Abstract
The social amoeba Dictyostelium discoideum is a versatile biological model widely used in drug discovery and studying cellular stress responses. However, its application for cytotoxicity evaluation of natural products, particularly algal-derived compounds, remains underutilized. In this study, we developed a high-throughput developmental assay in D. discoideum to analyze the cytotoxicity of acetone and methanol extracts from the Peruvian seaweed Glossophora kunthii. Our results showed that the acetone extract caused a transient delay in the social development of the amoeba. In contrast, the methanol extract exhibited no significant effects, even at high extract concentrations. UHPLC/Orbitrap/ESI/MS/MS analysis tentatively identified ten major compounds, including pachydictyol A and dictyotriol A diacetate. The presence of diterpenes, such as dictyotadiol and pachydictyol A, previously reported to exhibit moderate cytotoxic activity, likely explains the developmental delay observed with the acetone extract. This study highlights the utility of D. discoideum as a scalable cytotoxicity screening platform within algal pharmacognosy, facilitating the early identification of non-toxic marine natural products suitable for further biomedical and biotechnological development.
1. Introduction
Many compounds derived from natural products, which are produced by plants, microorganisms, and marine organisms, have been used as medicines in their original or semi-synthetic form [1,2]. Chemical structures exhibiting remarkable structural diversity from marine organisms represent one of the main pathways in the search for bioactive compounds with pharmacological properties [3]. For instance, macroalgae are rich in bioactive compounds that could be exploited as functional ingredients for both human and animal health applications. Bioactive compounds such as sulfated polysaccharides, furanones, bromophenols, phlorotannins, and terpenoids have been widely investigated and have demonstrated significant antioxidant, antimicrobial, antiviral, and anticancer properties [4,5,6].
Glossophora kunthii, considered the taxonomic synonym of Dictyota kunthii [7], is a brown alga (Phaeophyceae) belonging to the family Dictyotaceae. It has a membranous thallus of yellowish-brown or olive-brown color, presenting a darker coloration at the basal region. Its morphology is characterized by dichotomous branching and rounded, generally bifid apices [8]. This alga is distributed in South America (Peru and Chile), Australia, New Zealand, and Southeast Asia [9]. The literature has reported the pharmaceutical and health potential of bioactive compounds present in brown algae [5] and the isolation of various compounds contained in them, including 8β,ll-dihydroxypachydictyol A, dictyoxide, pachydictyol A, dilophol, dictyodial [10,11], 9-epidictyol B [12], xenicane derivative [13], dictyotriol A diacetate [14], and crenulide diterpenes [4]. However, few studies have focused on the structural characterization and chemical profiling of these species. Research on other species in the family Dictyotaceae has identified active compounds with antimalarial, antitubercular [15] and anti-leishmaniasis [16] properties, among others, suggesting promising biotechnological potential for Glossophora kunthii. Brown algae, in general, are highly valued for their nutraceutical properties and their commercial use in producing bioactive molecules, as well as in the extraction of alginates, carrageenans, and agar meant for its use in the food industry and other sectors [17,18]. Nonetheless, comprehensive studies integrating chemical profiling with functional biological evaluation remain scarce for South American brown algae, including G. kunthii.
Cytotoxicity evaluation is a crucial step in the discovery and validation of active compounds, and it is typically performed using mammalian cell lines. However, these systems present several disadvantages, including high maintenance costs and a significant risk of culture contamination, limiting their applicability in large-scale analyses [19]. For this reason, in this study, we propose the use of the amoeba Dictyostelium discoideum for cytotoxicity assays due to its importance for drug evaluation in humans, since this model shares several cellular processes and adjacent homologous genes, with the upper eukaryotic cell [20]. Through studies of social amoeba, Dictyostelium discoideum has been widely used as a model organism in cell and developmental biology, providing valuable insight into processes such as cell differentiation, chemotaxis, and signaling pathways [21]. Moreover, this amoeba has also been employed in pharmacological trials, where drugs that affect mammalian cells have shown similar effects in this model, although sometimes at higher concentrations [22].
Therefore, this study proposes the social amoeba D. discoideum as both an alternative model and a high-performance platform to perform a cytotoxicity screening of acetonic and methanolic extracts from Peruvian seaweed G. kunthii. In addition, the comparison and identification of the chemical profiling of these extracts were performed based on UHPLC/Orbitrap/ESI/MS/MS.
2. Results
2.1. Properties of Extracts from the Alga Glossophora kunthii
The extracts from G. kunthii (500 g; Figure 1) were obtained using acetone and methanol as solvents through sequential maceration, at room temperature (72 h each). When required, the process was supplemented with direct sonication and centrifugation. This approach yielded 13 g of the acetone extract and 22.5 g of the methanol extraction. Non-polar extracts were not prepared because they primarily contain lipophilic compounds, such as fats and pigments. In this study, we report the isolation and identification of two diterpenoids, known as pachydictyol A [11] and dictyotriol A diacetate, with 1H-NMR data identical to those previously reported [14].

Figure 1.
Glossophora kunthii.
Concerning cytotoxicity studies, pachydictyol A has shown some anticancer activities [23], moderate cytotoxic activity on different cell lines (HepG2, WI/38,VERO, MCF-7), and moderate antitumor activity through Ehrlich in vitro assay alongside weak to moderate antioxidant activity for ABTS [24]. It has also presented potent antithrombotic [25] and antiplatelet and anticoagulant activities [26]. Regarding the terpenoid dictyotriol A diacetate, it was isolated from Dictyota binghamiae [14] for the first time. There is no literature describing any biological tests for dictyotriol A diacetate.
2.2. Social Development Test of Amoeba D. discoideum for Cytotoxicity Assessment
We evaluated the cytotoxic activity of algal extracts by analyzing the social development cycle of the amoeba model Dictyostelium discoideum (Figure 2), following protocols previously established in our laboratory [27]. This assay was based on the principle that secondary metabolites, with cytotoxic activity present in the extracts, can inhibit or delay the social development of D. discoideum. In contrast, non-cytotoxic compounds allow for the normal progression and completion of their social cycle.
