Abstract
Marine sponges are multicellular and primitive animals that potentially represent a wealthy source of novel drugs. The genus Acanthella (family Axinellidae) is renowned to produce various metabolites with various structural characteristics and bioactivities, including nitrogen-containing terpenoids, alkaloids, and sterols. The current work provides an up-to-date literature survey and comprehensive insight into the reported metabolites from the members of this genus, as well as their sources, biosynthesis, syntheses, and biological activities whenever available. In the current work, 226 metabolites have been discussed based on published data from the period from 1974 to the beginning of 2023 with 90 references.
1. Introduction
Natural metabolites from various sources, including microbes, animals, minerals, and plants, have been traditionally utilized for treating various human illnesses [1,2,3,4,5,6]. Recently, the developments in high-throughput screening and spectroscopic and analytical technologies have significantly boosted natural drug discovery, including marine-based drugs [7]. The marine environment is a rich source of a vast group of structurally unparalleled metabolites with diverse pharmacological activities that are reported from different marine organisms such as tunicates, sponges, mollusks, and bryozoans [8]. These metabolites are potential candidates for biotechnological applications and a number of them are in clinical trials; therefore, their impact on the pharmaceutical industry is continually growing [9,10,11]. They have been found to display an array of bioactivities, primarily antimicrobial, immunosuppressive, antifouling, anticancer, anthelmintic, antiprotozoal, neuroprotection, antiviral, and anti-inflammatory [7,8]. Among the marine organisms that have been investigated, sponges (Porifera), which are soft-bodied and sessile organisms, have become the focal point of natural product investigations due to the vast range of structurally unique biometabolites separated from these organisms [12,13].
The Axinellidae family (class Demospongiae, order Bubarida, family Dictyonellidae) [14] members, including Axinella and Acanthella genera, produce structurally varied terpenes with various nitrogen-containing functionalities [15]. Marine sponges of the genus Acanthella (Schmidt, 1862) are present in large numbers in the world’s oceans, especially in the South China Sea [16]. The accepted species of this genus and their status are listed in Table 1.

Table 1.
List of the accepted species of the genus Acanthella and their status [14].
These sponges have been proven to be a prosperous source of sesquiterpenes and diterpenes featuring various nitrogen-containing groups, such as –NC (isonitrile), –NCS (isothiocyanate), –NCO (isocyanate), and –NHCHO (formamide) functionalities. Additionally, alkaloids and sterols are reportedly derived from these sponges. Some of these metabolites possess bioactivities such as antimalarial, cytotoxic, antimicrobial, anthelmintic, and antifouling [17,18,19,20,21,22,23,24,25]. Furthermore, some of them with promising bioactivities and unique structures have drawn the attention of chemists to investigate their synthesis in alignment with an in-depth biological evaluation for discovering new drug leads [26,27,28,29]. It is noteworthy that there are no available reported works that focus on the genus Acanthella. Therefore, this work aimed at highlighting the potential of this genus in terms of natural metabolite production. Herein, all reported studies on this genus are discussed, including secondary metabolites and their sources, as well as their biosynthesis, synthesis, and bioactivities whenever applicable.
2. Methodology
The reported studies on the genus Acanthella were collected by carrying out a literature search on different Publishers and databases, including Web of Science, PubMed, Scopus, Google-Scholar, Science Direct, Thieme, Bentham, Springer, Willey, and Taylor/Francis using the keywords: “Acanthella + compound” OR “Acanthella + NMR” OR “Acanthella + Biological activity” OR “Acanthella + nanoparticles” OR “Acanthella + Terpenoids” OR “Acanthella + Semi-synthesis”. This review involved investigations published in the English language in peer-reviewed journals in the period from 1974 to the beginning of 2023. A total of 85 published articles have been highlighted. The suggests irrelevant and non-reviewed journals’ published articles, as well as all non-English written articles, were not included. For the non-English work, the data were extracted from the English abstracts whenever available.
3. Secondary Metabolites of Genus Acanthella
Different metabolites were separated and characterized from different species of this genus using various spectroscopic and chromatographic techniques. The isolated metabolites are categorized according to their chemical classes into sesquiterpenes, diterpenes, alkaloids, steroid compounds, and others. Additionally, their reported biosynthetic and synthetic studies are also highlighted whenever applicable.
3.1. Sesquiterpenes
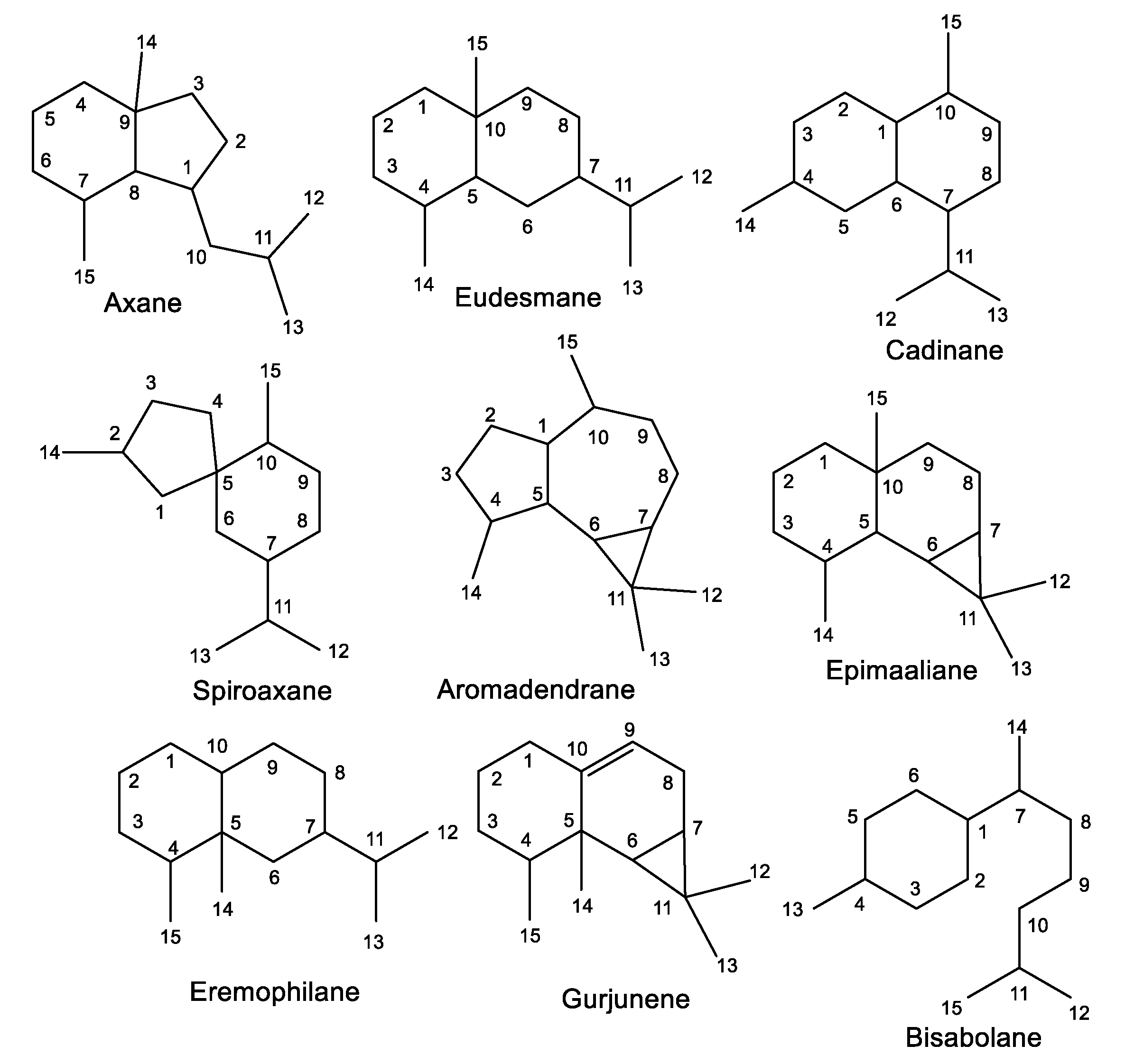
The reported investigations revealed the purification of various classes of sesquiterpenes that are substituted by isonitrile or isothiocyanate functionalities, including mono-, bi-, and tri-cyclic skeletons with 3-, 5-, 6-, and/or 7-membered rings (Figure 1 and Table 2). Frequently, formamide derivatives were reported along with both isothiocyanate and/or isonitrile moieties. Isonitrile-containing metabolites have been reported from some species belonging to Penicillium and Axinella genera [30]. Several reports stated their characterization from Acanthella. It was reported that A. cavernosa (Dendy, 1922) can convert cyanide and thiocyanate for isocyanide and isothiocyanate biosynthesis, which could be attributed to the presence of rhodanese or the equivalent enzyme [31]. Therefore, thiocyanate was postulated to be the precursor for the isothiocyanate moiety in terpenes by direct utilization or oxidative desulphurization of cyanide, conversion to isocyanide terpenes, and reinsertion of sulfur [31].
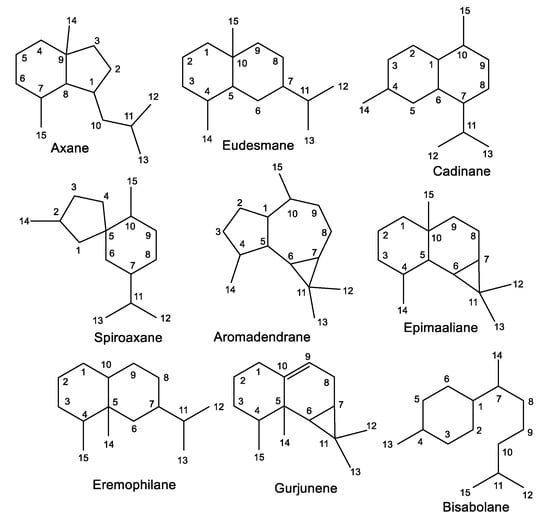
Figure 1.
Classes of sesquiterpenes reported from the genus Acanthella.

Table 2.
Sesquiterpenes from the genus Acanthella (molecular weight and formulae, chemical class, species, and sampling locations).
3.1.1. Aromadendrane-Type Sesquiterpenes
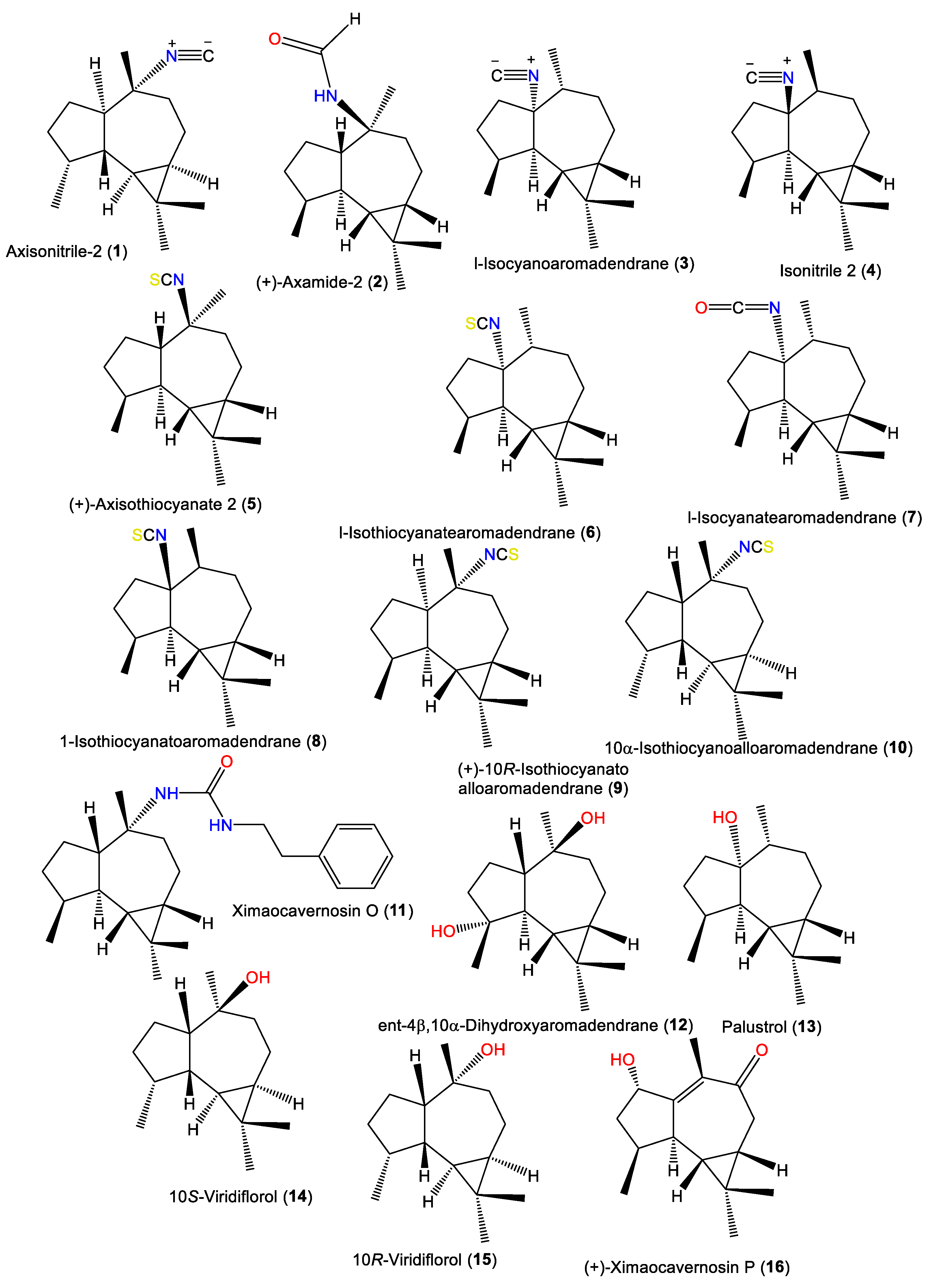
In 1987, l-isocyanoaromadendrane (3) was reported as a novel isonitrile sesquiterpene from the fish toxic CH2Cl2 fraction of A. acuta using SiO2CC (silica gel column chromatography), assigned by spectral and chemical methods [34].
Furthermore, 5 and 9 were isolated from Japanese A. cavernosa by SiO2 CC and RP-HPLC and identified by different spectroscopic methods [40]. Ximaocavernosin O (11) was isolated from an A. cavernosa Et2O fraction using silica gel/MCI/Sephadex LH-20/(RP)-HPLC/chiral-phase HPLC and was characterized by spectroscopy, X-ray diffraction, and QM-NMR analyses as well as Mosher’s method and TDDFT-ECD calculations [33]. Compound 11 is similar to 2 and was formerly reported from nudibranch Hexabranchus sanguineus with a C-10 phenyl urea fragment instead of a C-10 formamide in 2 [33]. New aromadendrane-type sesquiterpenoids, 16 and 12, were purified from the Hainan A. cavernosa using SiO2/Sephadex LH-20 CC/chiral-phase HPLC by Shen et al.. Their configurations were elucidated based on spectral, TDDFT-ECD, and X-ray analyses and optical rotation measurements. Compound 16 with a 2S/4S/5R/6S/7S configuration ([α]D +169.6]) is identical to 2β-hydroxyaromadendr-1(10)-en-9-one ([α]D _186]) except for the optical rotation (Figure 2) [33].
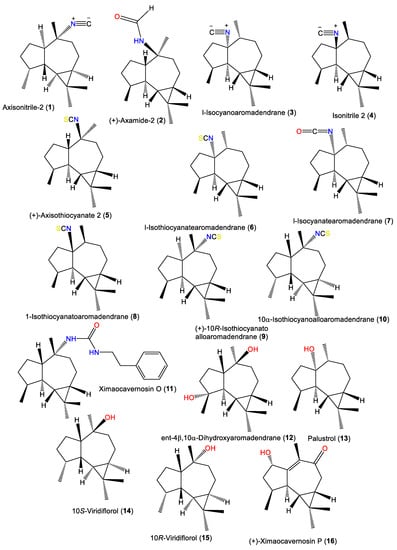
Figure 2.
Aromadendrane-type sesquiterpenes (1–16) reported from the genus Acanthella.
3.1.2. Spiroaxane-Type Sesquiterpenes
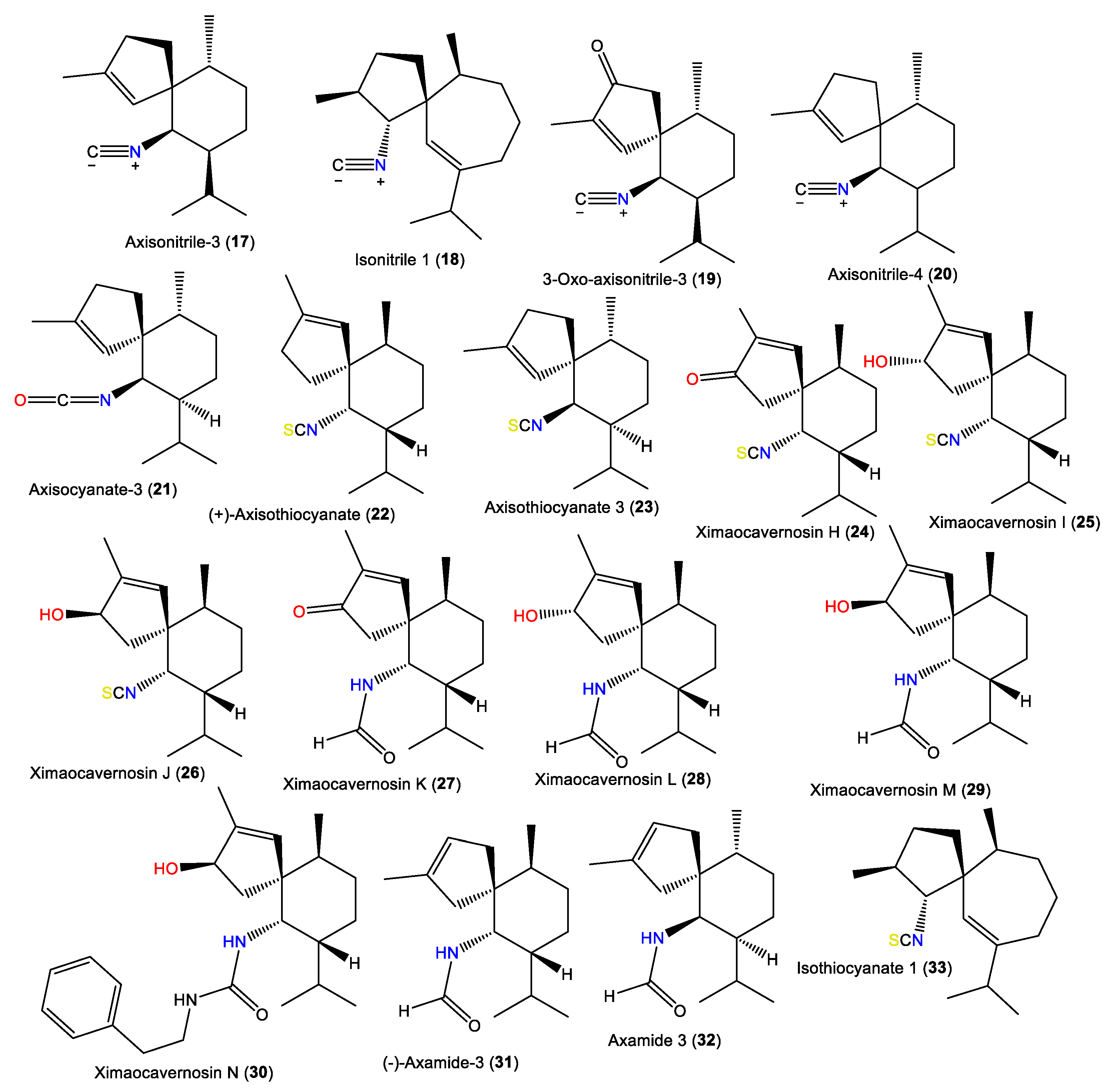
Spiroaxane skeletons containing sesquiterpenes are of rare natural occurrence. Compound 19, a new sesquiterpene isocyanide with a spiroaxane (spiro [5,6] decane) skeleton was obtained from Chinese Acanthella sp., which is a 3-oxo derivative of 17 [41]. Additionally, 23 is a spiroaxane sesquiterpene with a C-6 isocyanate and was purified and characterized by Jumaryatno et al. from A. cavernosa specimens collected from Coral gardens/Gneerings reef/Mooloolaba/Australia and from A. klethra collected from Pelorus Island, Queensland, in addition to 17 [19,37].
Additionally, 23–30 were isolated from A. cavernosa Et2O fractions using silica gel/MCI/Sephadex LH-20/(RP)-HPLC/chiral-phase HPLC and characterized by spectral, X-ray diffraction, and QM-NMR analyses as well as Mosher’s method and TDDFT-ECD calculations [33]. Compounds 23–30 are spiroaxane derivatives involving compounds with C-6 isothiocyanate (e.g., 23–25) and formamide or a 1-phenethyl urea fragment (e.g., 26–30) [33] (Figure 3). In 2019, Wu et al. reported the purification of axamide-3 (32) from the acetone fraction of A. cavernosa collected from Xidao Island (Hainan Province, China) which was characterized by NMR spectral data and optical rotation [48].
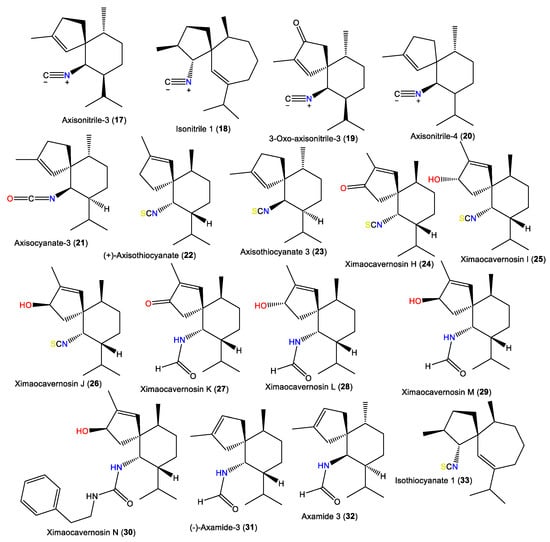
Figure 3.
Spiroaxane-type sesquiterpenes (17–33) reported from the genus Acanthella.
3.1.3. Eudesmane-Type Sesquiterpenes
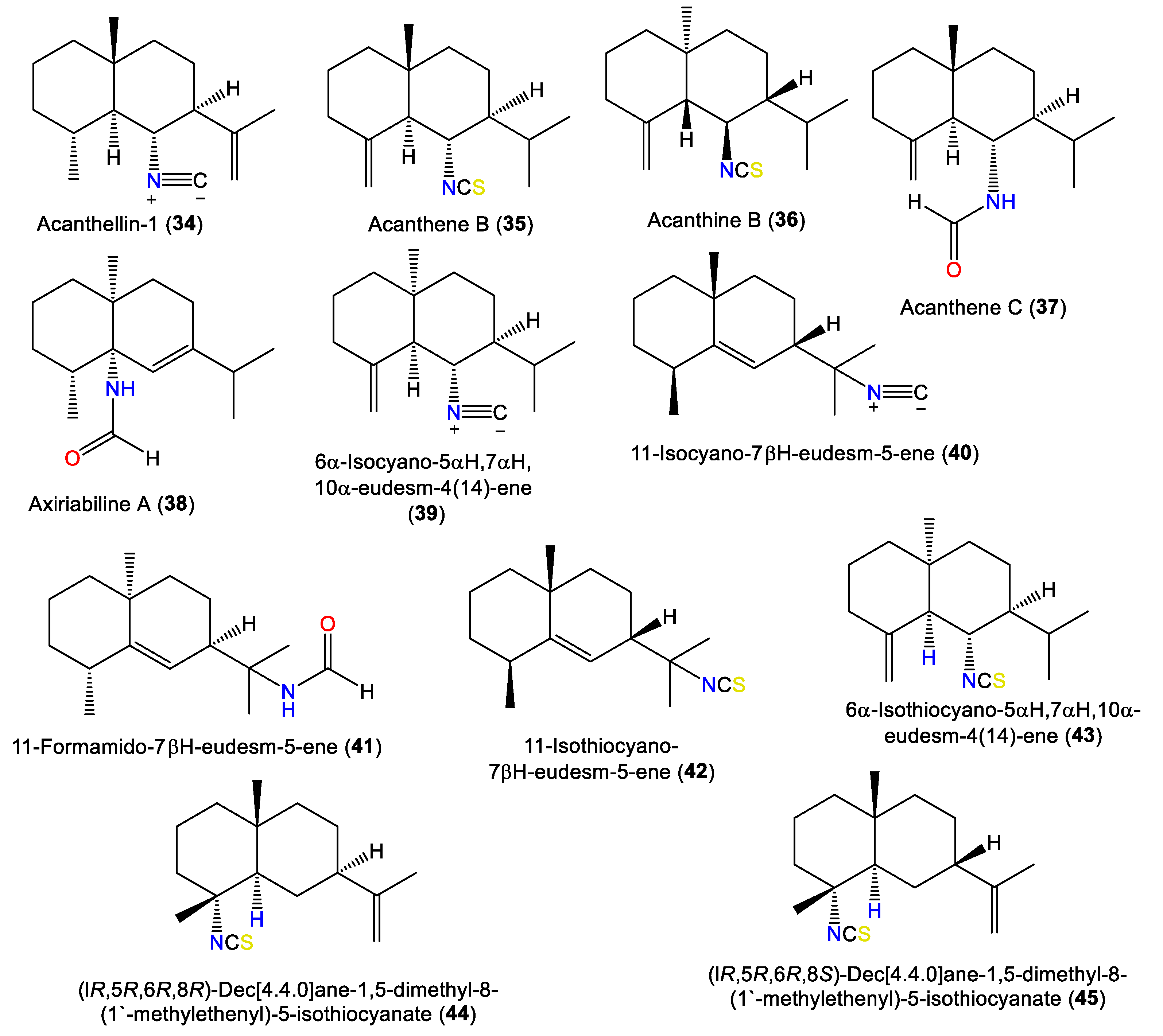
Acanthellin-1 (34) is a bicyclic sesquiterpene with isopropylidene and isonitrile moieties. It was separated as an optically active oil from the ether fraction of the acetone extract of A. acuta collected from the Bay of Naples using SiO2CC, and was characterized by NMR and chemical methods, as well as optical rotation [30] (Figure 4). A chromatographic investigation of A. klethra collected from Pelorus Island, Queensland, yielded sesquiterpenoids with isothiocyanate and isonitrile groups, i.e., 42, 44, and 45, that were assigned by spectral and X-ray analyses. Compounds 42, 44, and 45 are of eudesmane-type and are related to 34. Compounds 45 and 44 are different in stereo-configuration at C-7 [19,44]. Additionally, 39 and 43 are in the bicyclic cis-eudesmane class of sesquiterpenes, possessing isocyanate and isothiocyanate functionalities, respectively, and were purified and specified from A. acuta [47], whereas 35 is a stereoisomer of 5 [46].
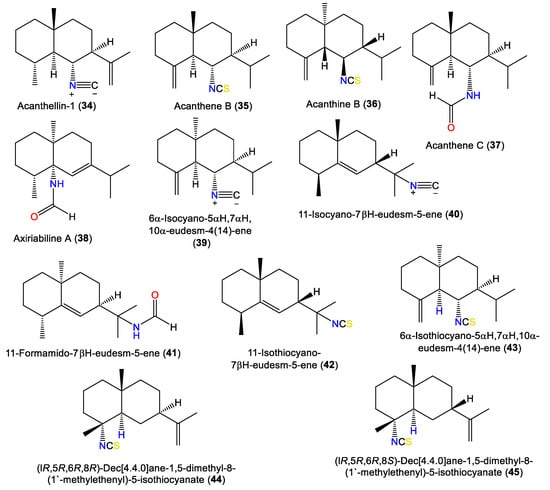
Figure 4.
