Abstract
Marine pyridoacridines are a class of aromatic chemicals that share an 11H-pyrido[4,3,2-mn]acridine skeleton. Pyridoacridine alkaloids display diverse biological activities including cytotoxicity, fungicidal and bactericidal properties, production of reactive oxygen species (ROS) and topoisomerase inhibition. These activities are often dependent on slight modifications to the pyridoacridine skeleton. Here we demonstrate that while structurally similar to neoamphimedine and amphimedine, the biological activity of deoxyamphimedine differs greatly. Deoxyamphimedine damages DNA in vitro independent of topoisomerase enzymes through the generation of reactive oxygen species. Its activity was decreased in low oxygen, with the removal of a reducing agent and in the presence of anti-oxidants. Deoxyamphimedine also showed enhanced toxicity in cells sensitive to single or double strand DNA breaks, consistent with the in vitro activity.
1. Introduction
Marine pyridoacridines are a class of planar aromatic molecules with an 11H-pyrido-[4,3,2mn]acridine skeleton that show affinity for DNA. Great interest in this class of compounds is evident from numerous synthetic studies and total synthesis reports [1–8]. In general, pyridoacridines are cytotoxic and some of them possess potent anti-viral, anti-fungal, anti-bacterial, anti-tumor and anti-parasitic activity [9]. For the majority of this class, cytotoxicity has been shown to be due to DNA-binding properties, topoisomerase inhibition and the production of reactive oxygen species (ROS) [9].
Deoxyamphimedine, neoamphimedine and amphimedine are pyridoacridines isolated from Xestospongia sponges collected in the Philippines and Micronesia (Figure 1). We previously reported that neoamphimedine is cytotoxic via topoisomerase 2-dependent DNA aggregation/catenation, while amphimedine is relatively non-toxic [10]. During the analysis of the bioactivities of neoamphimedine and amphimedine it was observed that deoxyamphimedine cleaved DNA in vitro in the absence of topoisomerase (1 or 2) [11]. In this paper we describe the ability of deoxyamphimedine to damage DNA through the production of ROS.
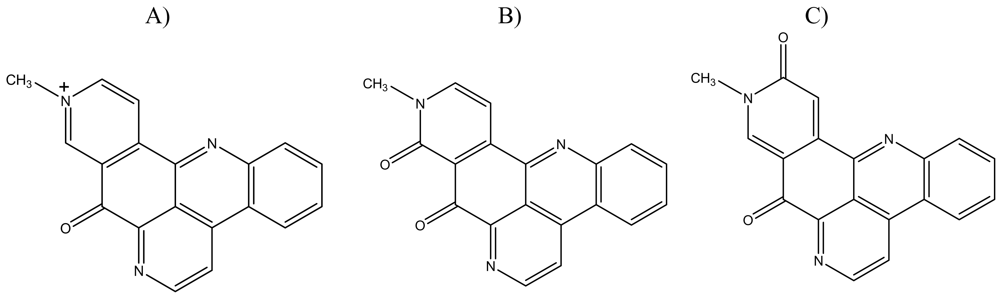
Figure 1.
Chemical structures of: A) deoxyamphimedine, B) neoamphimedine and C) amphimedine.
Reactive oxygen species, although produced during normal aerobic metabolism in mammalian cells, have been implicated in cell and tissue damage [12,13]. Free radicals produced by oxidative processes can attack DNA at bases or sugars, causing primarily single strand breaks, as well as, secondary double strand breaks and abasic sites [14–16]. Quinone functions are capable of redox cycling. One electron reduction of the quinone forms reactive semiquinones, allowing electron transfer to other species [17]. Higher redox potential of quinone molecules has been correlated with increased DNA cleavage [18]. This study demonstrates the cytotoxicity of deoxyamphimedine, its ability to intercalate and damage DNA and the attenuation of this activity when the generation of ROS was inhibited.
2. Results and Discussion
2.1. Cytotoxicity in Cultured Mammalian Cell Lines
Pyridoacridines are often cytotoxic in cultured cell lines. Deoxyamphimedine was originally found to be toxic in HCT-116 (human colon tumor) cells and shown to have enhanced toxicity (4-fold) against the EM9 strain of Chinese hamster ovary (CHO) cells, which are deficient in single strand (ss) DNA break repair when compared to the wild type AA8 strain [11]. The sensitivity observed in ssDNA break repair deficient cells typically correlates with compounds having topoisomerase 1 or reactive oxygen species (ROS) mediated DNA cleavage [19–21]. We further tested deoxyamphimedine in a panel of other cultured cell lines previously tested with neoamphimedine and amphimedine [10]. The IC50 values of deoxyamphimedine are reported in Table 1.

Table 1.
Cytotoxicity of Deoxyamphimedine.
The observed IC50 values were typically slightly lower than those reported for neoamphimedine, while amphimedine was not toxic up to 100 μM tested [10]. Deoxyamphimedine and neoamphimedine were cytotoxic in every tumor cell line examined, indicating their potential as anti-cancer drugs. Deoxyamphimedine was almost equally toxic to the A2780wt and its paired multi-drug resistant cell line, A2780AD, which exhibited resistance to doxorubicin (33 fold), M-AMSA (8 fold), and taxol (15 fold) when compared to the A2780wt cell line. The lack of significant fold difference for deoxyamphimedine in the A2780 cell lines indicates that it is likely not a substrate for the multi-drug resistant pump that often impedes the efficacy of cancer drugs in chemotherapy. In repeated experiments, deoxyamphimedine again was selectively more toxic to the mutant CHO cell lines, EM9 and xrs-6, than the wild type (AA8). The EM9 cell line has enhanced sensitivity to topoisomerase 1 poisons and ROS that cause DNA single-strand breaks. Sensitivity observed in the xrs-6 cell line is often associated with compounds that generate DNA double strand breaks via topoisomerase 2 or high amounts of single strand DNA cleavage [22]. These data confirm DNA strand breakage as a contributing mechanism of deoxyamphimedine cytotoxicity.
