20 (δC 14.3, Δ11 E configuration) and C-11, C-12 and C-13, and 1,1-ADEQUATE [23] cross-peaks (Figure 1), it was possible to link together the three proton spin systems into a continuous carbon chain to form the one ring within 3. HMBC and COSY correlations also confirmed two of the C=C double bonds (Δ2 and Δ4) were conjugated; H-2 to C-4, H-3 to C-4 and C-5, and H-5 to C-3, and that Δ2 had E configuration (H-2: δH 5.20 [dd, 15.3, 10.0 Hz]; H-3: δH 6.20 [d, 15.3 Hz]). Comparison of these data with literature values for cembrenene (7) previously isolated from Sinularia mayi [26], (Table 2) indicated the structures to be similar. The protons associated with the two remaining methyl groups CH3-16 and CH3-17 demonstrated long-range 1H-13C couplings to each others protons and carbon and to C-1 and C-15 giving rise to a gem-dimethyl constellation attached to C-1 with the hydroxyl moiety residing at C-15, and the planar structure as shown in 3, 15-hydroxy-cembrenene. Literature searches for this molecule yielded a report detailing the synthetic dehydration of 2-hydroxy-nepthenol to give 3 [27]. The current report, however, is the first time 3 has been isolated from a natural source.
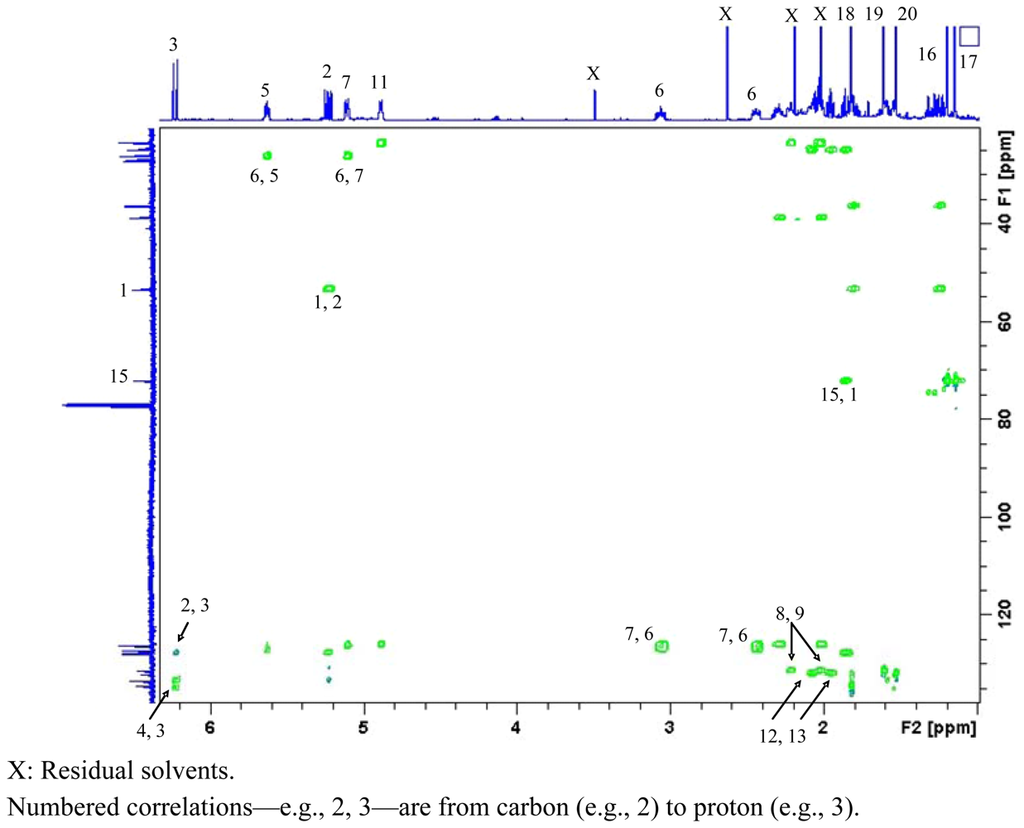
Figure 1.