Eudesmane-type sesquiterpenes (34–45) reported from the genus Acanthella.
Axiriabiline A (38) was obtained from the acetone fraction of A. cavernosa collected from Xidao Island (Hainan Province, China) and characterized by NMR spectral data and optical rotation [48]. Burgoyne et al. (1993) purified two new sesquiterpenoid acanthenes B and C (35 and 37) along with 40 and 42–44 from the hexane fraction of unidentified Acantbella species using SiO2 flash CC/HPLC. The compounds were characterized by spectral analyses [46].
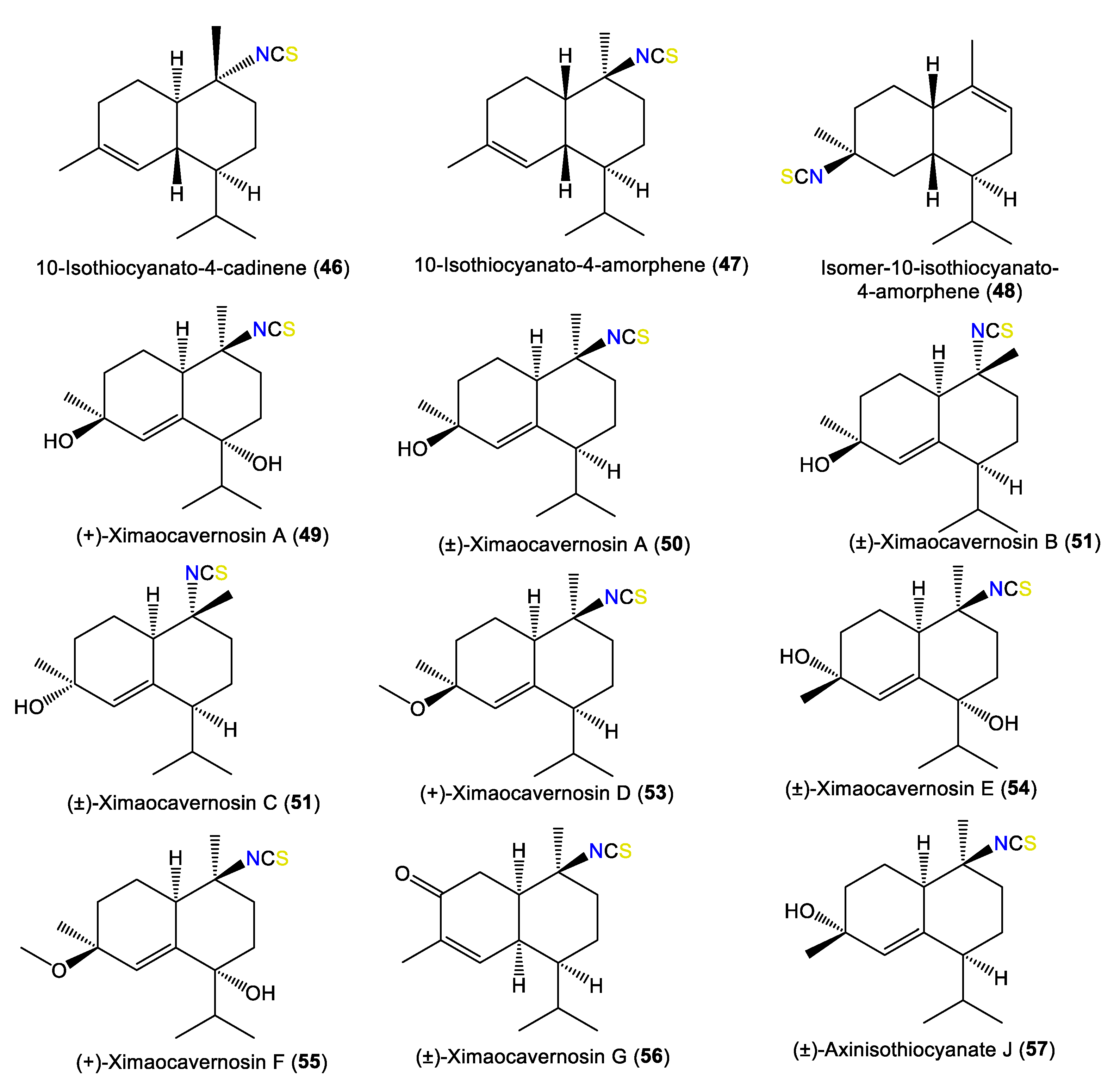
3.1.4. Cadinene-Type Sesquiterpenes
In 2000, Clark et al. isolated a new sesquiterpene, 46, that has a 1R/6R/7S/10R configuration and C-10 isothiocyanato functionality and [α]D +3 [45]. In addition, Nogata et al. purified a new sesquiterpene, 65, and the known 67 from A. cavernosa EtOH extracts utilizing SiO2/Sephadex LH-20/ODS HPLC. These compounds were assigned based on spectral data and chemical transformations [49] (Figure 5). Compound 65 has a C-10 formamido functionality instead of the C-10-OH in 67 [49].
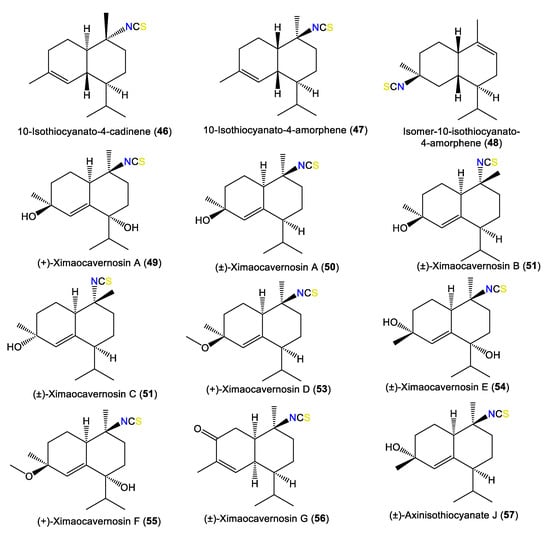
Figure 5.
Cadinene-type sesquiterpenes (46–57) reported from the genus Acanthella.
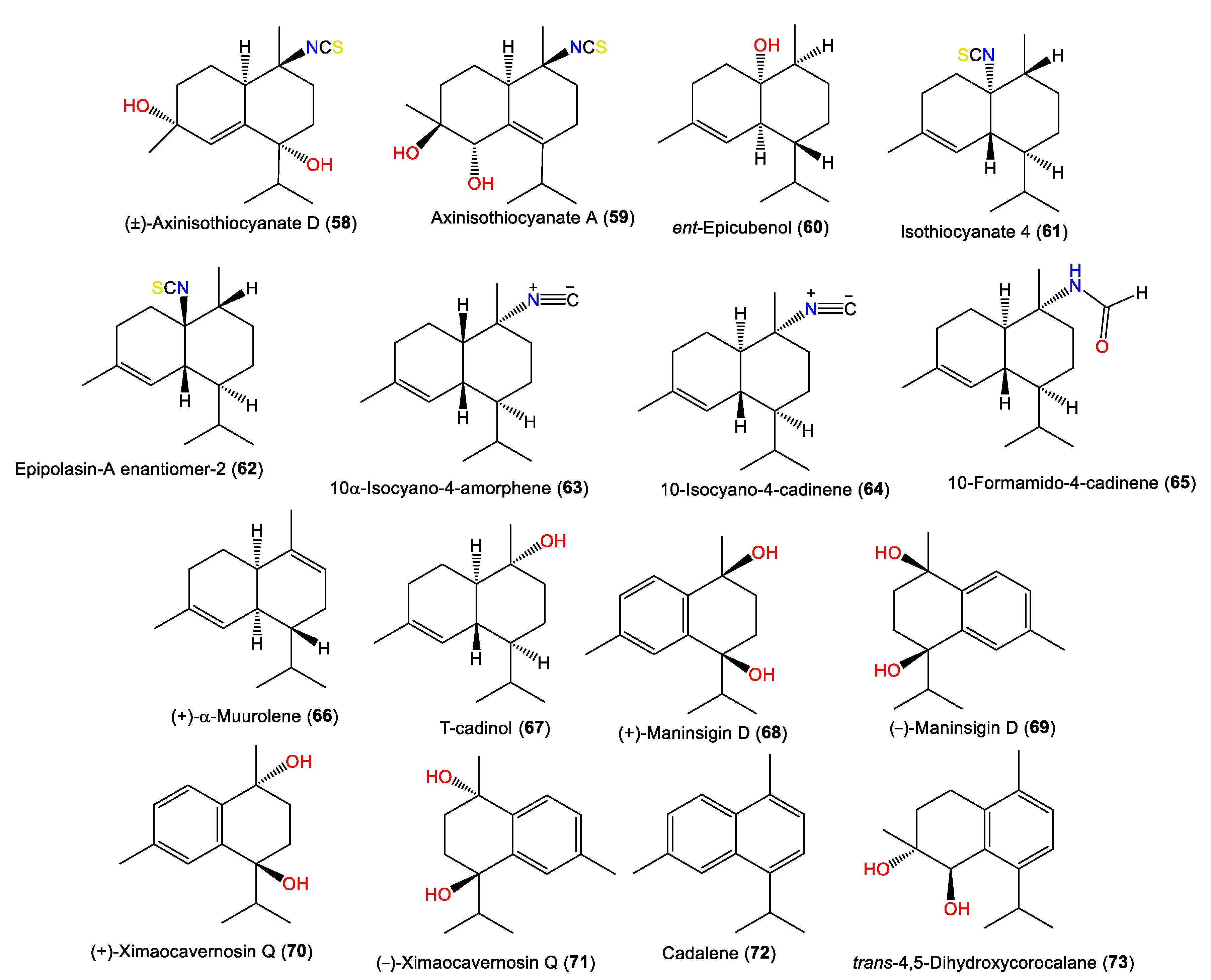
New cadinane-type sesquiterpenoids, ximaocavernosins A–G (49–56), were isolated from A. cavernosa Et2O fractions using SiO2/MCI/Sephadex LH-20/(RP)-HPLC/chiral-phase HPLC and characterized by spectroscopy, X-ray diffraction, and QM-NMR analyses as well as Mosher’s method and TDDFT-ECD calculations [33]. Compounds 49–56 have cadinane frameworks with a Δ5,6 double bond and a C-10 isothiocyanate but differ in stereochemistry and oxidation patterns [33]. In 2019, Wu et al. reported the purification of 10-formamido-4-cadinene (65) from the acetone fraction of A. cavernosa collected from Xidao Island (Hainan Province, China), which was characterized by NMR spectral data and optical rotation [48]. New cadinane-type sesquiterpenoids, 49 and 68–73, were purified from Hainan A. cavernosa using SiO2/Sephadex LH-20 CC/chiral-phase HPLC (Figure 6). Their configurations were elucidated based on spectral, TDDFT-ECD, and X-ray analyses and optical rotation measurements. Maninsigin D and ximaocavernosin Q were obtained as racemic forms, which were separated into their enantiomers [(+)-68/(−)-69 and (+)-70/(−)-71] using chiral-phase HPLC [43].
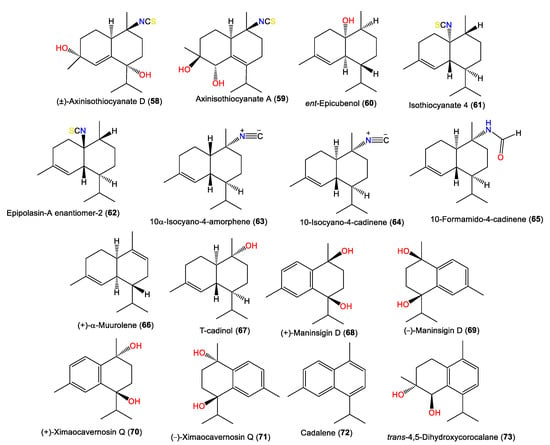
Figure 6.
Cadinene-type sesquiterpenes (58–73) reported from the genus Acanthella.
3.1.5. Other Sesquiterpenes
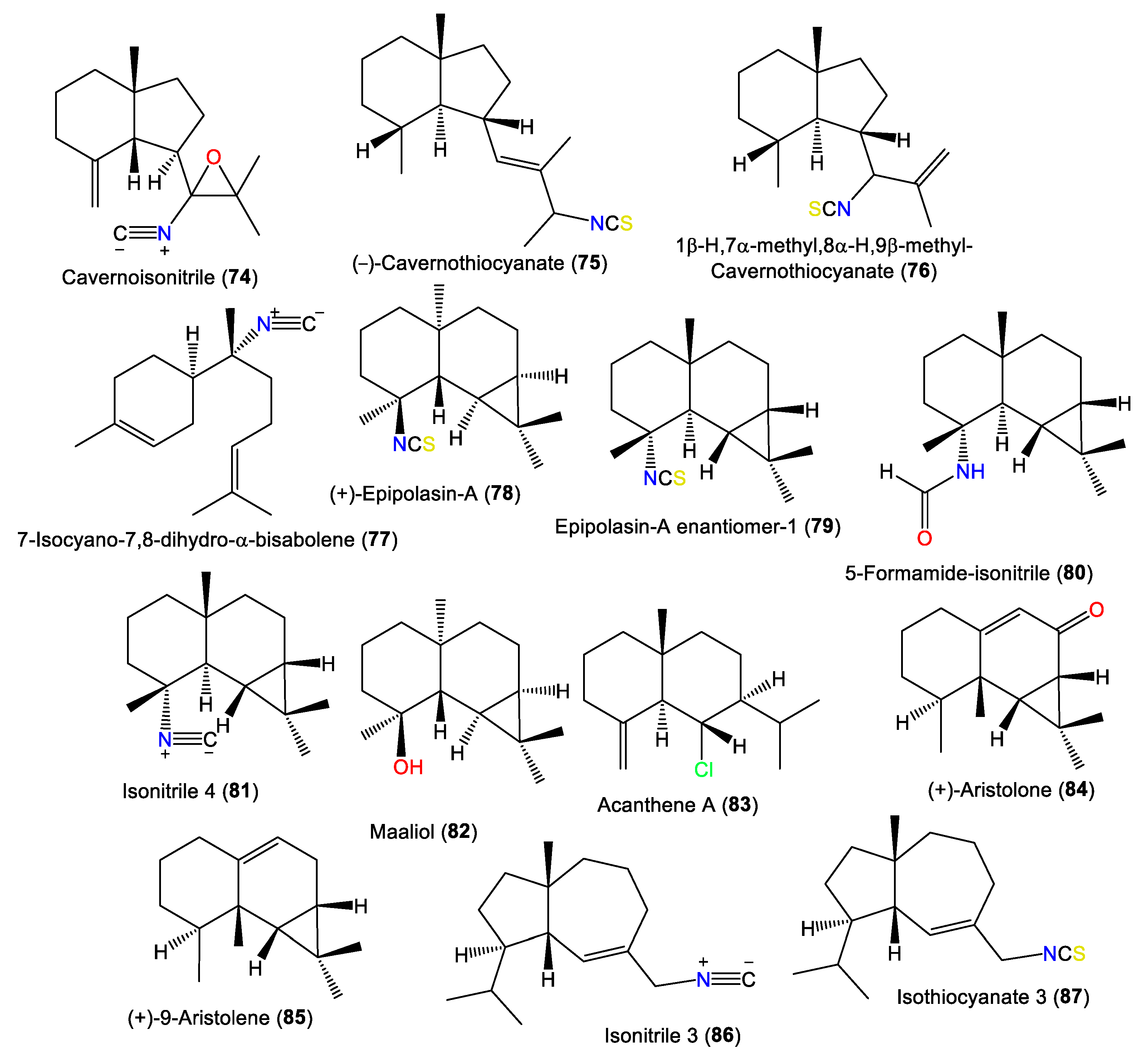
New axane sesquiterpenoids, 74 and 75, in addition to 77, were separated from the antifungal hexane fraction of A. cavernosa collected from the Hachijo-Jima Islands using flash CC/sephadex LH-20/HPLC. They were elucidated based on spectral data [23]. Compound 74 is a rare oxygenated tricyclic sesquiterpene cyanide belonging to axane-type sesquiterpenes [23]. Furthermore, 66, 75, 76, and 85 were isolated by SiO2 CC and RP-HPLC and identified by alpha-D, spectral data, and chemical methods from Japanese A. cavernosa [40]. Additionally, the new epimaaliane sesquiterpene 79, along with 78, were specified from the antimicrobial acetone extracts of A. pulcherrima using spectral and optical rotation measurements. Compound 79 is an enantiomer of 78 with an opposite [α]D value and differs at the ring junction [35]. Burgoyne et al. purified epimaaliane-type sesquiterpenes 80 and 81 from the hexane fraction of an unidentified Acantbella species using SiO2 flash CC and HPLC. The compounds were characterized by spectral analyses [46] (Figure 7).
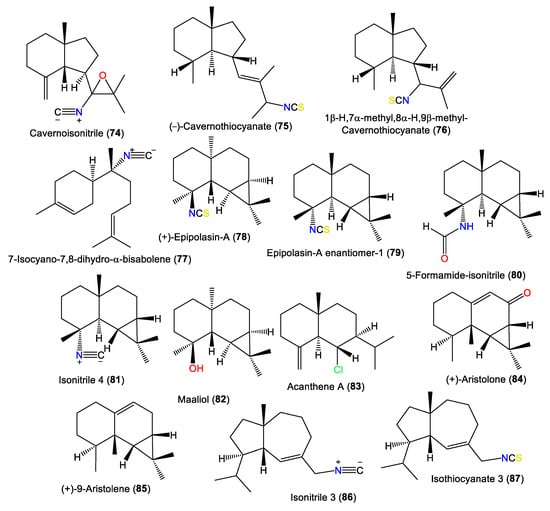
Figure 7.
Other sesquiterpenes (74–87) reported from the genus Acanthella.
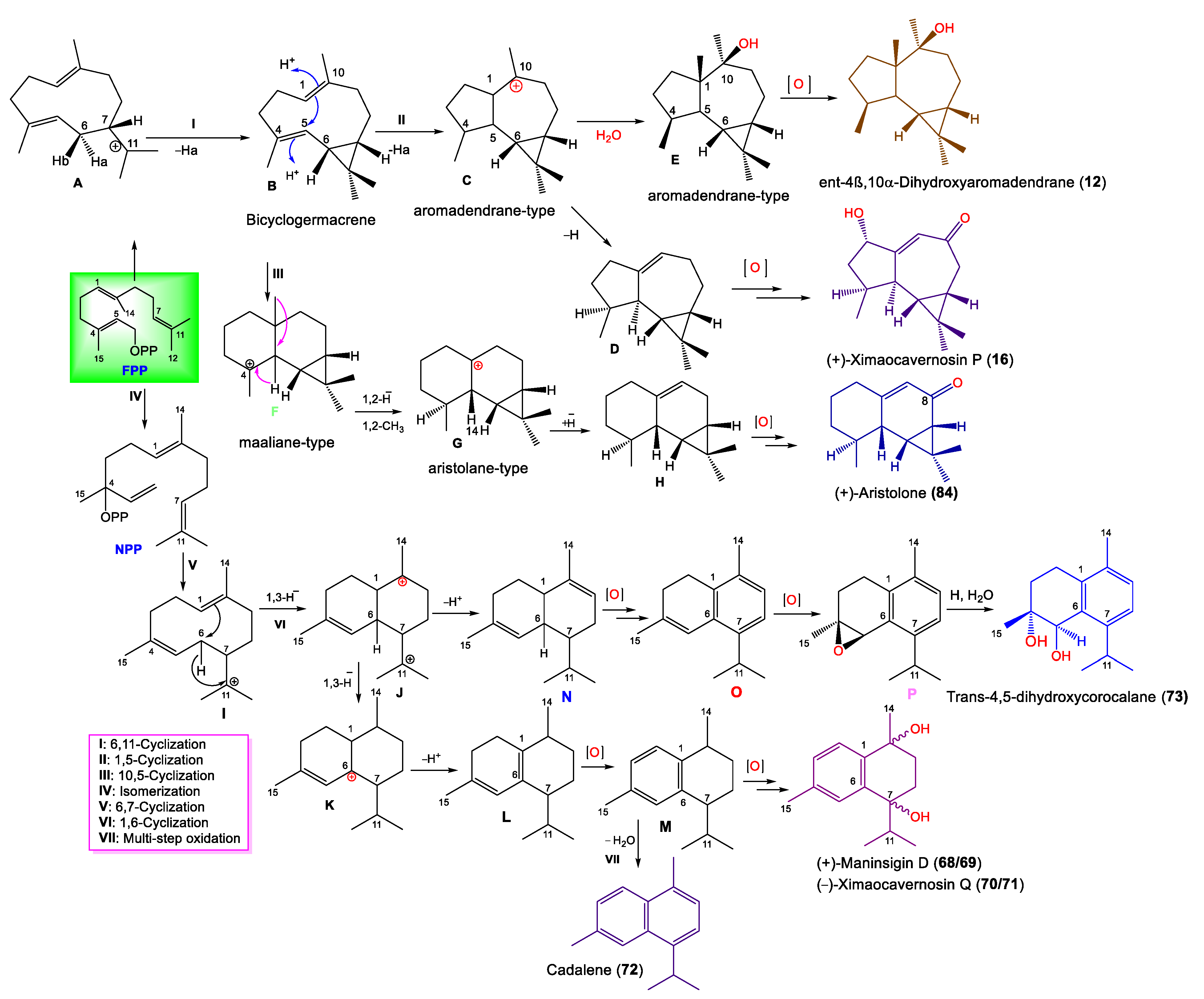
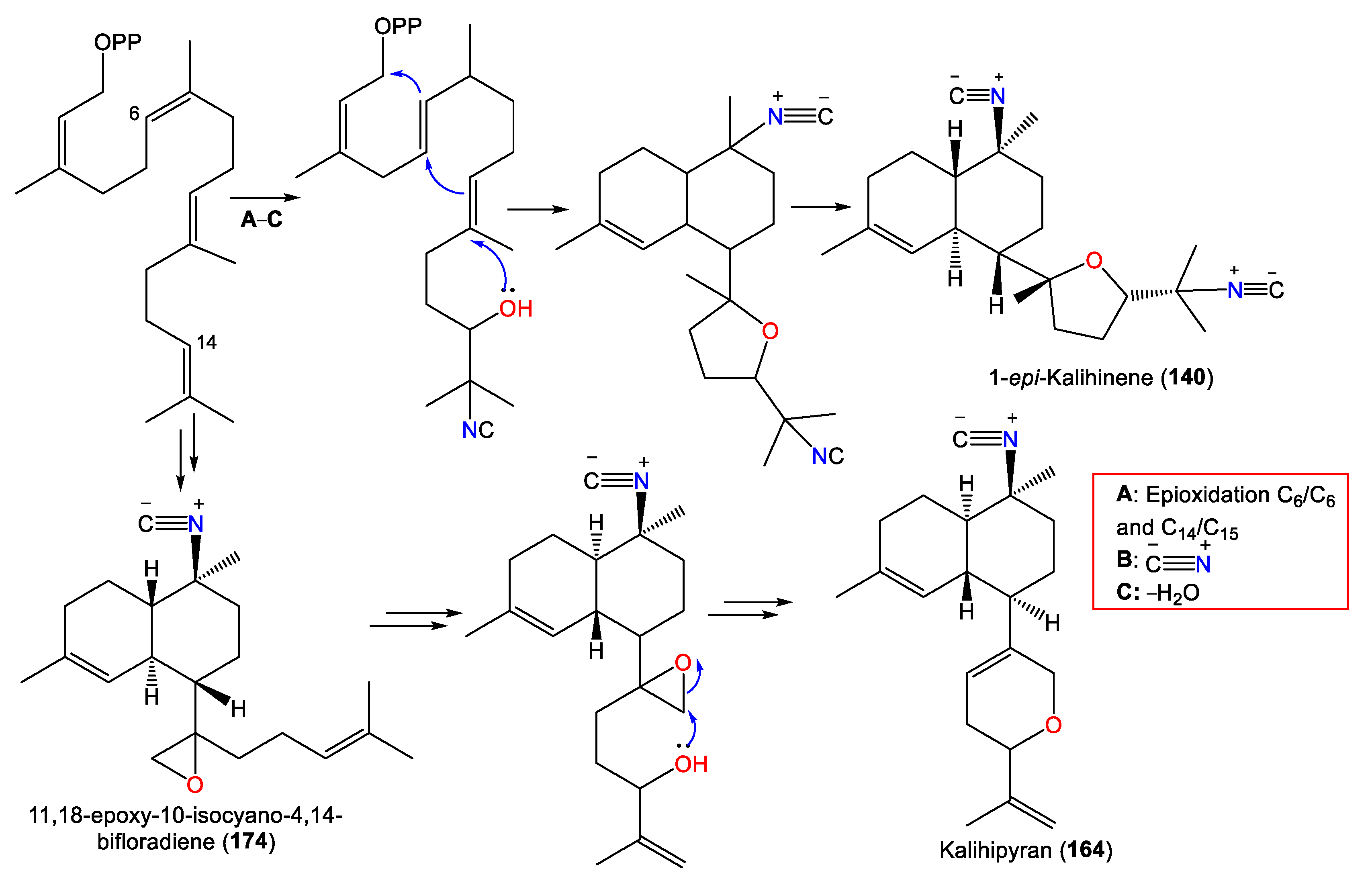
Notably, Shen et al. proposed that 12, 16, 49, 68–73, and 84 originate from E,E-farnesyl diphosphate (E,E-FPP), as illustrated in Scheme 1 [43].
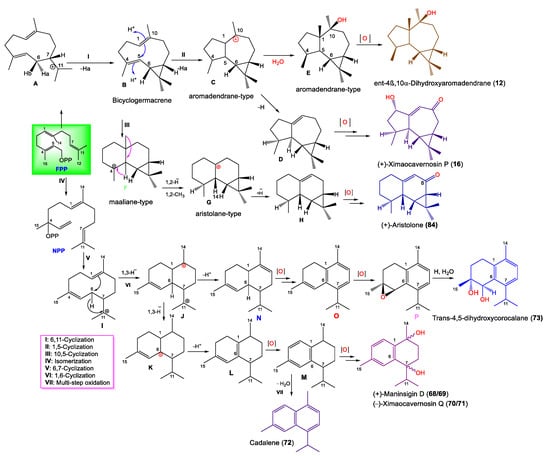
Scheme 1.
Biosynthesis pathway of 12, 16, 49, 68–73, and 84 [43].