2.2. Deoxyamphimedine Intercalates DNA
Many pyridoacridines have shown binding affinity for DNA, and this binding often correlates with the observed cytotoxicity [9]. To measure DNA intercalation, deoxyamphimedine was compared to amphimedine and neoamphimedine, in an ethidium bromide (EtBr) displacement assay. Deoxyamphimedine exhibited potent DNA intercalation, displacing 50% of EtBr at 1 μM, while neoamphimedine required approximately 100 μM to displace 50% of EtBr. Amphimedine was unable to significantly displace EtBr at concentrations up to 100 μM (Figure 2). These results suggest that deoxyamphimedine has a higher affinity for DNA than its analogs, as might be expected from its positive charge.
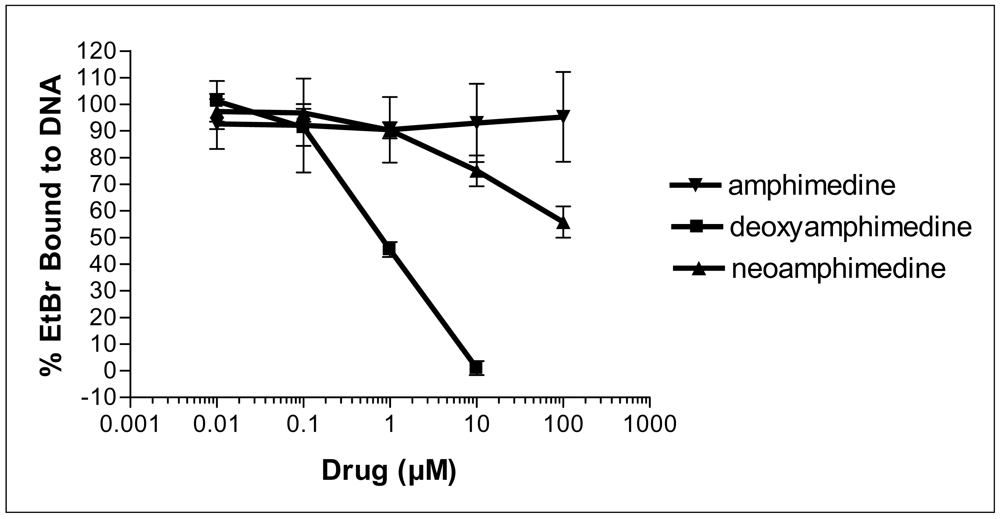
Figure 2.
EtBr displacement by the amphimedines as a measure of DNA intercalation. Values are the average of n=4 ± SD.
2.3. Deoxyamphimedine Cleaves DNA Independently of Topoisomerase 1 or 2
DNA intercalators often interfere with DNA metabolizing enzymes, such as the topoisomerases. Therefore, in vitro analyses to evaluate the potential importance of the topoisomerase enzymes for DNA damage were performed.
The topoisomerase 1 and 2 DNA cleavage assays (Figure 3) showed that amphimedine did not inhibit either of the topoisomerase enzymes, nor did it increase the amount of cleaved DNA observed in the gel. Neoamphimedine, as previously reported, has no effect on topoisomerase 1, but produced catenated DNA complexes in the presence of topoisomerase 2, inducing only minimal topoisomerase 2-mediated DNA cleavage [10]. Deoxyamphimedine was a potent cleaver of DNA in vitro as indicated by the increased intensity in the nicked (single strand break) and linear (double strand break) plasmid DNA bands. This activity was independent of the presence of topoisomerase 1 or 2.