Figure 2.
Social development of the amoeba Dictyostelium discoideum.
This study evaluated and compared two extracts (acetone and methanol) from the seaweed G. kunthii. The social development of the amoebae was monitored over six days, using three concentrations (25, 50, and 100 µg/mL) of each extract. Under normal conditions, D. discoideum completed its social cycle in approximately 48 h [28].
As shown in Figure 3, the acetone extract affected the social development of the D. discoideum in a concentration-dependent manner. At all tested concentrations, a significant delay (**** p < 0.0001) in the progression of the social cycle was observed, particularly during the first two days, when treated amoebae remained in the early aggregation and phagocytic plaque formation stages, while the control group had already reached the culmination phase. However, by the end of the six-day experimental period, all treated amoebae successfully completed their social cycle, indicating that the effect of the extract was transient and primarily associated with the initial stages of multicellular development.
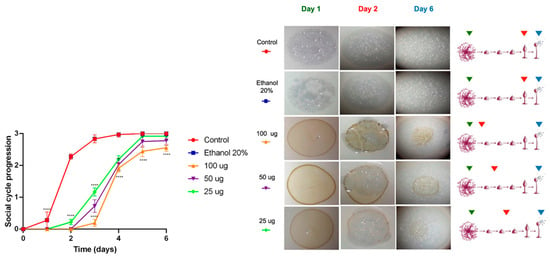
Figure 3.
Cytotoxicity evaluation of the acetonic extract of Ghossophora kunthii. Statistical analysis was performed using a two-way ANOVA test with multiple comparisons and Dunnet’s post-test (n = 6) (* = p < 0.05, ** = p < 0.005, *** = p < 0.001, **** = p < 0.0001).
In contrast, the methanolic extracts did not show significant effects on the social development of D. discoideum. Phagocytic plaques formed on the first day, similarly to the control group, and only a slight delay was observed at the highest concentration (100 µg/mL) during the first 48 h (**** p < 0.0001), as shown in Figure 4. From the third day onward, amoebae treated with all concentrations reached developmental stages comparable to the control, completing the social cycle within the six-day experimental period. Overall, both extracts did not exhibit substantial cytotoxicity; however, the acetone extract caused a moderate delay during the first two days compared with the methanolic extract, which showed a faster recovery in development. These differences were statistically significant only at higher concentration (100 µg/mL), indicating a transient and dose-dependent effect.
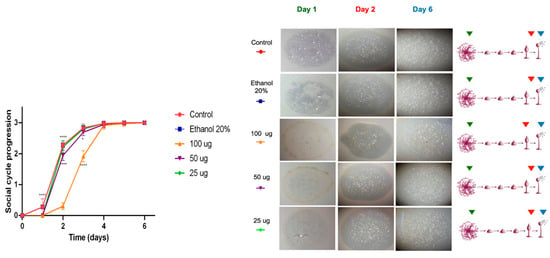
Figure 4.
Cytotoxicity test performed by monitoring the social development of D. Discoideum. Cytotoxicity evaluation of the methanolic extract of Ghossophora kunthii. Statistical analysis was performed using a two-way ANOVA test with Dunnet’s post-test for multiple comparisons (n = 6) (* = p < 0.05, ** = p < 0.005, *** = p < 0.001, **** = p < 0.0001).
Mass spectrometry analysis of the acetone extract from G. kunthii identified two diterpenes, pachydictyol A (peak 8) and dictyotriol A diacetate (peak 9), both previously reported to have moderate cytotoxic activity [29,30]. The absence of these compounds in the methanolic extract may account for the lack of significant effects on the social development of the amoebae, suggesting that dictyotriol A diacetate and pachydictyol A are likely responsible for the slight delay observed.
The Dictyostelium discoideum model has been shown to be a valuable system for the assessment of the cytotoxic potential of natural extracts. For example, Hernández et al. (2025) [27] employed this model to evaluate dichloromethane and methanolic extracts from various parts of Helenium aromaticum. In that study, the methanolic extracts did not exhibit cytotoxicity and allowed the amoebae to develop normally, while the dichloromethane extracts caused developmental delays in a concentration-dependent manner. Additionally, toxicogenomic studies have reinforced the relevance of D. discoideum in linking toxic effects, both teratogenic and non-teratogenic, to specific genetic responses [31].
2.3. Identification of the Compounds by UHPLC/Orbitrap/ESI/MS/MS of the Extracts of the Peruvian Seaweed G. kunthii
To compare and identify the secondary metabolites present in the methanol and acetone extracts of G. kunthii, a combination of diode array detection and high-resolution tandem mass spectrometry (UHPLC/Orbitrap/ESI-MS/MS) was performed. This was performed in positive mode, resulting in the tentative identification of ten compounds, as shown in both Table 1 and Figure S5.

Table 1.
Tentative identification of compounds contained in the methanol and acetone extracts from Ghossophora kunthii algae by UHPLC/Orbitrap/ESI/MS/MS.
Diterpenes
The results of the determination of the presence of terpenoids in the acetonic and methanolic extracts revealed a total of 10 compounds, belonging to prenylated-guaiane diterpenes with the perhydroazulene skeleton [32]. They were tentatively identified as pachydictyol A (peak 8, Figure S3) [33], dictyol C (peak 7) [33], hydroxypachydictyol A (peak 6) [34], dictyotriol A diacetate (peak 9, Figure S4) and 4β-acetoxydictyodial A (peak 10) [35]. The biosynthetic proposal [36] of these structures can be seen in Figure 5. Some possible precursors of diterpenes were tentatively identified as patchouliguaiol D (peak 2), showing the molecular ion [MH]+ at m/z 219.1760, two diagnostic ions at m/z 203.1442 [MH-C14H19O]+, and m/z 177.1274 [MH-C12H17O]+ [37]. Peak 5 was tentatively identified as hydroxyguaiane sucrose, while peak 1 remained unknown.