3.2. Diterpenoids
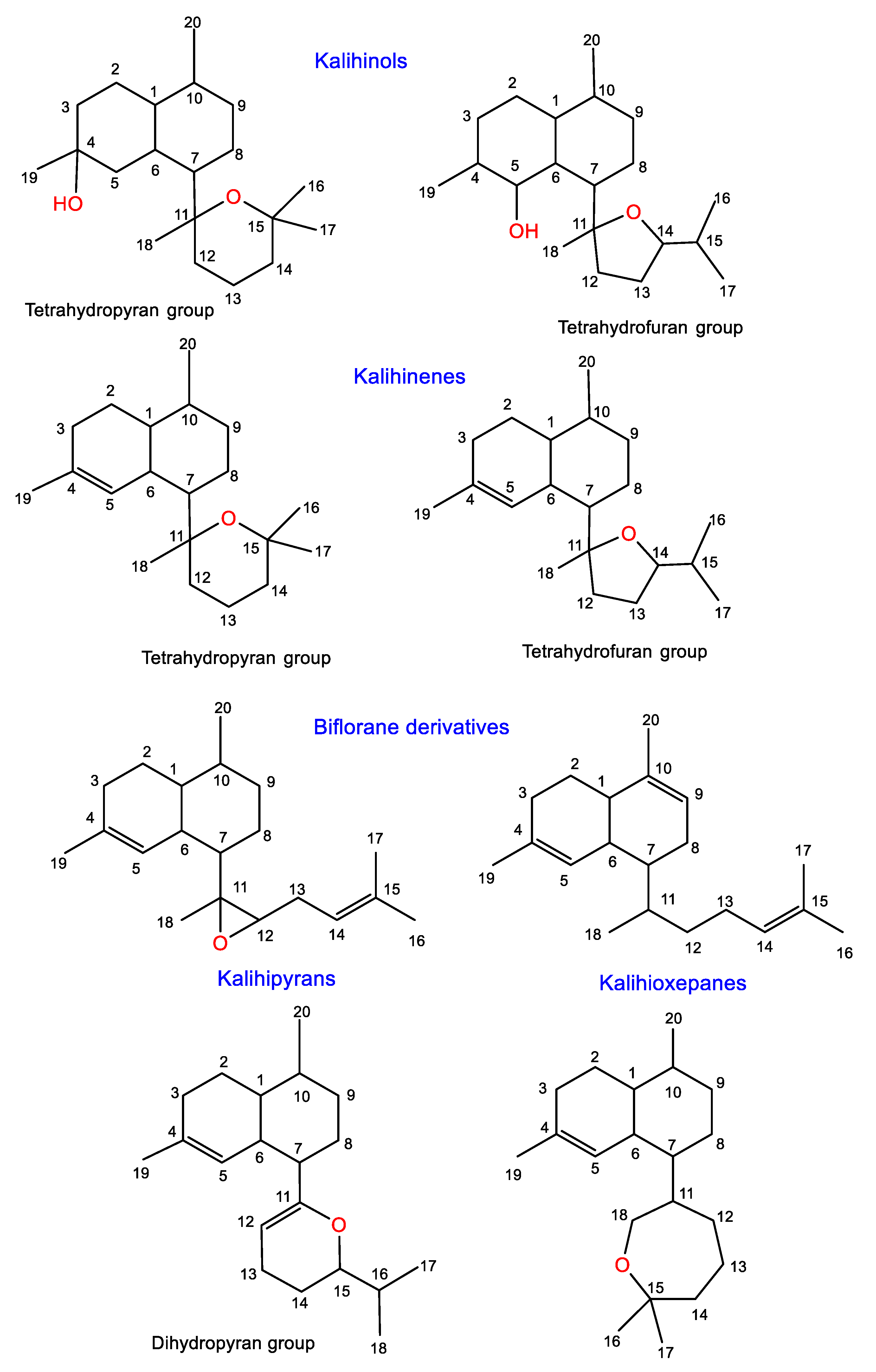
Diterpenoids are among the common metabolites reported from various Acanthella species. These compounds are characterized by the existence of nitrogenous functionalities such as isothiocyanato, isocyano, and/or formamido groups. These diterpenes are classified into two major classes, kalihinanes and biflorane derivatives, according to the 8C side chain (Figure 8 and Table 3). Kalihinanes have a decalin frame structure with C-7-attached dihydropyran, tetrahydropyran, or tetrahydofuran moiety. Additionally, these rings may carry various substituents such as OH, Cl, isothiocyanato, isocyano, and formamido groups or chlorine. They include kalihinenes, kalihinols, and kalihipyranes. Kalihinols are spilt into two main categories, tetrahydrofuran (I) and tetrahydropyran (II) groups, according to the C-7 substitution. Commonly, they have trans-decalin framework with a C-4 or C-5 tertiary alcohol and an isocyanate moiety at C-10 and/or C-5. The first group has a tetrahydrofuran moiety featuring NCS, NC, or Cl at C-15, or the gem-dimethyl is substituted by an isopropenyl moiety, whereas the tetrahydropyran group possesses Cl atom at C-14. Kalihinenes have a Δ4-trisubstituted double bond and possess similar structural features to kalihinols, while biflorane diterpenoids are a class of kalihinane diterpenes featuring a linear eight-carbon open chain substituent at C-7. Biosynthetically, these metabolites are proposed to result from the cyclization of the biflorane skeleton (trans or cis form) and geranylgeranyl pyrophosphate [51]. Their stereochemistry has been determined using spectroscopic, X-ray and/or CD analyses, as well as chemical and computational methods.
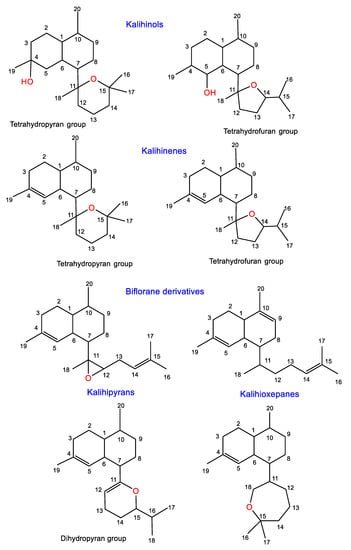
Figure 8.
Classes of diterpenes reported from genus Acanthella.

Table 3.
Diterpenes from the genus Acanthella (molecular weight and formulae, chemical class, species, and sampling locations).
3.2.1. Kalihinols
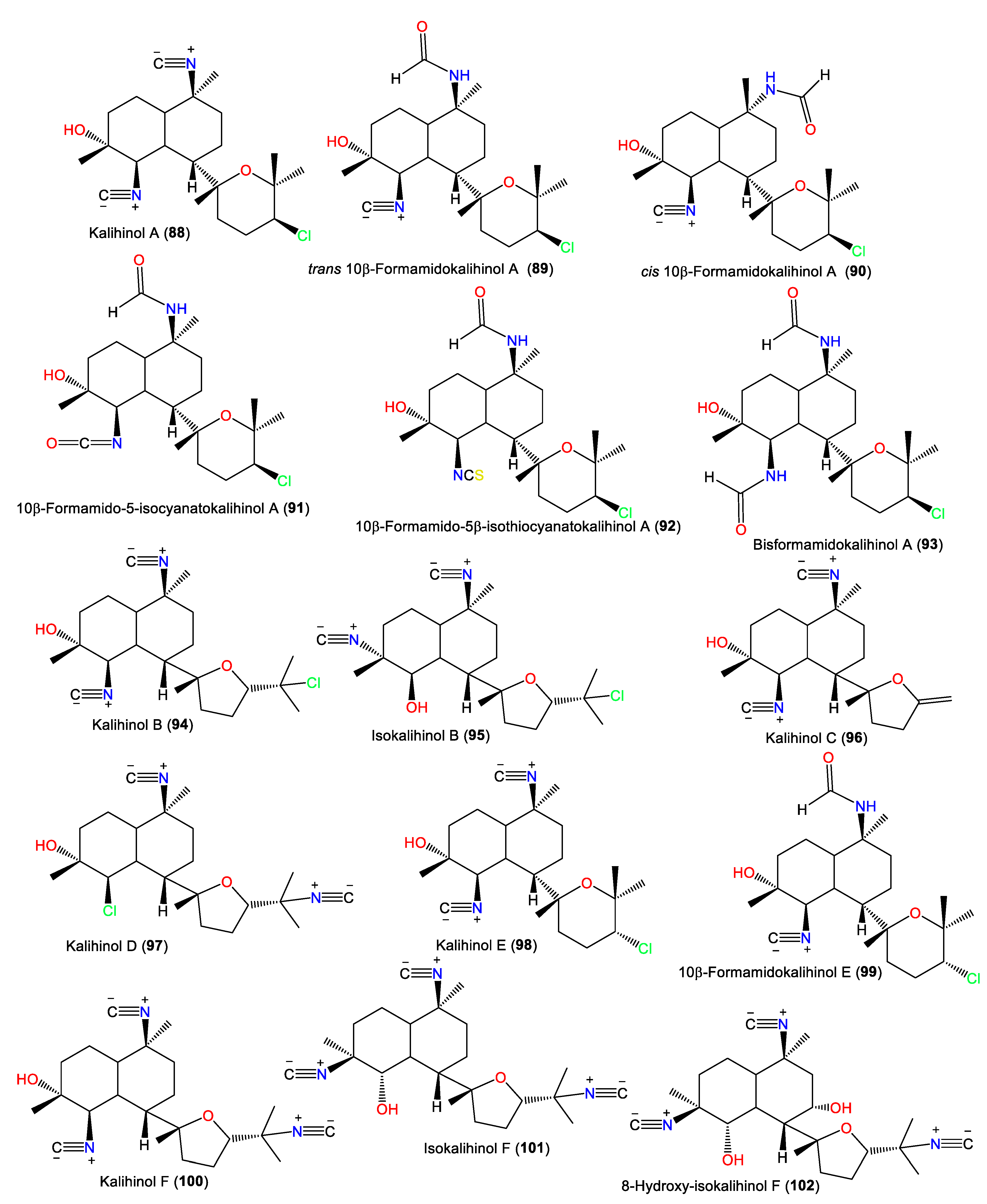
Kalihinol A (88) was the first reported member of this diterpenoid class in 1984 [52] (Table 3). It is a tricyclic diterpenoid belonging to group II, containing isocyano, hydroxyl, and chlorine moieties. It was separated from the CCl4 extract of Acanthella sp. and characterized by NMR and X-ray analyses [52] and its configuration was assigned as 1S/4R/5R/6S/7S/10S/11R/14S using the CD exciton chirality method [65] (Figure 9).
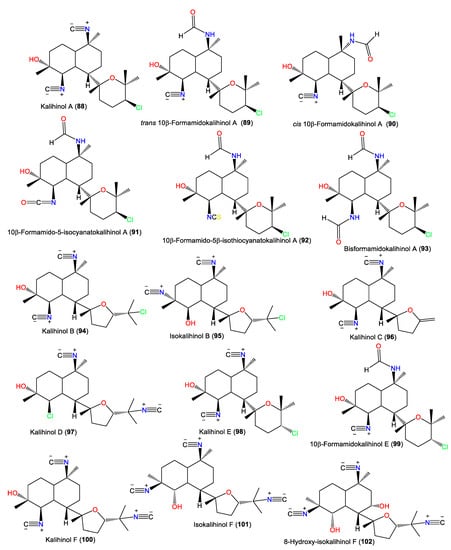
Figure 9.
Kalihinol diterpenes (88–102) reported from the genus Acanthella.
In 1987, Chang et al. purified and characterized isocyano-diterpenoids kalihinols A–H (88, 94, 96–98, 100, 108, and 111) and X–Z (125, 127, and 130) from Acanthella sp. obtained from Guam and Fiji using spectral and X-ray analyses. They differ in the C-7 attached moiety; the tetrahydropyran with C-14 chlorine (e.g., 25, 88, 98, 130, and 127); or the tetrahydro-furanyl moiety, with 15-NC (e.g., 97 and 100), 15-NCS (e.g., 108), 15-C1 (e.g., 94), or isopropenyl replacing gem-dimethyl (e.g., 96) [22] (Figure 10).
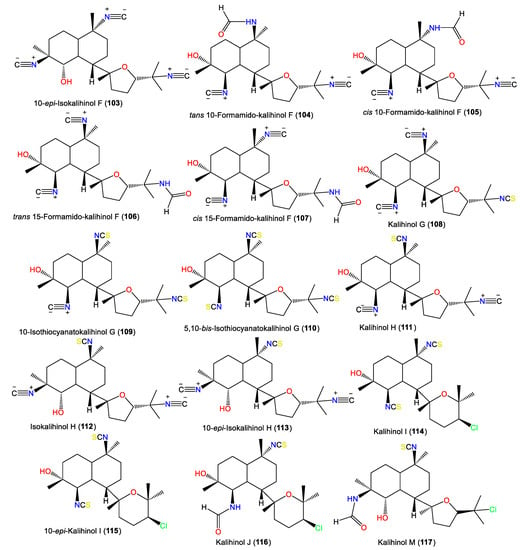
Figure 10.
Kalihinol diterpenes (103–117) reported from the genus Acanthella.
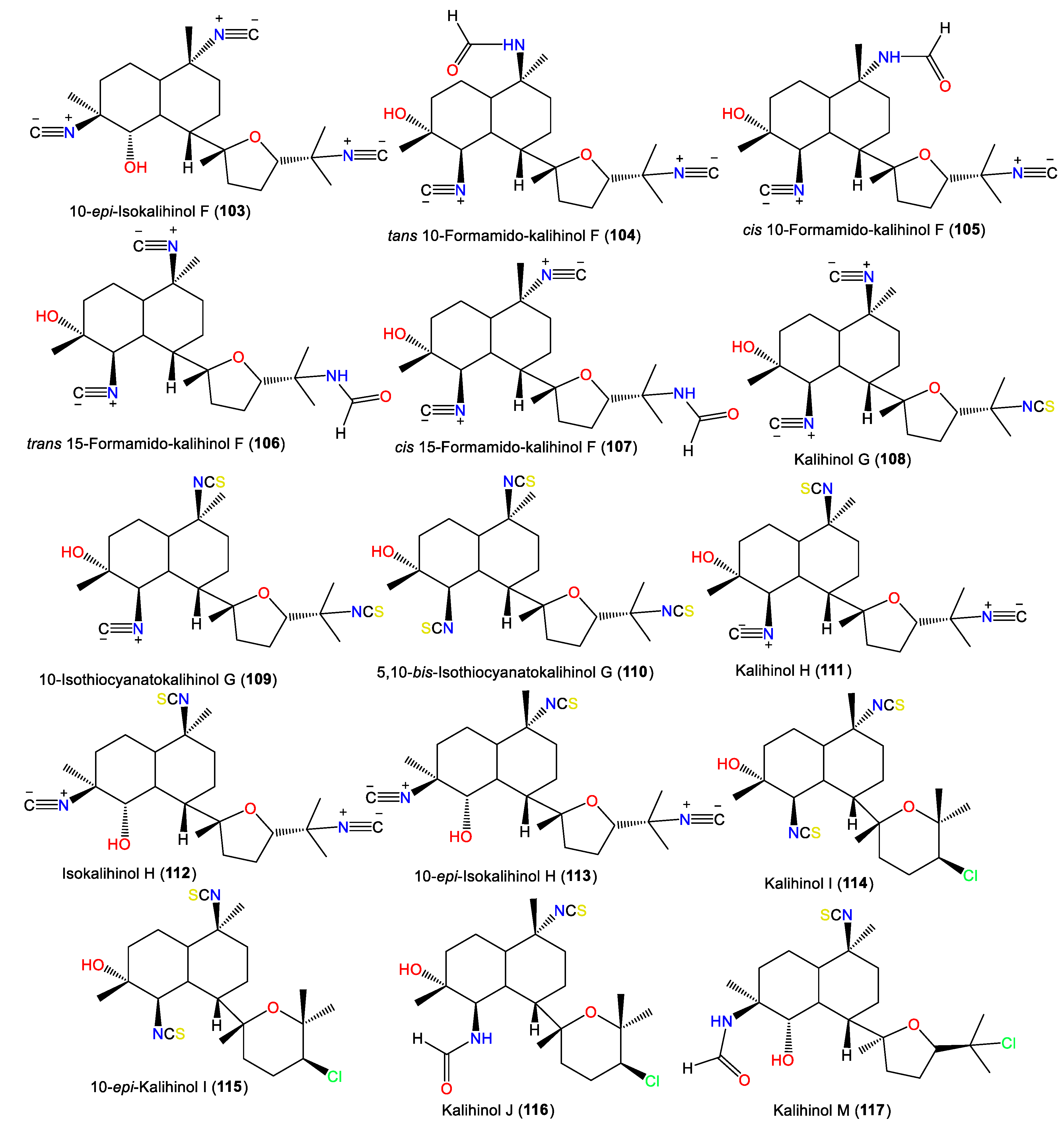
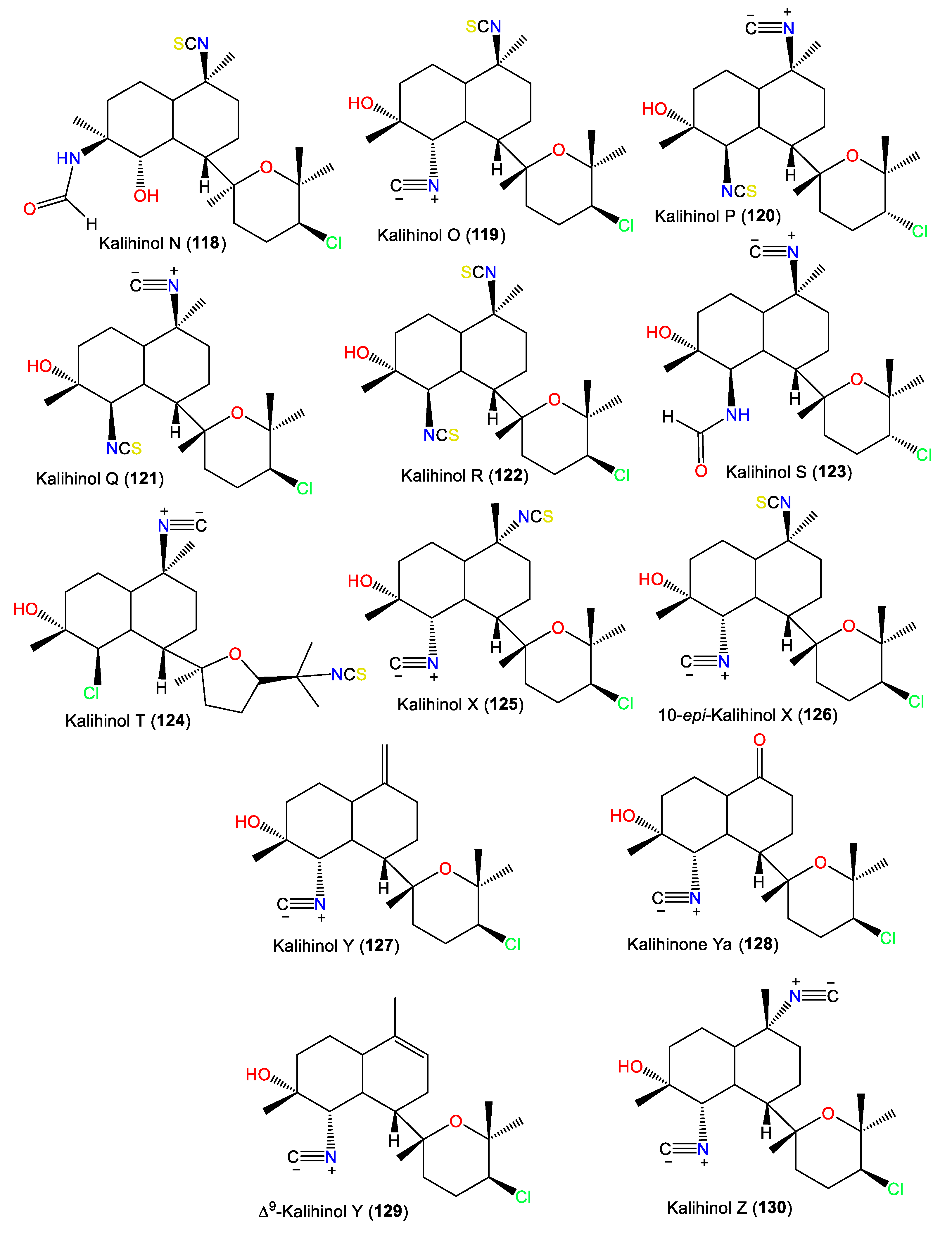
Additionally, 101 was isolated as colorless needles from A. carvenosa by flash chromatography and HPLC. It resembles 100 with a difference in the substitution at C4 and C5 [53]. Additionally, new members of the kalihinols family, 114 and 116, along with 125 and 127, were purified from A.cavernosa collected from Thailand by SiO2 CC and HPLC and identified by extensive NMR data. Compound 114 is similar to 125 with a C-5-N-formyl instead of the isothiocyanoate moiety in 125 [18]. Compounds 117 and 118 are two novel C-4 formamido analogs of isokalihinols reported from the South China Sea specimen of A. cavernosa. They have a trans-decalin ring at C-7 of the tetrahydrofuran and tetrahydropyran rings, respectively. Compound 117 possesses a C-15 chlorine atom and a C-10 isothiocyanato group, whereas 118 is an example of tetrahydropyran-type isokalihinol. They have 1S/4S/5S/6S/7S/10S/11R/14S and 1S/4S/5S/6S/7S/10S/11R/14R configurations, respectively [16]. From Okinawan Acanthella sp., new members of kalihinane-type diterpenes, 110, 115, and 129, were purified from the EtOAc fraction using SiO2 CC and HPLC. Compound 129 is of tetrahydropyran type and is closely similar to 127, with a trisubstituted olefinic bond in 129 instead of the exo-methylene group in 127, while 110 and 115 have three and two isothiocyano groups, respectively [57] (Figure 11).
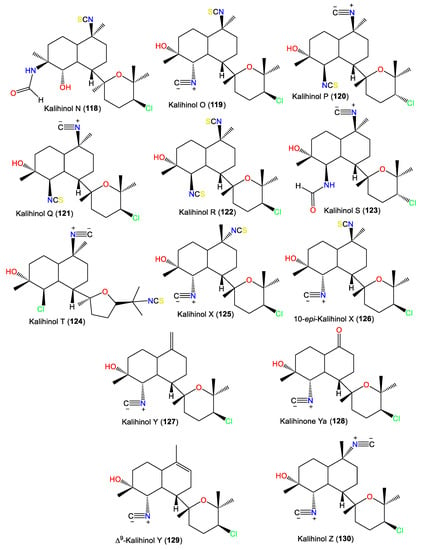
Figure 11.
Kalihinol diterpenes (118–130) reported from the genus Acanthella.
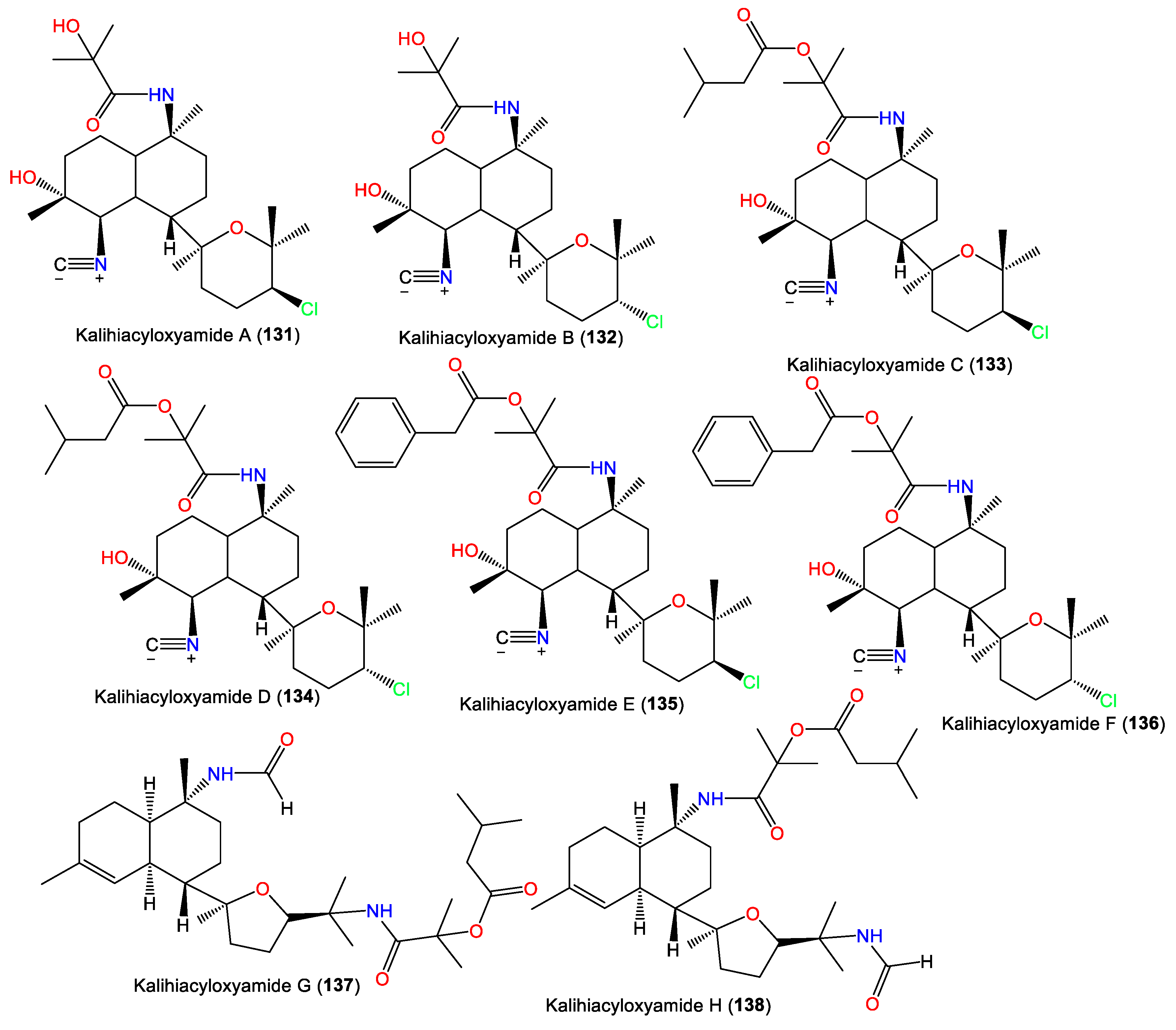
In 1994, Trimurtulu et al. reported new diterpene isonitriles, 103 and 113, from A. carvenosa collected from The Seychelles. Compound 103 differs from 101 in the trans-decalin ring system configuration, whereas 113 has an isothiocyanate group instead of one of the C-10 isonitrile groups of 103 [59]. Besides, Xu et al. reported new diterpenoids, kalihinols O–T (119–124), together with 88, 98, 109, 115, and 126, from A. cavernosa in the South China Sea using SiO2 and Sephadex LH-20 CC [16]. Their structures and stereo-structures were determined by NMR/CD/X-ray analyses [16]. Compound 119 is structurally similar to 98, with a C-10 isothiocyanate instead of a C-10 isonitrile in 98, whereas 120 is an isocyanato analog of 88 and 123 is a C-5 formamide analog of 126. Furthermore, 121 and 122 are the C-14 epimers of 120 and 115, respectively, and 124 is the C-15-isothiocyanato analog of 97 [16]. Clark et al. in 2000, purified a new kalihinol-type diterpenoid, 8-OH-isokalihinol F (102), from A. cavernosa obtained from Heron Island, Great Barrier Reef, Australia, which was structurally similar to 101 with an additional C-8 OH [45]. In addition, new formamide analogs, 104 and 106, were purified by Bugni et al. in 2004 from two Philippine A. cavernosa specimens. They featured formamide moieties at C-10 and C-15, respectively, instead of the isonitrile in 100 [21]. Furthermore, a new kalihinol diterpene, 126, was isolated from Hainan Acanthella sp. by SiO2 and Sephadex LH-20 CC and was assigned as a C-10 epimer of 127 [42]. Additionally, new α-acyloxy-amide-substituted diterpenoids, kalihiacyloxyamides A–H (131–138), were separated from South China Sea A. cavernosa EtOAc fractions using SiO2/Rp-18 CC/RP-HPLC that were elucidated based on spectral, X-ray, and CD analyses (Figure 12). These metabolites featured isobutyl amide (e.g., 131 and 132), iso-amyl ester (e.g., 133, 134, 137, and 138), and phenethyl ester (e.g., 135 and 136) groups [62].
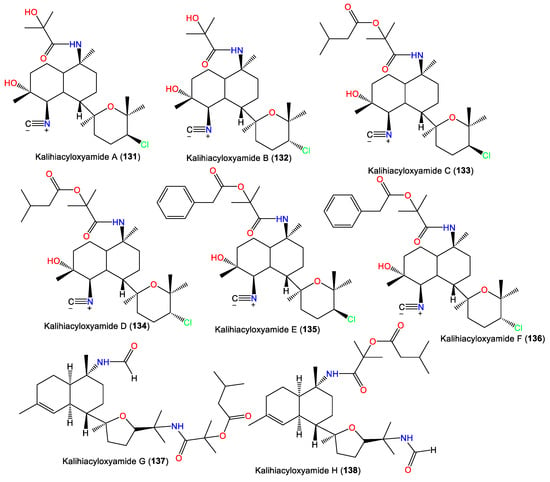
Figure 12.