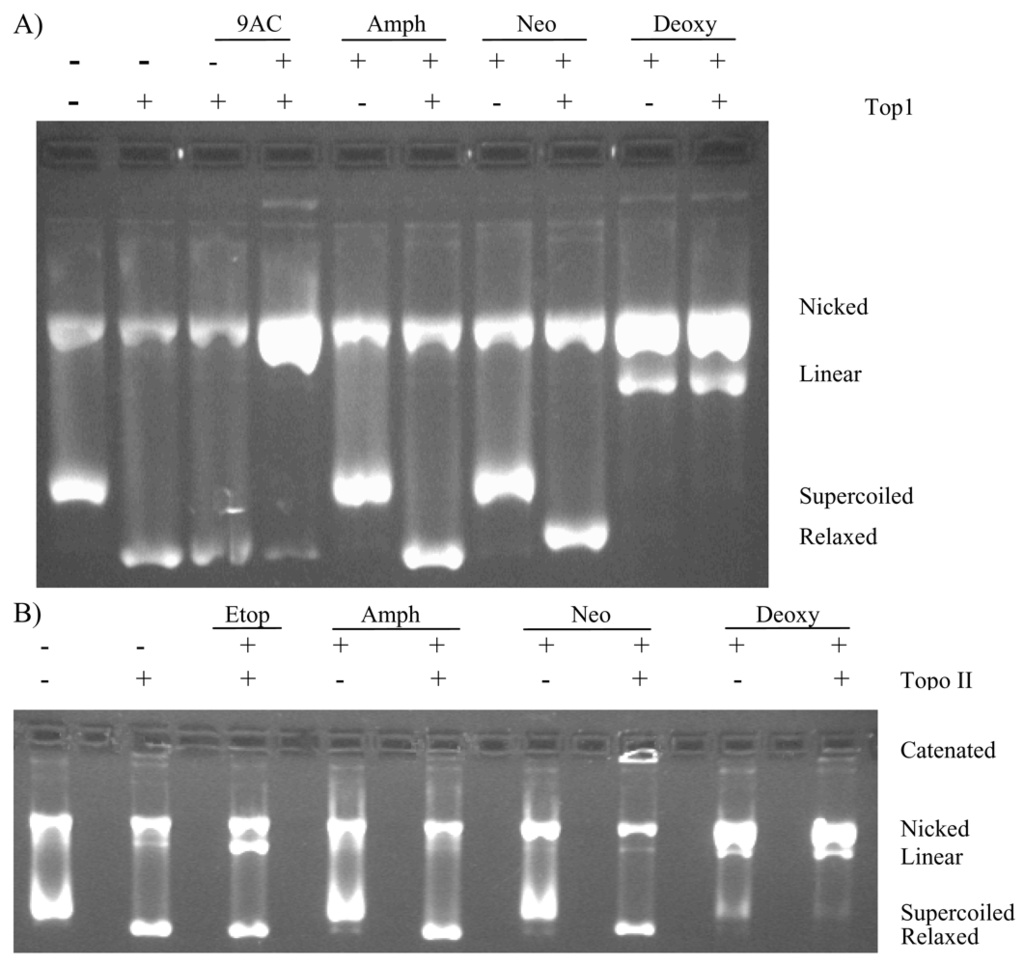
Figure 3.
Topoisomerase 1 and topoisomerase 2 DNA cleavage gels for amphimedine (Amph), neoamphimedine (Neo) and deoxyamphimedine (Deoxy). A) Plasmid DNA (supercoiled rf M13 mp 19 DNA) was used in the cleavage assay in the presence or absence of topoisomerase 1, with DMSO, 9-aminocamptothecin, 9AC (9 μM) that induces topoisomerase 1-mediated DNA single strand breaks, amphimedine (100 μM), neoamphimedine (100 μM), and deoxyamphimedine (100 μM). B) Plasmid DNA was used in the cleavage assay in the presence and absence of topoisomerase 2, etoposide (Etop, 100 μM) that induces topoisomerase 2-mediated DNA double strand breaks, amphimedine (100 μM), neoamphimedine (100 μM), and deoxyamphimedine (100 μM).
Further evaluation in the cleavage assay showed deoxyamphimedine could fully cleave DNA under aerobic conditions in experiments carried out in water to which only dithiothreitol (DTT, a reducing agent) and DNA were added. Figure 4A shows results for all of the amphimedines in the absence or presence of 5 mM DTT. While no catenation was observed with neoamphimedine, there was a slight increase in the nicked DNA band. The amount of cleaved DNA observed was greatly increased with deoxyamphimedine in the presence of DTT. The amount of deoxyamphimedine-induced DNA cleavage observed increased with increasing amounts of DTT (Figure 4B). However, under hypoxic conditions the amount of induced DNA cleavage was attenuated (Figure 4C). The extent of DNA cleavage increased with increasing deoxyamphimedine concentrations (Figure 4D) with detectable cleavage appearing at 5 μM and increasing to approximately 84% cleavage at 100 μM in this experimental system. Additional studies using the cleavage assay showed that deoxyamphimedine-induced DNA cleavage exhibited a time dependency (data not shown), with complete cleavage of the substrate in the presence of 5 mM DTT within 30 minutes. Deoxyamphimedine also cleaved plasmid DNA, regardless of its topological state. Specific reaction conditions, such as high salt or temperature, will reverse DNA damage detectable from stabilized drug-top1-DNA complexes, but not ROS-induced DNA damage. These conditions did not reverse deoxyamphimedine-induced DNA damage (data not shown).
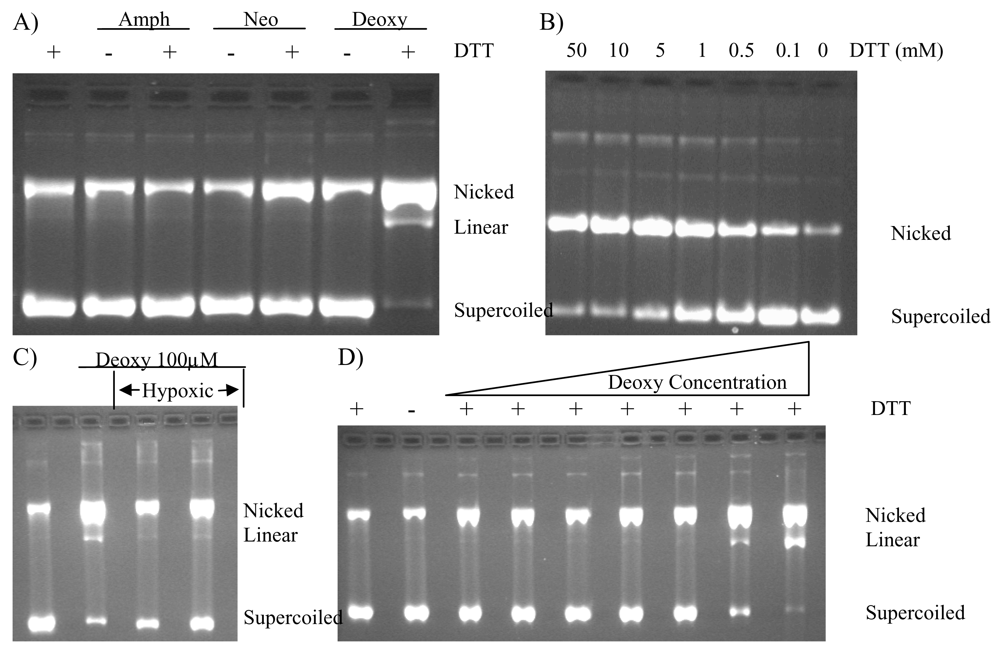
Figure 4.