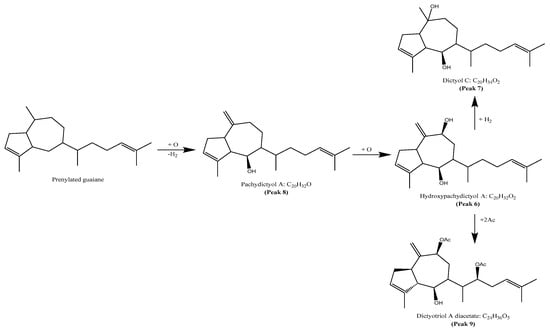
Figure 5.
Proposed biosynthetic of terpenes from Glossophora kunthii.
Unfortunately, the amount of extract obtained from the algal material was insufficient to allow the isolation and characterization of all these compounds; only two major compounds were isolated and characterized. Future work will be performed toward the isolation and structural elucidation of the remaining compounds.
3. Discussion
Marine algae have attracted growing scientific and commercial interest due to their exceptional metabolic capacity to synthesize a broad spectrum of bioactive compounds with diverse biotechnological applications. These metabolites include essential minerals, structural and storage carbohydrates, proteins, polyunsaturated fatty acids, amides, amines, antioxidants, photosynthetic pigments, and an ample repertoire of secondary metabolites with ecological and pharmacological relevance [38,39,40,41,42,43]. Marine macroalgae have emerged as a valuable reservoir for pharmacognosy, which is the study of natural products derived from biological sources with medicinal potential. This places macroalgae at the forefront of marine biotechnology and drug discovery.
Among these ones, brown algae of the genus Dictyota stand out due to their widespread distribution in tropical and subtropical marine ecosystems. These habitats are characterized by intense ecological interactions and selective pressures. Such environments have likely driven the evolution of complex chemical defense systems in Dictyota species, leading to the production of diverse and structurally intricate secondary metabolites, notably diterpenes. These compounds are known to serve multiple ecological functions: deterring herbivory, preventing microbial colonization, and potentially acting as infochemicals that modulate interactions between symbionts and specialized grazers [44,45].
Interestingly, some primary consumers may have evolved resistance to these deterrents and, in turn, exploit the same compounds for their defense, suggesting a sophisticated form of chemical mediation within the food web.
Diterpenes derived from Dictyota are also taxonomically informative, contributing to the chemotaxonomic resolution within the Dictyotaceae family. In this context, the detection of pachydictyol A and dictyotriol A diacetate in Glossophora kunthii aligns with prior reports from related taxa [11,14], reinforcing the ecological ubiquity and evolutionary conservation of these metabolites. Their identification not only supports the placement of G. kunthii within Dictyotaceae but also highlights the functional consistency of these compounds across phylogenetically related lineages.
Structurally, Dictyota-derived diterpenes are typically classified according to the cyclization pattern of their common precursor, geranylgeraniol. The diterpenoids identified in our study, featuring sesquiterpene like ring architectures belong to the prenylated guaiane type class [34,46]. These structures often include core motifs such as the perhydroazulene skeleton and hydroxyl substitutions, which have been linked to pronounced cytotoxic, antimicrobial, and antiviral activities. Minor modifications in stereochemistry, oxidation state, or side chain functionalization can drastically alter the biological properties of these compounds, suggesting that even closely related analogs may exhibit divergent pharmacological profiles. This diversity underscores the relevance of Dictyota diterpenes within the scope of marine pharmacognosy, where structure-activity relationships are central to identify new therapeutic leads. Furthermore, our comparison of acetone and methanol extracts reveals the pivotal role of solvent polarity in modulating metabolite recovery. The differential chemical profiles recovered under these conditions underscore the importance of the extraction strategy in natural product discovery workflows.
The action mechanism of structurally related compounds, including pachydictyol C, dictyol E, and 3,4-epoxy-7,18 delabelladiene, isolated from Dictyota spiralis, has been previously investigated. These studies demonstrated that, upon exposure to parasitic cells of Leishmania amazonensis and Trypanosoma cruzi, the mentioned compounds induce a reduction in mitochondrial membrane potential and intracellular ATP levels, chromatin condensation, accumulation of reactive oxygen species (ROS), maintenance of plasma membrane permeability, and marked morphological alterations. Collectively, these observations suggest a cell death mechanism consistent with apoptosis [47]. Moreover, other dictyol-type diterpenes have been reported to inhibit nitric oxide production, suppress inducible nitric oxide synthase (iNOS)m RNA expression, interleukin-6 (IL-6) and cyclooxygenase-2 (COX-2) expression in RAW264 cell [48]. Based on these findings, we propose that the isolated compounds may exert their biological activity through a similar mechanism; however, the present study has not yet elucidated their precise action mode.
Our work highlights the utility of Dictyostelium discoideum as a relevant and responsive eukaryotic model for preliminary cytotoxicity screening [22,27]. While this social amoeba does not replicate all aspects of mammalian cell physiology, it offers significant advantages, including genetic tractability, rapid growth, and cost efficiency. Recognized by the National Institutes of Health as a non-mammalian model organism in biomedical research [49]. D. discoideum enables early-stage toxicity assessments in a living system. This facilitates the prioritization of candidate compounds for further in vitro and in vivo validation. The observed cytotoxic responses in our assay further support its potential for preclinical screening of marine-derived secondary metabolites. The use of D. discoideum as a model organism to assess the toxicity of natural secondary metabolites has been previously described in pharmacological and toxicological studies [27].
Taken together, our findings contribute to the growing body of evidence demonstrating the chemodiversity and bioactivity of Dictyotaceae-derived diterpenes and advocate for the integration of marine chemical ecology, pharmacognosy, and alternative model systems in early-stage drug discovery pipelines.