Kalihinol diterpenes (131–138) reported from the genus Acanthella.
3.2.2. Kalihinenes
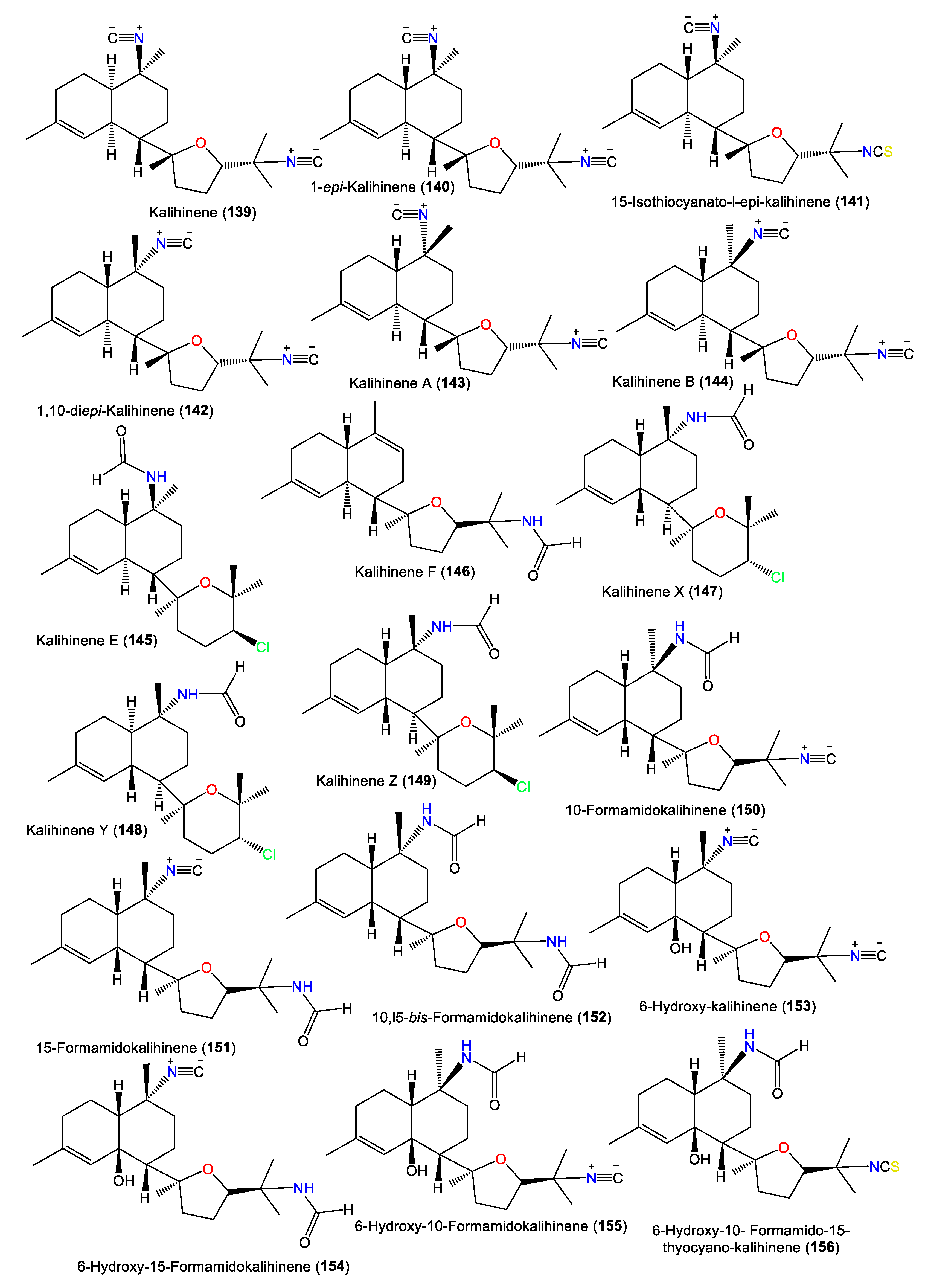
The first member of this group is kalihinene (139), which was purified from an A. klethra EtOH extract using SiO2 CC/Develosil ODS-5 CC/HPLC and assigned by NMR and X-ray analyses [23]. Furthermore, compounds 143 and 144 were reported as novel monounsaturated kalihinane class diterpenes derived from A. Cavernosa toxic CH2Cl2 extracts against Artemia salina and Lebistes reticulatus using VLC/Flash/Rp-18 CC. These two compounds are diastereoisomers of 139 that feature a trans-decalin skeleton instead of the trans-decalin skeleton of kalihinene. On the other hand, 143 and 144 are epimers at C-10 [54]. Additionally, 145 and 146 are tertrahydropyran/trans-decalin and tetrahydrofuran/cis-decalin analogs bearing formamido groups at C-10 and C-15, respectively (Figure 13). Kalihinene E (145) is a C-14 epimer of 148 with 1S/6S/7S/10S/11R/14R configuration [63]. In 1994, Rodríguez et al. purified new diterpenoids belonging to kalihinene and 6-OH kalihinene groups (148 and 150–156), along with 139 from A. cavernosa collected from a Fijian location. These compounds were characterized based on spectral and X-ray analyses as well as biogenetic evidence [51]. Additionally, 140–142 are new metabolites reported from A. carvenosa collected from The Seychelles [59]. Compound 140 is a C-1 isomer of 139, 141 has C-15 isothiocyanate instead of C-15 isonitrile in 140, and 142 is an isomer of 140 and 139 [59].
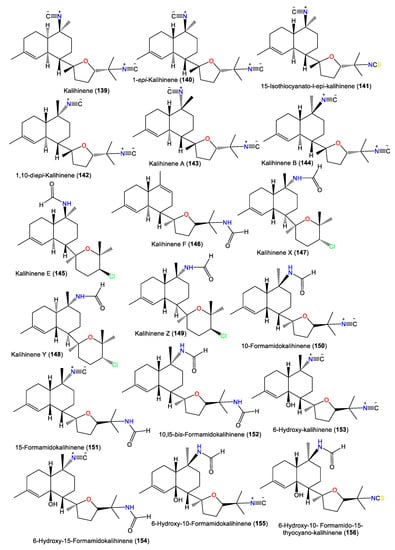
Figure 13.
Kalihinene diterpenes (139–156) reported from the genus Acanthella.
3.2.3. Kalihipyrans and Kalihioxepanes
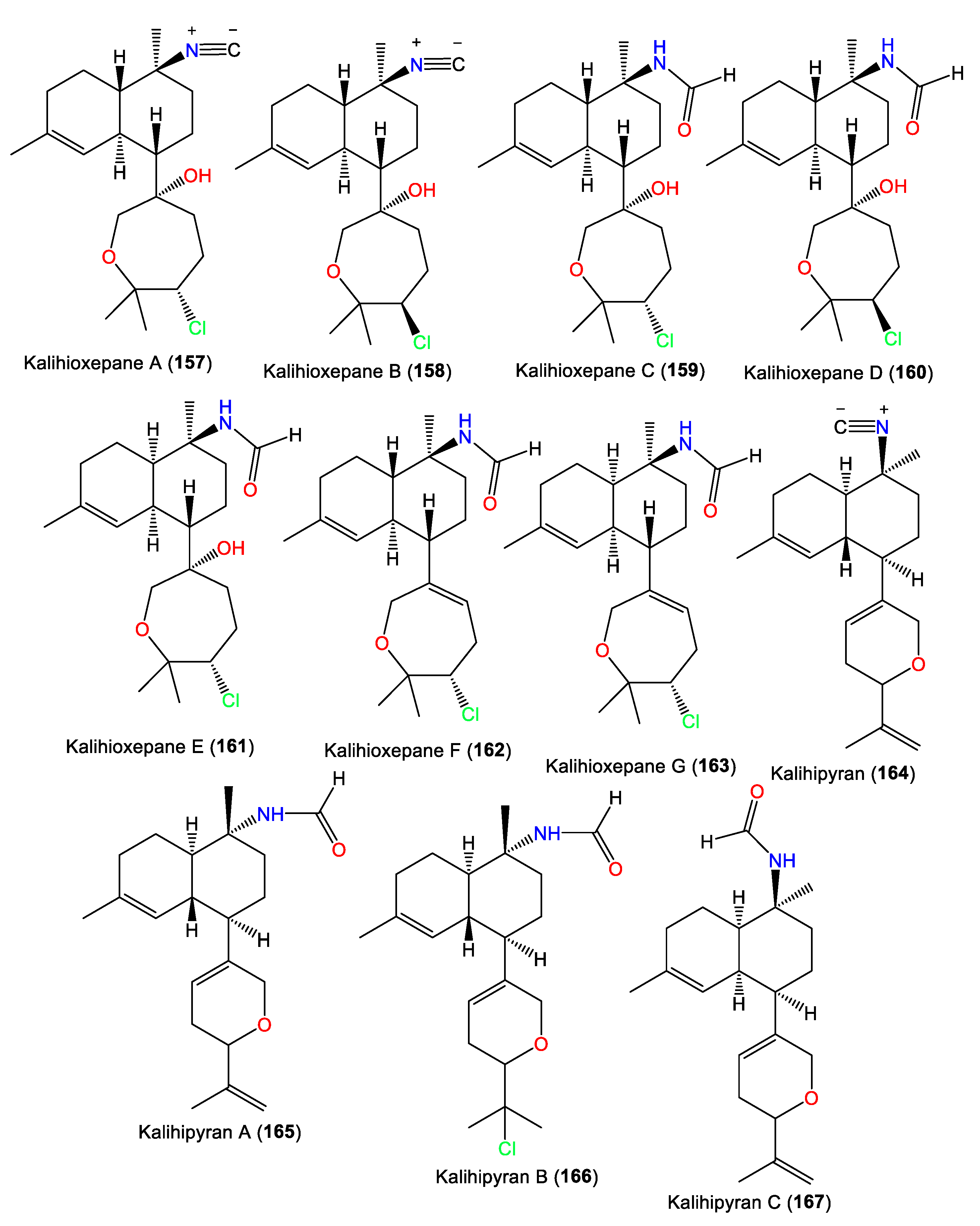
Kalihipyran (164) is a tricyclic kalihinene-type diterpene with a C-7 isopropenyl-containing dihydropyran moiety and a C-10 isonitrile [59]. Compound 167 is an isomer of 165 with cis-decalin [63], while 166 has a C-15 chlorine atom [55,56]. In 2022, Wang et al. purified new kalihinane diterpenoids, kalihioxepanes A–G (157–163), from South China Sea A. cavernosa by the means of SiO2/Sephadex LH-20/HPLC. The structures were elucidated by spectral and X-ray analyses, in addition to quantum chemical calculation methods [64] (Figure 14).
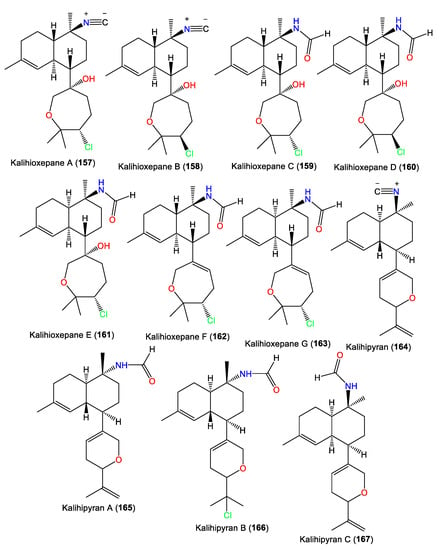
Figure 14.
Kalihioxepanes (157–163) and kalihipyrans (164–167) diterpenes reported from the genus Acanthella.
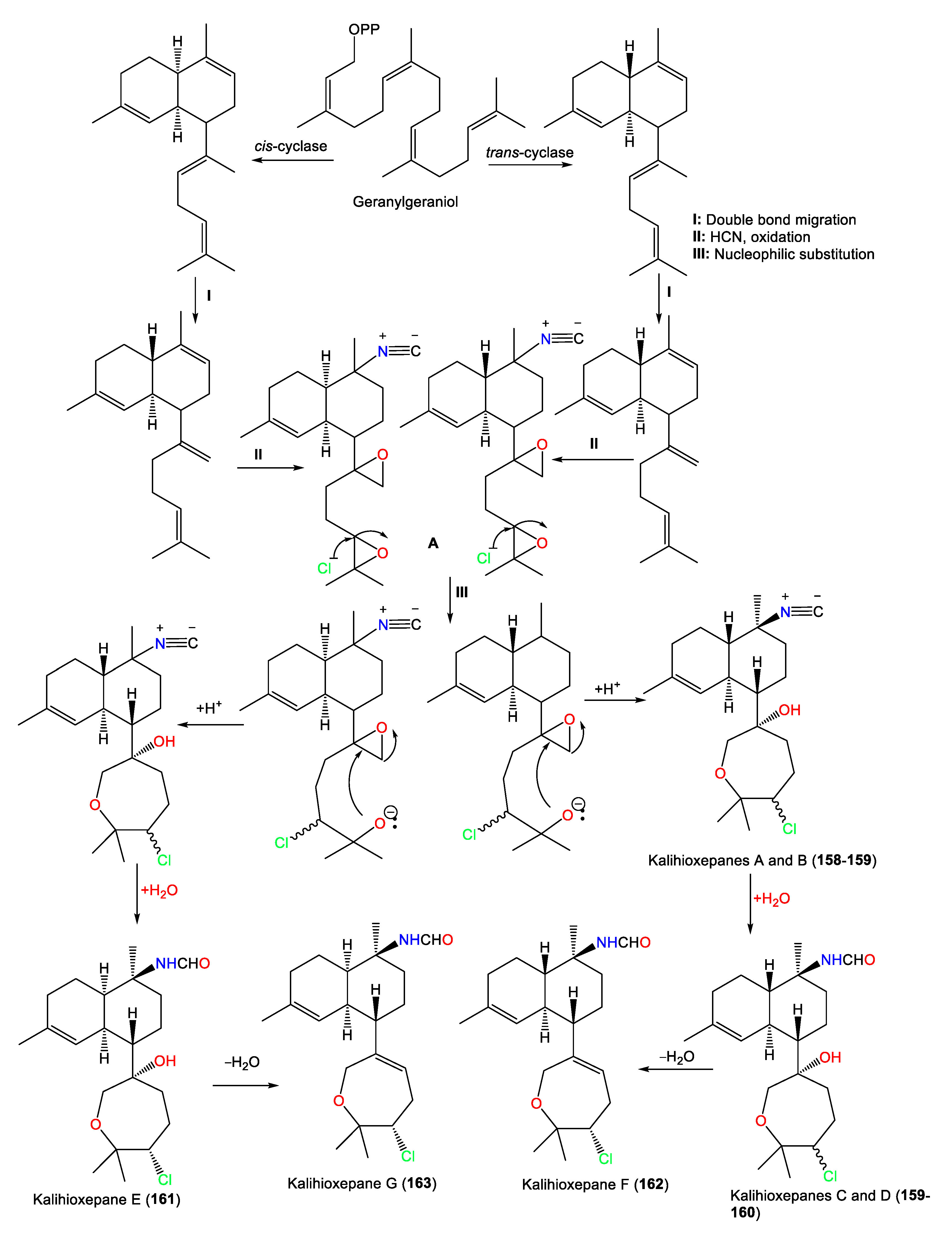
These metabolites possess a rare C-7-attached oxepane ring with a C-14 Cl atom. Compounds 157–160 have a trans decalin skeleton; however, 161–163 have a cis-decalin skeleton with C-10 isonitrile (e.g., 157 and 158) and formamide (e.g., 159–163) groups [64]. Wang et al. proposed that 157–163 are biosynthesized using geranylgeraniol as a precursor (Scheme 2). The latter undergoes a series of reactions, including cyclization, double-bond migration, nucleophilic addition, and oxidation reactions, to give epoxide biflorane (A, the key intermediate). Nucleophilic substitution of biflorane forms 157 and 158. After that, hydration generates 159–161 which are then dehydrated to give 162 and 163, respectively [64].
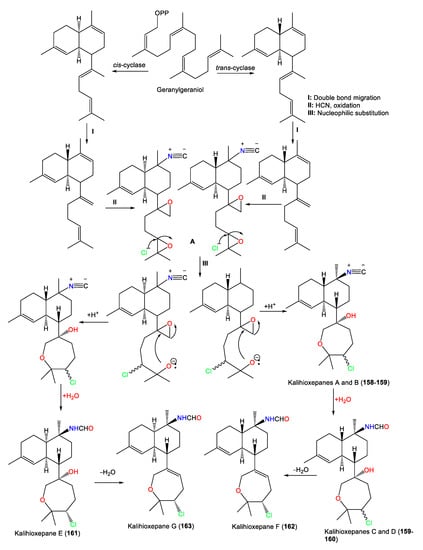
Scheme 2.
Biosynthesis of kalihioxepanes A–G (157–163) [64].
3.2.4. Biflorane Diterpenes
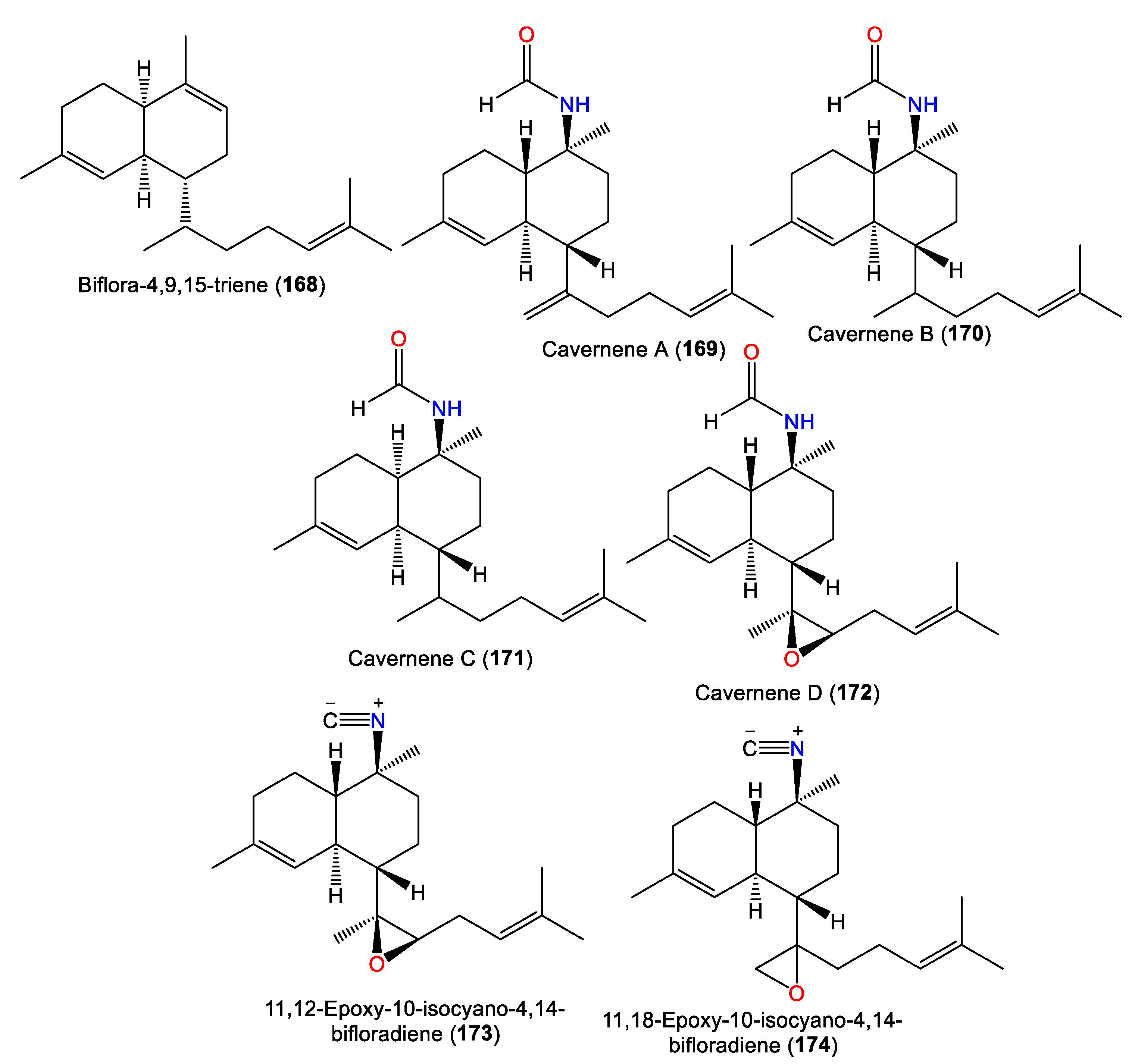
From the Japanese A. cavernosa, biflora-4,9,15-triene (168) was separated, which is a rare biflorane diterpene related to 66, by replacing the methyl hydrogen of the isopropyl group of 66 with a prenyl group [40]. In 2012, Xu et al. reported 169–172 from CH2Cl2 extracts of South China Sea A. cavernosa, bearing a C-10 formamide group that varied in the decalin moiety (cis or trans) configuration and nature of C-7-linked side chain [63] (Figure 15). Their structures were assigned by spectral and X-ray analyses. Compounds 169, 170, and 172 are trans-decalin derivatives, with a C-7 isoprenoid unit, a mono-olefinic isoprenoid sidechain, and a trisubstituted epoxide in the side chain, respectively. In contrast, 171 had a cis-decalin moiety [63].
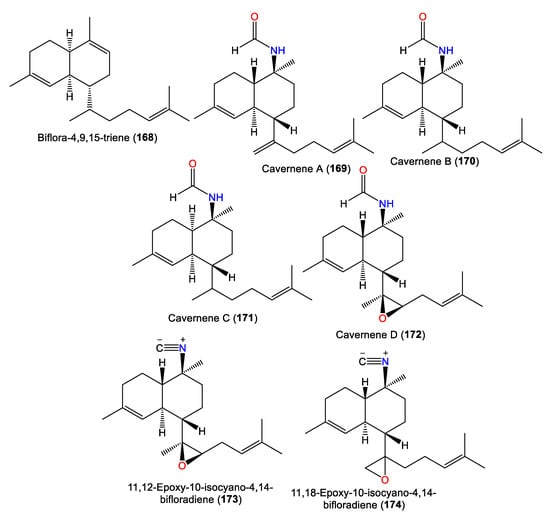
Figure 15.
Biflorane (168–174) diterpenes reported from the genus Acanthella.
Investigation of A. cavernosa DCM/MeOH extracts led to the separation of two oxirane analogs with a trans-decalin framework, 173 and 174, featuring a trisubstituted epoxide and a terminal epoxide group in the side chain, respectively. Compound 174 was suggested to be a precursor of the kalihipyran skeleton [45]. Clark et al. proposed that the biosynthesis of pyranyl and furanyl kalihinols involves epoxidation of the bifloradiene precursor’s terminal double bond by a nucleophilic attack at either epoxide end by a cyanide ion to form a hydroxyisocyanide. The latter initiates cyclisation to afford a bicyclic system (Scheme 3). Compounds 173 and 174 are alternative epoxidation products. Compound 174 was suggested to be a precursor of the kalihipyran skeleton [45].
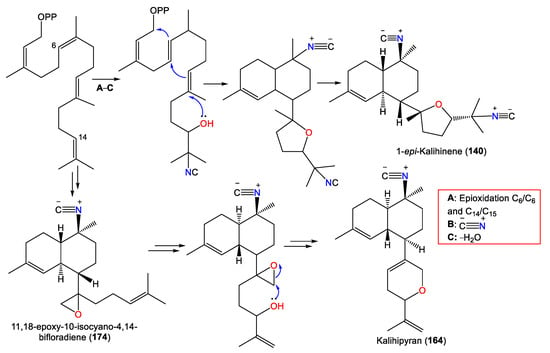
Scheme 3.
Biosynthesis of 140, 164, and 174 [45].
3.3. Alkaloids
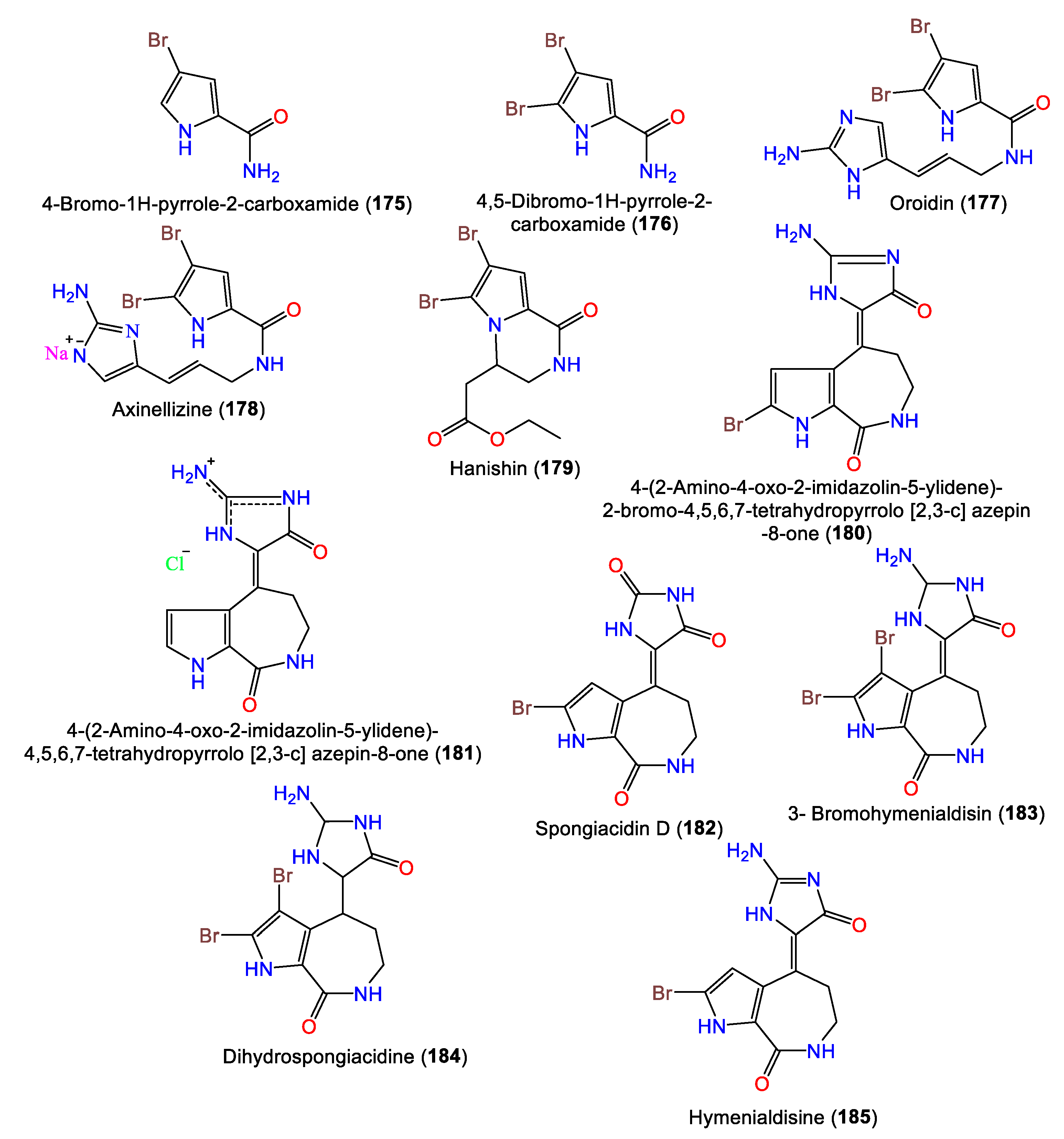
Several reports have stated the isolation of different classes of alkaloids from this genus. It is noteworthy that bromopyrrole alkaloids are the dominant type reported from the species of this genus (Table 4). Oroidin 177 is the first member of pyrrole 2-aminoimidazole alkaloids. These alkaloids were reported to have significant bioactivities, as well as chemical defense against predator fish.

