Antitumor Compounds from Marine Actinomycetes
Abstract
:1. Introduction
2. Polyketides
3. Non-Ribosomal Peptides
4. Mixed Polyketide-Nonribosomal Peptides
5. Isoprenoids
6. Indolocarbazoles
7. Others
8. Conclusions
Acknowledgements
References
- Ventura, M; Canchaya, C; Tauch, A; Chandra, G; Fitzgerald, GF; Chater, KF; van Sinderen, D. Genomics of Actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol Mol Biol Rev 2007, 71, 495–548. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, M; Williams, ST. Ecology of actinomycetes. Annu Rev Microbiol 1983, 37, 189–216. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, AJ; Williams, ST. Actinomycetes as agents of biodegradation in the environment-a review. Gene 1992, 115, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Stach, JE; Bull, AT. Estimating and comparing the diversity of marine actinobacteria. Antonie van Leeuwenhoek 2005, 87, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K. Volatile metabolites from actinomycetes. Chemosphere 1996, 32, 1427–1434. [Google Scholar] [CrossRef]
- Schrempf, H. Recognition and degradation of chitin by streptomycetes. Antonie van Leeuwenhoek 2001, 79, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Berdy, J. Bioactive microbial metabolites. J Antibiot 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Newman, DJ; Cragg, GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Olano, C; Méndez, C; Salas, JA. Antitumor compounds from actinomycetes: from gene clusters to new derivatives by combinatorial biosynthesis. Nat Prod Rep 2009, 26, 628–660. [Google Scholar] [CrossRef] [PubMed]
- Demain, AL; Sánchez, S. Microbial drug discovery: 80 years of progress. J Antibiot 2009, 62, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Baltz, RH. Renaissance in antibacterial discovery from actinomycetes. Curr Opin Pharmacol 2008, 8, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, NB; Sabry, SA; El-Sherif, ZM; Abu El-Ela, GA. Isolation and enumeration of marine actinomycetes from seawater and sediments in Alexandria. J Gen Appl Microbiol 2000, 46, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z; Zeng, W; Huang, Y; Yang, Z; Li, J; Cai, H; Su, W. Detection of antitumor and antimicrobial activities in marine organism associated actinomycetes isolated from the Taiwan Strait, China. FEMS Microbiol Lett 2000, 188, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, HP; Bruntner, C; Bull, AT; Ward, AC; Goodfellow, M; Potterat, O; Puder, C; Mihm, G. Marine actinomycetes as a source of novel secondary metabolites. Antonie van Leeuwenhoek 2005, 87, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ward, AC; Bora, N. Diversity and biogeography of marine actinobacteria. Curr Opin Microbiol 2006, 9, 1–8. [Google Scholar] [CrossRef]
- Fenical, W; Jensen, PR. Developing a new resource for drug discovery: marine actinomycete bacteria. Nat Chem Biol 2006, 2, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Panthom-Aree, W; Stach, JEM; Ward, AC; Horikoshi, K; Bull, AT; Goodfellow, M. Diversity of actinomycetes isolated from challenger deep sediment (10,898 m) from the Mariana Trench. Extremophiles 2006, 10, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bull, AT; Stach, JEM. Marine actinobacteria: new opportunities for natural product search and discovery. Trends Microbiol 2007, 15, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Gontang, EA; Fenical, W; Jensen, PR. Phylogenetic diversity of Gram-positive bacteria cultured from marine sediments. Appl Environ Microbiol 2007, 73, 3272–3282. [Google Scholar] [CrossRef] [PubMed]
- Bredholdt, H; Galatenko, OA; Engelhardt, K; Fjærvik, E; Terekhova, LP; Zotchev, SB. Rare actinomycete bacteria from the shallow water sediments of the Trodheim fjord, Norway: isolation, diversity and biological activity. Environ Microbiol 2007, 9, 2756–2764. [Google Scholar] [CrossRef] [PubMed]
- Jensen, PR; Lauro, FM. An assessment of actinobacterial diversity in the marine environment. Antonie van Leeuwenhoek 2008, 94, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Anzai, K; Nakashima, T; Kuwahara, N; Suzuki, R; Ohfuku, Y; Takeshita, S; Ando, K. Actinomycete bacteria isolated from the sediments at coastal and offshore area of Nagasaki Prefecture, Japan: diversity and biological activity. J Biosci Bioeng 2008, 106, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Hong, K; Gao, AH; Xie, QY; Gao, H; Zhuang, L; Lin, HP; Yu, HP; Li, J; Yao, XS; Goodfellow, M; Ruan, JS. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar Drugs 2009, 7, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, LA; Fragoso-Yánez, D; Pérez-García, A; Rosellón-Druker, J; Quintana, ET. Actinobacterial diversity from marine sediments collected in México. Antonie van Leeuwenhoek 2009, 95, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bernan, VS; Greenstein, M; Carter, GT. Mining marine microorganisms as a source of new antimicrobials and antifungals. Curr Med Chem Anti-Infective Agents 2004, 3, 181–195. [Google Scholar] [CrossRef]
- Piel, J. Metabolites from symbiotic bacteria. Nat Prod Rep 2004, 21, 519–538. [Google Scholar] [CrossRef] [PubMed]
- Kim, TK; Fuerst, JA. Diversity of polyketide synthase genes from bacteria associated with the marine sponge Pseudoceratina clavata: culture-dependent and culture-independent approaches. Environ Microbiol 2006, 8, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Blunt, JW; Copp, BR; Munro, MH; Northcote, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2006, 23, 26–78. [Google Scholar] [CrossRef] [PubMed]
- Mayer, AM; Rodríguez, AD; Berlinck, RG; Hamann, MT. Marine pharmacology in 2003–4 Marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp Biochem Physiol 2007, 145, 553–581. [Google Scholar]
- Williams, PG. Panning for chemical gold: marine bacteria as a source of new therapeutics. Trends Biotechnol 2009, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Blunt, JW; Copp, BR; Hu, WP; Munro, MH; Northcote, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2009, 26, 170–244. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W; Sethna, KM; Lloyd, GK. Marine microorganisms as a developing resource for drug discovery. Pharm News 2002, 9, 489–494. [Google Scholar]