1,1-ADEQUATE spectrum of 3 (600 MHz basic frequency, CDCl3).X: Residual solvents.Numbered correlations—e.g., 2, 3—are from carbon (e.g., 2) to proton (e.g., 3).

Table 2.
1H and 13C NMR data (125 MHz, CDCl3) for 15-hydroxy-cembrenene (3); 13C NMR data (125 MHz, CDCl3) for decaryiol (2), 2-hydroxy-nephthenol (4) and nephthenol (5); (22.6 MHz, CDCl3) for 3,4-epoxy-nephthenol (6), cembrenene (7) and arachidonic acid (8).
A second Nephthea sp., specimen B, was investigated for its anticancer activity with one of the active fractions yielding 4, having the same molecular formula as 2, C20H34O2. Comparison of the 1H and 13C NMR data of 4 with those of 2 showed it to contain only two oxygenated carbons (δC 71.1 d and 74.6 s) as compared to the three in 2, and three C=C double bonds (δC 139.5 s, 135.4 s, 133.3 s, 127.9 d, 124.9 d and 123.9 d) rather than two as found in 2, confirming it to be a monocyclic diol. Its 1D and 2D NMR data confirmed it to be 2-hydroxy-nephthenol [27]. After leaving 4 to stand for one week in CDCl3, it was found to have quantitatively rearranged to 3. Given this result, it is unclear whether 3, previously reported synthetically [27] and reported here from Nephthea sp. specimen A, was in fact a natural product, or a by-product of the isolation process [27]. However, close inspection of fractions from specimen A shortly after they were prepared did not reveal the presence of any 4, leading us to believe that 3 is in actual fact naturally occurring.
A second active compound, 5, was isolated from specimen B. The mass spectrum of 5 showed an [M + Na]+ ion in its HRESIMS consistent with the molecular formula C20H34O and four degrees of C=C unsaturation. The 1H and 13C NMR data of 5 showed it to contain three C=C double bonds (δC 134.1 s, 133.4 s, 133.0 s, 125.9 d, 125.8 d and 125.0 d) as well as one hydroxyl group (δC 75.1 s), making it a monocyclic alcohol. Comparison of its spectroscopic data with literature values confirmed 5 to be nephthenol [28].
Compound 8 was isolated as a yellow oil with the molecular formula of C20H32O2, as determined by HRESIMS measurement of its [M – H]− ion. Analysis of the 1H and 13C NMR spectral data of 8 in CDCl3 revealed signals consistent with the presence of a carboxyl group (δC 177.9 s) and eight sp2 methine carbons (δC 130.5 d, 129.0 d, 128.8 d, 128.6 d, 128.3 d, 128.1 d, 127.9 d and 127.5 d) accounting for all five of the C=C double bond equivalents of unsaturation within the molecule and showing it to be acyclic. Signals from three methylene carbons adjacent to cis double bonds were observed at δC 25.6, 25.6 and 25.6, as well as for seven other methylene carbons and one methyl group (δC 14.0). Comparison of these values with literature values confirmed 8 as the fatty acid arachidonic (20:4n-6) acid [29].
Compounds 1–5, and 8 were screened for their whole cell anticancer activity against three human tumor cell lines (SF-268 [CNS], MCF-7 [breast], H460 [lung]). All compounds demonstrated weak (GI50 > 100 μM) non-selective activity towards the three cell lines.