4. Materials and Methods
4.1. Preparation of the Seaweed Extracts of Ghossophora kunthii
4.1.1. Seaweed Collection
Ghossophora kunthii (C.Agardh) J.Agardh 1882, was collected in December 2017 by Elizabeth Figueroa-Valencia in the Ballenitas and Catarindo, located in the Islay, Arequipa Region, Perú (Figure 1). The sample was deposited with the voucher (N° 0172017) at The Herbario Sur Peruano (HSP)—Michael Owen Dillon Scientific Institute (IMOD), Arequipa, Peru.
4.1.2. Preparation of Algal Extracts
After the cleaning and selection, the seaweeds were dried in an oven at 37 °C. The sample was ground to obtain 500 g of material, and it was subsequently macerated at room temperature for 72 h, this procedure was repeated 3 times. The extraction solvents were filtered and concentrated to dryness by a rotary evaporator at 40 °C.
TLC (Kieselgel 60 GF254, Merck, Santiago, Chile) was developed primarily on n-hexane/EtOAc mixtures, and spots were revealed by spraying plates with anisaldehyde/sulfuric acid and heating at 105 °C. Silica gel (Kieselgel 60, Merck, Santiago, Chile, 0.063–0.200 mm) and Sephadex (LH-20, Sigma Aldrich, Santiago, Chile) were used in column chromatography (CC). Chromatotron model 4924 T was used for the isolation of terpenoids. The rotor was coated with a mixture of aluminum oxide 60 GF-254 and calcium sulfate hemi-hemihydrate in thin-layer chromatography; the layer thickness was 2 mm. Technical solvents used in chromatography processes were previously distilled and dried according to standard procedures.
4.2. Structure Analysis and Characterization of Isolated Compounds
The compounds were isolated from acetone extract obtained by maceration; the crude extract obtained after solvent evaporation was fractionated by flash column chromatography (SiO2). Each fraction was subjected to repeated permeation with Sephadex LH-20 under TLC monitoring. Finally, diterpene mixtures were separated by centrifugal Thing-Layer Chromatography (Chromatotron). The isolated compounds were dissolved in CDCl3 for spectroscopic analysis using Bruker Advance (400 MHz for 1H). TMS was used as an internal standard.
Compound 1 was obtained as a yellow viscous substance. HR-ESI/MS: m/z 289.2578 ([M + H]+, calc. for C20H33O 289.2531). 1H-NMR (CDCl3, 400 MHz): δ 2.67 (q, 1H, J = 10 Hz), 2.51 (m, 1H), 5.33 (br s, 1H), 2.28 (m, 1H), 3.92 (br d, 1H, J = 8 Hz), 1.55 (m), 1.51 (m), 2.63 (m, 1H), 1.25 (m), 2.25 (m), 2.04 m; 1.93 m, 5.09 (br t, 1H, J = 6 Hz), 1.68 (br s, 3H), 1.81 (m, 3H), 4.74 (br s, 1H), 4.72 (s, 1H), 0.96 (d, 3H, J = 7 Hz), 1.65 (br s, 3H). Proton chemical shifts suggest that compound 1 could correspond to an azulene-type diterpene, pachydictyol A, with the molecular formula C20H32O. This compound has previously been reported in several species of the genus Dictyota, such as D. dichotoma and D. menstrualis. The identification was confirmed by comparing the experimental data with those reported in the literature by Abou-El-Wafa et al. (2013) [50] (Figures S1 and S3).
Compound 2 was obtained as a white solid. HR-ESI/MS: m/z 403.2467 ([M + H]+, calc. for C24H35O5 403.2484). The 1H-NMR (CDCl3, 400 MHz) spectrum showed δ =2.79 (q, 1H, J = 10 Hz), 2.95 (br s, 1H), 2.49 (m, 1H), 5.34 (br s, 1H), 2.33 (m, 4H), 3.87 (dd, 1H, J = 3, 8 Hz) 3.04, 1H, 2.02 (dd, 1H, J = 3, 10 Hz), 1.92 (ddd, 1H, J = 2, 10, 15 Hz), 1.76 (dd, 1H, J = 6, 15 Hz), δ: 5.59 (dd, 1H, J = 2, 6 Hz), 1.86 (m, 1H), 4.92 (m, 1H), 5.10 (t, 1H, J = 7 Hz), 1.64 (br s, 3H), 1.83 (m, 3H),5.10 (s, 1H), 5.06 (s, 1H), 0.94 (d, 3H, J = 7 Hz), 1.71 (br s, 3H), 2.07 (s, 3H), 2.06 (s, 3H). Compound 2 was identified as the diterpene dictyotriol A diacetate by comparing its NMR data with those reported for Dictyota binghamiae by Pathirana et al. (1984) [14] (Figures S2 and S4). Compound 1 and 2 are shown in Figure 6.
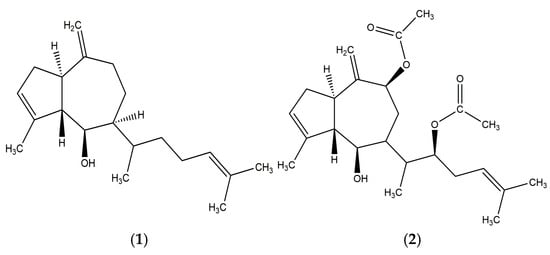
Figure 6.
Structures of the two major compounds isolated from the acetone extract of Glossophora kunthii: Pachydictyol A (1) and Dictyotriol A diacetate (2).
4.3. Identification by Using UHPLC Q/Orbitrap/ESI/MS/MS
The compounds present in the seaweed G. kunthii extracts were tentatively determined using UHPLC Q/Orbitrap/ESI/MS/MS in positive mode. These experiments were based on the previously tests reported by Salgado et al. (2017) [51]. The extracts (1 mg) were prepared with HPLC methanol and then injected into the UHPLC equipment.