- Tsueng, G; Teisan, S; Lam, KS. Defined salt formulations for the growth of Salinispora tropica strain NPS21184 and the production of salinosporamide A (NPI-0052) and related analogs. Appl Microbiol Biotechnol 2008, 78, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Janssen, PH; Yates, PS; Grinton, BE; Taylor, PM; Sait, M. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and. Verrucomicrobia Appl Environ Microbiol 2002, 68, 2391–2396. [Google Scholar] [CrossRef]
- Donadio, S; Monciardini, P; Alduina, R; Mazza, P; Chiocchini, C; Cavaletti, L; Sosio, M; Puglia, AM. Microbial technologies for the discovery of novel bioactive metabolites. J Biotechnol 2002, 99, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Bull, AT; Stach, JE. Marine actinobacteria: new opportunities for natural product search and discovery. Trends Microbiol 2007, 15, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Bull, AT; Stach, JE; Ward, AC; Goodfellow, M. Marine actinobacteria: perspectives, challenges, future directions. Antonie van Leeuwenhoek 2005, 87, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Lam, KS; Tsueng, G; McArthur, KA; Mitchell, SS; Potts, BC; Xu, J. Effects of halogens on the production of salinosporamides by the obligate marine actinomycete Salinispora tropica. J Antibiot 2007, 60, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Selvin, J; Shanmughapriya, S; Gandhimathi, R; Seghal Kiran, G; Rajeetha Ravji, T; Natarajaseenivasan, K; Hema, TA. Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08. Appl Microbiol Biotechnol 2009, 83, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Moore, BS; Kalaitzis, JA; Xiang, L. Exploiting marine actinomycete biosynthetic pathways for drug discovery. Antonie van Leeuwenhoek 2005, 87, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hou, YH; Wang, QF; Ding, L; Li, FC; Qin, S. attB site disruption in marine Actinomyces sp. M048 via DNA transformation of a site-specific integration vector. Biotechnol Appl Biochem 2008, 50, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Shen, B. Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms. Curr Opin Chem Biol 2003, 7, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, RS; Sankaranarayanan, R; Mohanty, D. Versatility of polyketide synthases in generating metabolic diversity. Curr Opin Struct Biol 2007, 17, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Williams, PG; Miller, ED; Asolkar, RN; Jensen, PR; Fenical, W. Arenicolides A-C, 26-membered ring macrolides from the marine actinomycete Salinispora arenicola. J Org Chem 2007, 72, 5025–5034. [Google Scholar] [CrossRef] [PubMed]
- Williams, PG; Asolkar, RN; Kondratyuk, T; Pezzuto, JM; Jensen, PR; Fenical, W. Saliniketals A and B, bicyclic polyketides from the marine actinomycete Salinispora arenicola. J Nat Prod 2007, 70, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Gerner, EW; Meyskens, FL, Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 2004, 4, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Cañedo, LM; Fernández-Puentes, JL; Baz, JP. IB-96212, a novel cytotoxic macrolide produced by a marine MicromonosporaII Physico-chemical properties and structure determination. J. Antibiot 2000, 53, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Chimeno, RI; Cañedo, L; Espliego, F; Grávalos, D; De La Calle, F; Fernández-Puentes, JL; Romero, F. IB-96212, a novel cytotoxic macrolide produced by a marine MicromonosporaI Taxonomy, fermentation, isolation and biological activities . J Antibiot 2000, 53, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Wu, SJ; Fotso, S; Li, F; Qin, S; Laatsch, H. Amorphane sesquiterpenes from a marine Streptomyces sp. J Nat Prod 2007, 70, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Asolkar, RN; Maskey, RP; Helmke, E; Laatsch, H. Chalcomycin B, a new macrolide antibiotic from the marine isolate Streptomyces sp. B7064. J. Antibiot 2002, 55, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Gupta, RS; Murray, W; Gupta, R. Cross resistance pattern towards anticancer drugs of a human carcinoma multidrug-resistant cell line. Br J Cancer 1988, 58, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ward, SL; Hu, Z; Schirmer, A; Reid, R; Revill, WP; Reeves, CD; Petrakovsky, OV; Dong, SD; Katz, L. Chalcomycin biosynthesis gene cluster from Streptomyces bikiniensis: novel features of an unusual ketolide produced through expression of the chm polyketide synthase in Streptomyces fradiae. Antimicrob Agents Chemother 2004, 48, 4703–4712. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, SS; Nicholson, B; Teisan, S; Lam, KS; Potts, BC. Aureoverticillactam, a novel 22-atom macrocyclic lactam from the marine actinomycete Streptomyces aureoverticillatus. J Nat Prod 2004, 67, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Kwon, HC; Kauffman, CA; Jensen, PR; Fenical, W. Marinomycins A-D, antitumor-antibiotics of a new structure class from a marine actinomycete of the recently discovered genus “Marinispora”. J Am Chem Soc 2006, 128, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Hara, M; Akasaka, K; Akinaga, S; Okabe, M; Nakano, H; Gomez, R; Wood, D; Uh, M; Tamanoi, F. Identification of Ras farnesyltransferase inhibitors by microbial screening. Proc Natl Acad Sci USA 1993, 90, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Sattler, I; Thiericke, R; Zeeck, A. The manumycin-group metabolites. Nat Prod Rep 1998, 15, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Li, F; Maskey, RP; Qin, S; Sattler, I; Fiebig, HH; Maier, A; Zeeck, A; Laatsch, H. Chinikomycins A and B: isolation, structure elucidation, and biological activity of novel antibiotics from a marine Streptomyces sp. isolate M045. J Nat Prod 2005, 68, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Asolkar, RN; Jensen, PR; Kauffman, CA; Fenical, W. Daryamides A-C, weakly cytotoxic polyketides from a marine-derived actinomycete of the genus Streptomyces strain CNQ-085. J Nat Prod 2006, 69, 1756–1759. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B; Ireland, CM. Lissoclinolide, the first non-nitrogenous metabolite from a Lissoclinum tunicate. J Nat Prod 1990, 53, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Gallo, GG; Coronelli, C; Vigevani, A; Lancini, GC. The structure of tetrenolin: a new antibiotic substance. Tetrahedron 1969, 25, 5677–5680. [Google Scholar] [CrossRef] [PubMed]