3. Experimental Section
3.1. General experimental
C18 flash vacuum chromatography was performed using Phenomenex C18 (50 μm). HPLC was performed employing a Phenomenex Luna C18 column (250 × 21 mm) attached to a Shimadzu HPLC system consisting of a Shimadzu SCL-10Avp system controller equipped with a Shimadzu LC-10AT pump, Shimadzu SPD-M10Avp photodiode array detector, Shimadzu FRC-10A fraction collector and Shimadzu SIL-10A auto sampler using Shimadzu Class-VP software. IR spectra were measured on a Nicolet Nexus FTIR. Optical rotations were collected on a Jasco 715 CD polarimeter. All NMR spectra were recorded on either a Bruker Avance 600 MHz NMR spectrometer complete with cryoprobe, or a Bruker Avance 300 MHz NMR spectrometer, with spectra referenced to residual 1H and 13C resonances in the deuterated solvents. Accurate mass spectrometric data were measured using a Bruker BioApex 47 FT mass spectrometer. All other details as previously published [30].
3.2. Animal material
Nephthea sp. specimen A was collected from Seribu Islands, DKI Jakarta, Indonesia, at a depth of 10 m, at 10:21 am, on the 22 July 2005; Nephthea sp. specimen B was collected from Seribu Islands, DKI Jakarta, Indonesia, at a depth of 15 m, at 1:10 pm, on the 22 June, 2005. Soft coral taxonomy was undertaken by K. Fabricius, AIMS. A voucher sample for each specimen has been lodged with the Indonesia Research Center for Marine and Fisheries Product Processing and Biotechnology, Jakarta, Indonesia.
3.3. Bioassay
Natural product samples were assayed against three cell lines; SF-268, MCF-7 and H460 cells, as described in a previous study [31]. In brief, natural product samples, solubilized in DMSO and serially diluted in RPMI 1640 medium, were added to SF-268, MCF-7 and H460 cells so that the final doses ranged from 1000 μg/mL to 1 μg/mL. Total cellular protein was measured using the sulforhodamine B (SRB) assay as an indicator of cell number. Inhibition of growth by 50% (GI50) was determined by comparing the sample treated values to those of vehicle only control and time 0 readings.
3.4. Extraction and isolation
Extract A: The organic solubles (0.84 g), obtained by employing repeated extraction of 100.00 g wet weight of Nephthea sp. specimen A with MeOH, were filtered through a plug of reversed phase C18 silica using MeOH as eluent. The MeOH was removed under reduced pressure and the resultant dry extract subjected to preparative RP-HPLC (9 mL/min, gradient elution from 15% MeCN:H2O to 100% MeCN; column 250 × 20 mm RP Luna C18 (2), Phenomenex, over 70 mins) to yield 63 fractions. Three of the 63 fractions, 27, 30 and 32, were found to be active in the applied bioassay systems. 1H NMR analysis of these fractions showed them to be a 1:1 mixture of 3 and 4 (10.0 mg, 1.19% organic extract), 8 (10.0 mg, 1.19% organic extract), and 5 (10.0 mg, 1.19% organic extract), respectively.
Extract B: The organic solubles (3.31 g), obtained by employing repeated extraction of 300.00 g wet weight of Nephthea sp. specimen B with MeOH, were filtered through a plug of reversed phase C18 silica using MeOH and DCM as eluents. The MeOH and DCM were removed under reduced pressure and the resultant dry extracts (1.49 g and 0.10 g, respectively) subjected to preparative RP-HPLC. The MeOH extract (9 mL/min, gradient elution from 15% MeCN:H2O to 100% MeCN; column 250 × 20 mm RP Luna C18 (2), Phenomenex, over 70 mins) yielded 57 fractions of which only one, fraction 35, was found to be active in the applied bioassay systems. 1H NMR analysis of this fraction showed it to be 2 (57.9 mg, 1.75% organic extract). The DCM extract (9 mL/min, gradient elution from 15% MeCN:H2O to 100% MeCN; column 250 × 20 mm RP Luna C18 (2), Phenomenex, over 70 mins) yielded 55 fractions of which two, fractions 16 and 18, were found to be active in the applied bioassay systems. 1H NMR analysis of these fractions showed them to be 3 (2.3 mg, 0.07% organic extract), and 1 (0.8 mg, 0.02% organic extract), respectively.