Deoxyamphimedine dependence on DTT, oxygen and concentration. A) Plasmid DNA was incubated in the presence of DTT (5 mM), amphimedine (Amph, 100 μM), neoamphimedine (Neo, 100 μM), and deoxyamphimedine (Deoxy, 100 μM). B) Plasmid DNA was incubated in the presence of deoxyamphimedine (100 μM) with varying amounts of DTT (as shown). C) Plasmid DNA was incubated in the presence of DTT (5 mM) and deoxyamphimedine (100 μM) as indicated. Hypoxic conditions were induced in the last 2 lanes by bubbling argon in the reaction vessel. D) Plasmid DNA was incubated in the presence or absence of DTT (5 mM) with varying amounts of deoxyamphimedine (0.1 μM, 0.5 μM, 1 μM, 5 μM, 10 μM, 50 μM, 100 μM). Increase in cleaved plasmid DNA over control in this experiment was quantifiable at 5 μM (12%), 10 μM (14%), 50 μM (67%), and 100 μM (84%).
2.4. Protection of DNA from Deoxyamphimedine Cleavage
Metal chelators and FeSO4 were used to determine if a Fenton type reaction could be responsible for deoxyamphimedine-induced DNA cleavage. Metal chelators were added to aerobic reactions containing deoxyamphimedine, DTT and DNA. DNA cleavage in the presence of deoxyamphimedine was not further stimulated when FeSO4 (100 μM) was added (data not shown). Ion coupled plasma-mass spectrometry (ICP-MS) was performed on deoxyamphimedine in DMSO to determine if it bound to metal ions in solution. The results suggest that some metal binding may be occurring (data not shown). However, only slight protection from DNA cleavage was sporadically detectable in the presence of deferoxamine mesylate (up to 50 mM) and ferrozine (up to 100 μM) (Figure 5A) or EDTA (100 μM) (data not shown). Overall, the addition of metals or metal chelators did not strongly alter the DNA cleaving ability of deoxyamphimedine.
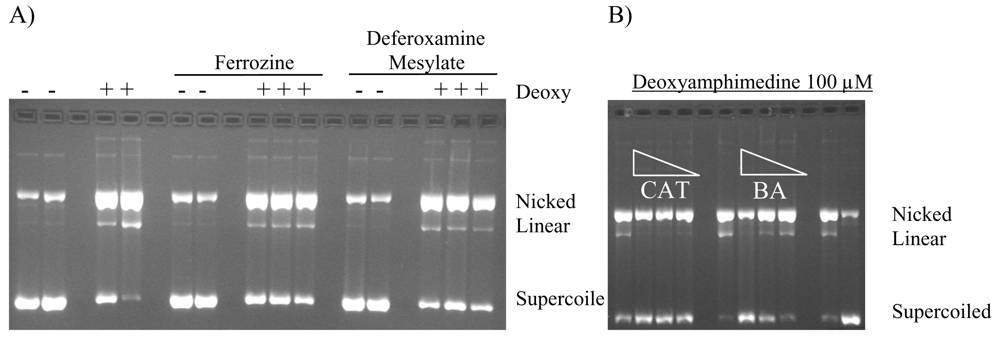
Figure 5.
A) Effects of chelators on deoxyamphimedine (Deoxy)-induced DNA cleavage. Plasmid DNA was incubated in the absence or presence of deoxyamphimedine (100 μM), ferrozine (100 μM) and deferoxamine mesylate (100 μM). B) Anti-oxidant enzymes and radical scavenger protection of deoxyamphimedine induced DNA cleavage. Plasmid DNA was incubated in the presence of deoxyamphimedine (100 μM) and varying amounts of catalase (CAT) (88 u, 8.8 u, 0.88 u) or the free radical scavenger benzoic acid (BA) (8.8 mM, 0.88 mM, 0.088 mM).
To determine if reactive oxygen species played a role in deoxyamphimedine’s DNA cleavage, an anti-oxidant enzyme and radical scavengers were employed to attenuate DNA cleavage. Figure 5B shows that catalase (CAT) and benzoic acid (BA) were able to inhibit cleavage by deoxyamphimedine. Other radical scavengers, glutathione (GSH) and N-acetylcholine (NAC), also attenuated deoxyamphimedine-induced DNA cleavage (data not shown). Superoxide dismutase (SOD), which converts superoxide to H2O2, did not decrease deoxyamphimedine-induced DNA cleavage. No additional protection was detected when SOD was used with catalase when compared to catalase alone (data not shown).