- Pagani, H; Lancini, G; Tamoni, G; Coronelli, C. Tetrenolin and SS/1018 A, antibacterial agents isolated from a strain of actinomycetales. J Antibiot 1973, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Richardson, AD; Ireland, CM. A profile of the in vitro antitumor activity of lissoclinolide. Toxicol Appl Pharmacol 2004, 195, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Oh, DC; Gontang, EA; Kauffman, CA; Jensen, PR; Fenical, W. Salinipyrones and pacificanones, mixed-precursor polyketides from the marine actinomycete Salinispora pacifica. J Nat Prod 2008, 71, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, GO; Williams, PG; Feling, RH; Kauffman, CA; Jensen, PR; Fenical, W. Sporolides A and B: structurally unprecedented halogenated macrolides from the marine actinomycete Salinispora tropica. Org Lett 2005, 7, 2731–2734. [Google Scholar] [CrossRef] [PubMed]
- Cho, JY; Kwon, HC; Williams, PG; Kauffman, CA; Jensen, PR; Fenical, W. Actinofuranones A and B, polyketides from a marine-derived bacterium related to the genus Streptomyces (actinomycetales). J Nat Prod 2006, 69, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Oh, DC; Williams, PG; Kauffman, CA; Jensen, PR; Fenical, W. Cyanosporasides A and B, chloro- and cyano-cyclopenta[a]indene glycosides from the marine actinomycete “Salinispora pacifica”. Org Lett 2006, 8, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y; Shirasaki, S; Kawasaki, T; Matsuo, Y; Adachi, K; Shizuri, Y. Structures of new cytotoxic antibiotics, piericidins C7 and C8. J Antibiot 2007, 60, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Morgenbesser, SD; Williams, BO; Jacks, T; DePinho, RA. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 1994, 371, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y; Shirasaki, S; Shiba, S; Kawasaki, T; Matsuo, Y; Adachi, K; Shizuri, Y. Piericidins C7 and C8, new cytotoxic antibiotics produced by a marine Streptomyces sp. J Antibiot 2007, 60, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Jeong, SY; Shin, HJ; Kim, TS; Lee, HS; Park, SK; Kim, HM. Streptokordin, a new cytotoxic compound of the methylpyridine class from a marine-derived Streptomyces sp. KORDI-3238. J. Antibiot 2006, 59, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Smith, WC; Xiang, L; Shen, B. Genetic localization and molecular characterization of the nonS gene required for macrotetrolide biosynthesis in Streptomyces griseus DSM40695. Antimicrob Agents Chemother 2000, 44, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Borrel, MN; Pereira, E; Fiallo, M; Garnier-Suillerot, A. Mobile ionophores are a novel class of P-glycoprotein inhibitors. The effects of ionophores on 4′-O-tetrahydropyranyl-adriamycin incorporation in K562 drug-resistant cells. Eur J Biochem 1994, 223, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T; Kinoshita, M; Wei, H; Kobayashi, M. Stereostructure of komodoquinone A, a neuritogenic anthracycline, from marine Streptomyces sp. KS3. Chem Pharm Bull 2003, 51, 1402–1404. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T; Kinoshita, M; Aoki, S; Kobayashi, M. Komodoquinone A, a novel neuritogenic anthracycline, from marine Streptomyces sp. KS3. J Nat Prod 2003, 66, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Wu, SJ; Fotso, S; Li, F; Qin, S; Kelter, G; Fiebig, HH; Laatsch, H. 39-N-carboxamido-staurosporine and selina-4(14),7(11)-diene-8,9-diol, new metabolites from a marine Streptomyces sp. J Antibiot 2006, 59, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Butler, MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep 2008, 25, 475–516. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z; Jakobi, K; Welzel, K; Hertweck, C. Biosynthesis of the antitumor agent chartreusin involves the oxidative rearrangement of an anthracyclic polyketide. Chem Biol 2005, 12, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Lorico, A; Long, BH. Biochemical characterisation of elsamicin and other coumarin-related antitumour agents as potent inhibitors of human topoisomerase II. Eur J Cancer 1993, 29A, 1985–1991. [Google Scholar] [PubMed]
- McGovren, JP; Neil, GL; Crampton, SL; Robinson, MI; Douros, JD. Antitumor activity and preliminary drug disposition studies on chartreusin (NSC 5159). Cancer Res 1977, 37, 1666–1672. [Google Scholar] [PubMed]
- Maskey, RP; Helmke, E; Laatsch, H. Himalomycin A and B: isolation and structure elucidation of new fridamycin type antibiotics from a marine Streptomyces isolate. J Antibiot 2003, 56 , 942–949. [Google Scholar] [PubMed]
- Masuma, R; Tanaka, K. New antitumor antibiotics, OS-4742 A1, A2, B1 and B2 produced by a strain of. Streptomyces J Antibiot 1977, 30, 908–916. [Google Scholar] [CrossRef]