Table 4.
Alkaloids reported from the genus Acanthella (molecular weight and formulae, chemical class, species, and sampling locations).
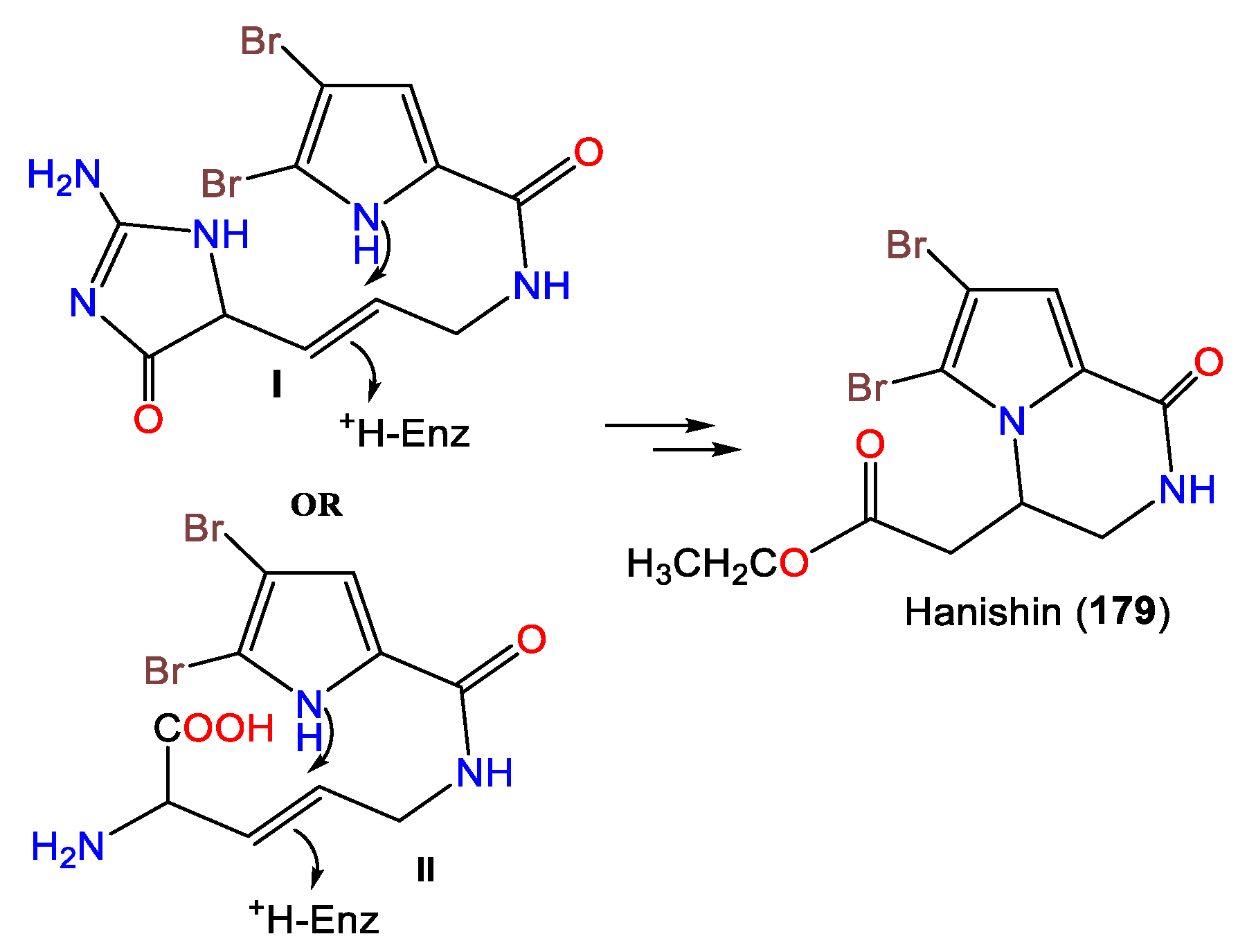
In 2010, Hammami et al. purified a novel bromopyrolimidazole analog, 178, along with 177 from Tunisian A. acuta diethyl ether extracts [25]. Four bromo-pyrrole alkaloids, including novel alkaloid hanishin (179) in addition to 175–177, were isolated from A. carteri collected from the northern coast of Hanish Island, Yemen, South Red Sea, by Mancini et al. Compounds 177 and 179 are members of the oroidin family of alkaloids that are considered condensation products of prolines. Compound 179 was proposed to be derived from aminoimidazolinone (I) or amino acid (II) intermediates through 1N-C9 cyclization with subsequent side-chain oxidative breakdown [66] (Scheme 4).
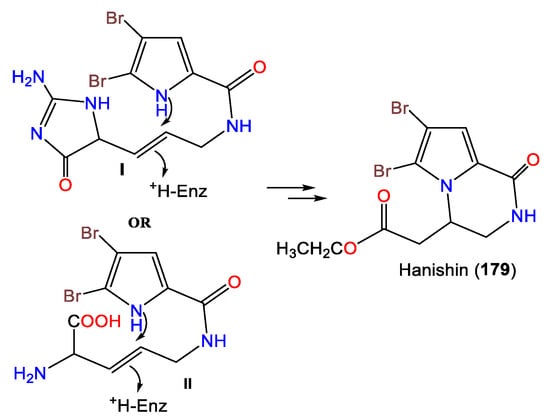
Scheme 4.
Proposed biosynthesis of 179 [66].
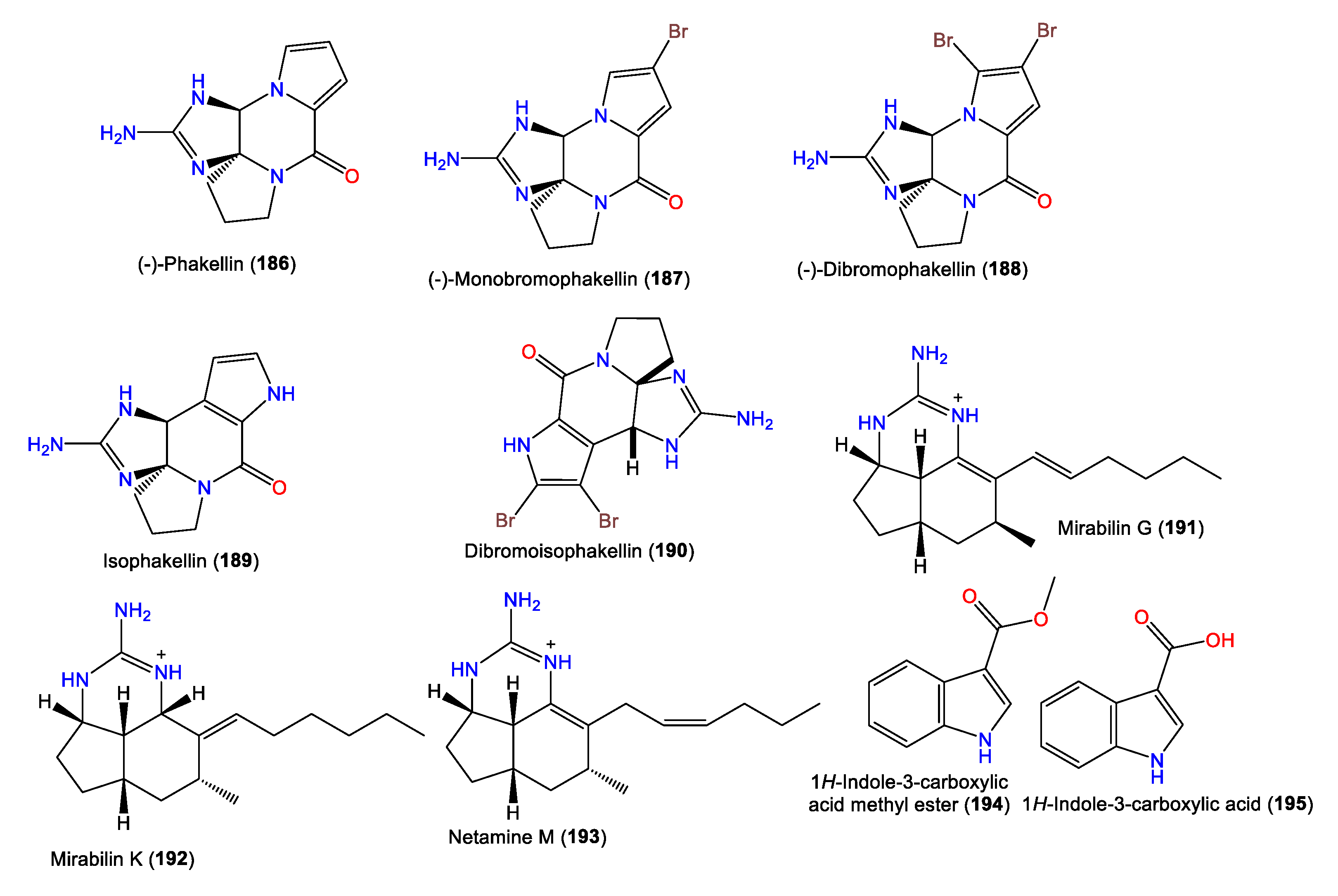
Mattia et al. purified 180 as a brominated alkaloid from Red Sea A. Aurantiaca BuOH extracts. The compound features an aminooxodihydroimidazole ring linked to a pyrroloazepine group via a double bond (Figure 16) [68]. Compounds 180 and 181 were obtained from A. aurantiaca BuOH extracts using Sephadex LH-20 and crystallization and were characterized by spectral and X-ray analyses [67]. In 2014, Macabeo and Guce reported the bromopyrrole-imidazole alkaloids 182–184 from CH2Cl2-MeOH extracts of A. carteri from The Philippines [17], while 185 is a pyrrole alkaloid isolated from the n-BuOH fraction of Acanthella sp. using Sephadex LH-20/Rp-18 CC [69].
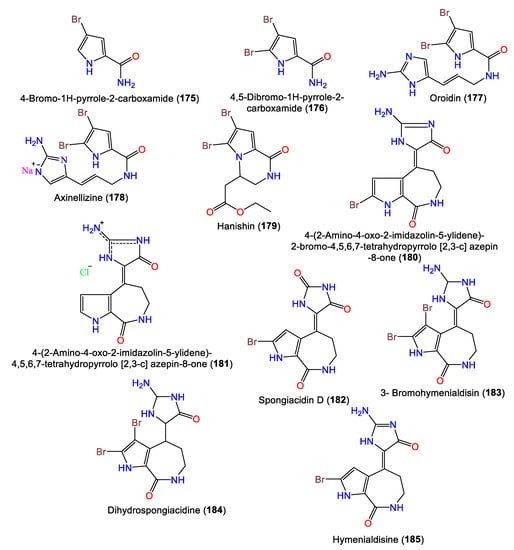
Figure 16.
Alkaloids (175–185) reported from genus Acanthella.
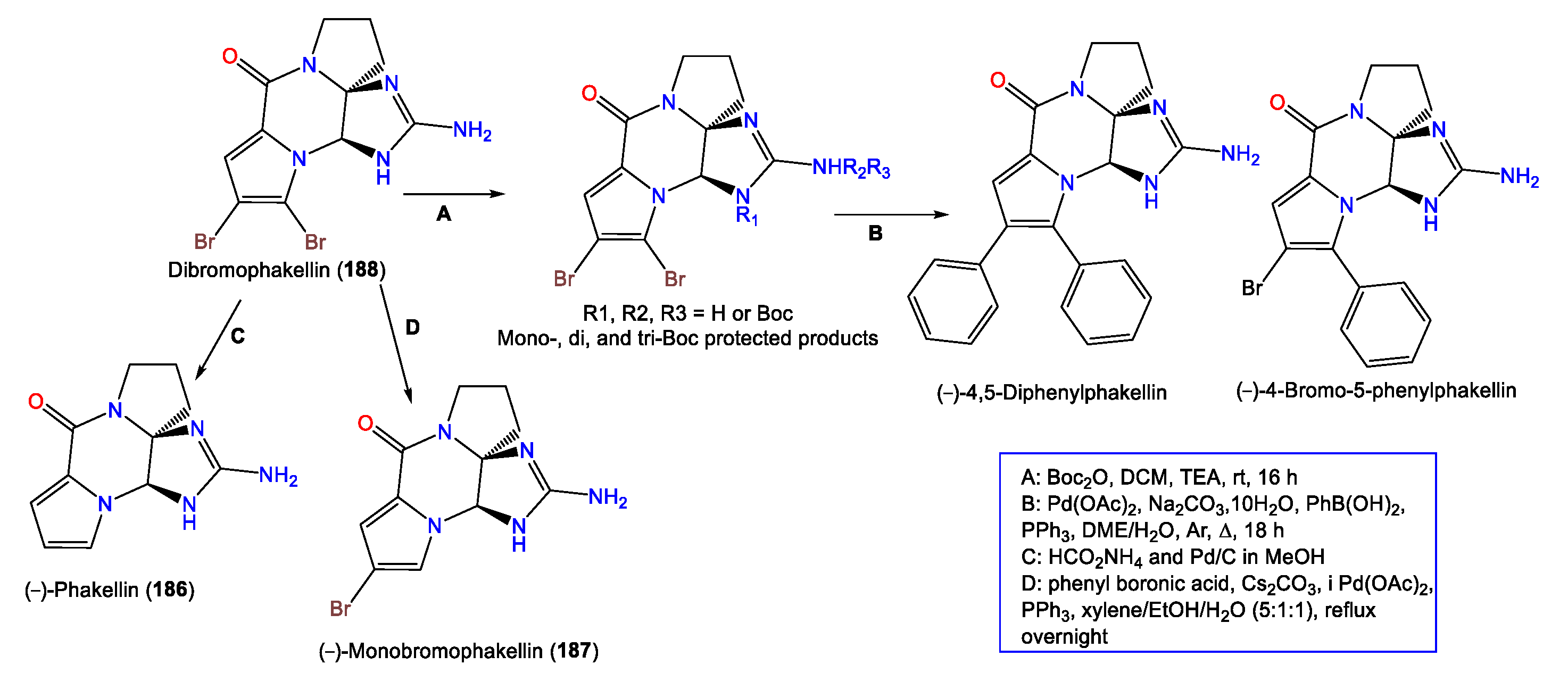
A series of synthetic reactions including Suzuki–Miyaura coupling and debromination resulted in natural analogs 186 and 187, in addition to new synthetic derivatives (−)-4-bromo-5-phenylphakellin and (−)-4,5-diphenylphakellin. It was found that the C-5 Br substitution with phenyl or H led to a loss in activity, revealing that the C-5 Br is important for α2B adrenoceptor agonistic activity (Scheme 5) [70].
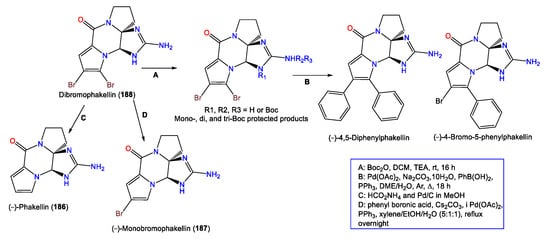
Scheme 5.
Semisynthesis of (−)-dibromophakellin (188) derivatives [70].
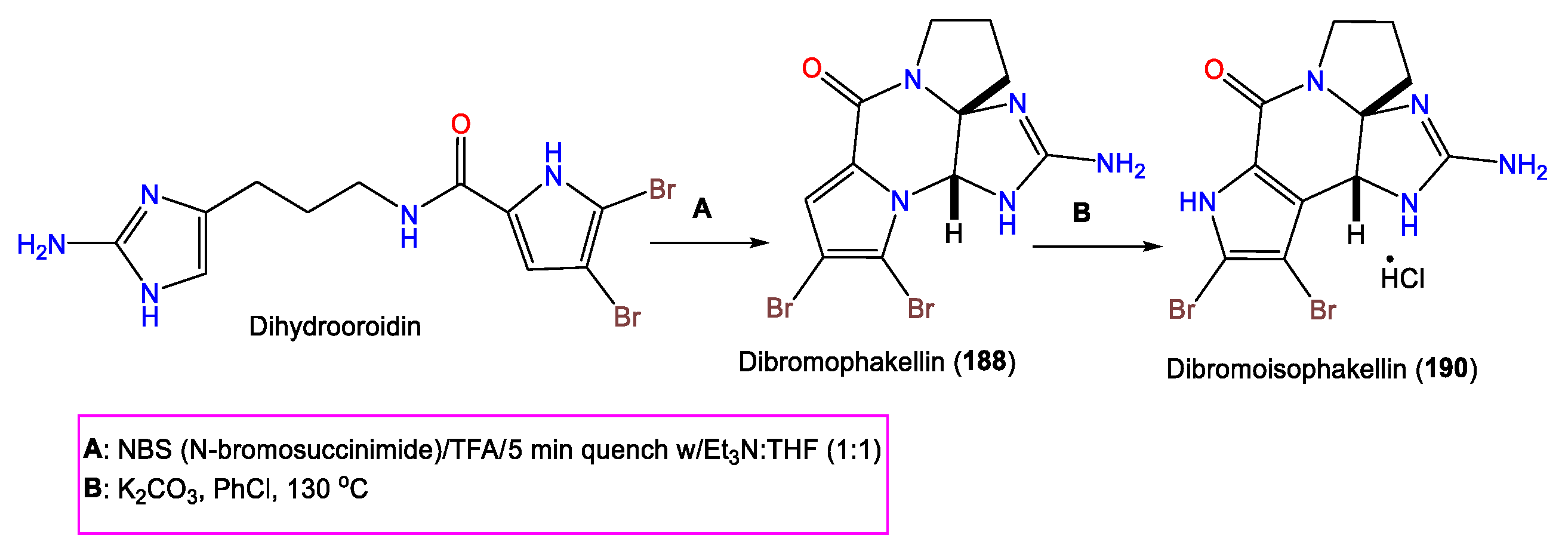
Furthermore, 190 was purified from A. carteri using Sephadex LH-20/SiO2 CC, giving a bright-orange color with a diazotized benzidine. The compound was characterized by NMR and X-ray analyses, as well as chemical methods. Compound 190 is a 6R/10S brominated alkaloid with a fused C-C pyrrole linkage to the cyclic guanidine core belonging to the 189 series [71].
In 2002, Wiese and his group reported the synthesis of 190 using dihydrooroidin that is converted to 188 (Scheme 6). Then, thermal rearrangement of 188 in the presence of K2CO3 produces 190 [73].
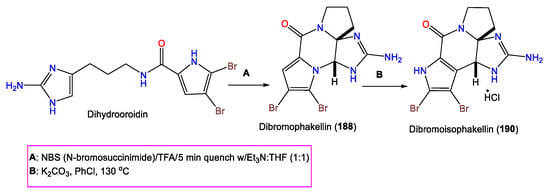
Scheme 6.
Synthesis of 190 using dihydrooroidin [73].
Additionally, Grkovic et al. were able to separate tricyclic-guanidine-containing alkaloids, including a new analog mirabilin K (192), along with 191 and 193, from A. cavernosa collected in Southwestern Australia using diol flash chromatography/Rp-18/HPLC. The compounds were characterized by spectroscopic analyses and optical rotation measurements. Compound 192 has a 4S*/7S*/9R*/11S*/12R* configuration, which differs from 191 in the C9-CH3 group and with the presence of a N-substituted methine group (Figure 17) [24]. Furthermore, 194 and 195 were obtained by Fan et al. from the acetone extracts of A. cavernosa collected from the South China Sea [50].
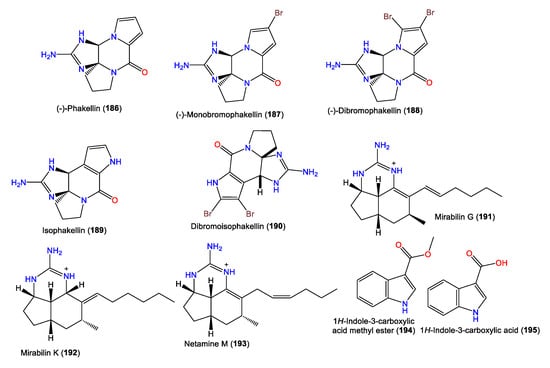
Figure 17.
Alkaloids (186–195) reported from the genus Acanthella.
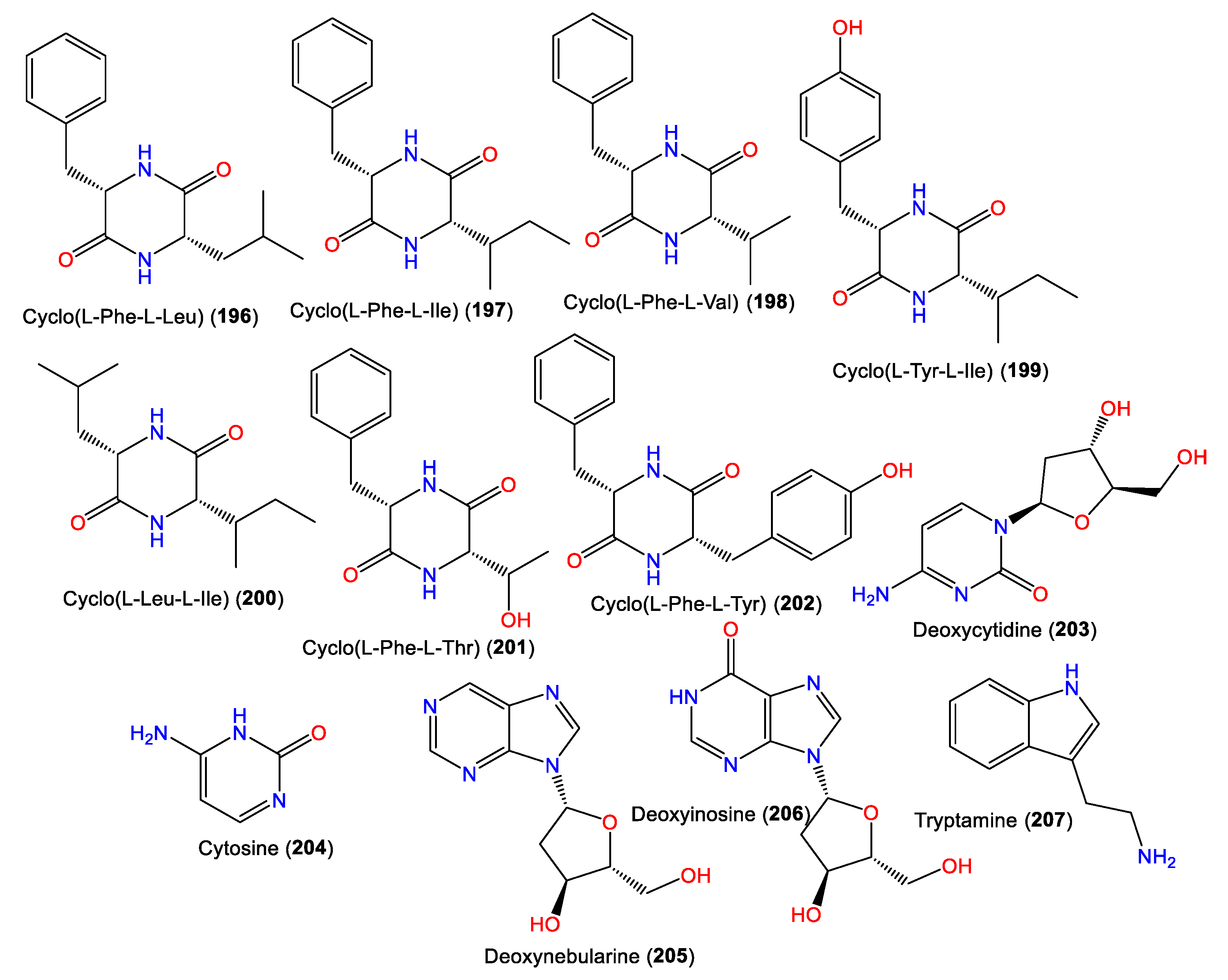
Diketopiperazines, including the rare cyclo(L-Phe-L-Thr) and cyclo(L-Tyr-L-Ile) (196–202), along with decarboxylated amino acid 207 and deoxyribonucleotides 203–206, were reported and characterized from Fijian A. cavernosa (Figure 18). Their L-L absolute configuration was assigned based on an NMR and CD comparison with synthetic L-L analogs, as well as optical rotation measurements [72].
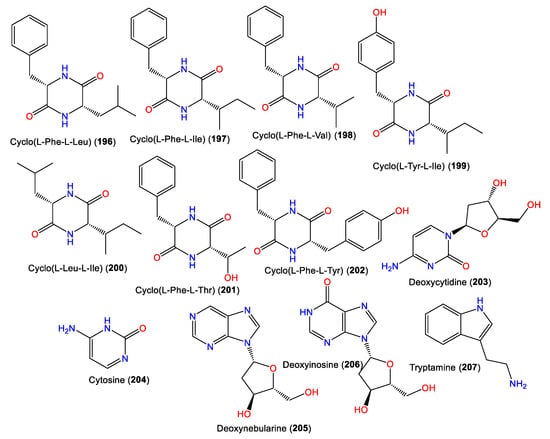
Figure 18.
Alkaloids (196–207) reported from the genus Acanthella.
3.4. Steroid Compounds
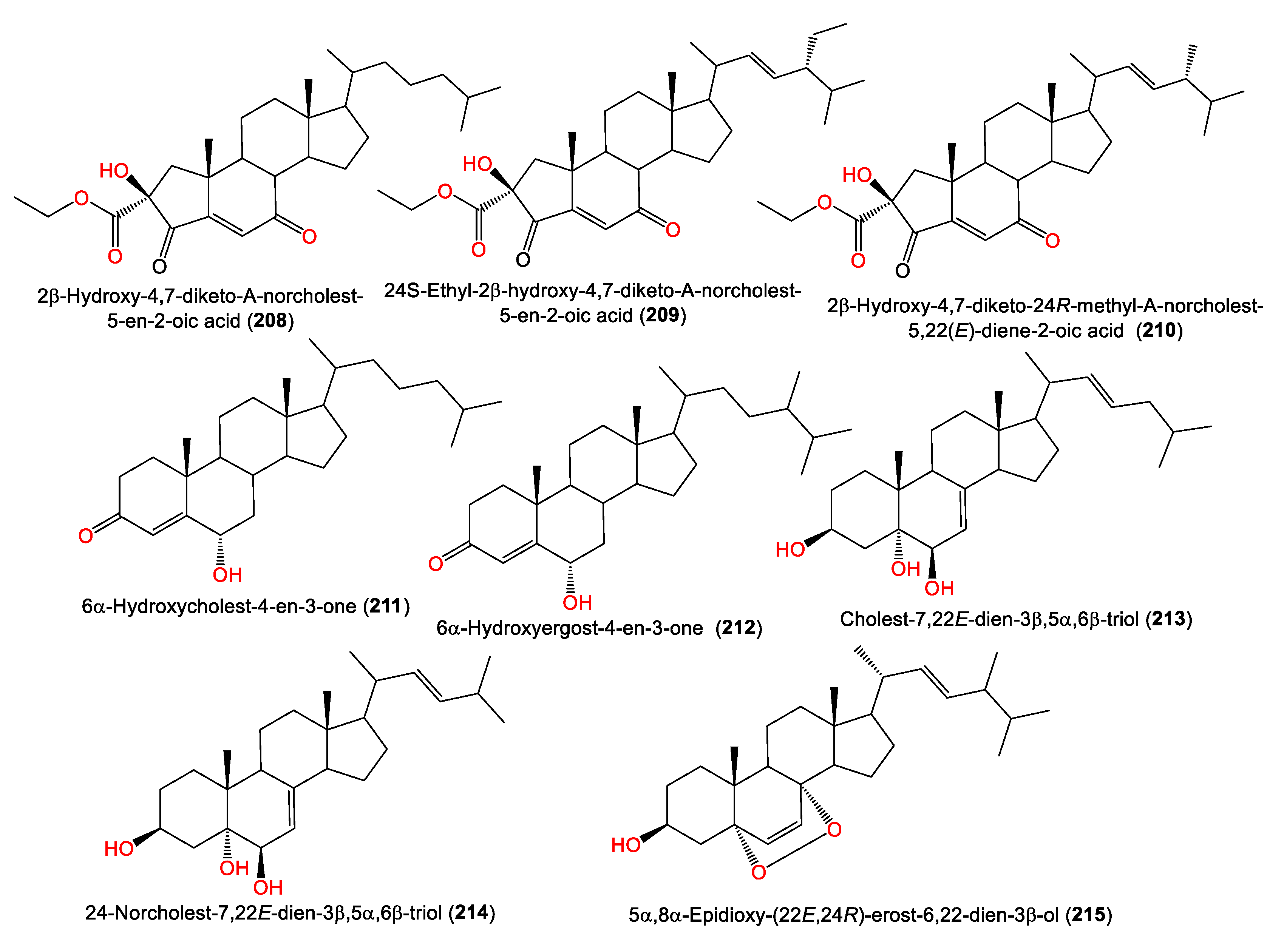
In 2008, Qui et al. reported the purification of three new nor-steroids, 208–210, along with the known steroids 211–214 from the petroleum ether fraction of A. cavernosa obtained from Hainan Island, China, using SiO2 CC/HPLC. The new steroids are related to A-ring-contracted steroid analogs featuring carbonyl and ketone groups located at C-3 and C-4; they differ in their C-17 side chains [74] (Table 5 and Figure 19). In addition, 215 was obtained from the acetone extract of the same sponge collected from the South China Sea [50].