2.5. Discussion
Although amphimedine, deoxyamphimedine and neoamphimedine are chemically very similar (see Figure 1), our data shows that their minor structural differences significantly alter their biological activities. Amphimedine was relatively inactive compared to deoxyamphimedine and neoamphimedine, both of which demonstrated considerable DNA-directed activity. Amphimedine did not significantly intercalate DNA at the concentrations tested and was not cytotoxic at doses up to 100 μM. Neoamphimedine and deoxyamphimedine displace EtBr from DNA, although deoxyamphimedine is more effective than neoamphimedine. Both deoxyamphimedine and neoamphimedine are cytotoxic, although by differing mechanisms. Deoxyamphimedine is a positively charged compound, which differentiates it from amphimedine and neoamphimedine. As mentioned above, it interacts with DNA avidly and is the most cytotoxic of the three amphimedines tested. Several other marine pyridoacridines (e.g., dercitin, cystodytin J, ascididemin and diplamine) have previously been shown to intercalate DNA [23–25].
Deoxyamphimedine facilitated DNA cleavage in vitro, unlike amphimedine or neoamphimedine. Under aerobic conditions, the reducing agent DTT was the sole requirement for full activity. Low oxygen conditions reduced the amount of DNA cleaved and the total absence of DTT yielded undetectable levels of DNA damage. The DNA cleavage was time dependent, requiring more time to reach maximal DNA damage than is typically needed by topoisomerase 1-DNA cleavable complex stabilizing drugs (DNA, topoisomerases and topoisomerase poisons form the ternary complex very rapidly).
In our experimental systems, deoxyamphimedine produced results very similar to ascididemin, another pyridoacridine that produces damage to DNA through ROS [24,26]. Anti-oxidants and anti-oxidant enzymes protected DNA from deoxyamphimedine-induced cleavage, indicating a ROS-mediated mechanism. Extensive protection against DNA cleavage by the anti-oxidant enzyme catalase, suggested H2O2 as a likely intermediate ROS. In addition, anti-oxidants and reactive scavengers including glutathione, BHA, NAC and benzoic acid all protected against DNA damage. Therefore, the production of −OH most likely occurs. Previous work using electron paramagnetic resonance spectroscopy with ascididemin and analogs yielded a complex signal that indicated multiple iminoquinone radical species were likely formed [24]. We speculate that this would be the case for deoxyamphimedine as well.
Quinones and iminoquinones are capable of redox cycling. One electron reduction of the quinone forms a reactive semiquinone, allowing electron transfer to other species [27]. Higher redox potential of quinone molecules has been correlated with increased DNA cleavage [18,28]. Moreover, evidence suggests that doxorubicin and daunorubicin, both with quinone moieties, owe some of their clinical activity to ROS generation [27,29–33]. We hypothesize that it is the iminoquinone portion of deoxyamphimedine that is responsible for the redox cycling and ROS generation. Redox cycling and ROS generation require a source of oxygen. Under hypoxic conditions the ability of deoxyamphimedine to cleave DNA diminishes. Direct reduction of deoxyamphimedine to the semi-iminoquinone species likely facilitates the production of H2O2 and DNA damaging free radicals. It will be beneficial to further characterize the redox potential of deoxyamphimedine when more of it is available, current supplies being exhausted. All together, these data strongly implicate ROS as the mediator of deoxyamphimedine-induced DNA damage.
3. Experimental
3.1. Chemicals and Reagents
Isolation and chemical characterization of amphimedine, deoxyamphimedine and neoamphimedine have been described [11,34]; all were greater than 95% pure. They were isolated from two Xestospongia sp. sponges collected from Surigao, Philippines and Palau. All chemicals and reagents were obtained from Sigma Chemical Co. (St. Louis, MO, USA) or Baker Chemical Co. (Springfield, NJ, USA) unless otherwise noted. Drug standards were purchased from Sigma Chemical Co. Radioactive thymidine was purchased from New England Nuclear (Boston, MA, USA). Restriction enzymes and buffers were purchased from New England Biolabs (Ipswich, MA, USA). Radiolabeled (4.4 × 103 cpm/g) 3H replicative form (rf) of M13 mp19 was isolated by the alkaline lysis method as described [35,36]. Human topoisomerase 1 and topoisomerase 2 were isolated as described [36,37].
3.2. Assessment of DNA Intercalation
DNA intercalation was measured as the ability of drug to displace ethidium bromide (EtBr) from DNA. Based on the protocol of McDonald et al. [25], 10 serial dilutions (nM-μM) of drug were added with 0.5 μM EtBr to 0.5 μM salmon testes DNA (Sigma Chemical Co.) in a 96-well microtiter plate and incubated for 30 min at room temperature. A fluorimeter was used to quantify the fluorescence of each reaction, (280 nm excitation wavelength; displacement of EtBr was measured as a decreased fluorescence at 600 nm).
3.3. Quantitation of DNA and Cleavage
DNA cleavage assays were performed as previously described [10,36]. In brief, 20 μL volumes containing 50 mM Tris-HCl (pH 7.5), 85 mM KCl, 10 mM MgCl2, 0.5 mM EDTA, 30 μg/mL bovine serum albumin (Atlanta Biologicals, Atlanta, GA), 2 mM DTT, 500 ng of radiolabeled and supercoiled rf M13 mp 19 DNA and 100–150 ng purified topoisomerase 1 or 80–120 ng purified topoisomerase 2 were treated with 1–2 μL drug (in DMSO) and incubated at 30°C for 30 min. The reactions were stopped by the addition of 2 μL of 1.5 mg/mL proteinase K in 0.5% SDS and incubated at 37°C for 60 min. The DNA was fractionated by electrophoresis in 0.8% agarose (containing 50 ng EtBr/mL TAE) to separate the nicked, linear, relaxed and supercoiled isomers.