Compound 1 (3,4-Epoxy-nephthenol acetate). A yellow oil; [α]D Sample decomposed prior to measurement; 1H (600 MHz, CDCl3), and 13C (150 MHz, CDCl3) NMR data see Table 1; HRESIMS m/z found 371.2563 for [M + Na]+ (calcd for C22H36O3Na 371.2557).
Compound 2 (Decaryiol). A yellow oil [α]D 20 + 27.2° (c 0.01), cf + 69.0° [25]; 13C (150 MHz, CDCl3) NMR data see Table 2; HRESIMS m/z found 329.2458 for [M – H]+ (calcd for C20H34O2Na 329.2451); and all remaining data as previously published [25].
Compound 3 (15-Hydroxy-cembrenene). A yellow oil; [α]D Sample decomposed prior to measurement; 1H (600 MHz, CDCl3) and 13C (150 MHz, CDCl3) NMR data see Table 2; HRESIMS m/z found 311.2330 for [M – H]+ (calcd for C20H32ONa 311.2345); and all remaining data as previously published [27].
Compound 4 (2-Hydroxy-nephthenol). A clear oil 13C (150 MHz, CDCl3) NMR data see Table 2; HRESIMS m/z found 329.2452 for [M + Na]+ (calcd for C20H34O2Na 329.2451); and all remaining data as previously published [27].
Compound 5 (Nephthenol). A clear oil; 13C (150 MHz, CDCl3) NMR data see Table 2; HRESIMS m/z found 313.2517 for [M – H]+ (calcd for C20H34ONa 313.2502); and all remaining data as previously published [28].
Compound 8 (Arachidonic acid). A yellow oil; 13C (150 MHz, CDCl3) NMR data see Table 2; HRESIMS m/z found 303.2325 for [M – H2O + Na]+ (calcd for C20H31O2 303.2330); and all remaining data as previously published [29].
Acknowledgements
This study was supported by AusAID through PSLP-Indonesia project ROU 37118. Many thanks go to our Marine Biotechnology team at the Indonesian Research Center for Marine and Fisheries Product Processing and Biotechnology for their collaborations in sample collection. Our thanks also go to A. Sabdono and O. Karnaradjasa, Diponegoro University, Indonesia, for provision of samples from Bali and J. Neilson, AIMS, for technical assistance with HPLC separations.
- Samples Availability: Available from the authors.
References
- Hay, ME; Duffy, JE; Pfister, AP; Fenical, W. Chemical defense against different marine herbivores: are amphipods insect equivalents? Ecology 1986, 68, 1567–1580. [Google Scholar]
- De Nys, R; Steinberg, PD; Willemsen, P; Dworjanyn, SA; Gabelish, CL; King, RJ. Broad spectrum effects of secondary metabolites from the red alga Delisea pulchra in antifouling assays. Biofouling 1995, 8, 259–271. [Google Scholar]
- De Nys, R; Steinberg, PD. Fingerman, M, Nagabhushanam, R, Thompson, M-F, Eds.; Role of secondary metabolites from algae and seagrasses in biofouling control. In Recent Advances in Marine Biotechnology: Biofilms, Bioadhesion, Corrosion and Biofouling; Science Publishers: Enfield, NH, USA, 1999; Volume 3, pp. 223–244. [Google Scholar]
- Pennings, SC; Pablo, SR; Paul, VJ. Chemical defenses of the tropical, benthic marine cyanobacterium Hormothamnion enteromorphoides: Diverse consumers and synergisms. Limnol. Oceanogr 1997, 42, 911–917. [Google Scholar]
- Wright, AD; Wang, H; Gurrath, M; König, GM; Kocak, G; Neumann, G; Loria, P; Foley, M; Tilley, L. Inhibition of heme (FP) detoxification processes underlies the antimalarial activity of terpene isonitrile compounds from marine sponges. J. Med. Chem 2001, 44, 873–885. [Google Scholar]