Table 5.
Sterols and other metabolites reported from the genus Acanthella (molecular weight and formulae, chemical class, species, and sampling locations).
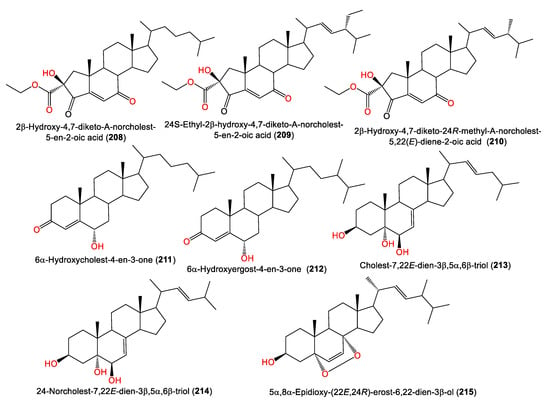
Figure 19.
Steroid compounds (208–215) reported from the genus Acanthella.
3.5. Other Metabolites
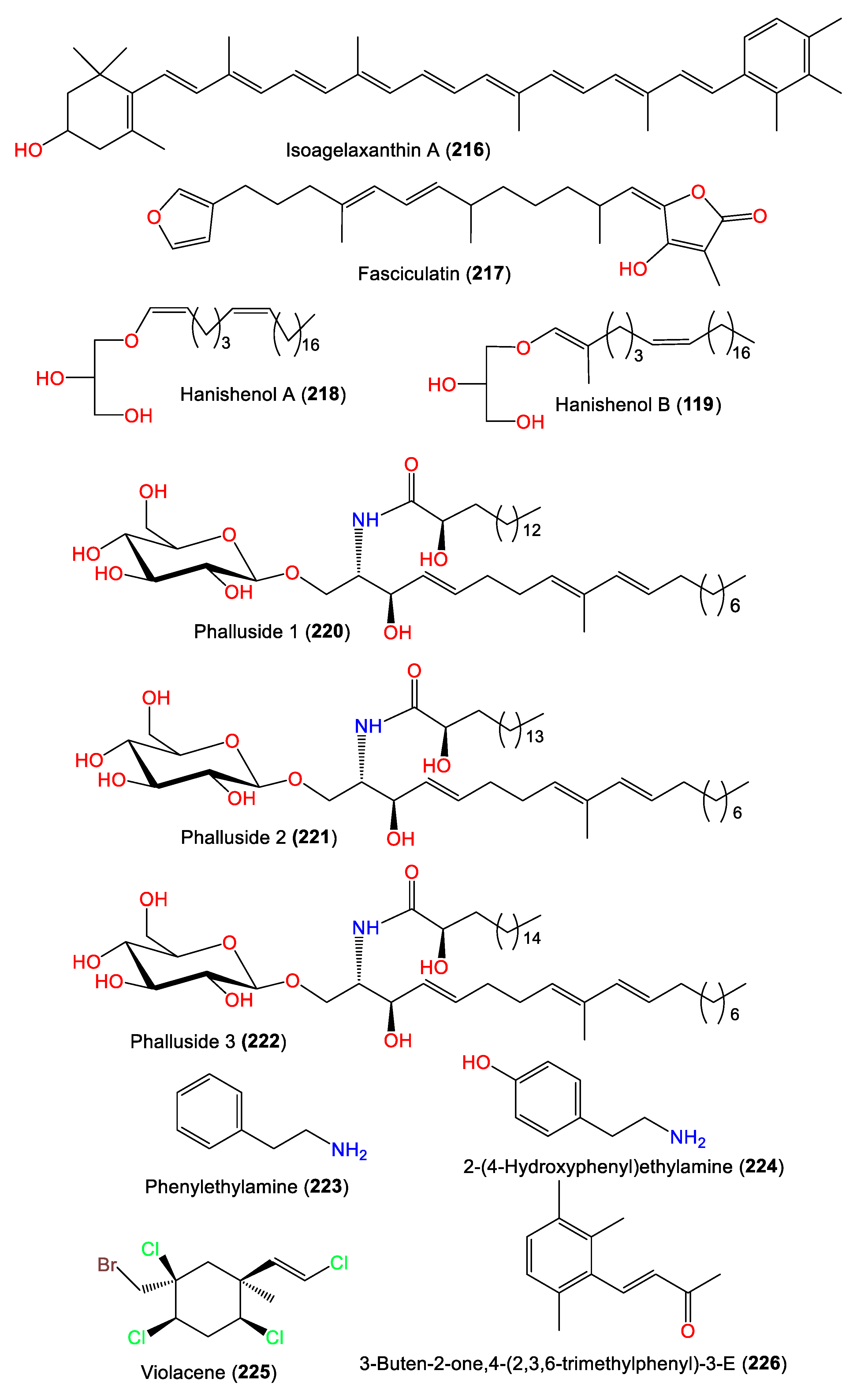
Compound 216 was separated from A. vulgata acetone extracts using an MgO column and crystallization from petroleum ether. The compound belongs to carotenoids, as it has a polyene chain with terminal aromatic moieties on both ends [75] (Figure 20). Mancini et al. were able to purify and characterize 219, a novel methyl-branched glycerol enol ether, and the related linear analog 218 from A. carteri obtained from Southern Red Sea Hanish Islands by utilizing flash CC/HPLC and spectral and chemical methods [76]. Compound 219 has an additional methyl group at C-2 of the sidechain compared to 218, and both have a 2`S configuration [76]. In 2010, Hammami et al. separated the sesterterpene 217 and cerebrosides 220–222 from Tunisian A. acuta diethyl ether extracts [25], whereas 226 was purified from the Chinese A. cavernosa by Fan et al. [50].
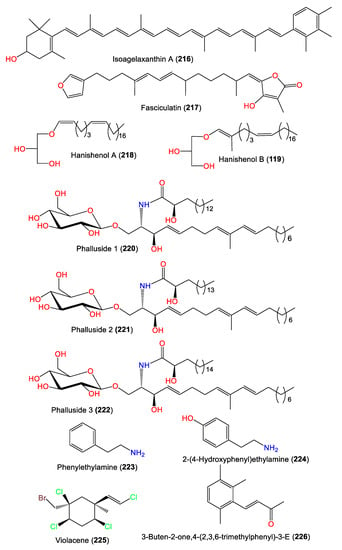
Figure 20.
Other metabolites (216–226) reported from the genus Acanthella.
4. Biological Activities of Acanthella Species and Their Metabolites
Various Acanthella species and their metabolites have been found to display various bioactivities. The reported investigations are highlighted below, and some results are listed in Table 6.
4.1. Antimicrobial and Antifouling Activities
McCaffrey and Endean reported that A. kleutha’s toluene/methanol (1:3 v/v) and CH2Cl2 extracts displayed antimicrobial potential comparable with penicillin G and streptomycin versus B. subtilis, K. pneumoniae, and S. aureus [77]. The A. carteri MeOH extract that was collected from Ras Nusrani in the Gulf of Aqaba was significantly effective versus B. subtilis, S. aureus, P. vulgaris, E. coli, C. tropicalis, and C. albicans (inhibition zone 9.0–23.3 mm) [78]. On the other hand, the n-BuOH fractions of A. acuta showed more promising antimicrobial potential versus A. niger, C. albicans, and S. aureus than CH3Cl fractions [79]. The MeOH extract of Acanthella elongata caused 100% and 87.5% inhibition of marine fish pathogens Aeromonas hydrophila, Pseudomonas aeruginosa, Vibrio alginolyticus, V. anguillarum, V. fischeri, V. fluvialis, V. pelagius, and V. vulnificus at 30°C and 20°C [20]. Additionally, A. cavernosa and A. ramosa from the Bay of Bengal exhibited activity versus the virulent fish pathogens Edwardsiella tarda, A. hydrophila, P. aeruginosa, V. alginolyticus, and P. fluorescens [80]. Rajendran et al. reported that the A. elongata CH2Cl2 fraction had antimicrobial properties versus V. alginolyticus (fish pathogen) and R. solani, while the EtOH extract prohibited Vibrio fisherii and Micrococcus sp. [81]. Nogata et al. reported that among the tested 86 Japanese sponge species, the EtOH extract of A. cavernosa obtained from Atami, Shizuoka prefecture, Tokyo, displayed 100% inhibition of B. amphitrite larval settlements with no toxicity [49]. Additionally, Okino et al. reported that the EtOH extract of A. cavernosa obtained from Yakushima Island had antifouling potential as it prohibited metamorphosis and larval settlement of the Balanus amphitrite barnacle [56].

Table 6.
Biological activity of reported metabolites from the genus Acanthella.
Table 6.
Biological activity of reported metabolites from the genus Acanthella.
| Compound Name | Biological Activity | Assay, Organism or Cell Line | Biological Results | Ref. | |
|---|---|---|---|---|---|
| Compound | Positive Control | ||||
| (+)-10(R)-Isothiocyanatoalloaromadendrane (9) | Cytotoxicity | MTT/A549 | 1.98 µg/mL (IC50) | - | [42] |
| Axisonitrile-3 (17) | Antimalarial | Plasmodium falciparum-D6/Microculture radioisotope | 142.0 ng/mL (IC50) | Chloroquine 1.95 ng/mL (IC50) | [19] |
| Plasmodium falciparum-W2/Microculture radioisotope | 16.5 ng/mL (IC50) | Chloroquine 22.8 ng/mL (IC50) | [19] | ||
| Cytotoxicity | MTT/A549 | 2.44 µg/mL (IC50) | - | [42] | |
| 11-Isothiocyano-7βH-eudesm-5-ene (42) | Antimalarial | Plasmodium falciparum-D6/Microculture radioisotope | 2240.0 ng/mL (IC50) | Chloroquine 1.95 ng/mL (IC50) | [19] |
| Plasmodium falciparum-W2/Microculture radioisotope | 610.0 ng/mL (IC50) | Chloroquine 22.8 ng/mL (IC50) | [19] | ||
| (lR,5R,6R,8S)-Dec[4.4.0]ane-1,5-dimethyl-8-(1′-methylethenyl)-5-isothiocyanate (45) | Antimalarial | Plasmodium falciparum-D6/Microculture radioisotope | 4000.0 ng/mL (IC50) | Chloroquine 1.95 ng/mL (IC50) | [19] |
| Plasmodium falciparum-W2/Microculture radioisotope | 550.0 ng/mL (IC50) | Chloroquine 22.8 ng/mL (IC50) | [19] | ||
| Kalihinol A (88) | Antibacterial | Microbroth dilution/B. subtilis PY79 | 50.0 μg/mL (MIC) | -Trimethoprim 1.0 μg/mL (MIC) -Ciprofloxacin 1.0 μg/mL (MIC) -Rifampin 1.0 μg/mL (MIC) -Sulfamethoxazole 4.0 μg/mL (MIC) -Chloramphenicol 16.0 μg/mL (MIC) -Polymyxin B 16.0 μg/mL (MIC) | [21] |
| Disc-diffusion/Ruegeria CtaxMed-2 | 0.67 mm (IZD) at 10 μg/Disc | Streptomycin 0.83 mm (IZD) at 10 μg/Disc | [58] | ||
| Disc-diffusion/Vibrio sp. (NAP-4) | 1.0 mm (IZD) at 10 μg/Disc | Streptomycin 1.83 mm (IZD) at 10 μg/Disc | [58] | ||
| Disc-diffusion/Vibrio furnissii | 6.33 mm (IZD) at 10 μg/Disc | Streptomycin 2.50 mm (IZD) at 10 μg/Disc | [58] | ||
| Antifouling | Settlement inhibition/Balanus amphitrite | 0.087 µg/mL (IC50) | CuSO4 0.15 µg/mL (IC50) | [55,56] | |
| Settlement inhibition/Balanus amphitrite | 0.92 µM (EC50) | Seawater + DMSO | [16] | ||
| Cytotoxicity | MTT/HCT-116 | 17.40 µM (IC50) | - | [16] | |
| trans and cis 10β-Formamidokalihinol A (89/90) | Antibacterial | Disc-diffusion/Vibrio sp. (NAP-4) | 1.0 mm (IZD) at 10 μg/Disc | Streptomycin 1.83 mm (IZD) at 10 μg/Disc | [58] |
| Disc-diffusion/Vibrio furnissii | 5.67 mm (IZD) at 10 μg/Disc | Streptomycin 2.50 mm (IZD) at 10 μg/Disc | [58] | ||
| Antifouling | Settlement inhibition/Balanus amphitrite | 1.37 µM (EC50) | Seawater + DMSO | [16] | |
| Cytotoxicity | MTT/CT-26 | 28.82 µM (IC50) | - | [16] | |
| 10β-Formamido-5β-isothiocyanatokalihinol A (92) | Antifouling | Settlement inhibition/Balanus amphitrite | 0.41 µM (EC50) | Seawater + DMSO | [16] |
| Isokalihinol B (95) | Cytotoxicity | MTT/P388 | 0.8 μg/mL | - | [23] |
| Kalihinol D (97) | Cytotoxicity | MTT/A549 | 3.17 μg/mL (IC50) | - | [42] |
| Kalihinol E (98) | Antifouling | Settlement inhibition/Balanus amphitrite | 1.85 µM (EC50) | Seawater + DMSO | [16] |
| Cytotoxicity | MTT/HCT-116 | 18.31 µM (IC50) | - | [16] | |
| Kalihinol F (100) | Antibacterial | Microbroth dilution/B. subtilis PY79 | 12.5 μg/mL (MIC) | -Trimethoprim 1.0 μg/mL (MIC) -Ciprofloxacin 1.0 μg/mL (MIC) -Rifampin 1.0 μg/mL (MIC) -Sulfamethoxazole 4.0 μg/mL (MIC) -Chloramphenicol 16.0 μg/mL (MIC) -Polymyxin B 16.0 μg/mL (MIC) | [21] |
| Trans 10-Formamido-kalihinol F (104/105) | Antibacterial | Microbroth dilution/B. subtilis PY79 | 50.0 μg/mL (MIC) | -Trimethoprim 1.0 μg/mL (MIC) -Ciprofloxacin 1.0 μg/mL (MIC) -Rifampin 1.0 μg/mL (MIC) -Sulfamethoxazole 4.0 μg/mL (MIC) -Chloramphenicol 16.0 μg/mL (MIC) -Polymyxin B 16.0 μg/mL (MIC) | [21] |
| 15-Formamido-kalihinol F (106/107) | Antibacterial | Microbroth dilution/B. subtilis PY79 | 50.0 μg/mL (MIC) | -Trimethoprim 1.0 μg/mL (MIC) -Ciprofloxacin 1.0 μg/mL (MIC) -Rifampin 1.0 μg/mL (MIC) -Sulfamethoxazole 4.0 μg/mL (MIC) -Chloramphenicol 16.0 μg/mL (MIC) -Polymyxin B 16.0 μg/mL (MIC) | [21] |
| Kalihinol G (108) | Antibacterial | Microbroth dilution/B. subtilis PY79 | 3.12 μg/mL (MIC) | -Trimethoprim 1.0 μg/mL (MIC) -Ciprofloxacin 1.0 μg/mL (MIC) -Rifampin 1.0 μg/mL (MIC) -Sulfamethoxazole 4.0 μg/mL (MIC) -Chloramphenicol 16.0 μg/mL (MIC) -Polymyxin B 16.0 μg/mL (MIC) | [21] |
| 10-epi-Kalihinol I (115) | Antifouling | Settlement inhibition/Balanus amphitrite | 0.27 µM (EC50) | Seawater + DMSO | [16] |
| Cytotoxicity | MTT/HCT-116 | 28.67 µM (IC50) | - | [16] | |
| Kalihinol J (116) | Antibacterial | Microbroth dilution/B. subtilis PY79 | 50.0 μg/mL (MIC) | -Trimethoprim 1.0 μg/mL (MIC) -Ciprofloxacin 1.0 μg/mL (MIC) -Rifampin 1.0 μg/mL (MIC) -Sulfamethoxazole 4.0 μg/mL (MIC) -Chloramphenicol 16.0 μg/mL (MIC) -Polymyxin B 16.0 μg/mL (MIC) | [21] |
| Kalihinol O (119) | Antifouling | Settlement inhibition/Balanus amphitrite | 1.43 µM (EC50) | Seawater + DMSO | [16] |
| Cytotoxicity | MTT/HCT-116 | 5.97 µM (IC50) | - | [16] | |
| Kalihinol P (120) | Antifouling | Settlement inhibition/Balanus amphitrite | 0.72 µM (EC50) | Seawater + DMSO | [16] |
| Cytotoxicity | MTT/HCT-116 | 10.68 µM (IC50) | - | [16] | |
| MTT/H1299 | 26.21 µM (IC50) | - | [16] | ||
| Kalihinol Q (121) | Antifouling | Settlement inhibition/Balanus amphitrite | 1.48 µM (EC50) | Seawater + DMSO | [16] |
| Cytotoxicity | MTT/HCT-116 | 20.55 µM (IC50) | - | [16] | |
| Kalihinol R (122) | Antifouling | Settlement inhibition/Balanus amphitrite | 1.16 µM (EC50) | Seawater + DMSO | [16] |
| Cytotoxicity | MTT/HCT-116 | 13.44 µM (IC50) | - | [16] | |
| Kalihinol S (123) | Antifouling | Settlement inhibition/Balanus amphitrite | 0.53 µM (EC50) | Seawater + DMSO | [16] |
| Kalihinol T (124) | Antifouling | Settlement inhibition/Balanus amphitrite | 0.74 µM (EC50) | Seawater + DMSO | [16] |
| 10-epi-Kalihinol X (126) | Cytotoxicity | MTT/A549 | 9.30 µg/mL (IC50) | - | [42] |
| Antifouling | Settlement inhibition/Balanus amphitrite | 0.69 µM (EC50) | Seawater + DMSO | [16] | |
| Cytotoxicity | MTT/HCT-116 | 8.21 µM (IC50) | - | [16] | |
| Kalihiacyloxyamide C (133) | Cytotoxicity | SRB/L-02 | 14.6 μM (IC50) | Doxorubicin not detected | [62] |
| MTT/K562 | 6.4 μM (IC50) | Doxorubicin ˂ 1.04 μM (IC50) | [62] | ||
| SRB/ASPC-1 | 19.0 μM (IC50) | Doxorubicin ˂ 1.04 μM (IC50) | [62] | ||
| SRB/H69AR | 12.0 μM (IC50) | Doxorubicin 15.1 μM (IC50) | [62] | ||
| SRB/MDA-MB-231 | 12.5 μM (IC50) | Doxorubicin ˂ 1.04 μM (IC50) | [62] | ||
| Kalihiacyloxyamide D (134) | Cytotoxicity | SRB/L-02 | 8.0 μM (IC50) | Doxorubicin not detected | [62] |
| MTT/K562 | 6.3 μM (IC50) | Doxorubicin ˂ 1.04 μM (IC50) | [62] | ||
| SRB/MDA-MB-231 | 7.3 μM (IC50) | Doxorubicin ˂ 1.04 μM (IC50) | [62] | ||
| Kalihiacyloxyamide G (137) | Cytotoxicity | SRB/L-02 | 19.2 μM (IC50) | Doxorubicin not detected | [62] |
| MTT/K562 | 15.0 μM (IC50) | Doxorubicin ˂ 1.04 μM (IC50) | [62] | ||
| SRB/MDA-MB-231 | 13.4 μM (IC50) | Doxorubicin ˂ 1.04 μM (IC50) | [62] | ||
| Kalihiacyloxyamide H (138) | Cytotoxicity | SRB/H69AR | 16.8 μM (IC50) | Doxorubicin 15.1 μM (IC50) | [62] |
| SRB/MDA-MB-231 | 12.5 μM (IC50) | Doxorubicin ˂ 1.04 μM (IC50) | [62] | ||
| Kalihinene (139) | Cytotoxicity | MTT/P388 | 1.2 μg/mL | - | [23] |
| Antibacterial | Microbroth dilution/B. subtilis PY79 | 6.25 μg/mL (MIC) | -Trimethoprim 1.0 μg/mL (MIC) -Ciprofloxacin 1.0 μg/mL (MIC) -Rifampin 1.0 μg/mL (MIC) -Sulfamethoxazole 4.0 μg/mL (MIC) -Chloramphenicol 16.0 μg/mL (MIC) -Polymyxin B 16.0 μg/mL (MIC) | [21] | |
| Kalihinene E (145) | Cytotoxicity | MTT/HCT-116 | 14.36 μM (IC50) | Camptothecin 9.25 μM (IC50) | [63] |
| MTT/HeLa | 13.36 μM (IC50) | Camptothecin 6.98 μM (IC50) | [63] | ||
| MTT/QGY-7701 | 17.78 μM (IC50) | Camptothecin 4.05 μM (IC50) | [63] | ||
| MTT/MDA-MB-231 | 12.84 μM (IC50) | Camptothecin 0.50 μM (IC50) | [63] | ||
| Kalihinene X (147) | Antifouling | Settlement inhibition/Balanus amphitrite | 0.49 µg/mL (IC50) | CuSO4 0.15 µg/mL (IC50) | [55,56] |
| Antibacterial | Microbroth dilution/B. subtilis PY79 | 1.56 μg/mL (MIC) | -Trimethoprim 1.0 μg/mL (MIC) -Ciprofloxacin 1.0 μg/mL (MIC) -Rifampin 1.0 μg/mL (MIC) -Sulfamethoxazole 4.0 μg/mL (MIC) -Chloramphenicol 16.0 μg/mL (MIC) -Polymyxin B 16.0 μg/mL (MIC) | [21] | |
| Cytotoxicity | MTT/HCT-116 | 12.25 μM (IC50) | Camptothecin 9.25 μM (IC50) | [63] | |
| MTT/A549 | 8.55 μM (IC50) | Camptothecin 2.32 μM (IC50) | [63] | ||
| MTT/HeLa | 10.59 μM (IC50) | Camptothecin 6.98 μM (IC50) | [63] | ||
| MTT/QGY-7701 | 13.02 μM (IC50) | Camptothecin 4.05 μM (IC50) | [63] | ||
| MTT/MDA-MB-231 | 7.46 μM (IC50) | Camptothecin 0.50 μM (IC50) | [63] | ||
| Kalihinene Y (148) | Antifouling | Settlement inhibition/Balanus amphitrite | 0.45 µg/mL (IC50) | CuSO4 0.15 µg/mL (IC50) | [55,56] |
| Antibacterial | Microbroth dilution/B. subtilis PY79 | 1.56 μg/mL (MIC) | -Trimethoprim 1.0 μg/mL (MIC) -Ciprofloxacin 1.0 μg/mL (MIC) -Rifampin 1.0 μg/mL (MIC) -Sulfamethoxazole 4.0 μg/mL (MIC) -Chloramphenicol 16.0 μg/mL (MIC) -Polymyxin B 16.0 μg/mL (MIC) | [21] | |
| Cytotoxicity | MTT/A549 | 16.12 μM (IC50) | Camptothecin 2.32 μM (IC50) | [63] | |
| MTT/HeLa | 10.05 μM (IC50) | Camptothecin 6.98 μM (IC50) | [63] | ||
| MTT/QGY-7701 | 14.41 μM (IC50) | Camptothecin 4.05 μM (IC50) | [63] | ||
| MTT/MDA-MB-231 | 15.23 μM (IC50) | Camptothecin 0.50 μM (IC50) | [63] | ||
| Kalihinene Z (149) | Antifouling | Settlement inhibition/Balanus amphitrite | 1.1 µg/mL (IC50) | CuSO4 0.15 µg/mL (IC50) | [55,56] |
| 10-Formamidokalihinene (150) | Antifouling | Settlement inhibition/Balanus amphitrite | 0.095 µg/mL (IC50) | CuSO4 0.15 µg/mL (IC50) | [55,56] |
| Cytotoxicity | MTT/A549 | 6.98 μM (IC50) | Camptothecin 2.32 μM (IC50) | [63] | |
| MTT/HeLa | 13.30 μM (IC50) | Camptothecin 6.98 μM (IC50) | [63] | ||
| MTT/QGY-7701 | 14.53 μM (IC50) | Camptothecin 4.05 μM (IC50) | [63] | ||
| MTT/MDA-MB-231 | 6.84 μM (IC50) | Camptothecin 0.50 μM (IC50) | [63] | ||
| 15-Formamidokalihinene (151) | Antifouling | Settlement inhibition/Balanus amphitrite | 0.14 µg/mL (IC50) | CuSO4 0.15 µg/mL (IC50) | [55] |
| MTT/A549 | 17.53 μM (IC50) | Camptothecin 2.32 μM (IC50) | [63] | ||
| MTT/HeLa | 14.74 μM (IC50) | Camptothecin 6.98 μM (IC50) | [63] | ||
| MTT/QGY-7701 | 16.39 μM (IC50) | Camptothecin 4.05 μM (IC50) | [63] | ||
| Kalihipyran A (165) | Antifouling | Settlement inhibition/Balanus amphitrite | 1.3 µg/mL (IC50) | CuSO4 0.15 µg/mL (IC50) | [55] |
| Cytotoxicity | MTT/A549 | 13.09 μM (IC50) | Camptothecin 2.32 μM (IC50) | [63] | |
| MTT/HeLa | 11.19 μM (IC50) | Camptothecin 6.98 μM (IC50) | [63] | ||
| MTT/QGY-7701 | 13.53 μM (IC50) | Camptothecin 4.05 μM (IC50) | [63] | ||
| Kalihipyran B (166) | Antifouling | Settlement inhibition/Balanus amphitrite | 0.85 µg/mL (IC50) | CuSO4 0.15 µg/mL (IC50) | [55] |
| Cavernene A (169) | Cytotoxicity | MTT/HCT-116 | 6.31 μM (IC50) | Camptothecin 9.25 μM (IC50) | [63] |
| Cytotoxicity | MTT/HCT-116 | 8.99 μM (IC50) | Camptothecin 9.25 μM (IC50) | [63] | |
| Biflora-4,9,15-triene (168) | Antifouling | Settlement inhibition/Balanus amphitrite | 9.7 µg/mL (IC50) | CuSO4 0.15 µg/mL (IC50) | [55] |
| Hymenialdisine (185) | Cytotoxicity | AlamarBlue/A2780S | 146.8 μM (IC50) | Cisplatin 31.4 μM (IC50) | [82] |
| 2β-Hydroxy-4,7-diketo-A-norcholest-5-en-2-oic acid (208) | Antifouling | Settlement inhibition/Balanus albicostatus | 8.2 μg/mL EC50) | Capsaicin 1.32 μg/mL (EC50) | [74] |
| 24S-Ethyl-2β-hydroxy-4,7-diketo-A-norcholest-5-en-2-oic acid (209) | Antifouling | Settlement inhibition/Balanus albicostatus | 23.5 μg/mL (EC50) | Capsaicin 1.32 μg/mL (EC50) | [74] |
| 2β-Hydroxy-4,7-diketo-24R-methyl-A-norcholest-5,22(E)-diene-2-oic acid (210) | Antifouling | Settlement inhibition/Balanus albicostatus | 31.6 μg/mL (EC50) | Capsaicin 1.32 μg/mL (EC50) | [74] |
Additionally, 100 displayed antimicrobial effectiveness versus S. aureus, B. subtilis, and C. albicans [83]. Compound 125 was found to have notable antimicrobial potential against S. aureus, C. albicans, and T. mentagrophytes [18]. Bugni et al. assessed the antibacterial activity of 88, 100, 104–108, 116, 125, 127, and 139 through in vitro inhibition of B. subtilis PY79 growth, as well as inhibition of bacterial folate biosynthesis using agar diffusion/microbroth dilution and luminescence rescue assays, respectively [21]. The results showed that the pyranyl-type 127 and 125 (MICs of 1.56 µg/mL) revealed a potent antibacterial potential; however, they only weakly inhibited the folate biosynthesis, suggesting an additional mechanism of action for these pyranyl derivatives. On the other hand, the furanyl-type 100, 108, and 139 displayed a more selective folate biosynthesis inhibition than pyranyl-type kalihinols. The existence of a formamido moiety at any position markedly decreased the activity, which could be due to a reduced cellular uptake. Additionally, the C-10 substitution pattern greatly affected the potency, which was evident by loss of activity in 88, which differs in the C-10 isonitrile group orientation from the potent 127 and 125 (which have an exo-methylene and isothiocyanate groups, respectively) [21]. Xu et al. reported that 151 showed antifungal activity against T. rubrum and M. gypseum (MICs of 8.0 and 32.0 μg/mL, respectively), while 150 had activity against C. albicans, C. neoformans, T. rubrum, and M. gypseum (MICs of 4.0–8.0 μg/mL). It was noted that the isonitrile functionalities had a substantial role in antifungal activity [63]. The antifungal effects of 20, 177, 178, 217, and 220–222 against phytopathogenic fungi Fusarium oxysporum f. sp. niveum, F. solani f. sp. cucurbitae, Pythium ultimum, and Alternaria solani were assessed. Compounds 177, 178, and 220–222 had antifungal activities against A. solani, whereas 20 was the most active against F. oxysporom, F. solani, and A. solani (IZDs of 11.5 to 25.0 mm) [25]. None of them exhibited activity towards P. ultimum [25].