The UHPLC system used was a Thermo Scientific Dionex Ultimate 3000 with quaternary RS pump and column oven as part of Thermo Scientific Dionex Ultimate 3000 Series TCC-3000RS, equipped with an autosampler and a high-speed PDA detector using Chromeleon 7.2 software: Thermo Fisher Scientific, Waltham, MA, USA and Dionex Softron GmbH, Bremen, Germany. Analytical chromatography was performed on a UHPLC C18 column (150 mm × 4.6 mm ID, Thermo Fisher Scientific, Bremen, Germany) at a temperature of 25 °C. Four UV detection systems at 254, 280, 320, and 440 nm were used, together with the PDA detector in the 180–800 nm range. The mobile phases were a 1% formic acid solution in water (A) and acetonitrile (B). The elution gradient (time (min), % B) was as follows: (0.00, 5); (5.00, 5); (10.00, 30); (15.00, 30); (20.00, 70); (25.00, 70); (35.00, 5), with 12 min allowed for column equilibration The flow rate was 1.00 mL/min, and the injection volume was 10 μL. The HESI II and Orbitrap focus spectrometer parameters were optimized, as previously reported by Garneau et al. (2013) and Simirgiotis et al. (2016) [52,53]. The hybrid instrument was equipped with quadrupole and orbitrap, with a resolution of 70.000 at m/z 200) and HCD (high-resolution) cell, using independent variable data acquisition (vDIA) for untargeted analysis. This allowed for the identification of unknown compounds. Daughter ions were produced in the HCD cell at a collision energy of 5 eV, resulting in high sensitivity and accurate mass measurements, with mass errors ranging between 0.1 and 10 ppm. All ions were formed after the heated electrospray probe (HESI II), which are detected in the orbitrap and fragmented in the HCD cell. After detection, the neutral losses were analyzed using the base peak and full TIC spectra with daughter MS2 ions through Thermo Xcalibur 3.1 software.
4.4. Dictyostelium Discoideum Strain and Culture Conditions
The strain D. discoideum used in this work corresponds to the axenic strain AX4 (DBS0302402) obtained from the Dicty Stock Center (Figure 2) [54]. It was grown according to the protocols described by [28]. Briefly, the amoeba D. discoideum was maintained at 23 °C in SM agar (10 g/L glucose, 10 g/L peptone, 1 g/L yeast extract, 1 g/L MgSO4* 7H2O, 1.9 g/L of KH2PO4, 0.6 g/L of K2HPO4, 20 g/L of Bacto agar, pH 6.5) containing a lawn of K. aerogenes DBS0305928 (previously grown in LB; 37 °C, 180 rpm, 18 h). Before the tests, amoebas were grown at 23 °C with stirring (180 rpm) in HL5 medium (14 g/L tryptone, 7 g/L yeast extract, 0.35 g/L NaHPO4, 1.2 g/L of KH2PO4 and 14 g/L of glucose, pH 6.3) in the absence of bacteria (axenic culture) and supplemented with ampicillin (Amp) 100 µg/mL and streptomycin (Stp) 300 µg/mL.
The amoebas were harvested in the early exponential phase (1–2 × 106 cells/mL). Viable amoeba cells were determined with Trypan Gibco® blue (Thermo Fisher Scientific) and counted in the Neubauer chamber. Subcultures (S3 to S5) of the amoeba D. discoideum in the exponential phase were used for cytotoxicity tests of Ghossophora kunthii algal extracts.
4.5. Social Development Test of the Amoeba Dictyostelium discoideum for Cytotoxic Evaluation of Algal Extracts
SM agar plates and a lawn of K. aerogenes were used to perform social development assays of D. discoideum. To assess the cytotoxicity of extracts, 25, 50, and 100 μg doses were tested, following protocols similar to those used in cell line assays [55,56]. A 20% ethanol solution served as the negative control. Ten thousand amoebas in the exponential phase were inoculated [57], and the plates were incubated at 23 °C. The developmental cycle of D. discoideum was monitored daily for 6 days, focusing on the aggregation, culmination, and final culmination stages [58]. Scores from 1 to 3 were assigned based on the stage reached [57]. Data were recorded every 24 h and analyzed using GraphPad Prism 6. This assay effectively detects the cytotoxic activity of compounds by evaluating their impact on the viability and cellular differentiation of D. discoideum.
5. Conclusions
This study introduces a practical and cost effective platform for early cytotoxicity screening of marine algal extracts using the social development assay of Dictyostelium discoideum. By leveraging the organism’s sensitivity to chemical perturbations, this approach enables the rapid evaluation of bioactivity under physiologically relevant conditions. The assay proved to be suitable for detecting differential effects among solvent extracts, highlighting its potential to distinguish between cytotoxic and non-cytotoxic samples in a high-throughput format.
Moreover, the chemical characterization of Glossophora kunthii extracts led to the successful isolation of two diterpenes, pachydictyol A and dictyotriol A diacetate, alongside the tentative identification of additional metabolites through high-resolution mass spectrometry. These findings underscore the pharmacognostic value of Dictyotaceae species and support the integration of ecological screening models with advanced analytical chemistry to accelerate the discovery of marine-derived bioactive compounds.
Altogether, this work contributes a robust methodological framework to marine natural product research and reinforces the utility of non-mammalian systems in the early stages of drug development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md23110442/s1, Figure S1: 1H-NMR spectrum of compound 1 (CDCl3, 400 MHz); Figure S2: 1H-NMR spectrum of compound 2 (CDCl3, 400 MHz); Figure S3: Mass spectra of pachydictyol A (compound 1); Figure S4: Mass spectra of dictyotriol A diacetate (compound 2); Figure S5: UHPLC-Q/Orbitrap/ESI/MS/MS chromatograms of the acetonic (green) and methanolic (red) extracts of the alga Glossophora kunthii.