- König, GM; Wright, AD; Linden, A. Antiplasmodial and Cytotoxic Metabolites from the Maltese Sponge Agelas oroides. Planta Med 1998, 64, 443–447. [Google Scholar]
- Rinehart, KL; Morales, JJ; Reid, J; Reymundo, I; Floriano, P; Gravalos, LG. ETM-775 metabolite of ecteinascidin 743. US Patent 6,316,214 B1, 13 November 2001. [Google Scholar]
- Coll, JC; La Barre, S; Sammarco, PW; Williams, WT; Bakus, GJ. Chemical defence in soft corals of the Great Barrier Reef: A study of comparative toxicities. Mar. Ecol. Prog. Ser 1988, 8, 271–278. [Google Scholar]
- Coll, JC; Bowden, BF; Tapiolas, DM; Dunlap, WC. In situ isolation of allelochemicals from soft corals (Coelenterata: Octocorallia): a totally submersible sampling apparatus. J. Exp. Mar. Biol. Ecol 1982, 60, 292–299. [Google Scholar]
- Schmitz, FJ; Hollenbeak, KH; Prasad, RS. Marine natural products: Cytotoxic spermidine derivatives from the soft coral Sinularia brongersmai. Tetrahedron Lett 1979, 20, 3387–3390. [Google Scholar]
- Weinheimer, AJ; Matson, JA; Hossain, MB; van der Helm, D. Marine anticancer agents: Sinularin and dihydrosinularin, new cembranolides from the soft coral, Sinularia flexibilis. Tetrahedron Lett 1977, 34, 2923–2926. [Google Scholar]
- Kobayashi, J; Ohizumi, Y; Nakamura, H; Yamakado, T; Matsuzaki, T; Hirata, Y. Ca-antagonistic substance from soft coral of the genus Sarcophyton. Experientia 1983, 39, 67–69. [Google Scholar]
- Handayani, D; Edrada, RA; Proksch, P; Wray, V; Witte, L; van Ofwegen, L; Kunzmann, A. New Oxygenated Sesquiterpenes from the Indonesian Soft Coral Nephthea chabrolii. J. Nat. Prod 1997, 60, 716–718. [Google Scholar]
- Morris, LA; Christie, EM; Jaspars, M; van Ofwegen, L. A bioactive secosterol with unusual A-and B-ring oxygenation pattern isolated from an Indonesian soft coral Lobophytum sp. J. Nat. Prod 1998, 61, 538–541. [Google Scholar]
- Anta, C; Gonzalez, N; Santafe, G; Rodriguez, J; Jimenez, C. New Xenia diterpenoids from the Indonesian soft coral Xenia sp. J. Nat. Prod 2002, 65, 766–768. [Google Scholar]
- Anta, C; Gonzalez, N; Rodriguez, J; Jimenez, C. A new secosterol from the Indonesian octocoral Pachyclavularia violacea. J. Nat. Prod 2002, 65, 1357–1359. [Google Scholar]
- Fattorusso, E; Romano, A; Taglialatela-Scafati, O; Irace, C; Maffettone, C; Bavestrello, G; Cerrano, C. Oxygenated cembranoids of the decaryiol type from the Indonesian soft coral Lobophytum sp. Tetrahedron 2009, 65, 2898–2904. [Google Scholar]
- Wang, W; Lee, J-S; Ukai, K; Mangindaan, REP; Wewengkang, DS; Rotinsulu, H; Kobayashi, H; Tsukamoto, S; Namikosh, M. (25S)-Cholesten-26-oic acid derivatives from an Indonesian soft coral Minabea sp. Steroids 2009, 74, 758–760. [Google Scholar]
- Januar, HI; Chasanah, E; Tapiolas, DM; Motti, CA; Wright, AD. Cembranes from two different Nephthea sp., collected from Indonesian waters. the 50th Annual Meeting of the American Society of Pharmacognosy, Honolulu, HI, USA, 27 June 2009–1 July 2009; Conference Abstracts. p. 111.