Fouling is the deposition and accumulation of living organisms (biofouling) and certain non-living materials on hard surfaces, most frequently in the aquatic environment, which results in serious economic problems [74]. Organotin compounds are the widely used chemicals for controlling these sessile organisms. These compounds are under criticism due to environmental and ecosystem concerns that necessitate the need for the development of nontoxic alternatives. Some soft corals and marine sponges produce environmentally friendly antifoulant secondary metabolites that provide chemical defenses against biofouling.
Compounds 5, 9, 23, 32, 66, 75, 76, 85, 89, 91, 92, 98, 99, and 168 were examined for their antifouling potential through the prohibition of the settlement of the barnacle Balanus amphitrite larvae. Compounds 32, 89, 91, 92, 98, and 99 inhibited metamorphosis and larval settlement by 100%. Additionally, 89, 91, 92, 98, and 99 showed 100% inhibition of the larval settlement and metamorphosis (at concentrations of 0.05 to 5.0 μg/mL). Isocyanate and isothiocyanate analogs 91 and 92 (EC50 ca. 0.05 μg/mL) had high antifouling capacities [40]. Compounds 65 and 67 (EC50s 0.50 and 0.53 µg/mL, respectively) prohibited B. amphitrite larval settlement with much less toxicity to larvae than CuSO4, while 65 had a much higher toxicity than 67 [49]. In addition, the related metabolites with isocyano and isothiocyanato moieties (47, 63, and 64) had marked antifouling capacities, with ED50 values of 7.2, 0.70, and 0.14 µg/mL, respectively [49]. In 2006, compounds 88–90 were evaluated against bacterial strains which induce Hydroides elegans larval settlement (Micrococcus luteus, Loktanella hongkongensis, Pseudoalteromonas sp., Ruegeria CtaxMed-2, Rhodovulum sp. MB-253, Staphylococcus haemolyticus, Vibrio halioticoli, Vibrio sp. NAP4, and Vibrio fluvialis) and marine pathogenic bacteria (Shewanella algae, Moraxella phenylpyruvica, S. aureus, Vibrio vulnificus, and Vibrio furnissii) using a disc diffusion assay [58]. These compounds strongly prohibited the growth of larval settlement-promoting strains S. haemolyticus, L. hongkongensis, and M. luteus (IZDs of 8.17–10.67 mm) compared to streptomycin. Additionally, they possessed powerful antibacterial efficacies versus pathogenic strains V. furnissii and S. aureus (IZDs of 5–7 mm) compared to streptomycin (IZD of 0.83–2.50 mm). Compound 88 was found to effectively suppress larval settlement (EC50 0.5 μg/mL), and was further incorporated in Phytagel®. This compound slightly reduced thet bacterial abundance, but it modified the bacterial species composition in the biofilm, suggesting its capacity to change the bacterial community composition, which in turn modulates the larval settlement of fouling organisms [58]. Compounds 88, 89, 92, 98, 115, 119–124, and 126 displayed significant antifouling activities against B. amphitrite larvae (EC50s of 0.41 to 1.43 μM) [16]. It was found that the compounds with an equatorial-Cl and isothiocyanate group had more activity. Compound 92 had a significant antifouling activity without cytotoxicity, which could indicate its potential as an antifouling agent [16]. Compounds 88, 147–151,165, 166, and 168 were assessed for their antifouling activity against cyprid larvae of the barnacle Balanus amphitrite. The novel compounds 165 and 166 suppressed larval settlement and metamorphosis of the barnacle Balanus amphitrite (IC50s 1.3 and 0.85 µg/mL, respectively), which was comparable to 147–149 (IC50s of 0.49, 0.45, and 1.1 µg/mL, respectively) with low toxicity, whereas the isocyano derivatives 88, 150, and 151 (IC50s of 0.087, 0.095, and 0.14 µg/mL, respectively) were more efficient. Interestingly, 168 was moderately active (IC50 4.6 µg/mL) and 88 and 150 were more active than CuSO4 (IC50 0.15 µg/mL) [55,56]. Besides, 185 was found to have a significant antifouling capacity against P. viridis (EC50 31.77 μg/mL in a mussel bioassay), B. neritina (EC50 3.43 μg/mL in a bryozoan bioassay), and U. prolifera (EC50 8.31 μg/mL in an algal bioassay). These results suggest that 185 may play a role in chemical defense against fouling in Acanthella sp. [69]. In addition to the potent anti-fouling diterpenes reported from A. cavernosa, Qui et al. stated that the nor-steroids 208–210 biosynthesized by A. cavernosa also demonstrated marked antifouling potentials (EC50s of 8.2, 23.5, and 31.6 μg/mL, respectively) towards the Balanus albicostatus larval settlement compared to capsaicin (EC50 of 1.32 μg/mL) in a settlement inhibition assay [74].
4.2. Cytotoxic Activity
The MeOH extracts of A. elongata from fishing nets of Kanyakumari revealed a toxicity (LC50 of 0.51 µg/mL) on Artemia salina in a brine shrimp bioassay [84]. In the MTT assay, A. carteri hydro-EtOH extracts had promising activities against HT-29, T47D, and Casky cell lines (IC50s of 37.56, 482.84, and 37.64 μg/mL, respectively) [85], while A. acuta MeOH extracts displayed notable cytotoxic activities versus LS174 and HeLa cell lines (IC50s of 9.92 and 29.51 μg/mL, respectively) [86].
Compounds 95 and 139 exhibited a cytotoxic efficacy on P388 murine leukemia cells (IC50s of 0.8 and 1.2 µg/mL, respectively) [61]. Compound 100 is a tetrahydrofuran-containing kalihinol with three isonitrile functionalities at C-5, C-10, and C-15 and was obtained from Acanthella sp. from the Cape Sada coast (Ehime Prefecture, Japan). This compound was found to have topoisomerase-I inhibition capacity by prohibiting the chromosome separation in Asterina pectinifera (fertilized starfish) eggs, while it did not affect topoisomerase II and DNA polymerases (α, β, and γ) [61]. In an MTT assay, 9, 17, 97, and 126 exhibited moderate in vitro cytotoxic capacities versus A549 (IC50s of 1.98–9.30 µg/mL) [42], whilst 88, 98, 115, 119–121, and 126 demonstrated cytotoxic potential against HCT-116 (IC50s of 5.97 to 28.67 μM) [16]. Compounds 169 and 170 showed potent cytotoxic potential against HCT-116 (IC50s of 6.31 and 8.99 μM, respectively) compared to camptothecin (IC50 of 9.25 μM), whereas 145 had activity against HCT-116, HeLa, QGY-7701, and MDA-MB-231 (IC50 of 12.84 to 17.78 μM) [63]. Furthermore, 157 was found to demonstrate a potent cytotoxic potential (IC50s of 6.57 and 3.60 µM, respectively) towards K562 and H69 cells, whereas 158 revealed moderate (IC50 of 8.73 µM) capacity against K562 cells compared to doxorubicin (IC50s of 0.252 and 0.980 µM, respectively) [64]. Compounds 133 and 135 exhibited cytotoxicity against K562 cell lines (IC50s of 6.4 and 6.3 μM, respectively), while 135 and 138 were active against MDA-MB-231 cell lines (IC50s of 7.3 and 7.9 μM, respectively) compared to doxorubicin (IC50 of ˂1 μM) [62]. Compounds 175–177 and 179 showed in vitro cytotoxic capacity against NSCLC-N6 (IC50s ranging from 4.8 to 11.2 μg/mL) [66]. In 2021, Abdullah et al. reported that 185 was less toxic versus A2780S cells (IC50 of 146.8 μM) and had no activity versus A2780CP compared to cisplatin (IC50 of 31.4 μM for A2780S and 76.9 μM for A2780CP) in an alamarBlue® assay [82]. The guanidine-containing alkaloids 191–193 were evaluated for the cellular stability of PDCD4 (Programmed cell death 4) using HEK-293 cells [24]. Compounds 191 and 193 were found to suppress TPA (tetradecanoylphorbol-13-acetate)-induced degradation of PDCD4 (EC50s of 1.8 and 2.8 μg/mL, respectively) compared to rapamycin (EC50 of 0.02 μg/mL), while 192 had no activity. It is noteworthy that 191 and 193 are the first marine natural metabolites that stabilized PDCD4 under tumor-promoting conditions [24].
4.3. Larvicidal and Antimalarial Activities
The larvicidal potential of A. elongata (doses of 0.01% to 10.0% for 24h) collected from Kanyakumari was assessed against s mosquito vector of filariasis, Culex sp. 3rd instar larvae. Its MeOH extract exhibited potent larvicidal capacity (LC50 of 0.066 mg/mL), suggesting the potential of A. elongata extracts for the development of novel larvicidal agents [87]. Whilst the n-BuOH, n-hexane, aqueous, and EtOAc fractions (IC50s of 9.5, 4.6, 15.0, and 3.3 µg/mL, respectively) of A. cavernosa extracts had the ability to prohibit heme polymerization, revealing their anti-malarial potential [88].
Compounds 17, 23, 42, and 45 demonstrated in vitro, dose-dependent antimalarial activity against P. falciparum (IC50s of 142 to 12340 ng/mL for D6 and 16.5 to 3110 ng/mL for W2) compared to chloroquine (IC50 of 1.95 ng/mL for D6 and 22.8 ng/mL for W2), where 17 was the most active. It was found that the isonitrile group was crucial and isothiocyanate derivative 23 was 500-fold less active than 17. The eudesmane isothiocyanates 42 and 45 have lower potential than 17 [19]. Compounds 88, 108, 110, 111, 114, 115, 127, 129, 139, and 153 were assessed for their antimalarial activity [57]. Among the tested compounds, 88 was found to display potent in vitro (EC50 of 1.2 × 10−9 M) and selective (SI of 317) antimalarial effectiveness, whereas 115, 110, 139, and 153 had EC50s from 8.0 × 10−8 M to ˃ 1.8 × 10−6 M against P. falciparum compared to mefloquine (EC50 of 3.2 × 10−8 M) [57].
4.4. Cyclooxygenase Inhibitory and α2B Adrenoceptor Agonistic Activities
Compound 84 displayed promising dose-dependent anti-inflammatory activity through inhibition of TNF-α and CCL2 (% inhibition ratios of 74.1% and 64.1%, respectively) at a concentration of 1 μM in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages [43]. Yan et al. reported that 88 revealed significant COX-2 (IC50 of 1.07 µM) inhibitory activity [41]. In 2009, Davis et al. stated that 188 showed α2B adrenoceptor agonist activity (EC50 of 4.2 μM) compared to noradrenaline in a α2B adrenoceptor FLIPR (fluorescence imaging plate reader) assay in HEK 293 cells [70].
4.5. Insecticidal and Anthelmintic Activities
Hammami et al. evaluated the insecticidal effect of 34 against Tribolium confusum Duv (major pest of stored products) using the direct contact application method; it was found to prohibit Tribolium confusum Duv larvae growth (45% mortality at a concentration of 10 mg/mL) [25]. An in vitro anthelmintic assay of A. carvenosa-derived kalihinols against Nippostrongylus brasilliensis (gastrointestinal roundworm) (at 50 µg/mL) demonstrated that 127 had extreme activity; however, 114, 88, 125, and 130 were potent and 101 was inactive [18,53].
4.6. Antioxidant Activity
A. carteri hydro-EtOH extracts revealed a DPPH scavenging ability (IC50 of 56.94 μg/mL) compared to ascorbic acid (IC50 of 0.67 μg/mL) [85]. Putra et al. stated that n-BuOH fractions of A. cavernosa had the largest phenolic content, followed by EtOAc, aqueous, and n-hexane fractions. These fractions demonstrated antioxidant potential with the % inhibition of DPPH radicals ranging from 16.40 to 40.57%, whereas the n-hexane fraction displayed the most powerful DPPH radical suppression (at a concentration of 171.86 µg/mL) [88].
5. Nanoparticles
Synthesis of nanoparticles (NPs) with green technology is beneficial over chemical procedures because of their lower environmental impacts [89,90]. The use of biological extracts of living organisms, such as actinomycetes, bacteria, yeast, plants, marine sponges, and fungi, in green synthetic processes indicates their considerable potential for NP synthesis [89]. Some researchers have reported the biosynthesis of NPs using species of the genus Acanthella that are cost-effective and compatible with pharmaceutical and biomedical applications and could be utilized commercially for large-scale production. In 2010, Inbakandan et al. synthesized highly stable AuNPs (gold nanoparticles) using an A. elongata extract by reducing aqueous AuCl4−, suggesting that this sponge is a perfect candidate for AuNP synthesis [91]. In another study in 2012, AgNPs were synthesized using the H2O-soluble extract of A. elongata. These NPs were characterized by UV, XRD (X-ray diffraction), TEM (transmission electron microscopy), and FTIR (Fourier transform infrared spectroscopy). It was found that amines of the sponge extract were accountable for the bio-reduction of the silver salt to the AgNps [90].
6. Conclusions
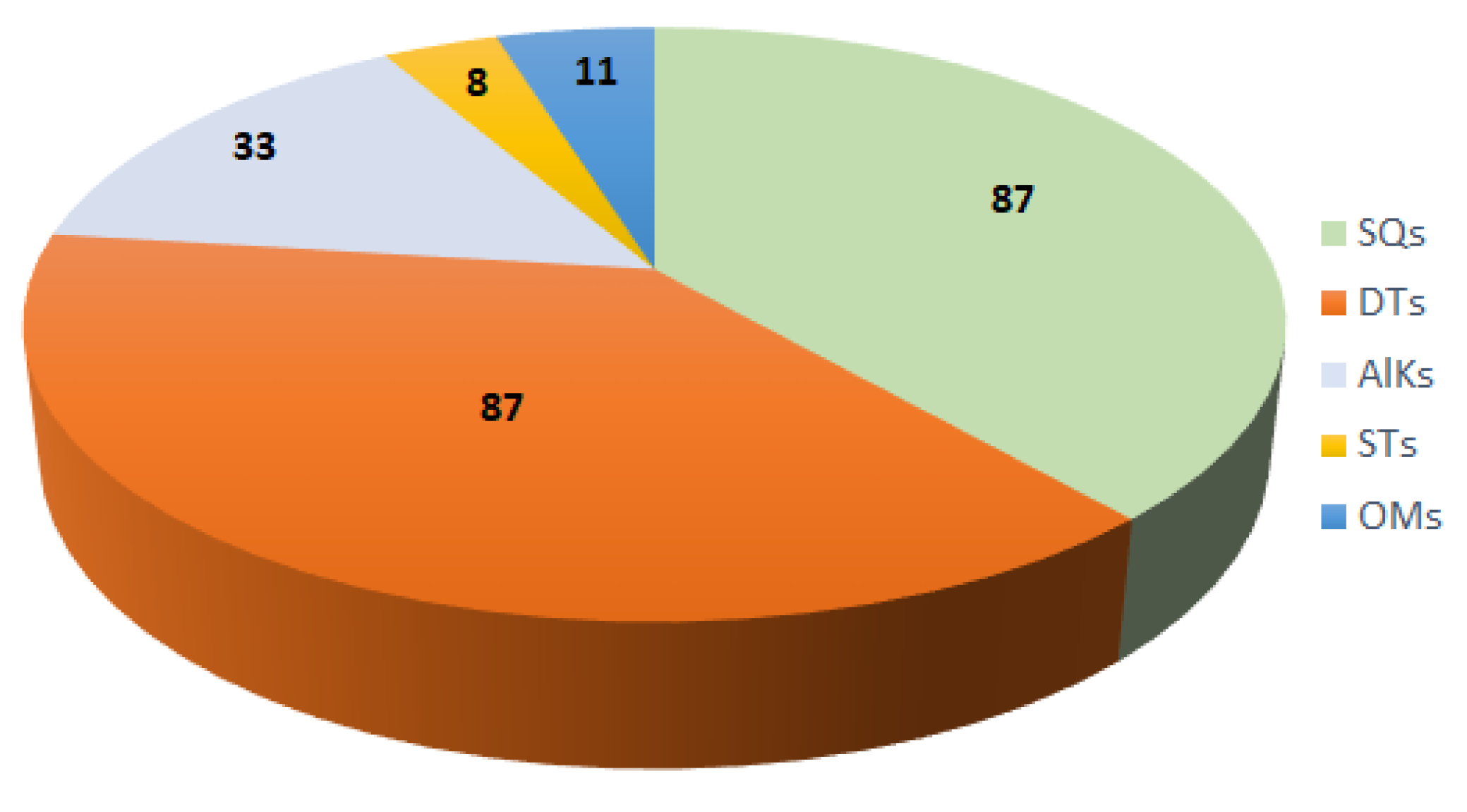
The current work presents extensive documentation of the reported studies on the genus Acanthella with a special focus on their diverse chemical classes of metabolites and their bioactivities. The sponges of this genus were obtained from various marine environments. A total of 226 metabolites from various species of this genus were reported in the period from 1974 to the beginning of 2023. These metabolites illustrated in this work belong mainly to terpene (sesqui- and di-terpenes), alkaloid, and steroid chemical classes (Figure 21).
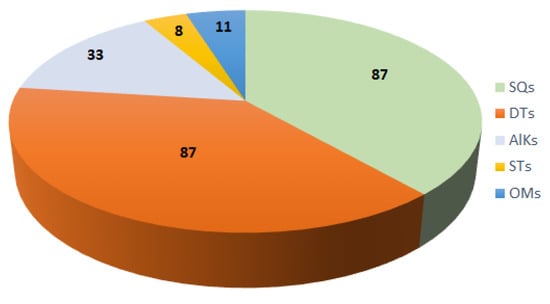
Figure 21.
Different classes of metabolites reported from the genus Acanthella. SQs: sesquiterpenes; DTs: diterpenes; AlKs: alkaloids; STs: steroid compounds; OMs: other metabolites.
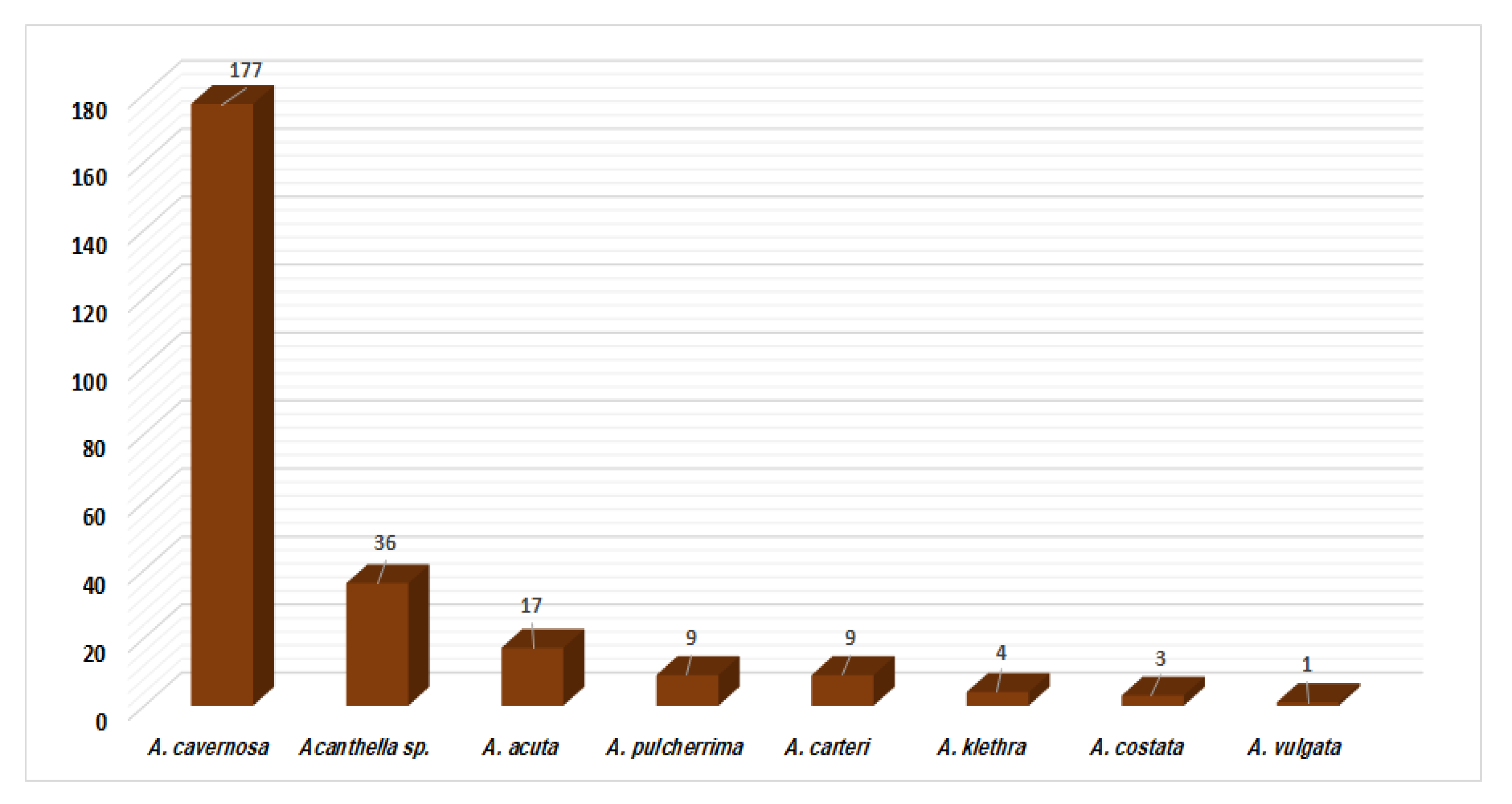
Metabolites have been reported from A. cavernosa, A. pulcherrima, A. klethra, A. acuta, A. carteri, A. costata, A. vulgate, and unidentified Acanthella species. A. cavernosa (177 compounds), Acanthella sp. (36 compounds), and A. acuta (17 compounds) are frequently studied members of this genus in terms of the number of isolated compounds and have proven to be rich in terpenes and alkaloids (Figure 22). Interestingly, kalihinane-type diterpenoids are commonly purified from A. carvenosa, which could be a chemotaxonomic marker for this sponge.
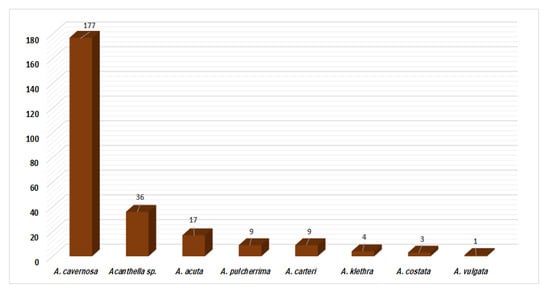
Figure 22.
Number of compounds reported from each studied Acanthella species.
In addition, bioactivity investigations of some species extracts are detailed in the literature. Sesquiterpene- and diterpene-containing nitrogen and alkaloids were the dominant metabolites reported from the species of genus Acanthella. These metabolites were mainly assessed for antifouling, antimicrobial, and cytotoxic activities. Limited studies have reported the larvicidal, antimalarial, cyclooxygenase inhibitory, α2B adrenoceptor agonistic, insecticidal, and anthelmintic capacities of these compounds. It is noteworthy that some kalihinol and kalihinene diterpenes have marked antifouling potentials. Additionally, 88 demonstrated notable antifouling, cytotoxic, antimalarial, COX-2 inhibition, and anthelmintic activities. The diverse structural features and bioactivities demonstrated by some of these metabolites make them attractive biological targets that are worthy of further investigation.