- Maskey, RP; Helmke, E; Fiebig, HH; Laatsch, H. Parimycin: isolation and structure elucidation of a novel cytotoxic 2,3-dihydroquinizarin analogue of gamma-indomycinone from a marine streptomycete isolate. J Antibiot 2002, 55, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Maskey, RP; Helmke, E; Kayser, O; Fiebig, HH; Maier, A; Busche, A; Laatsch, H. Anti-cancer and antibacterial trioxacarcins with high anti-malaria activity from a marine streptomycete and their absolute stereochemistry. J Antibiot 2004, 57, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Maskey, RP; Sevvana, M; Usón, I; Helmke, E; Laatsch, H. Gutingimycin: a highly complex metabolite from a marine streptomycete. Angew Chem Int Ed Engl 2004, 43, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, A; Frauendorf, H; Laatsch, H; Diederichsen, U. Formation of gutingimycin: analytical investigation of trioxacarcin A-mediated alkylation of dsDNA. Anal Bioanal Chem 2008, 390, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Huang, YF; Tian, L; Fu, HW; Hua, HM; Pei, YH. One new anthraquinone from marine Streptomyces sp. FX-58. Nat Prod Res 2006, 20, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Gorajana, A; Kurada, BV; Peela, S; Jangam, P; Vinjamuri, S; Poluri, E; Zeeck, A. 1-Hydroxy-1-norresistomycin, a new cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. J Antibiot 2005, 58, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Kock, I; Maskey, RP; Biabani, MA; Helmke, E; Laatsch, H. 1-Hydroxy-1-norresistomycin and resistoflavin methyl ether: new antibiotics from marine-derived streptomycetes. J Antibiot 2005, 58, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Martin, P; Rodier, S; Mondon, M; Renoux, B; Pfeiffer, B; Renard, P; Pierre, A; Gesson, JP. Synthesis and cytotoxic activity of tetracenomycin D and of saintopin analogues. Bioorg Med Chem 2001, 10, 253–260. [Google Scholar]
- Hutchinson, CR. Biosynthetic studies of daunorubicin and tetracenomycin C. Chem Rev 1997, 97, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Shiono, Y; Shiono, N; Seo, S; Oka, S; Yamazaki, Y. Effects of polyphenolic anthrone derivatives, resistomycin and hypercin, on apoptosis in human megakaryoblastic leukemia CMK-7 cell line. Z Naturforsch 2002, 57, 923–929. [Google Scholar]
- Jakobi, K; Hertweck, C. A gene cluster encoding resistomycin biosynthesis in Streptomyces resistomycificus; exploring polyketide cyclization beyond linear and angucyclic patterns. J Am Chem Soc 2004, 126, 2298–2289. [Google Scholar] [CrossRef] [PubMed]
- Gorajana, A; Venkatesan, M; Vinjamuri, S; Kurada, BV; Peela, S; Jangam, P; Poluri, E; Zeeck, A. Resistoflavine, cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. Microbiol Res 2007, 162, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Martin, GD; Tan, LT; Jensen, PR; Dimayuga, RE; Fairchild, CR; Raventos-Suarez, C; Fenical, W. Marmycins A and B, cytotoxic pentacyclic C-glycosides from a marine sediment-derived actinomycete related to the genus Streptomyces. J Nat Prod 2007, 70, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Imamura, N; Kakinuma, K; Ikekawa, N; Tanaka, H; Omura, S. Biosynthesis of vineomycins A1 and B2. J Antibiot 1982, 37, 602–607. [Google Scholar]
- Kitahara, T; Naganawa, H; Okazaki, T; Okami, Y; Umezawa, H. The structure of SS-228 Y, an antibiotic from Chainia sp. J Antibiot 1975, 28, 280–285. [Google Scholar] [CrossRef]
- Okazaki, T; Kitahara, T; Okami, Y. Studies on marine microorganisms. IV. A new antibiotic SS-228 Y produced by Chainia isolated from shallow sea mud. J Antibiot 1975, 28, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Malet-Cascón, L; Romero, F; Espliego-Vázquez, F; Grávalos, D; Fernández-Puentes, JL. IB-00208, a new cytotoxic polycyclic xanthone produced by a marine-derived Actinomadura. I. Isolation of the strain, taxonomy and biological activites. J Antibiot 2003, 56, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, JC; Fernández-Puentes, JL; Baz, JP; Canedo, LM. IB-00208, a new cytotoxic polycyclic xanthone produced by a marine-derived Actinomadura. II. Isolation, physico-chemical properties and structure determination. J Antibiot 2003, 56, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Tresselt, D; Eckardt, K; Ihn, W. Antibiotics from Actinomycetes - chemical composition of antibiotic griseorhodin A. Tetrahedron 1978, 34 , 2693–2699. [Google Scholar] [PubMed]
- Ueno, T; Takahashi, H; Oda, M; Mizunuma, M; Yokoyama, A; Goto, Y; Mizushina, Y; Sakaguchi, K; Hayashi, H. Inhibition of human telomerase by rubromycins: Implication of spiroketal system of the compounds as an active moiety. Biochemistry 2000, 39, 5995–6002. [Google Scholar] [CrossRef] [PubMed]
- Shay, JW; Zou, Y; Hiyama, E; Wright, WE. Telomerase and cancer. Hum Mol Genet 2001, 10, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Li, A; Piel, J. A gene cluster from a marine Streptomyces encoding the biosynthesis of the aromatic spiroketal polyketide griseorhodin A. Chem Biol 2002, 9, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Walsh, CT. Polyketide and nonribosomal peptide antibiotics: modularity and versatility. Science 2004, 303, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Sieber, SA; Marahiel, MA. Molecular mechanisms underlying nonribosomal peptide synthesis: approaches to new antibiotics. Chem Rev 2005, 105, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, MA; Walsh, CT. Assembly-line enzymology for polyketide and nonribosomal Peptide antibiotics: logic, machinery, and mechanisms. Chem Rev 2006, 106, 3468–3496. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, HP; Bruntner, C; Riedlinger, J; Bull, AT; Knutsen, G; Goodfellow, M; Jones, A; Maldonado, L; Pathom-aree, W; Beil, W; Schneider, K; Keller, S; Sussmuth, RD. Proximicin A, B and C, novel aminofuran antibiotic and anticancer compounds isolated from marine strains of the actinomycete Verrucosispora. J Antibiot 2008, 61, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Riedlinger, J; Reicke, A; Zähner, H; Krismer, B; Bull, AT; Maldonado, LA; Ward, AC; Goodfellow, M; Bister, B; Bischoff, D; Süssmuth, RD; Fiedler, HP. Abyssomicins, inhibitors of the para-aminobenzoic acid pathway produced by the marine Verrucosispora strain AB-18-032. J Antibiot 2004, 57, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K; Keller, S; Wolter, FE; Röglin, L; Beil, W; Seitz, O; Nicholson, G; Bruntner, C; Riedlinger, J; Fiedler, HP; Süssmuth, RD. Proximicins A, B, and C-antitumor furan analogues of netropsin from the marine actinomycete Verrucosispora induce upregulation of p53 and the cyclin kinase inhibitor p21. Angew Chem Int Ed Engl 2008, 47, 3258–3261. [Google Scholar] [CrossRef] [PubMed]