- Januar, HI; Chasanah, E; Tapiolas, DM; Motti, CA; Wright, AD. Cytotoxic cembranes from Indonesian specimens of the soft coral Nephthea sp. the 51st Annual Meeting of the American Society of Pharmacognosy, St. Petersburg Beach, Florida, USA, 10–14 July 2010; Conference Abstracts. p. 50.
- Chasanah, E; Januar, HI; Bourne, D; Liptrot, C; Wright, AD. Screening of Anti-cancer Activity of Fungi Derived from Indonesia Marine Sponges. World Ocean Conference Side Event, International Symposium on Ocean Science, Technology and Policy, Manado, North Sulawesi, Indonesia, 12–14 May 2009; Conference Abstracts. Chapter IV-B, p. 10.
- Januar, HI; Chasanah, E; Liptrot, C; Doyle, J; Nielson, J; Tapiolas, D; Motti, C; Wright, AD. Dereplication of Active Extracts Obtained from Indonesian Marine Organisms. the 48th Annual Meeting of the American Society of Pharmacognosy, Portland, ME, USA, 14–18 July 2007. Congress Abstracts, P-012M.
- Köck, M; Reif, B; Gerlach, M; Reggelin, M. Application of the 1,n-ADEQUATE experiment in the assignment of highly substituted aromatic compounds. Molecules 1996, 1, 41–45. [Google Scholar]
- Greenland, GJ; Bowden, BF. Cembranoid Diterpenes Related to Sarcophytol A from the Soft Coral Sarcophyton trocheliophorum (Alcyonacea). Aust. J. Chem 1994, 47, 2013–2021. [Google Scholar]
- Carmely, S; Groweiss, A; Kashman, Y. Decaryiol, a New Cembrane from the Marine Soft Coral Sarcophyton decaryi. J. Org. Chem 1981, 46, 4279–4284. [Google Scholar]
- Uchio, Y; Nabeya, H; Nakayama, M; Hayashi, S; Hase, T. Cembrenene and mayol, two new cembranoid diterpenes from the soft coral Sinularia mayi. Tetrahedron Lett 1981, 22, 1689–1690. [Google Scholar]
- Tursch, B; Braekman, JC; Daloze, D. Chemical studies of marine invertebrates. XIII. 2-Hydroxynephthenol, a novel cembrane diterpene from the soft coral Litophyton viridis (Coelenterata, Octocorallia, Alcyonacea). Bull. Soc. Chim. Belg 1975, 84, 767–774. [Google Scholar]
- Schmitz, FJ; Vanderah, DJ; Ciereszko, LS. Marine natural products. nephthenol and epoxynephthenol acetate, cembrene derivatives from a soft coral. J. Chem. Soc. Chem. Commun 1974, 10, 407–408. [Google Scholar]
- Jyothirmayi, N; Ramadoss, CS; Divakar, S. Nuclear magnetic resonance studies of cylodextrin complexes of linoleic acid and arachidonic acid. J. Agric. Food Chem 1991, 39, 2123–2127. [Google Scholar]
- Tapiolas, DM; Bowden, B; Motti, CA; Willis, RH; Abou-Mansour, E; Bourne, D; Doyle, JR; Llewellyn, L; Wright, AD. Eusynstyelamides A, B and C from the Ascidian Eusynstyela latericus and Revision of the Structure of Eusynstyelamide. J. Nat. Prod 2009, 72, 1115–1120. [Google Scholar]
- Wright, AD; Nielson, JL; Tapiolas, DM; Motti, CA; Ovenden, SPB; Kearns, PS; Liptrot, CH. Detailed NMR, including 1,1-ADEQUATE, and anticancer studies of compounds from the echinoderm Colobometra perspinosa. Mar. Drugs 2009, 7, 565–575. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).