Author Contributions
Conceptualization, S.J.F.-V., M.H., F.P.C. and C.A.; Methodology, S.J.F.-V., M.H., G.C., I.P., A.A., E.F.-V. and T.C.d.T.; Investigation, S.J.F.-V. and M.H.; Data curation, S.J.F.-V., M.H. and G.C.; Writing—original draft, S.J.F.-V. and M.H.; Writing—review and editing, G.C., E.F.-V., F.P.C. and C.A.; Formal analysis, I.P., A.A., F.P.C. and C.A.; Reviewed draft, A.A. and E.F.-V.; Supervision, T.C.d.T., F.P.C. and C.A.; Resources, T.C.d.T. and E.F.-V.; Sourced the funding, F.P.C. and C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Universidad Nacional de San Agustin de Arequipa-UNSA (grant contract number 0160-2016-UNSA).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study will be made available on request from corresponding author.
Acknowledgments
Carlos Areche acknowledges ANID (Fondecyt Regular 1230414).
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the correspondence contact information. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| UHPLC | Ultra-High Performance Liquid Chromatography |
| ESI | Electrospray Ionization |
| MS | Mass Spectrometry |
| NMR | Nuclear Magnetic Resonance |
| HepG2 | Human Hepatocellular Carcinoma Cell Line |
| WI/38 | Human diploid lung fibroblast cell line |
| VERO | African green monkey kidney epithelial cell line |
| MCF-7 | Michigan Cancer Foundation-7 |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| ANOVA | Analysis of Variance |
| TLC | Thin Layer Chromatography |
| LH-20 | Lipophilic Hydroxypropylated (pore size 20) |
| CC | Column Chromatography |
| TCC | Thermostatted Column Compartment |
| RS | Rapid Separation |
| PDA | Photodiode Array Detector |
| UV | Ultraviolet |
| HESI II | Heated Electrospray Ionization (version II) |
| HCD | Higher-energy Collisional Dissociation |
| vDIA | Variable Data—Independent Acquisition |
| TIC | Total Ion Chromatogram |
| TMS | Tetramethylsilane |
| SM | Standard Medium |
| RAW264 | Murine macrophage cell line |
References
- Das, K.; Tiwari, R.K.S.; Shrivastava, D.K. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J. Med. Plants Res. 2010, 4, 104–111. [Google Scholar]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef]
- Cappello, E.; Nieri, P. From Life in the Sea to the Clinic: The Marine Drugs Approved and under Clinical Trial. Life 2021, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Arrieche, D.; Carrasco, H.; Olea, A.F.; Espinoza, L.; San-Martín, A.; Taborga, L. Secondary Metabolites Isolated from Chilean Marine Algae: A Review. Mar. Drugs 2022, 20, 337. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef]
- Bogaert, K.A.; Delva, S.; De Clerck, O. Concise review of the genus Dictyota J.V. Lamouroux. J. Appl. Phycol. 2020, 32, 1521–1543. [Google Scholar] [CrossRef]
- Tapia, L. Biodiversity Guide N 4. Vol. I Macrofauna and Marine Algae; Universidad de Antofagasta: Antofagasta, Chile, 2002; Volume 1, p. 17. [Google Scholar]
- Guiry, M.D. Dictyota kunthii (C.Agardh) Greville: AlgaeBase. Available online: https://www.algaebase.org/search/species/detail/?species_id=20205 (accessed on 12 October 2024).
- Nys, R.D.; Wright, A.D.; König, G.M.; Sticher, O. A diterpene from the marine alga Glossophora kunthii. Phytochemistry 1993, 32, 463–465. [Google Scholar] [CrossRef]
- Rivera, A.P.; Astudillo, L.A.; Gonzalez, A.G.; Manta, E.; Cataldo, F. Two new bicyclic diterpenoids from the brown alga Glossophora kuntii. J. Nat. Prod. 1987, 50, 965–967. [Google Scholar] [CrossRef]
- Arroyo, P.; Norte, M.; Vázquez, J.T.; Nakanishi, K. Absolute Configuration of Hydroazulenoid Diterpenes Based on Circular Dichroism. J. Org. Chem. 1991, 56, 2671–2675. [Google Scholar] [CrossRef]
- Norte, M.; González, A.G.; Arroyo, P.; Zárraga, M.; Pérez, C.; Rodriguez, M.L.; Ruiz-Perez, C.; Dorta, L. New xenicane diterpenes from the brown algae of dictyotaceae. Tetrahedron 1990, 46, 6125–6132. [Google Scholar] [CrossRef]
- Pathirana, C.; Andersen, R.J. Diterpenoids from the brown alga Dictyota binghamiae. Can. J. Chem. 1984, 62, 1666–1671. [Google Scholar] [CrossRef]
- Jongaramruong, J.; Kongkam, N. Novel diterpenes with cytotoxic, anti-malarial and anti-tuberculosis activities from a brown alga Dictyota sp. J. Asian Nat. Prod. Res. 2007, 9, 743–751. [Google Scholar] [CrossRef]
- Lira, M.L.F.; Lopes, R.; Gomes, A.P.; Barcellos, G.; Verícimo, M.; Osako, K.; Ortiz-Ramirez, F.A.; Ramos, C.J.B.; Cavalcanti, D.N.; Teixeira, V.L.; et al. Anti-leishmanial activity of Brazilian green, brown, and red algae. J. Appl. Phycol. 2016, 28, 591–598. [Google Scholar] [CrossRef]
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.R.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal proteins: Production strategies and nutritional and functional properties. Bioresour. Technol. 2021, 332, 125125. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D. Brown algae carbohydrates: Structures, pharmaceutical properties, and research challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef]