EtBr stained DNA was visualized by its fluorescence under UV light, and the bands were sliced out of the gel. To quantify the DNA in the bands the gel slices were placed in scintillation vials with 1 mL water, melted in a microwave oven and mixed with 10 mL Opti-Fluor (Packer Co., Meridien, CT, USA) while molten. Radioactivity determined by standard liquid scintillation counting [37]. The percentage of cleaved DNA, represented by radioactivity in the combined nicked and linear bands, was determined relative to the total radioactivity in the reactions, following subtraction of radioactivity due to endogenously cleaved DNA. The percentage of catenated DNA was determined similarly. All experiments were repeated three times, representative results are presented.
3.4. Cell Culture
The human epidermoid-nasopharyngeal tumor cell line (KB), human melanoma cell line (SK-mel-5), human breast cancer cell line (MCF7), human ovarian multi-drug resistant cell lines (A2780 and A2780AD), and Chinese hamster ovary cell lines (CHO): AA8 (wild type), EM9 (single strand break repair deficient) and xrs-6 (double strand break repair deficient) were used. KB, SK-mel-5, MCF7, EM9 and AA8 cell lines were purchased from ATCC, Rockville, MD). A2780 and A2780AD cell lines were provided by Dr. Jindrich Kopecek (University of Utah, UT, USA) and the xrs-6 cell line was a generous gift from Dr. Penny A. Jeggo and co-workers (University of Sussex, U.K.). All cell lines were maintained in Minimal Essential Medium alpha modification (α-MEM), Atlanta Biologicals. CHO cell lines were supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals), and the others were supplemented with 2.5% FBS and 7.5% calf sera (Atlanta Biologicals), 100 u/mL penicillin and 100 μg/mL streptomycin (Sigma Chemical Co.). Cells were grown at 37°C as monolayers in 75 cm2 culture flasks and detached with trypsin before seeding into microtiter culture plates.
3.5. MTT Cell Cytotoxicity Assay
Cytotoxicity was established in a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as performed by Mossman [38] and modified by others [22, 39, 40]. Drugs were dissolved in 100% DMSO at initial concentrations of 10 mM and serially diluted. The final concentration of DMSO in the cell culture wells was 1% or less. Cells were seeded in 200 μL of growth media in Corning 96-well microtiter plates at 30,000 cells/well (AA8), 40,000 cells/well (xrs-6 or EM9), or 20,000 cells/well (human cell lines). Four hours after seeding, cells were treated, each dose in quadruplicate, with 2 μL of drug (CHO cell lines) or 1 μL of drug (all other cell lines). CHO cell lines were refed at 18 hours. After 72 hours, all cultures were refed with 100 μL McCoy’s medium and 11 μL MTT (5 mg/mL in PBS, pH 7.4) was added to each well. The plates were incubated for 4 hours at 37°C. Viable cells reduced MTT to a purple formazan product that was solubilized by the addition of 100 μL DMSO to aspirated culture wells. The absorbance at 540 nm was measured for each well using a Bio-Rad MP450 plate reader (Bio-Rad, Hercules, CA). Following subtraction of absorbance from blank culture cells, the average absorbance for each set of drug-treated wells was compared to the average absorbance of the control wells to determine the fractional survival at any particular drug concentration. The inhibitory concentration 50 (IC50) was defined as the drug concentration that yielded a fractional survival of 50%.
3.6. Time and Concentration Dependence of DNA Cleavage
To determine the time and concentration dependency of deoxyamphimedine cleavage in vitro, DNA cleavage assays were performed as described above (3.3) using different time points of incubation (0 to 60 minutes) and increasing concentrations of deoxyamphimedine (0.1 to 500 μM).
3.7. DNA Cleavage Protection Assays
Protection from deoxyamphimedine-induced DNA cleavage was determined as described [24]. Metal chelators (i.e. EDTA, ferrozine, deferoxamine mesylate), the metal salt, FeSO4, radical scavengers [i.e. glutathione (GSH), N-acetylcysteine (NAC) and benzoic acid (BA)] or anti-oxidant enzymes [i.e. catalase (CAT)] were added to reactions described above to further define the potential mechanisms of DNA cleavage.
3.8. Assessment of DNA Cleavage under Hypoxic Conditions
DNA cleavage reactions were carried out as described above (3.3); however, these reactions were modified to be hypoxic based on the work of Radisky et al. [41]. Drug and reaction mix containing DNA were bubbled with argon for several minutes prior to the addition of drug to the reaction mix. At this point, the reaction mix was bubbled under argon for 1 min, lids were closed and it was then incubated at 37 °C for 30 min before being run out on an agarose gel, as described above.
Acknowledgements
The authors would like to thank Dr. Joseph A. Holden (Department of Pathology, University of Utah) for support and helpful discussions. Research supported by ICBG grant UO1 TW006671 and RO1 CA 36622.
- Samples Availability: Available from the authors.