- Cho, JY; Williams, PG; Kwon, HC; Jensen, PR; Fenical, W. Lucentamycins A-D, cytotoxic peptides from the marine-derived actinomycete Nocardiopsis lucentensis. J Nat Prod 2007, 70, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, K; Matsuo, Y; Adachi, K; Imagawa, H; Nishizawa, M; Shizuri, Y. Mechercharmycins A and B, cytotoxic substances from marine-derived Thermoactinomyces sp. YM3-251. J Antibiot 2005, 58, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Romero, F; Espliego, F; Pérez, Baz J; García de Quesada, T; Grávalos, D; de la Calle, F; Fernández-Puentes, JL. Thiocoraline, a new depsipeptide with antitumor activity produced by a marine Micromonospora. I. Taxonomy, fermentation, isolation, and biological activities. J Antibiot 1997, 50, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Pérez Baz, J; Canedo, LM; Fernández Puentes, JL; Silva Elipe, MV. Thiocoraline, a novel depsipeptide with antitumor activity produced by a marine Micromonospora. II. Physico-chemical properties and structure determination. J Antibiot 1997, 50, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Erba, E; Bergamaschi, D; Ronzoni, S; Faretta, M; Taverna, S; Bonfanti, M; Catapano, CV; Faircloth, G; Jimeno, J; D’Incalci, M. Mode of action of thiocoraline, a natural marine compound with anti-tumour activity. Br J Cancer 1999, 80, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S; Malkinson, JP; Paumier, D; Searcey, M. Bisintercalator natural products with potential therapeutic applications: isolation, structure determination, synthetic and biological studies. Nat Prod Rep 2007, 24, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Lombó, F; Velasco, A; Castro, A; de la Calle, F; Brana, AF; Sánchez-Puelles, JM; Méndez, C; Salas, JA. Deciphering the biosynthesis pathway of the antitumor thiocoraline from a marine actinomycete and its expression in two Streptomyces species. ChemBioChem 2006, 7, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Asolkar, RN; Freel, KC; Jensen, PR; Fenical, W; Kondratyuk, TP; Park, EJ; Pezzuto, JM. Arenamides A-C, cytotoxic NF-κB inhibitors from the marine actinomycete Salinispora arenicola. J Nat Prod 2009, 72, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, BB; Sethi, G; Nair, A; Ichikawa, H. Nuclear factor-κB A holy grail in cancer prevention and therapy. Curr Signal Transduction Ther 2006, 1, 25–52. [Google Scholar] [CrossRef]
- Melisi, D; Chiao, PJ. NF-κB as a target for cancer therapy. Expert Opin Ther Targets 2007, 11, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Miller, ED; Kauffman, CA; Jensen, PR; Fenical, W. Piperazimycins: cytotoxic hexadepsipeptides from a marine-derived bacterium of the genus. Streptomyces J Org Chem 2007, 72, 323–330. [Google Scholar] [CrossRef]
- Feling, RH; Buchanan, GO; Mincer, TJ; Kauffman, CA; Jensen, PR; Fenical, W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew Chem Int Ed Engl 2003, 42, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Udwary, DW; Zeigler, L; Asolkar, RN; Singan, V; Lapidus, A; Fenical, W; Jensen, PR; Moore, BS. Genome sequencing reveals complex secondary metabolome in the marine actinomycete Salinispora tropica. Proc Natl Acad Sci USA 2007, 104, 10376–10381. [Google Scholar] [CrossRef] [PubMed]
- Jensen, PR; Williams, PG; Oh, DC; Zeigler, L; Fenical, W. Species-specific secondary metabolite production in marine actinomycetes of the genus Salinispora. Appl Environ Microbiol 2007, 73, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Miller, CP; Ban, K; Dujka, ME; McConkey, DJ; Munsell, M; Palladino, M; Chandra, J. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood 2007, 110, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Beer, LL; Moore, BS. Biosynthetic convergence of salinosporamides A and B in the marine actinomycete Salinispora tropica. Org Lett 2007, 9, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Williams, PG; Buchanan, GO; Feling, RH; Kauffman, CA; Jensen, PR; Fenical, W. New cytotoxic salinosporamides from the marine actinomycete Salinispora tropica. J Org Chem 2005, 70, 6196–6203. [Google Scholar] [CrossRef] [PubMed]
- Reed, KA; Manam, RR; Mitchell, SS; Xu, J; Teisan, S; Chao, TH; Deyanat-Yazdi, G; Neuteboom, ST; Lam, KS; Potts, BC. Salinosporamides D-J from the marine actinomycete Salinispora tropica, bromosalinosporamide, and thioester derivatives are potent inhibitors of the 20S proteasome. J Nat Prod 2007, 70, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lam, KS; Tsueng, G; McArthur, KA; Mitchell, SS; Potts, BC; Xu, J. Effects of halogens on the production of salinosporamides by the obligate marine actinomycete Salinispora tropica. J Antibiot 2007, 60, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Eustáquio, AS; Moore, BS. Mutasynthesis of fluorosalinosporamide, a potent and reversible inhibitor of the proteasome. Angew Chem Int Ed Engl 2008, 47, 3936–3938. [Google Scholar] [CrossRef] [PubMed]
- McGlinchey, RP; Nett, M; Eustáquio, AS; Asolkar, RN; Fenical, W; Moore, BS. Engineered biosynthesis of antiprotealide and other unnatural salinosporamide proteasome inhibitors. J Am Chem Soc 2008, 130, 7822–7823. [Google Scholar] [CrossRef] [PubMed]
- Manam, RR; Macherla, VR; Tsueng, G; Dring, CW; Weiss, J; Neuteboom, ST; Lam, KS; Potts, BC. Antiprotealide is a natural product. J Nat Prod 2009, 72, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Manam, RR; Teisan, S; White, DJ; Nicholson, B; Grodberg, J; Neuteboom, ST; Lam, KS; Mosca, DA; Lloyd, GK; Potts, BC. Lajollamycin, a nitro-tetraene spiro-beta-lactone-gamma-lactam antibiotic from the marine actinomycete Streptomyces nodosus. J Nat Prod 2005, 68, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C; Ju, J; Christenson, SD; Smith, WC; Song, D; Zhou, X; Shen, B; Deng, Z. Utilization of the methoxymalonyl-acyl carrier protein biosynthesis locus for cloning the oxazolomycin biosynthetic gene cluster from Streptomyces albus JA3453. J. Bacteriol 2006, 188, 4142–4147. [Google Scholar] [CrossRef] [PubMed]