- Castaño, M.E.; Zapata, J.C. Cell Cultures; In Virol Principles; Biogenesis; Fondo Editorial Biogenesis, Universidad de Antioquia: Medellín, Colombia, 2000; pp. 49–64. [Google Scholar]
- Williams, J.G.; Noegel, A.A.; Eichinger, L. Manifestations of multicellularity: Dictyostelium reports in. Trends Genet. 2005, 21, 392–398. [Google Scholar] [CrossRef]
- Mathavarajah, S.; Flores, A.; Huber, R.J. Dictyostelium discoideum: A model system for cell and developmental biology. Curr. Protoc. Essent. Lab. Tech. 2017, 15, 14.1.1–14.1.19. [Google Scholar] [CrossRef]
- Bravo-Toncio, C.; Álvarez, J.A.; Campos, F.; Ortíz-Severín, J.; Varas, M.; Cabrera, R.; Lagos, C.F.; Chávez, F.P. Dictyostelium discoideum as a surrogate host-microbe model for antivirulence screening in Pseudomonas aeruginosa PAO1. Int. J. Antimicrob. Agents 2016, 47, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Attia, E.Z.; Saber, H.; Saber, A.A.; Bringmann, G.; Abdelmohsen, U.R. The Biodiversity of the Genus Dictyota: Phytochemical and Pharmacological Natural Products Prospectives. Molecules 2022, 27, 672. [Google Scholar] [CrossRef]
- Ayyad, S.E.N.; Makki, M.S.; Al-Kayal, N.S.; Basaif, S.A.; El-Foty, K.O.; Asiri, A.M.; Alarif, W.M.; Badria, F.A. Cytotoxic and protective DNA damage of three new diterpenoids from the brown alga Dictoyota dichotoma. Eur. J. Med. Chem. 2011, 46, 175–182. [Google Scholar] [CrossRef]
- Pereira, R.C.C.; Lourenço, A.L.; Terra, L.; Abreu, P.A.; Teixeira, V.L.; Castro, H.C. Marine Diterpenes: Molecular Modeling of Thrombin Inhibitors with Potential Biotechnological Application as an Antithrombotic. Mar. Drugs 2017, 15, 79. [Google Scholar] [CrossRef]
- De Andrade Moura, L.; Marqui de Almeida, A.C.; Domingos, T.F.S.; Ortiz-Ramirez, F.; Cavalcanti, D.N.; Teixeira, V.L.; Fuly, A.L. Antiplatelet and Anticoagulant Effects of Diterpenes Isolated from the Marine Alga, Dictyota menstrualis. Mar. Drugs 2014, 12, 2471–2484. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Areche, C.; Castañeta, G.; Rojas, D.; Varas, M.A.; Marcoleta, A.E.; Chávez, F.P. Dictyostelium discoideum-assisted pharmacognosy of plant resources for discovering antivirulence molecules targeting Klebsiella pneumoniae. Nat. Prod. Res. 2025, 39, 5179–5186. [Google Scholar] [CrossRef]
- Fey, P.; Kowal, A.S.; Gaudet, P.; Pilcher, K.E.; Chisholm, R.L. Protocols for growth and development of Dictyostelium discoideum. Nat. Protoc. 2007, 2, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Ayyad, S.E.N.; Abdel-Halim, O.B.; Shier, W.T.; Hoye, T.R. Cytotoxic hydroazulene diterpenes from the brown alga Cystoseira myrica. Z. fur Naturforsch-Sect. C J. Biosci. 2003, 58, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Gedara, S.R.; Abdel-Halim, O.B.; El-Sharkawy, S.H.; Salama, O.M.; Shier, T.W.; Halim, A.F. Cytotoxic hydroazulene diterpenes from the brown alga Dictyota dichotoma. Z. fur Naturforsch-Sect. C J. Biosci. 2003, 58, 17–22. [Google Scholar] [CrossRef]
- Baines, R.P.; Wolton, K.; Thompson, C.R.L. Dictyostelium discoideum: An Alternative Nonanimal Model for Developmental Toxicity Testing. Toxicol. Sci. 2021, 183, 302–318. [Google Scholar] [CrossRef]
- Xia, X.; Li, B.; Hou, Y.; Zhang, J.; Yan, X. Diterpenes From the Marine Brown Algae of the Genus Dilophus. Nat. Prod. Commun. 2020, 15, 14. [Google Scholar] [CrossRef]
- Siless, G.E.; García, M.; Pérez, M.; Blustein, G.; Palermo, J.A. Large-scale purification of pachydictyol A from the brown alga Dictyota dichotoma obtained from algal wash and evaluation of its antifouling activity against the freshwater mollusk Limnoperna fortunei. J. Appl. Phycol. 2018, 30, 629–636. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Zhao, Z.; Xia, X.; Li, B.; Zhang, J.; Yan, X. Diterpenes from the Marine Algae of the Genus Dictyota. Mar. Drugs 2018, 16, 159. [Google Scholar] [CrossRef] [PubMed]
- De-Paula, J.C.; Lopes-Filho, E.A.P.; Salgueiro, F.; Yoneshigue-Valentin, Y.; Cavalcanti, D.N.; Villaça, R.C.; Teixeira, V.L. Diterpenes content of the brown alga Dictyota ciliolata (Dictyotales, Phaeophyceae) and recognition of a Brazilian haplotype based on psbA sequences. N. Z. J. Bot. 2018, 56, 415–429. [Google Scholar] [CrossRef]
- Obando, J.M.C.; Dos Santos, T.C.; Bernardes, M.; Nascimento, N.; Villaça, R.C.; Teixeira, V.L.; Barbarino, E.; Cavalcanti, D.N. Chemical variation and analysis of diterpenes from seaweed Dictyota menstrualis under controlled conditions. Algal Res. 2022, 62, 102637. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, M.; Li, X.; Peng, C.; Lin, D.; Li, X.; He, Y.; Xiong, L. Absolute Configurations and Bioactivities of Guaiane-Type Sesquiterpenoids Isolated from Pogostemon cablin. J. Nat. Prod. 2018, 81, 1919–1927. [Google Scholar] [CrossRef]