References and Notes
- Brahic, C; Darro, F; Belloir, M; Bastide, J; Kiss, R; Delfourne, E. Synthesis and cytotoxic evaluation of analogues of the marine pyridoacridine amphimedine. Bioorg Med Chem 2002, 10, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Delfourne, E; Darro, F; Bontemps-Subielos, N; Decaestecker, C; Bastide, J; Frydman, A; Kiss, R. Synthesis and characterization of the antitumor activities of analogues of meridine, a marine pyridoacridine alkaloid. J Med Chem 2001, 44, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Delfourne, E; Darro, F; Portefaix, P; Galaup, C; Bayssade, S; Bouteille, A; Le Corre, L; Bastide, J; Collignon, F; Lesur, B; Frydman, A; Kiss, R. Synthesis and in vitro antitumor activity of novel ring D analogues of the marine pyridoacridine ascididemin: structure-activity relationship. J Med Chem 2002, 45, 3765–3771. [Google Scholar] [CrossRef] [PubMed]
- Delfourne, E; Kiss, R; Le Corre, L; Dujols, F; Bastide, J; Collignon, F; Lesur, B; Frydman, A; Darro, F. Synthesis and in vitro antitumor activity of phenanthrolin-7-one derivatives, analogues of the marine pyridoacridine alkaloids ascididemin and meridine: structure-activity relationship. J Med Chem 2003, 46, 3536–3545. [Google Scholar] [CrossRef] [PubMed]
- Delfourne, E; Kiss, R; Le Corre, L; Dujols, F; Bastide, J; Collignon, F; Lesur, B; Frydman, A; Darro, F. Synthesis and in vitro antitumor activity of ring C and D-substituted phenanthrolin-7-one derivatives, analogues of the marine pyridoacridine alkaloids ascididemin and meridine. Bioorg Med Chem 2004, 12, 3987–3994. [Google Scholar] [CrossRef] [PubMed]
- Delfourne, E; Kiss, R; Le Corre, L; Merza, J; Bastide, J; Frydman, A; Darro, F. Synthesis and in vitro antitumor activity of an isomer of the marine pyridoacridine alkaloid ascididemin and related compounds. Bioorg Med Chem 2003, 11, 4351–4356. [Google Scholar] [CrossRef] [PubMed]
- Feliu, L; Vera-Luque, P; Albericio, F; Alvarez, M. Advances in solid-phase cycloadditions for heterocyclic synthesis. J Comb Chem 2007, 9, 521–565. [Google Scholar] [CrossRef] [PubMed]
- LaBarbera, DV; Bugni, TS; Ireland, CM. The total synthesis of neoamphimedine. J Org Chem 2007, 72, 8501–8505. [Google Scholar] [CrossRef] [PubMed]
- Marshall, KM; Barrows, LR. Biological activities of pyridoacridines. Nat Prod Rep 2004, 21, 731–751. [Google Scholar] [CrossRef] [PubMed]
- Marshall, KM; Matsumoto, SS; Holden, JA; Concepcion, GP; Tasdemir, D; Ireland, CM; Barrows, LR. The anti-neoplastic and novel topoisomerase II-mediated cytotoxicity of neoamphimedine, a marine pyridoacridine. Biochem Pharmacol 2003, 66, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, D; Marshall, KM; Mangalindan, GC; Concepcion, GP; Barrows, LR; Harper, MK; Ireland, CM. Deoxyamphimedine, a new pyridoacridine alkaloid from two tropical Xestospongia sponges. J Org Chem 2001, 66, 3246–3248. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Biological effects of the superoxide radical. Arch Biochem Biophys 1986, 247, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B; Gutteridge, JMC. Free Radicals in Biology and Medicine, 2nd ed; Clarendon Press: Oxford, USA, 1989. [Google Scholar]
- Spencer, JP; Jenner, A; Aruoma, OI; Cross, CE; Wu, R; Halliwell, B. Oxidative DNA damage in human respiratory tract epithelial cells. Time course in relation to DNA strand breakage. Biochem Biophys Res Commun 1996, 224, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Sarker, AH; Watanabe, S; Seki, S; Akiyama, T; Okada, S. Oxygen radical-induced single-strand DNA breaks and repair of the damage in a cell-free system. Mutat Res 1995, 337, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E; Gattavecchia, E; Feroci, G; Battino, M. Interaction between reactive oxygen species and coenzyme Q10 in an aprotic medium: a cyclic voltammetry study. Mol Aspects Med 1994, 15(Suppl), s83–s88. [Google Scholar] [PubMed]
- Crawford, PW; Gross, J; Lawson, K; Cheng, CC; Dong, Q; Liu, DF; Luo, YL; Szczepankiewicz, BG; Heathcock, CH. Electrochemical properties of some biologically active quinone derivatives: furanquinones, pyridoquinones, and diplamine, a cytotoxic pyridoacridine alkaloid. J Electrochem Soc 1997, 144, 3710–3715. [Google Scholar]
- Malins, DC; Holmes, EH; Polissar, NL; Gunselman, SJ. The etiology of breast cancer. Characteristic alteration in hydroxyl radical-induced DNA base lesions during oncogenesis with potential for evaluating incidence risk. Cancer 1993, 71, 3036–3043. [Google Scholar] [PubMed]
- Cortes, F; Pinero, J; Palitti, F. Cytogenetic effects of inhibition of topoisomerase I or II activities in the CHO mutant EM9 and its parental line AA8. Mutat Res 1993, 288, 281–289. [Google Scholar] [PubMed]