- Dairi, T. Studies on biosynthetic genes and enzymes of isoprenoids produced by actinomycetes. J Antibiot 2005, 58, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A; Ikeda, D; Nakamura, H; Naganawa, H; Kurasawa, S; Okami, Y; Takeuchi, T; Iitaka, Y. Altemicidin, a new acaricidal and antitumor substance. II. Structure determination. J Antibiot 1989, 42, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A; Kurasawa, S; Ikeda, D; Okami, Y; Takeuchi, T. Altemicidin, a new acaricidal and antitumor substance. I. Taxonomy, fermentation, isolation and physico-chemical and biological properties. J Antibiot 1989, 42, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, C; Jensen, PR; Fenical, W. Marinone and debromomarinone: Antibiotic sesquiterpenoid naphthoquinones of a new structure class from a marine bacterium. Tetrahedron Lett 1992, 33, 7663–7666. [Google Scholar] [CrossRef]
- Hardt, IH; Jensen, PR; Fenical, W. Neomarinone, and new cytotoxic marinone derivatives, produced by a marine filamentous bacterium (actinomycetales). Tetrahedron Lett 2000, 41, 2073–2076. [Google Scholar] [CrossRef]
- Kalaitzis, JA; Hamano, Y; Nilsen, G; Moore, BS. Biosynthesis and structural revision of neomarinone. Org Lett 2003, 5, 4449–4452. [Google Scholar] [CrossRef] [PubMed]
- Ding, L; Pfoh, R; Rühl, S; Qin, S; Laatsch, H. T-muurolol sesquiterpenes from the marine Streptomyces sp. M491 and revision of the configuration of previously reported amorphanes. J Nat Prod 2009, 72, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Soria-Mercado, IE; Prieto-Davo, A; Jensen, PR; Fenical, W. Antibiotic terpenoid chloro-dihydroquinones from a new marine actinomycete. J Nat Prod 2005, 68, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, DS; Mynderse, JS; Baker, PJ; Berry, DM; Boeck, LD; Yao, RC; Mertz, FP; Nakatsukasa, WM; Mabe, J; Ott, J; Counter, FT; Ensminger, PW; Allen, NE; Alborn, WE; Hobbs, JN. A80915, a new antibiotic complex produced by Streptomyces aculeolatus. Discovery, taxonomy, fermentation, isolation, characterization, and antibacterial evaluation. J Antibiot 1990, 43, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Saha, M; Jaisankar, P; Das, S; Sarkar, KK; Roy, S; Besra, SE; Vedasiromani, JR; Ghosh, D; Sana, B; Mukherjee, J. Production and purification of a bioactive substance inhibiting multiple drug resistant bacteria and human leukemia cells from a salt-tolerant marine Actinobacterium sp. isolated from the Bay of Bengal. Biotechnol Lett 2006, 28, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C; Méndez, C; Salas, JA. Indolocarbazole natural products: occurrence, biosynthesis, and biological activity. Nat Prod Rep 2006, 23, 1007–1045. [Google Scholar] [CrossRef] [PubMed]
- Omura, S; Iwai, Y; Hirano, A; Nakagawa, A; Awaya, J; Tsuchya, H; Takahashi, Y; Masuma, R. A new alkaloid AM-2282 of Streptomyces origin taxonomy, fermentation, isolation and preliminary characterization. J Antibiot 1977, 30, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Onaka, H; Taniguchi, S; Igarashi, Y; Furumai, T. Cloning of the staurosporine biosynthetic gene cluster from Streptomyces sp. TP-A0274 and its heterologous expression in Streptomyces lividans. J Antibiot 2002, 55, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Salas, AP; Zhu, L; Sánchez, C; Brana, AF; Rohr, J; Méndez, C; Salas, JA. Deciphering the late steps in the biosynthesis of the anti-tumour indolocarbazole staurosporine: sugar donor substrate flexibility of the StaG glycosyltransferase. Mol Microbiol 2005, 58, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C; Méndez, C; Salas, JA. Engineering biosynthetic pathways to generate antitumor indolocarbazole derivatives. J Ind Microbiol Biotechnol 2006, 33, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C; Zhu, L; Brana, AF; Salas, AP; Rohr, J; Méndez, C; Salas, JA. Combinatorial biosynthesis of antitumor indolocarbazole compounds. Proc Natl Acad Sci USA 2005, 102, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Salas, JA; Méndez, C. Indolocarbazole antitumour compounds by combinatorial biosynthesis. Curr Opin Chem Biol 2009, 13, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Hernández, LM; Blanco, JA; Baz, JP; Puentes, JL; Millán, FR; Vázquez, FE; Fernández-Chimeno, RI; Grávalos, DG. 4′-N-methyl-5′-hydroxystaurosporine and 5′-hydroxystaurosporine, new indolocarbazole alkaloids from a marine Micromonospora sp. strain. J Antibiot 2000, 53, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Han, X; Cui, C; Gu, Q; Zhu, W; Liu, H; Gu, J; Osada, H. ZHD-0501, a novel naturally occurring staurosporine analog from Actinomadura sp. 007. Tetrahedron Lett 2005, 46, 6137–6140. [Google Scholar] [CrossRef]
- Liu, R; Zhu, T; Li, D; Gu, J; Xia, W; Fang, Y; Liu, H; Zhu, W; Gu, Q. Two indolocarbazole alkaloids with apoptosis activity from a marine-derived actinomycete Z2039-2. Arch Pharm Res 2007, 30, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Robey, RW; Shukla, S; Steadman, K; Obrzut, T; Finley, EM; Ambudkar, SV; Bates, SE. Inhibition of ABCG2-mediated transport by protein kinase inhibitors with a bisindolylmaleimide or indolocarbazole structure. Mol Cancer Ther 2007, 6, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- McArthur, KA; Mitchell, SS; Tsueng, G; Rheingold, A; White, DJ; Grodberg, J; Lam, KS; Potts, BC. Lynamicins A-E, chlorinated bisindole pyrrole antibiotics from a novel marine actinomycete. J Nat Prod 2008, 71, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Boonlarppradab, C; Kauffman, CA; Jensen, PR; Fenical, W. Marineosins A and B, cytotoxic spiroaminals from a marine-derived actinomycete. Org Lett 2008, 10, 5505–5508. [Google Scholar] [CrossRef] [PubMed]
- Liu, R; Cui, CB; Duan, L; Gu, QQ; Zhu, WM. Potent in vitro anticancer activity of metacycloprodigiosin and undecylprodigiosin from a sponge-derived actinomycete Saccharopolyspora sp. nov. Arch Pharm Res 2005, 28, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, HH; Rodgers, GC; Keith, DD. Metacycloprodigiosin, a tripyrrole pigment from Streptomyces longisporusruber. J Am Chem Soc 1969, 91, 1263–1264. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, HH; Keith, DD; Rodgers, GC. The structure of metacycloprodigiosin. Tetrahedron 1976, 32, 1855–1861. [Google Scholar] [CrossRef]