Author Contributions
Conceptualization, S.R.M.I. and G.A.M.; methodology, S.R.M.I., G.A.M., S.G.A.M., K.F.G., S.F.M. and D.F.A.; software, K.F.G., S.F.M. and D.F.A.; writing—original draft preparation, S.R.M.I., S.G.A.M. and G.A.M.; writing—review and editing, S.G.A.M., K.F.G., S.F.M. and D.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| A549 | Human lung adenocarcinoma epithelial cell line |
| A2780s | Human ovarian cancer cell line |
| A2780CP | Human ovarian cancer cell line |
| ASPC-1 | Human pancreatic cancer cell line |
| CCL2 | C–C motif chemokine ligand 2 |
| CD | Circular dichroism |
| CH2Cl2 | Dichloromethane |
| CHCl3 | Chloroform |
| COX-2 | Cyclooxygenase-2 |
| CT-26 | Murine colorectal carcinoma cell line |
| DKPs | Diketopiperazines |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EC50 | Half maximal effective concentration |
| EtOAc | Ethyl acetate |
| H69 | Chemo-sensitive human small cell lung cancer cell line |
| H69AR | Chemo-resistant human small cell lung cancer cell line |
| H1299 | Human non-small cell lung carcinoma cell line |
| HCT-116 | Human colon cancer cell line |
| HeLa | Human cervical epithelioid carcinoma cell line |
| HPLC | High-performance liquid chromatography |
| HRESIMS | High-resolution electrospray ionization mass spectroscopy |
| HT-29 | Human colon cancer cell line |
| IZD | Inhibition zone diameter |
| K562 | Human erythroleukemic cell line |
| KB | Human oral epidermoid carcinoma cell line |
| L02 | Human liver cell line |
| LC50 | Lethal concentration that kills 50% |
| LD50 | Half maximal lethal concentration |
| LD100 | Maximal lethal concentration |
| IR | Infrared |
| LPS | Lipopolysaccharide |
| Ls174T | Human colorectal cancer cell line |
| MDA-MB-231 | Human breast cancer cell line |
| MeOH | Methanol |
| MRC-5 | Human lung fibroblasts |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| n-BuOH | n-butanol |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NMR | Nuclear magnetic resonance |
| P 388 | Human leukemia cell line |
| PC-3 | Human prostatic testosterone-independent cell line |
| PDCD4 | Programmed cell death 4 |
| QGY-7701 | Human hepatocellular carcinoma cell line |
| QM-NMR | Quantum mechanical nuclear magnetic resonance |
| RP-18 | Reversed phase-18 |
| SiO2 CC | Silica gel column chromatography |
| T47D | Human hormone-dependent breast cancer cell line |
| TDDFT-ECD | Time-dependent density functional theory/electronic circular dichroism |
| TNF-α | Tumor necrosis factor alpha |
| TPA | Tetradecanoylphorbol-13-acetate |
References
- Ibrahim, S.R.M.; Fadil, S.A.; Fadil, H.A.; Eshmawi, B.A.; Mohamed, S.G.A.; Mohamed, G.A. Fungal Naphthalenones; Promising Metabolites for Drug Discovery: Structures, Biosynthesis, Sources, and Pharmacological Potential. Toxins 2022, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Mohamed, G.A.; Ibrahim, S.R. Lansium domesticum—A Fruit with Multi-Benefits: Traditional Uses, Phytochemicals, Nutritional Value, and Bioactivities. Nutrients 2022, 14, 1531. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Choudhry, H.; Asseri, A.H.; Elfaky, M.A.; Mohamed, S.G.; Mohamed, G.A. Stachybotrys chartarum—A Hidden Treasure: Secondary Metabolites, Bioactivities, and Biotechnological Relevance. J. Fungi 2022, 8, 504. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.; Mohamed, G.A. Bright Side of Fusarium oxysporum: Secondary Metabolites Bioactivities and Industrial Relevance in Biotechnology and Nanotechnology. J. Fungi 2021, 7, 943. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Fadil, S.A.; Fadil, H.A.; Hareeri, R.H.; Abdallah, H.M.; Mohamed, G.A. Ethnobotanical Uses, Phytochemical Composition, Biosynthesis, and Pharmacological Activities of Carpesium abrotanoides L. (Asteraceae). Plants 2022, 11, 1598. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khedr, A.I.M.; Zayed, M.F.; El-Kholy, A.A.S. Genus Hylocereus: Beneficial Phytochemicals, Nutritional Importance, and Biological Relevance—A Review. J. Food Biochem. 2018, 42, e12491. [Google Scholar] [CrossRef]
- Shinde, P.; Banerjee, P.; Mandhare, A. Marine Natural Products as Source of New Drugs: A Patent Review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 283–309. [Google Scholar] [CrossRef]
- Banerjee, P.; Mandhare, A.; Bagalkote, V. Marine Natural Products as Source of New Drugs: An Updated Patent Review (July 2018–July 2021). Expert Opin. Ther. Pat. 2022, 32, 317–363. [Google Scholar] [CrossRef] [PubMed]
- Kollár, P.; Rajchard, J.; Balounová, Z.; Pazourek, J. Marine Natural Products: Bryostatins in Preclinical and Clinical Studies. Pharm. Biol. 2014, 52, 237–242. [Google Scholar] [CrossRef]
- Rangel, M.; Falkenberg, M. An Overview of the Marine Natural Products in Clinical Trials and on the Market. J. Coast. Life Med. 2015, 3, 421–428. [Google Scholar]
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528. [Google Scholar] [CrossRef]
- Khan, S.; Al-Fadhli, A.A.; Tilvi, S. Discovery of Cytotoxic Natural Products from Red Sea Sponges: Structure and Synthesis. Eur. J. Med. Chem. 2021, 220, 113491. [Google Scholar] [CrossRef]
- Maslin, M.; Gaertner-Mazouni, N.; Debitus, C.; Joy, N.; Ho, R. Marine Sponge Aquaculture Towards Drug Development: An Ongoing History of Technical, Ecological, Chemical Considerations and Challenges. Aquacult. Rep. 2021, 21, 100813. [Google Scholar] [CrossRef]
- de Voogd, N.J.; Alvarez, B.; Boury-Esnault, N.; Carballo, J.L.; Cárdenas, P.; Díaz, M.-C.; Dohrmann, M.; Downey, R.; Goodwin, C.; Hajdu, E.; et al. World Porifera Database. Acanthella Schmidt, 1862. 2023. Available online: https://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=165648 (accessed on 8 April 2023).
- Emsermann, J.; Kauhl, U.; Opatz, T. Marine Isonitriles and their Related Compounds. Mar. Drugs 2016, 14, 16. [Google Scholar] [CrossRef]
- Xu, Y.; Li, N.; Jiao, W.; Wang, R.; Peng, Y.; Qi, S.; Song, S.; Chen, W.; Lin, H. Antifouling and Cytotoxic Constituents from the South China Sea Sponge Acanthella cavernosa. Tetrahedron 2012, 68, 2876–2883. [Google Scholar] [CrossRef]
- Macabeo, A.P.G.; Guce, F.d. Bromopyrrole-Imidazole Alkaloids from Acanthella carteri Dendy (Axinellidae). Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 720–723. [Google Scholar]
- Alvi, K.A.; Tenenbaum, L.; Crews, P. Anthelmintic Polyfunctional Nitrogen-Containing Terpenoids from Marine Sponges. J. Nat. Prod. 1991, 54, 71–78. [Google Scholar] [CrossRef]
- Angerhofer, C.K.; Pezzuto, J.M.; König, G.M.; Wright, A.D.; Sticher, O. Antimalarial Activity of Sesquiterpenes from the Marine Sponge Acanthella klethra. J. Nat. Prod. 1992, 55, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Annie Selva Sonia, G.; Lipton, A.P.; Paul Raj, R. Antibacterial Activity of Marine Sponge Extracts Against Fish Pathogenic Bacteria. Isr. J. Aquac.—Bamidgeh 2008, 60, 172–176. [Google Scholar] [CrossRef]
- Bugni, T.S.; Singh, M.P.; Chen, L.; Arias, D.A.; Harper, M.K.; Greenstein, M.; Maiese, W.M.; Concepción, G.P.; Mangalindan, G.C.; Ireland, C.M. Kalihinols from Two Acanthella cavernosa Sponges: Inhibitors of Bacterial Folate Biosynthesis. Tetrahedron 2004, 60, 6981–6988. [Google Scholar] [CrossRef]
- Chang, C.W.; Patra, A.; Baker, J.A.; Scheuer, P.J. Kalihinols, Multifunctional Diterpenoid Antibiotics from Marine Sponges Acanthella Spp. J. Am. Chem. Soc. 1987, 109, 6119–6123. [Google Scholar] [CrossRef]
- Fusetani, N.; Yasumuro, K.; Kawai, H.; Natori, T.; Brinen, L.; Clardy, J. Kalihinene and Isokalihinol B, Cytotoxic Diterpene Isonitriles from the Marine Sponge Acanthella klethra. Tetrahedron Lett. 1990, 31, 3599–3602. [Google Scholar] [CrossRef]
- Grkovic, T.; Blees, J.S.; Bayer, M.M.; Colburn, N.H.; Thomas, C.L.; Henrich, C.J.; Peach, M.L.; McMahon, J.B.; Schmid, T.; Gustafson, K.R. Tricyclic Guanidine Alkaloids from the Marine Sponge Acanthella cavernosa that Stabilize the Tumor Suppressor PDCD4. Mar. Drugs 2014, 12, 4593–4601. [Google Scholar] [CrossRef]
- Hammami, S.; Bergaoui, A.; Boughalleb, N.; Romdhane, A.; Khoja, I.; Kamel, M.B.H.; Mighri, Z. Antifungal Effects of Secondary Metabolites Isolated from Marine Organisms Collected from the Tunisian Coast. C. R. Chim. 2010, 13, 1397–1400. [Google Scholar] [CrossRef]
- Nishikawa, K.; Umezawa, T.; Garson, M.J.; Matsuda, F. Confirmation of the Configuration of 10-Isothiocyanato-4-Cadinene Diastereomers through Synthesis. J. Nat. Prod. 2012, 75, 2232–2235. [Google Scholar] [CrossRef] [PubMed]
- White, R.D.; Keaney, G.F.; Slown, C.D.; Wood, J.L. Total Synthesis of (±)-Kalihinol C. Org. Lett. 2004, 6, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, H.; Abe, Y.; Sekiya, N.; Mitome, H.; Kawashima, E. Total Synthesis of Antimalarial Diterpenoid (+)-Kalihinol A. Chem. Comm. 2012, 48, 901–903. [Google Scholar] [CrossRef] [PubMed]
- White, R.D.; Wood, J.L. Progress Toward the Total Synthesis of Kalihinane Diterpenoids. Org. Lett. 2001, 3, 1825–1827. [Google Scholar] [CrossRef]
- Minale, L.; Riccio, R.; Sodano, G. Acanthellin-1, an Unique Isonitrile Sesquiterpene from the Sponge Acanthella acuta. Tetrahedron 1974, 30, 1341–1343. [Google Scholar] [CrossRef]
- Dumdei, E.J.; Flowers, A.E.; Garson, M.J.; Moore, C.J. The Biosynthesis of Sesquiterpene Isocyanides and Isothiocyanates in the Marine Sponge Acanthella cavernosa (Dendy); Evidence for Dietary Transfer to the Dorid Nudibranch Phyllidiella pustulosa. Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 1385–1392. [Google Scholar] [CrossRef]
- Fusetani, N.; Wolstenholme, H.J.; Shinoda, K.; Asai, N.; Matsunaga, S.; Onuki, H.; Hirota, H. Two Sesquiterpene Isocyanides and a Sesquiterpene Thiocyanate from the Marine Sponge Acanthella Cf. cavernosa and the Nudibranch Phyllidia ocellata. Tetrahedron Lett. 1992, 33, 6823–6826. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Z.; Yao, L.; Wang, J.; Guo, Y.; Li, X. Nitrogenous Sesquiterpenoids from the South China Sea Nudibranch Hexabranchus sanguineus and its Possible Sponge-Prey Acanthella Cavernosa: Chiral Separation, Stereochemistry and Chemical Ecology. Chin. J. Chem. 2022, 40, 235–246. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Deneubourg, F.; Huysecom, J.; Vandevyver, G. I-Isocyanoaromadendrane, A New Isonitrile Sesquiterpene from the Sponge Acanthella acuta. Bull. Sociétés Chim. Belg. 1987, 96, 539–543. [Google Scholar] [CrossRef]
- Capon, R.J.; MacLeod, J.K. New Isothiocyanate Sesquiterpenes from the Australian Marine Sponge Acanthella pulcherrima. Aust. J. Chem. 1988, 41, 979–983. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Moussiaux, B.; Stoller, C.; Deneubourg, F. Sponge Secondary Metabolites: New Results. Pure Appl. Chem. 1989, 61, 509–512. [Google Scholar] [CrossRef]
- Jumaryatno, P.; Rands-Trevor, K.; Blanchfield, J.T.; Garson, M.J. Isocyanates in Marine Sponges: Axisocyanate-3, a New Sesquiterpene from Acanthella cavernosa. ARKIVOC 2007, vii, 157–166. [Google Scholar] [CrossRef]
- Jumaryatno, P.; Stapleton, B.L.; Hooper, J.N.; Brecknell, D.J.; Blanchfield, J.T.; Garson, M.J. A Comparison of Sesquiterpene Scaffolds Across Different Populations of the Tropical Marine Sponge Acanthella cavernosa. J. Nat. Prod. 2007, 70, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Mayol, L.; Piccialli, V.; Sica, D. Nitrogenous Sesquiterpenes from the Marine Sponge Acanthella acuta: Three New Isocyanide-Isothiocyanate Pairs. Tetrahedron 1987, 43, 5381–5388. [Google Scholar] [CrossRef]
- Hirota, H.; Tomono, Y.; Fusetani, N. Terpenoids with Antifouling Activity Against Barnacle Larvae from the Marine Sponge Acanthella cavernosa. Tetrahedron 1996, 52, 2359–2368. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, X.; Yu, J.; Jin, D.; Guo, Y.; Mollo, E.; Cimino, G. 3-Oxo-Axisonitrile-3, a New Sesquiterpene Isocyanide from the Chinese Marine Sponge Acanthella Sp. J. Asian Nat. Prod. Res. 2006, 8, 579–584. [Google Scholar] [CrossRef]
- Sun, J.; Chen, K.; Yao, L.; Liu, H.; Guo, Y. A New Kalihinol Diterpene from the Hainan Sponge Acanthella Sp. Arch. Pharm. Res. 2009, 32, 1581–1584. [Google Scholar] [CrossRef]
- Shen, S.; Yang, Q.; Zang, Y.; Li, J.; Liu, X.; Guo, Y. Anti-Inflammatory Aromadendrane-and Cadinane-Type Sesquiterpenoids from the South China Sea Sponge Acanthella cavernosa. Beilstein J. Org. Chem. 2022, 18, 916–925. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Wright, A.D.; Sticher, O.; Fronczek, F.R. Two New Sesquiterpene Isothiocyanates from the Marine Sponge Acanthella klethra. J. Nat. Prod. 1992, 55, 633–638. [Google Scholar] [CrossRef]
- Clark, R.J.; Stapleton, B.L.; Garson, M.J. New Isocyano and Isothiocyanato Terpene Metabolites from the Tropical Marine Sponge Acanthella cavernosa. Tetrahedron 2000, 56, 3071–3076. [Google Scholar] [CrossRef]
- Burgoyne, D.L.; Dumdei, E.J.; Andersen, R.J. Acanthenes A to C: A Chloro, Isothiocyanate, Formamide Sesquiterpene Triad Isolated from the Northeastern Pacific Marine Sponge Acanthella Sp. and the Dorid Nudibranch Cadlina luteomarginata. Tetrahedron 1993, 49, 4503–4510. [Google Scholar] [CrossRef]
- Ciminiello, P.; Magno, S.; Mayol, L.; Piccialli, V. Cis-Eudesmane Nitrogenous Metabolites from the Marine Sponges Axinella Cannabina and Acanthella acuta. J. Nat. Prod. 1987, 50, 217–220. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, W.; Li, S.; Ye, J.; Huan, X.; Gavagnin, M.; Yao, L.; Wang, H.; Miao, Z.; Li, X. Cytotoxic Nitrogenous Terpenoids from Two South China Sea Nudibranchs Phyllidiella pustulosa, Phyllidia Coelestis, and their Sponge-Prey Acanthella cavernosa. Mar. Drugs 2019, 17, 56. [Google Scholar] [CrossRef]
- Nogata, Y.; Yoshimura, E.; Shinshima, K.; Kitano, Y.; Sakaguchi, I. Antifouling Substances Against Larvae of the Barnacle Balanus Amphitrite from the Marine Sponge, Acanthella cavernosa. Biofouling 2003, 19, 193–196. [Google Scholar] [CrossRef]
- Fan, W.; Wang, X.; Cai, H.; Sun, L.; Yang, L.; Nie, S. Chemical Analysis of the South China Sea Spine Body Sponge Acanthella cavernosa. J. Pharm. Pract. 2016, 34, 138–141, 166. [Google Scholar]
- Rodríguez, J.; Nieto, R.M.; Hunter, L.M.; Diaz, M.C.; Crews, P.; Lobkovsky, E.; Clardy, J. Variation among Known Kalihinol and New Kalihinene Diterpenes from the Sponge Acanthella cavernosa. Tetrahedron 1994, 50, 11079–11090. [Google Scholar] [CrossRef]
- Chang, C.W.; Patra, A.; Roll, D.M.; Scheuer, P.J.; Matsumoto, G.K.; Clardy, J. Kalihinol-A, a Highly Functionalized Diisocyano Diterpenoid Antibiotic from a Sponge. J. Am. Chem. Soc. 1984, 106, 4644–4646. [Google Scholar] [CrossRef]
- Omar, S.; Albert, C.; Fanni, T.; Crews, P. Polyfunctional Diterpene Isonitriles from Marine Sponge Acanthella carvenosa. J. Org. Chem. 1988, 53, 5971–5972. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Gregoire, F.; Popov, S.; van Soest, R. Two New Kalihinenes from the Marine Sponge Acanthella cavernosa. Bull. Soc. Chim. Belg. 1994, 103, 187–191. [Google Scholar] [CrossRef]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. New Antifouling Kalihipyrans from the Marine Sponge Acanthella vavernosa. J. Nat. Prod. 1996, 59, 1081–1083. [Google Scholar] [CrossRef]
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. Antifouling Kalihinenes from the Marine Sponge Acanthella cavernosa. Tetrahedron Lett. 1995, 36, 8637–8640. [Google Scholar] [CrossRef]
- Miyaoka, H.; Shimomura, M.; Kimura, H.; Yamada, Y.; Kim, H.; Yusuke, W. Antimalarial Activity of Kalihinol A and New Relative Diterpenoids from the Okinawan Sponge, Acanthella Sp. Tetrahedron 1998, 54, 13467–13474. [Google Scholar] [CrossRef]
- Yang, L.H.; Lee, O.O.; Jin, T.; Li, X.C.; Qian, P.Y. Antifouling Properties of 10β-Formamidokalihinol-A and Kalihinol A Isolated from the Marine Sponge Acanthella cavernosa. Biofouling 2006, 22, 23–32. [Google Scholar] [CrossRef]
- Trimurtulu, G.; Faulkner, D.J. Six New Diterpene Isonitriles from the Sponge Acanthella cavernosa. J. Nat. Prod. 1994, 57, 501–506. [Google Scholar] [CrossRef]
- Karuso, P.; Scheuer, P.J. Biosynthesis of Isocyanoterpenes in Sponges. J. Org. Chem. 1989, 54, 2092–2095. [Google Scholar] [CrossRef]
- Ohta, E.; Ohta, S.; Hongo, T.; Hamaguchi, Y.; Andoh, T.; Shioda, M.; Ikegami, S. Inhibition of Chromosome Separation in Fertilized Starfish Eggs by Kalihinol F, a Topoisomerase I Inhibitor obtained from a Marine Sponge. Biosci. Biotechnol. Biochem. 2003, 67, 2365–2372. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Han, X.; Zhang, D.; Hou, H.; Xiao, L.; Li, G. Kalihiacyloxyamides AH, A-Acyloxy Amide Substituted Kalihinane Diterpenes Isolated from the Sponge Acanthella cavernosa Collected in the South China Sea. Phytochemistry 2023, 206, 113512. [Google Scholar] [CrossRef]
- Xu, Y.; Lang, J.; Jiao, W.; Wang, R.; Peng, Y.; Song, S.; Zhang, B.; Lin, H. Formamido-Diterpenes from the South China Sea Sponge Acanthella cavernosa. Mar. Drugs 2012, 10, 1445–1458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, X.; Liu, G.; Zhang, D.; Hou, H.; Xiao, L.; de Voogd, N.J.; Tang, X.; Li, P.; Li, G. Kalihioxepanes A—G: Seven Kalihinene Diterpenoids from Marine Sponge Acanthella cavernosa Collected Off the South China Sea. Chin. J. Chem. 2022, 40, 1785–1792. [Google Scholar] [CrossRef]
- Shimomura, M.; Miyaoka, H.; Yamada, Y. Absolute Configuration of Marine Diterpenoid Kalihinol A. Tetrahedron Lett. 1999, 40, 8015–8017. [Google Scholar] [CrossRef]
- Mancini, I.; Guella, G.; Amade, P.; Roussakis, C.; Pietra, F. Hanishin, a Semiracemic, Bioactive C9 Alkaloid of the Axinellid Sponge Acanthella ccarteri from the Hanish Islands. A Shunt Metabolite? Tetrahedron Lett. 1997, 38, 6271–6274. [Google Scholar] [CrossRef]
- Cimino, G.; De Rosa, S.; De Stefano, S.; Mazzarella, L.; Puliti, R.; Sodano, G. Isolation and X-Ray Crystal Structure of a Novel Bromo-Compound from Two Marine Sponges. Tetrahedron Lett. 1982, 23, 767–768. [Google Scholar] [CrossRef]
- Mattia, C.A.; Mazzarella, L.; Puliti, R. 4-(2-Amino-4-Oxo-2-Imidazolin-5-Ylidene)-2-Bromo-4, 5, 6, 7-Tetrahydropyrrolo [2, 3-C] Azepin-8-One Methanol Solvate: A New Bromo Compound from the Sponge Acanthella aurantiaca. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 1982, 38, 2513–2515. [Google Scholar] [CrossRef]
- qing Feng, D.; Qiu, Y.; Wang, W.; Wang, X.; gang Ouyang, P.; huan Ke, C. Antifouling Activities of Hymenialdisine and Debromohymenialdisine from the Sponge Axinella Sp. Int. Biodeterior. Biodegrad. 2013, 85, 359–364. [Google Scholar] [CrossRef]
- Davis, R.A.; Fechner, G.A.; Sykes, M.; Garavelas, A.; Pass, D.M.; Carroll, A.R.; Addepalli, R.; Avery, V.M.; Hooper, J.N.; Quinn, R.J. (−)-Dibromophakellin: An α2B Adrenoceptor Agonist Isolated from the Australian Marine Sponge, Acanthella costata. Bioorg. Med. Chem. 2009, 17, 2497–2500. [Google Scholar] [CrossRef] [PubMed]
- Fedoreyev, S.A.; Utkina, N.K.; Ilyin, S.G.; Reshetnyak, M.V.; Maximov, O.B. The Structure of Dibromoisophakellin from the Marine Sponge Acanthella carteri. Tetrahedron Lett. 1986, 27, 3177–3180. [Google Scholar] [CrossRef]
- Laville, R.; Nguyen, T.B.; Moriou, C.; Petek, S.; Debitus, C.; Al-Mourabit, A. Marine Natural Occurring 2, 5-Diketopiperazines: Isolation, Synthesis and Optical Properties. Heterocycles 2015, 90, 1351–1366. [Google Scholar]
- Wiese, K.J.; Yakushijin, K.; Horne, D.A. Synthesis of Dibromophakellstatin and Dibromoisophakellin. Tetrahedron Lett. 2002, 43, 5135–5136. [Google Scholar] [CrossRef]
- Qiu, Y.; Deng, Z.W.; Xu, M.; Li, Q.; Lin, W.H. New A-nor Steroids and their Antifouling Activity from the Chinese Marine Sponge Acanthella cavernosa. Steroids 2008, 73, 1500–1504. [Google Scholar] [CrossRef]
- Tanaka Yoshito; Ito Yoshihito; Katayama Teruhisa. The Structure of Isoagelaxanthin a in Sea Sponge Acanthella vulgata. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 1169–1171. [Google Scholar] [CrossRef]
- Mancini, I.; Guella, G.; Pietra, F.; Amade, P. Hanishenols A-B, Novel Linear or Methyl-Branched Glycerol Enol Ethers of the Axinellid Sponge Acanthella carteri (= Acanthella aurantiaca) from the Hanish Islands, Southern Red Sea. Tetrahedron 1997, 53, 2625–2628. [Google Scholar] [CrossRef]
- McCaffrey, E.J.; Endean, R. Antimicrobial Activity of Tropical and Subtropical Sponges. Mar. Biol. 1985, 89, 1–8. [Google Scholar] [CrossRef]
- Gab-AUa, A.A.A.; Kilada, R.W.; Shalaby, I.M.; Helmy, T. Antimicrobial Activity of some Sponges from the Gulf of Aqaba. Egypt. J. Biol. 2000, 2, 28–33. [Google Scholar]
- Xue, S.; Zhang, H.T.; Wu, P.C.; Zhang, W.; Yuan, Q. Study on Bioactivity of Extracts from Marine Sponges in Chinese Sea. J. Exp. Mar. Biol. Ecol. 2004, 298, 71–78. [Google Scholar] [CrossRef]
- Choudhury, S.; Pattnaik, P.; Sree, A.; Bapuji, M.; Mukherjee, S.C. Antibacterial Activity of Sponge Extracts Against Fish Pathogens. Aquacult. Res. 2003, 34, 1075–1077. [Google Scholar] [CrossRef]
- Rajendran, I.; Sobhana, K.S.; Annie Selva Sonia, G.; Chakraborty, K.; Vijayan, K.K.; Vijayagopal, P. Antibacterial and Antifungal Properties of Southeast Indian Coastal Sponges. J. Mar. Biol. Assoc. India 2011, 53, 272–274. [Google Scholar]
- Abdullah, N.; Al Balushi, N.; Hasan, S.I.; Al Bahlani, S.; Dobretsov, S.; Tamimi, Y.; Burney, I.A. Hymenialdisine is Cytotoxic Against Cisplatin-Sensitive but Not Against Cisplatin-Resistant Cell Lines. Sultan Qaboos Univ. Med. J. 2021, 21, 632. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Chang, C.W.; Scheuer, P.J.; Van Duyne, G.D.; Matsumoto, G.K.; Clardy, J. An Unprecedented Triisocyano Diterpenoid Antibiotic from a Sponge. J. Am. Chem. Soc. 1984, 106, 7981–7983. [Google Scholar] [CrossRef]
- Annie Selva Sonia, G.; Lipton, A.P.; Paulraj, R. Lethal Concentration of Methanol Extract of Sponges to the Brine Shrimp, Artemia salina. J. Mar. Biol. Assoc. India 2009, 51, 122–125. [Google Scholar]
- Abdillah, S.; Nurhayati, A.P.D.; Nurhatika, S.; Setiawan, E.; Heffen, W.L. Cytotoxic and Antioxidant Activities of Marine Sponge Diversity at Pecaron Bay Pasir Putih Situbondo East Java. Indonesia J. Pharm. Res. 2013, 6, 685–689. [Google Scholar] [CrossRef]
- Stanojković, T.; Milović, S.; Matić, I.; Grozdanić, N.; Kljajić, Z. In Vitro Ispitivanje Antitumorske Aktivnosti Ekstrakta Sunđera Acanthella acuta. Lek. Sirovine 2015, 89–101. [Google Scholar] [CrossRef]
- Annie Selva Sonia, G.; Lipton, A.P. Mosquito Larvicidal Activity of Marine Sponge Metabolites. Glob. J. Pharmacol. 2012, 6, 1–3. [Google Scholar]
- Putra, M.Y.; Murniasih, T.; Wibowo, J.T.; Hadi, T.A.; Untari, F.; Nisa, A.C.; Swasono, R.T. Phenolic Content, Anti-Oxidant, Anti-Plasmodium and Cytotoxic Properties of the Sponge Acanthella cavernosa. Asian Pac. J. Trop. Dis. 2016, 6, 811–815. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Inbakandan, D.; Sivaleela, G.; Peter, D.M.; Kiurbagaran, R.; Venkatesan, R.; Khan, S.A. Marine Sponge Extract Assisted Biosynthesis of Silver Nanoparticles. Mater. Lett. 2012, 87, 66–68. [Google Scholar] [CrossRef]
- Inbakandan, D.; Venkatesan, R.; Khan, S.A. Biosynthesis of Gold Nanoparticles Utilizing Marine Sponge Acanthella Elongata (Dendy, 1905). Colloids Surf. B Biointerfaces 2010, 81, 634–639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).