- Myklestad, S.M.; Granum, E. Biology of (1,3)-β-Glucans and Related Glucans in Protozoans and Chromistans. In Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides; Academic Press: San Diego, CA, USA, 2009; pp. 353–385. [Google Scholar]
- Miazek, K.; Iwanek, W.; Remacle, C.; Richel, A.; Goffin, D. Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review. Int. J. Mol. Sci. 2015, 16, 23929–23969. [Google Scholar] [CrossRef]
- Fawcett, D.; Verduin, J.J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A Review of Current Research into the Biogenic Synthesis of Metal and Metal Oxide Nanoparticles via Marine Algae and Seagrasses. J. Nanosci. 2017, 4, 8013850. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef] [PubMed]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Rana, N.F.; Menaa, F. Green and Cost-Effective Synthesis of Metallic Nanoparticles by Algae: Safe Methods for Translational Medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, P.A.U.I.; Thiripuranathar, G.; Uzair, B.; Iqbal, H.; Khan, B.A.; Menaa, B. Ecological and Industrial Implications of Dynamic Seaweed-Associated Microbiota Interactions. Mar. Drugs 2020, 18, 641. [Google Scholar] [CrossRef]
- Taylor, R.B.; Lindquist, N.; Kubanek, J.; Hay, M.E. Intraspecific variation in palatability and defensive chemistry of brown seaweeds: Effects on herbivore fitness. Oecologia 2003, 136, 412–423. [Google Scholar] [CrossRef]
- Siamopoulou, P.; Bimplakis, A.; Iliopoulou, D.; Vagias, C.; Cos, P.; Vanden, D.; Roussis, V. Diterpenes from the brown algae Dictyota dichotoma and Dictyota linearis. Phytochemistry 2004, 65, 2025–2030. [Google Scholar]
- Kelecom, A.; Laneuville Teixeira, V. Diterpenes of marine brown algae of the family dictyotaceae: Their possible role as defense compounds and their use in chemotaxonomy. Sci. Total Environ. 1986, 58, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Chiboub, O.; Sifaoui, I.; Abderrabba, M.; Mejri, M.; Fernández, J.J.; Díaz-Marrero, A.R.; Lorenzo-Morales, J.; Piñero, J.E. Apoptosis-like cell death upon kinetoplastid induction by compounds isolated from the brown algae Dictyota spiralis. Parasites Vectors 2021, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Shiiba, N.; Kumagai, M.; Endo, H.; Tsuruta, T.; Nishikawa, K.; Morimoto, Y. Anti-inflammatory diterpenoids from the brown alga Dictyota coriacea. Biosci. Biotechnol. Biochem. 2025, 89, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Gräf, R.; Daunderer, C.; Schulz, I. Molecular and Functional Analysis of the Dictyostelium Centrosome. Int. Rev. Cytol. 2004, 241, 155–202. [Google Scholar]
- Abou-El-Wafa, G.S.; Shaaban, M.; Shaaban, K.A.; El-Naggar, M.E.; Maier, A.; Fiebig, H.H.; Laatsch, H. Pachydictyols B and C: New diterpenes from Dictyota dichotoma Hudson. Mar. Drugs 2013, 11, 3109–3123. [Google Scholar] [CrossRef]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary Metabolite Profiling of Species of the Genus Usnea by UHPLC-ESI-OT-MS-MS. Molecules 2017, 23, 54. [Google Scholar] [CrossRef]
- Garneau, F.X.; Collin, G.J.; Jean, F.I.; Gagnon, H.; Arze, J.B.L. Essential oils from Bolivia. XII. Asteraceae: Ophryosporus piquerioides (D.C.) Benth. ex Baker. J. Essent. Oil Res. 2013, 25, 388–394. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Schmeda-Hirschmann, G.; Avendaño, M.; Sepúlveda, B.; Winterhalter, P. Fast high resolution Orbitrap MS fingerprinting of the resin of Heliotropium taltalense Phil. from the Atacama Desert. Ind. Crops Prod. 2016, 85, 159–166. [Google Scholar] [CrossRef]
- Basu, S.; Fey, P.; Pandit, Y.; Dodson, R.; Kibbe, W.A.; Chisholm, R.L. DictyBase 2013: Integrating multiple Dictyostelid species. Nucleic Acids Res. 2013, 41, 676–683. [Google Scholar] [CrossRef]
- Díaz García, A.; Hermis Rodríguez, I. Cytotoxicity of medicinal plant extracts on the human lung carcinoma cell line A549. Rev. Cuba. Farm. 2011, 45, 101–108. [Google Scholar]
- Guillen Luna, G.M.; Hernández Guzmán, E.D. Determination of the Sub-Chronic Toxicity of the N-Hexane Extract of Calea Urticifolia (Juanislama) Leaves in NIH Mice. Bachelor’s Thesis, Universidad del Salvador, San Salvador, El Salvador, 2016. [Google Scholar]
- Marcoleta, A.E.; Varas, M.A.; Ortiz-Severín, J.; Vásquez, L.; Berríos-Pastén, C.; Sabag, A.V.; Chávez, F.P.; Allende, M.L.; Santiviago, C.A.; Monasterio, O.; et al. Evaluating Different Virulence Traits of Klebsiella pneumoniae Using Dictyostelium discoideum and Zebrafish Larvae as Host Models. Front. Cell. Infect. Microbiol. 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Varas, M.A.; Riquelme-Barrios, S.; Valenzuela, C.; Marcoleta, A.E.; Berríos-Pastén, C.; Santiviago, C.A.; Chávez, F.P. Inorganic polyphosphate is essential for Salmonella Typhimurium Virulence and survival in Dictyostelium discoideum. Front. Cell. Infect. Microbiol. 2018, 8, 326426. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).