- Pérez-Tomás, R; Montaner, B; Llagostera, E; Soto-Cerrato, V. The prodigiosins, proapoptotic drugs with anticancer properties. Biochem Pharmacol 2003, 66, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Shin, HJ; Kim, TS; Lee, HS; Park, JY; Choi, IK; Kwon, HJ. Streptopyrrolidine, an angiogenesis inhibitor from a marine-derived Streptomyces sp. KORDI-3973. Phytochemistry 2008, 69, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Hughes, CC; MacMillan, JB; Gaudêncio, SP; Jensen, PR; Fenical, W. The ammosamides: structures of cell cycle modulators from a marine-derived Streptomyces species. Angew Chem Int Ed Engl 2009, 48, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Bugni, TS; Woolery, M; Kauffman, CA; Jensen, PR; Fenical, W. Bohemamines from a marine-derived Streptomyces sp. J Nat Prod 2006, 69, 1626–1628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q; Schrader, KK; ElSohly, HN; Takamatsu, S. New cell-cell adhesion inhibitors from Streptomyces sp. UMA-044. J Antibiot 2003, 56, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, T; Blanco, FJ. Inhibitors targeting the LFA-1/ICAM-1 cell-adhesion interaction: design and mechanism of action. Curr Pharm Des 2008, 14, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Cossío, FP; Mendoza, ML; Zubia, A; Valcárcel, M; Vara, YI; Solaun, MS; López, JJ; San Sebastian, E. Novel inhibitors of the LFA-1/ICAM-1 interaction, and uses thereof. WIPO Patent WO/2007/054128 2007. [Google Scholar]
- Sánchez López, JM; Martínez Insua, M; Pérez Baz, J; Fernández Puentes, JL; Canedo Hernández, LM. New cytotoxic indolic metabolites from a marine Streptomyces. J Nat Prod 2003, 66, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Shin, HJ; Jeong, HS; Lee, HS; Park, SK; Kim, HM; Kwon, HJ. Isolation and structure determination of streptochlorin, an antiproliferative agent from a marine-derived Streptomyces sp. 04DH110. J Microbiol Biotechnol 2007, 17, 1403–1406. [Google Scholar] [PubMed]
- Park, C; Shin, HJ; Kim, GY; Kwon, TK; Nam, TJ; Kim, SK; Cheong, J; Choi, IW; Choi, YH. Induction of apoptosis by streptochlorin isolated from Streptomyces sp. in human leukemic U937 cells. Toxicol In Vitro 2008, 22, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Shin, DY; Shin, HJ; Kim, GY; Cheong, J; Choi, IW; Kim, SK; Moon, SK; Kang, HS; Choi, YH. Streptochlorin isolated from Streptomyces sp. Induces apoptosis in human hepatocarcinoma cells through a reactive oxygen species-mediated mitochondrial pathway. J Microbiol Biotechnol 2008, 18, 1862–1868. [Google Scholar] [PubMed]
- Choi, IK; Shin, HJ; Lee, HS; Kwon, HJ. Streptochlorin, a marine natural product, inhibits NF-κB activation and suppresses angiogenesis in vitro. J. Microbiol. Biotechnol 2007, 17, 1338–1343. [Google Scholar] [PubMed]
- Hohmann, C; Schneider, K; Bruntner, C; Irran, E; Nicholson, G; Bull, AT; Jones, AL; Brown, R; Stach, JE; Goodfellow, M; Beil, W; Krämer, M; Imhoff, JF; Süssmuth, RD; Fiedler, HP. Caboxamycin, a new antibiotic of the benzoxazole family produced by the deep-sea strain Streptomyces sp. NTK 937. J Antibiot 2009, 62, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, DH; Zhu, TJ; Liu, HB; Fang, YC; Gu, QQ; Zhu, WM. Four butenolides are novel cytotoxic compounds isolated from the marine-derived bacterium, Streptoverticillium luteoverticillatum11014. Arch Pharm Res 2006, 29, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Cho, KW; Lee, HS; Rho, JR; Kim, TS; Mo, SJ; Shin, J. New lactone-containing metabolites from a marine derived bacterium of the genus. Streptomyces J Nat Prod 2001, 64, 664–667. [Google Scholar] [CrossRef]
- Mukku, VJ; Speitling, M; Laatsch, H; Helmke, E. New butenolides from two marine streptomycetes. J Nat Prod 2000, 63, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Tsujibo, H; Sato, T; Inui, M; Yamamoto, H; Inamori, Y. Intracellular accumulation of phenazine antibiotics produced by an alkalophilic actinomycete. I. Taxonomy, isolation and identification of the phenazine antibiotics. Agric Biol Chem 1988, 52, 301–306. [Google Scholar] [CrossRef]
- Breitmaier, E; Hollstein, U. Carbon-13 nuclear magnetic resonance chemical shifts of substituted phenazines. J Org Chem 1976, 41, 2104–2108. [Google Scholar] [CrossRef]
- Hasegawa, K; Ueno, Y. The carbon-13 NMR spectra and electronic structure of 3H-phenoxazin-3-ones. Bull Chem Soc 1985, 58, 2832–2839. [Google Scholar] [CrossRef]
- Maskey, RP; Li, F; Qin, S; Fiebig, HH; Laatsch, H. Chandrananimycins A-C: production of novel anticancer antibiotics from a marine Actinomadura sp. isolate M048 by variation of medium composition and growth conditions. J Antibiot 2003, 56, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Cui, CB; Liu, HB; Gu, JY; Gu, QQ; Cai, B; Zhang, DY; Zhu, TJ. Echinosporins as new cell cycle inhibitors and apoptosis inducers from marine-derived Streptomyces albogriseolus. Fitoterapia 2007, 78, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Sato, T; Kawamoto, I; Oka, T; Okachi, R. A new antibiotic echinosporin (XK-213)-producing organism, isolation and characterization. J Antibiot 1982, 35, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Dübeler, A; Krastel, P; Floss, HG; Zeeck, A. Biosynthesis of the antibiotic echinosporin by a novel branch of the shikimate pathway. Eur J Org Chem 2002, 6, 983–987. [Google Scholar]
- Morimoto, M; Imai, R. Antitumor activity of echinosporin. J Antibiot 1985, 38, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, HK; Lee, DS; Lim, J; Kim, JS; Im, KS; Jung, JH. Topoisomerase I inhibitors from the Streptomyces sp. strain KM86-9B isolated from a marine sponge. Arch Pharm Res 1998, 21, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N; Sabaratnam, V; Abdullah, N; Ho, AS; Teo, SH; Lee, HB. Light-activated cytotoxic compounds from Malaysian microorganisms for photodynamic therapy of cancer. Antonie van Leeuwenhoek 2009, 95, 179–188. [Google Scholar] [CrossRef] [PubMed]


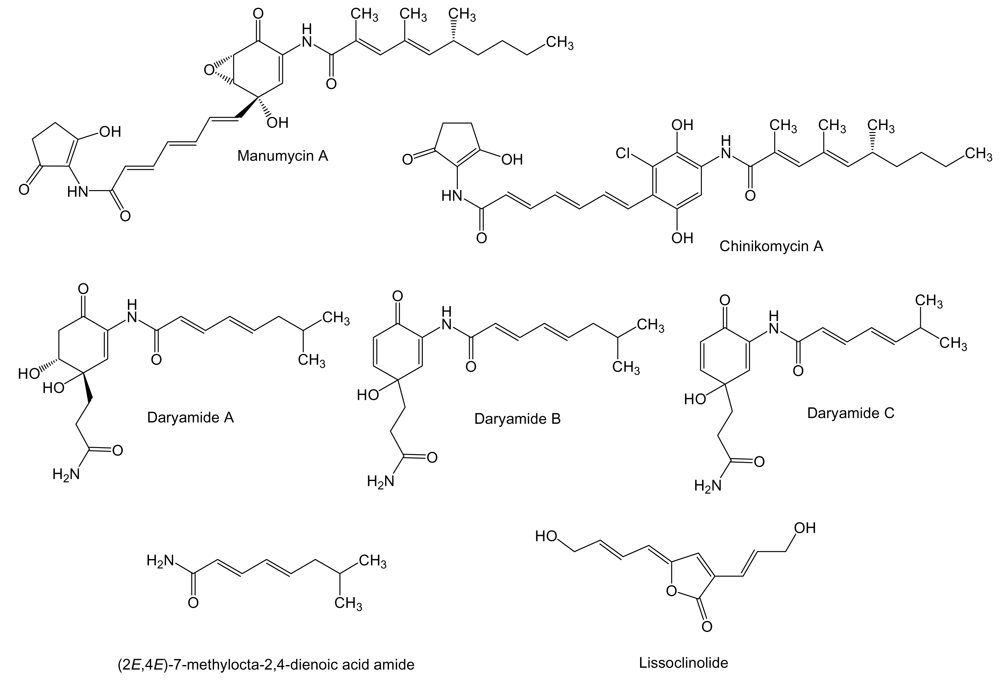

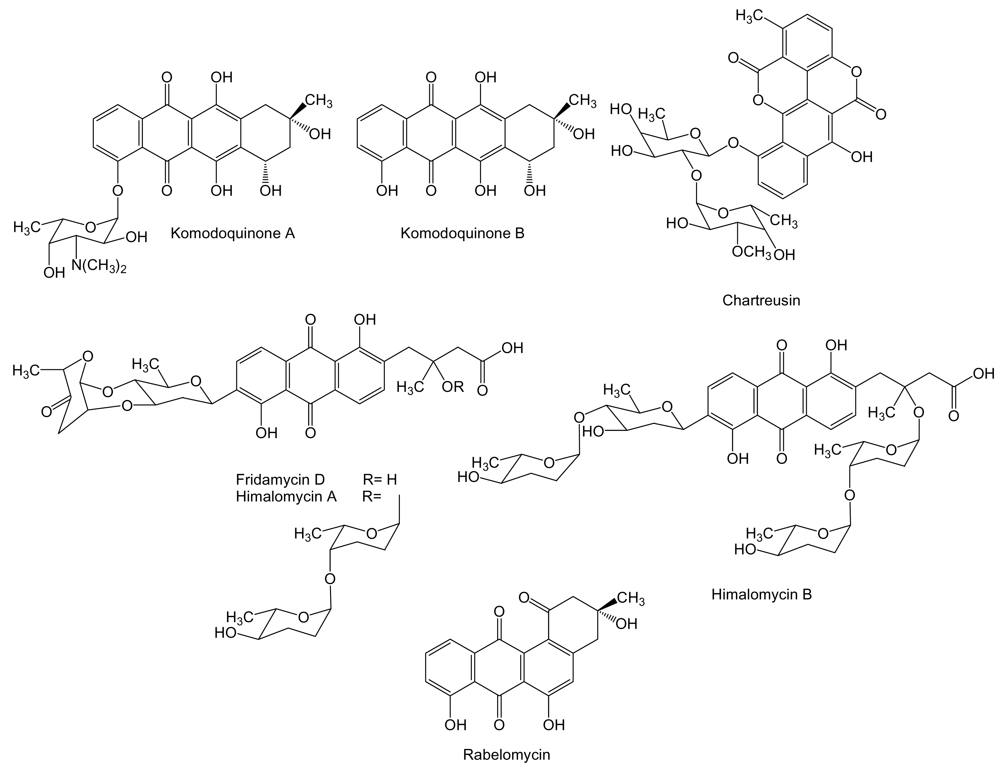

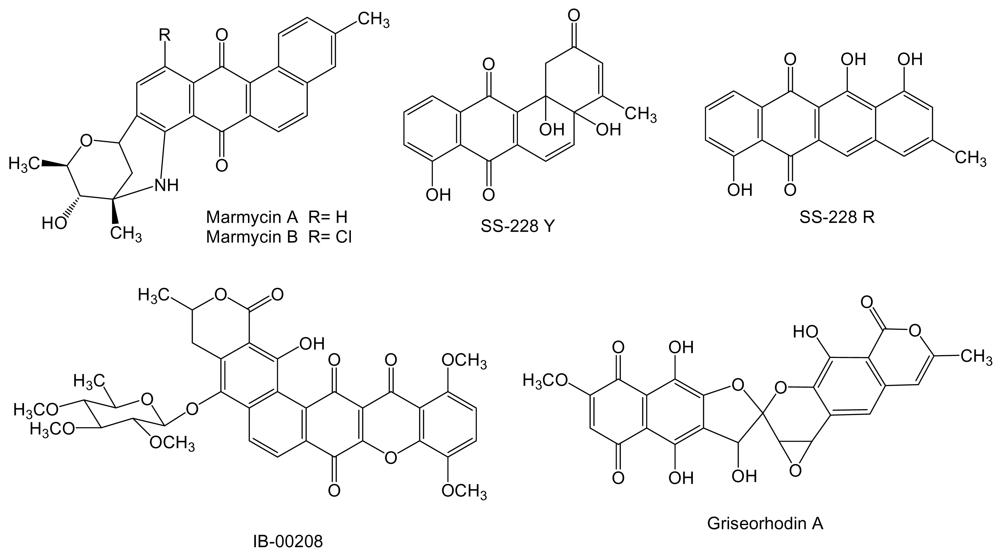
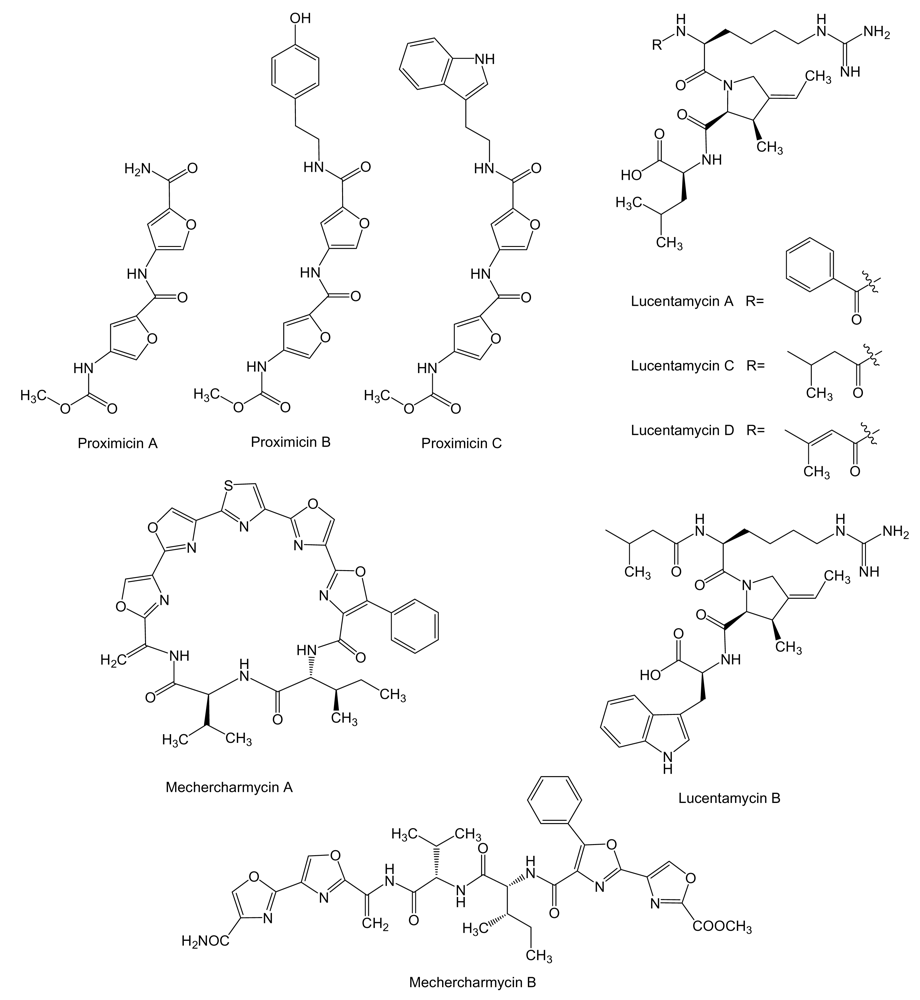




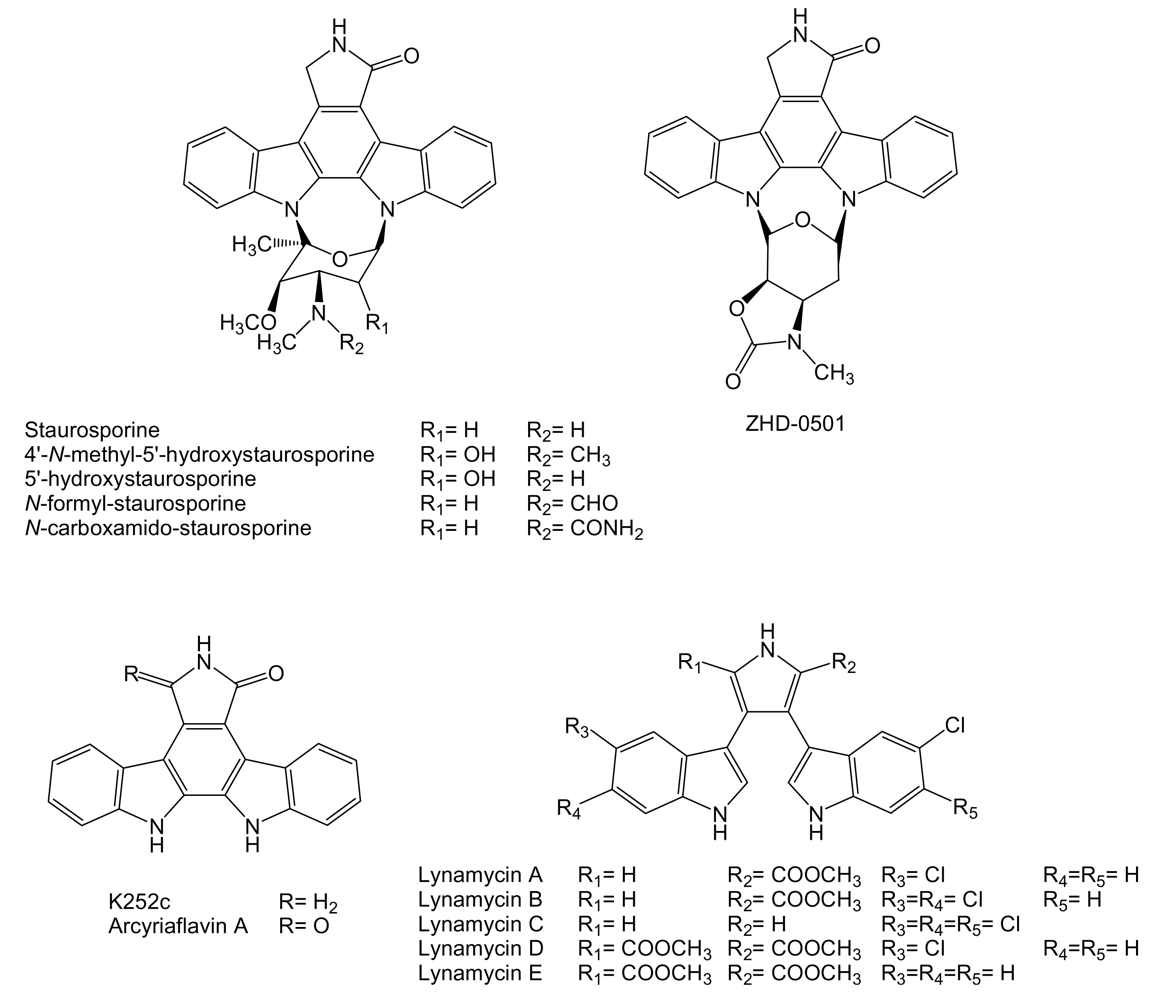