- Cantoni, O; Murray, D; Meyn, RE. Induction and repair of DNA single-strand breaks in EM9 mutant CHO cells treated with hydrogen peroxide. Chem Biol Interact 1987, 63, 29–38. [Google Scholar] [PubMed]
- Messer, J; Reynolds, M; Stoddard, L; Zhitkovich, A. Causes of DNA single-strand breaks during reduction of chromate by glutathione in vitro and in cells. Free Radic Biol Med 2006, 40, 1981–1992. [Google Scholar] [PubMed]
- Jeggo, PA; Caldecott, K; Pidsley, S; Banks, GR. Sensitivity of Chinese hamster ovary mutants defective in DNA double strand break repair to topoisomerase II inhibitors. Cancer Res 1989, 49, 7057–7063. [Google Scholar] [PubMed]
- Ding, Q; Chichak, K; Lown, JW. Pyrroloquinoline and pyridoacridine alkaloids from marine sources. Curr Med Chem 1999, 6, 1–27. [Google Scholar] [PubMed]
- Matsumoto, SS; Biggs, J; Copp, BR; Holden, JA; Barrows, LR. Mechanism of ascididemin-induced cytotoxicity. Chem Res Toxicol 2003, 16, 113–122. [Google Scholar] [PubMed]
- McDonald, LA; Eldredge, GS; Barrows, LR; Ireland, CM. Inhibition of topoisomerase II catalytic activity by pyridoacridine alkaloids from a Cystodytes sp. ascidian: a mechanism for the apparent intercalator-induced inhibition of topoisomerase II. J Med Chem 1994, 37, 3819–3827. [Google Scholar] [PubMed]
- Matsumoto, SS; Sidford, MH; Holden, JA; Barrows, LR; Copp, BR. Mechanism of action studies of cytotoxic marine alkaloids: ascididemin exhibits thiol-dependent oxidative DNA cleavage. Tetrahedron Lett 2000, 41, 1667–1670. [Google Scholar]
- Herman, EH; Ferrans, VJ. Reduction of chronic doxorubicin cardiotoxicity in dogs by pretreatment with (+/−)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF-187). Cancer Res 1981, 41, 3436–3440. [Google Scholar] [PubMed]
- Malins, DC. Identification of hydroxyl radical-induced lesions in DNA base structure: biomarkers with a putative link to cancer development. J Toxicol Environ Health 1993, 40, 247–261. [Google Scholar] [PubMed]
- Doroshow, JH. Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones. Proc Natl Acad Sci USA 1986, 83, 4514–4518. [Google Scholar] [PubMed]
- Doroshow, JH; Davies, KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem 1986, 261, 3068–3074. [Google Scholar] [PubMed]
- Doroshow, JH; Locker, GY; Ifrim, I; Myers, CE. Prevention of doxorubicin cardiac toxicity in the mouse by N-acetylcysteine. J Clin Invest 1981, 68, 1053–1064. [Google Scholar] [PubMed]
- Doroshow, JH; Locker, GY; Myers, CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest 1980, 65, 128–135. [Google Scholar] [PubMed]
- Herman, EH; Ferrans, VJ; Jordan, W; Ardalan, B. Reduction of chronic daunorubicin cardiotoxicity by ICRF-187 in rabbits. Res Commun Chem Pathol Pharmacol 1981, 31, 85–97. [Google Scholar] [PubMed]
- de Guzman, FS; Carte, B; Troupe, N; Faulkner, DJ; Harper, MK; Concepcion, GP; Mangalindan, GC; Matsumoto, SS; Barrows, LR; Ireland, CM. Neoamphimedine: a new pyridoacridine topoisomerase II inhibitor which catenates DNA. J Org Chem 1999, 64 , 1400–1402. [Google Scholar] [PubMed]
- Englund, PT. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell 1978, 14, 157–168. [Google Scholar] [PubMed]
- Matsumoto, SS; Haughey, HM; Schmehl, DM; Venables, DA; Ireland, CM; Holden, JA; Barrows, LR. Makaluvamines vary in ability to induce dose-dependent DNA cleavage via topoisomerase II interaction. Anticancer Drugs 1999, 10, 39–45. [Google Scholar] [PubMed]
- Holden, JA; Rolfson, DH; Low, RL. DNA topoisomerase I from human placenta. Biochim. Biophys. Acta 1990, 1049, 303–310. [Google Scholar] [PubMed]
- Mossman, T. Rapid colorimetric assay for cell growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983, 65, 55–63. [Google Scholar] [PubMed]
- Kokoshka, JM; Capson, TL; Holden, JA; Ireland, CM; Barrows, LR. Differences in the topoisomerase I cleavage complexes formed by camptothecin and wakayin, a DNA-intercalating marine natural product. Anticancer Drugs 1996, 7, 758–765. [Google Scholar] [PubMed]
- Bonnard, I; Bontemps, N; Lahmy, S; Banaigs, B; Combaut, G; Francisco, C; Colson, P; Houssier, C; Waring, MJ; Bailly, C. Binding to DNA and cytotoxic evaluation of ascididemin, the major alkaloid from the Mediterranean ascidian Cystodytes dellechiajei. Anticancer Drug Des 1995, 10, 333–346. [Google Scholar] [PubMed]
- Radisky, DC; Radisky, E; Barrows, LR; Copp, BR; Kramer, RA; Ireland, CM. Novel cytotoxic topoisomerase II inhibiting pyrroloiminoquinones from Fijian sponges of the Genus Zyzzya. J Am Chem Soc 1993, 115, 1632–1638. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).