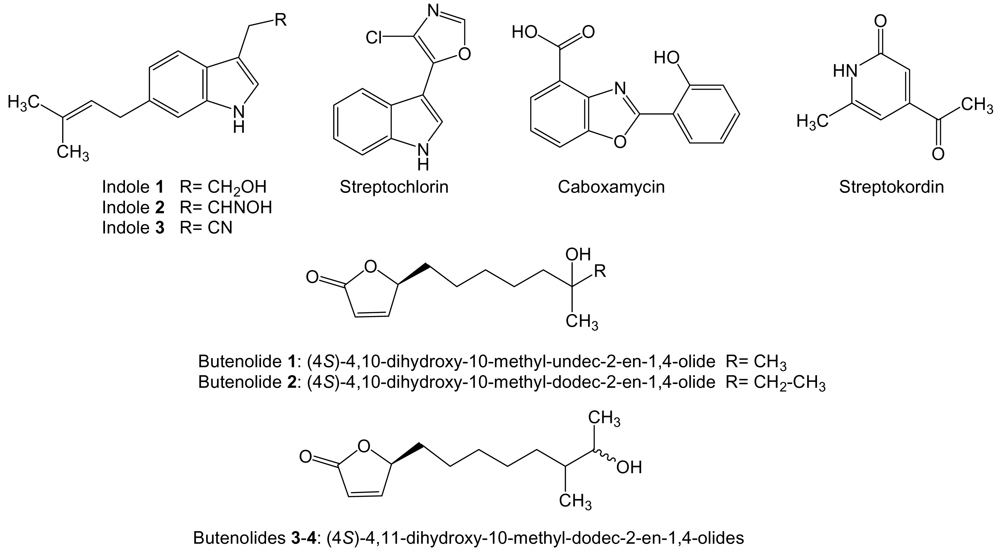
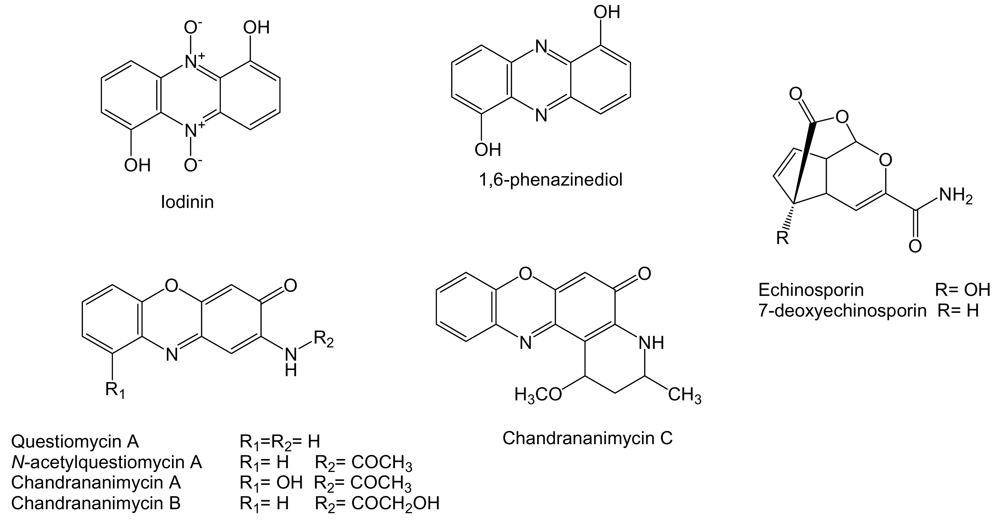
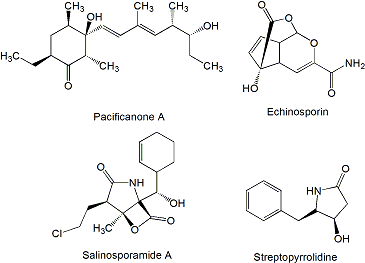
| Compound | Structural type | Organism | Ref. |
|---|---|---|---|
| 1-hydroxy-1-norresistomycin | Polyketide | Streptomyces chinaensis AUBN1/7 | 87 |
| Streptomyces sp. B8005 | 88 | ||
| 1,6-phenazinediol | Phenazine | Actinomadura sp. M048 | 116 |
| 1,8-dihydroxy-2-ethyl-3- methylanthraquinone | Polyketide | Streptomyces sp. FX-58 | 86 |
| 3,6-disubstituted indoles | Indole | Streptomyces sp. BL-49-58-005 | 167 |
| 4a,8a-dimethyl-6-(2-methyl- propenyloxy)- 3,4,4a,4b,5,6,8a,9-octahydro- 1H-phenanthren-2-one | Isoprenoid | Actinobacterium sp. MS1/7 | 143 |
| Actinofuranones | Polyketide | Streptomyces sp. CNQ766 | 65 |
| Altemicidin | Isoprenoid | Streptomyces sioyaensis SA-1758 | 135, 136 |
| Ammosamides | Pyrroloiminoquinone | Streptomyces sp. CNR-698 | 162 |
| Arcyriaflavin A | Indolocarbazole | Actinomycete sp. Z2039-2 | 153 |
| Arenamides | Non-ribosomal peptide | Salinispora arenicola CNT-088 | 117 |
| Arenicolides | Polyketide | Salinispora arenicola CNR-005 | 44 |
| Aureoverticillactam | Polyketide | Streptomyces aureoverticillatus NPS001583 | 53 |
| Bohemamines | Pyrrolizidine | Streptomyces sp. CNQ-583 | 163 |
| Butenolides | Butenolide | Streptoverticillium luteoverticillatum 11014 | 173 |
| Caboxamycin | Benzoxazole | Streptomyces sp. NTK 937 | 172 |
| Chalcomycin | Polyketide | Streptomyces sp. M491 | 49 |
| Chalcomycin B | Polyketide | Streptomyces sp. B7064 | 50 |
| Streptomyces sp. M491 | 49 | ||
| Chandrananimycins | Phenoxazin-3-one | Actinomadura sp. M048 | 179 |
| Chartreusin | Polyketide | Streptomyces sp. QD518 | 75 |
| Chinikomycins | Polyketide | Streptomyces sp. M045 | 57 |
| Chlorinated dihydroquinones | Isoprenoid | Actinomycete isolate CNQ-525 | 141 |
| Cyanosporasides | Polyketide | Salinispora pacifica CNS103 | 66 |
| Daryamides | Polyketide | Streptomyces sp. CNQ-085 | 58 |
| Echinosporins | Acetal-lactone | Streptomyces albogriseolus A2002 | 180 |
| Fridamycin D | Polyketide | Streptomyces sp. B6921 | 80 |
| Griseorhodin A | Polyketide | Streptomyces sp. JP95 | 100, 103 |
| Gutingimycin | Polyketide | Streptomyces sp. B8652 | 84 |
| Himalomycins | Polyketide | Streptomyces sp. B6921 | 80 |
| IB-0028 | Polyketide | Actinomadura sp. BL-42-PO13-046 | 98, 99 |
| IB-96212 | Polyketide | Micromonospora sp. L-25-ES25-008 | 47, 48 |
| Iodinin | Phenazine | Actinomadura sp. M048 | 179 |
| K252c | Indolocarbazole | Actinomycete strain Z2039-2 | 153 |
| Komodoquinones | Polyketide | Streptomyces sp. KS3 | 73, 74 |
| Lajollamycin | Polyketide/non-ribosomal peptide | Streptomyces nodosus NPS007994 | 132 |
| Lucentamycins | Non-ribosomal peptide | Nocardiopsis lucentensis CNR-712 | 110 |
| Lynamycins | Indolocarbazole | Marinispora sp. NPS12745 | 155 |
| Manumycin A | Polyketide | Streptomyces sp. M045 | 57 |
| Marinomycins | Polyketide | Marinispora sp. CNQ-140 | 54 |
| Marinones | Isoprenoid | Actinomycete isolate CNH-099 | 137–139 |
| Marineosins | Polypyrrole | Streptomyces sp. CNQ-617 | 156 |
| Marmycins | Polyketide | Streptomyces sp. CNH990 | 94 |
| Mechercharmycins | Non-ribosomal peptide | Thermoactinomyces sp. YM3-251 | 111 |
| Metacycloprodigiosin | Prodigiosin | Saccharopolyspora sp. nov. | 157 |
| Nonactin | Polyketide | Streptomyces sp. KORDI-3238 | 70 |
| Pacificanones | Polyketide | Salinispora pacifica CNS-237 | 63 |
| Parimycin | Polyketide | Streptomyces sp. B8652 | 82 |
| Piericidins | Polyketide | Streptomyces sp. YM14-060 | 67, 69 |
| Piperazimycins | Non-ribosomal peptide | Streptomyces sp. CNQ-593 | 120 |
| Proximicins | Non-ribosomal peptide | Verrucosispora sp. MG-37 | 107, 109 |
| Verrucosispora maris AB-18-032 | 108, 109 | ||
| Questiomycins | Phenoxazin-3-one | Actinomadura sp. M048 | 179 |
| Rabelomycin | Polyketide | Streptomyces sp. B6921 | 80 |
| Resitoflavine | Polyketide | Streptomyces chinaensis AUBN1/7 | 87, 93 |
| Streptomyces sp. B8005 | 88 | ||
| Resistomycin | Polyketide | Streptomyces sp. B8005 | 88 |
| Streptomyces sp. B4842 | 88 | ||
| Saliniketals | Polyketide | Salinispora arenicola CNR-005 | 45 |
| Salinipyrones | Polyketide | Salinispora pacifica CNS-237 | 63 |
| Salinosporamides | Polyketide/non-ribosomal peptide | Salinispora tropica CNB-392 | 64, 121, |
| Salinispora tropica CNB-440 | 126 | ||
| Salinispora tropica CNB-476 | 122, 131 | ||
| Salinispora tropica NPS000456 | 125, 131, 128 | ||
| Sporolides | Polyketide | Salinispora tropica CNB-392 | 64 |
| SS-228 Y | Polyketide | Chainia sp. SS-228 | 95, 97 |
| Staurosporins | Indolocarbazole | Streptomyces sp. KS3 | 74 |
| Micromonospora sp. L-31-CLCO-002 | 151 | ||
| Streptomyces sp. QD518 | 75 | ||
| Streptochlorin | Indole | Streptomyces sp. 04DH110 | 168–171 |
| Streptokordin | Methylpyridine | Streptomyces sp. KORDI-3238. | 70 |
| Streptopyrrolidine | Tetrahydropyrrole | Streptomyces sp. KORDI-3973 | 161 |
| Tetracenomycin D | Polyketide | Streptomyces sp. B8005 | 88 |
| Thiocoraline | Non-ribosomal peptide | Micromonospora sp. L-13-ACM2-092 | 112, 113 |
| T-Muurolol | Isoprenoid | Streptomyces sp. M491 | 49, 140 |
| Trioxacarcins | Polyketide | Streptomyces sp. isolate B8652 | 83 |
| Undecylprodigiosin | Prodigiosin | Saccharopolyspora sp. nov. | 157 |
| ZHD-0501 | Indolocarbazole | Actinomadura sp. 007 | 152 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Olano, C.; Méndez, C.; Salas, J.A. Antitumor Compounds from Marine Actinomycetes. Mar. Drugs 2009, 7, 210-248. https://doi.org/10.3390/md7020210
Olano C, Méndez C, Salas JA. Antitumor Compounds from Marine Actinomycetes. Marine Drugs. 2009; 7(2):210-248. https://doi.org/10.3390/md7020210
Chicago/Turabian StyleOlano, Carlos, Carmen Méndez, and José A. Salas. 2009. "Antitumor Compounds from Marine Actinomycetes" Marine Drugs 7, no. 2: 210-248. https://doi.org/10.3390/md7020210
APA StyleOlano, C., Méndez, C., & Salas, J. A. (2009). Antitumor Compounds from Marine Actinomycetes. Marine Drugs, 7(2), 210-248. https://doi.org/10.3390/md7020210