Abstract
A polyoxygenated and halogenated labdane, spongianol (1); a polyoxygenated steroid, 3β,5α,9α-trihydroxy-24S-ethylcholest-7-en-6-one (2); a rare seven-membered lactone B ring, (22E,24S)-ergosta-7,22-dien-3β,5α-diol-6,5-olide (3); and an α,β-unsaturated fatty acid, (Z)-3-methyl-9-oxodec-2-enoic acid (4) as well as five known compounds, 10-hydroxykahukuene B (5), pacifenol (6), dysidamide (7), 7,7,7-trichloro-3-hydroxy-2,2,6-trimethyl-4-(4,4,4-trichloro-3-methyl-1-oxobu-tylamino)-heptanoic acid methyl ester (8), and the primary metabolite 2’-deoxynucleoside thymidine (9), have been isolated from the Red Sea sponge Spongia sp. The stereoisomer of 3 was discovered in Ganoderma resinaceum, and metabolites 5 and 6, isolated previously from red algae, were characterized unprecedentedly in the sponge. Compounds 7 and 8 have not been found before in the genus Spongia. Compounds 1–9 were also assayed for cytotoxicity as well as antibacterial and anti-inflammatory activities.
1. Introduction
Sponges of the genus Spongia are known to be abundant sources of chemical constituents with diverse structures and bioactivity [1,2]. So far, the 3,4-seco-3,19-dinorditerpenes [3], 5,5,6,6,5-pentacyclic diterpenes [4], furanoterpenes [5,6], spongian diterpenes [7,8], scalarane sesterterpenoids [9,10], sesquiterpene quinones [11,12], diverse terpenes [13,14], sterols [15,16], and macrolides [14,17], along with fatty acids [18,19] and halides [20,21], have been isolated from the genus Spongia sp. Our preliminary studies on the Red Sea sponge Spongia sp. resulted in the isolation of a series of new compounds, including one 5,5,6,6,5-pentacyclic diterpene, two new furanotrinorsesquiterpenoid acids, and a furanyl trinorsesterpenoid [22]. Our continuous investigation of the chemical constituents of this sponge has again afforded four new compounds, including spongianol (1), 3β,5α,9α-trihydroxy-24S-ethylcholest-7-en-6-one (2), (22E,24S)-ergosta-7,22-dien-3β,5α-diol-6,5-olide (3), and (Z)-3-methyl-9-oxodec-2-enoic acid (4) (Figure 1), along with five known metabolites: 10-hydroxykahukuene B (5) [23], pacifenol (6) [24], dysidamide (7) [25], 7,7,7-trichloro-3-hydroxy-2,2,6-trimethyl-4-(4,4,4-trichloro-3-methyl-1-oxobutylamino)-heptanoic acid methyl ester (8) [25], and the primary metabolite 2’-deoxynucleoside thymidine (9) [26]. The molecular structures of 1–9 were established by MS, IR, and detailed NMR spectroscopic analysis (Supplementary Figures S1–S45) and by comparison with the reported spectral data of related known compounds. The cytotoxicity of hepatocellular carcinoma (HCC) Huh7 cells and the anti-inflammatory and antibacterial activity of 1–9 were also evaluated.
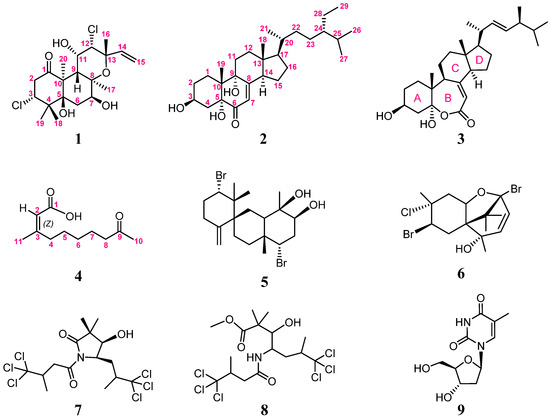
Figure 1.
Structures of metabolites 1–9.
2. Results and Discussion
Compound 1 was obtained as a colorless oil. The HRESIMS (Supplementary Figure S1) of 1 established the molecular formula C20H3035Cl2O5, implying five degrees of unsaturation. The IR spectrum of 1 revealed the presence of the hydroxyl, carbonyl, and olefin from absorptions at 3420, 1698, and 1646 cm–1, respectively. The 13C NMR spectroscopic data of 1 exhibited 20 carbon signals (Table 1), which were designated with the assistance of the DEPT spectrum as five methyls (δC 28.7, 25.9, 24.0, 20.1, and 18.9), three methylenes (including δC 116.7, 45.1, and 27.7), six methines (including four oxymethines, δC 70.9, 75.3, 68.9, and 65.9; one terminal vinyl group methine, δC 139.9; and δC 41.7), and five quaternary carbons (including one ketone carbon, δC 209.2; three oxygenated quaternary carbons, δC 82.1, 77.4, and 76.2; and δC 45.2). The NMR data of 1 (Table 1) showed the appearance of a vinyl group (δC 139.9, CH and 116.7, CH2; δH 6.78, 1H, dd, J = 17.5 and 11.5 Hz; and δH 5.35, 1H, d, J = 17.5 Hz, and 5.21, 1H, d, J = 11.5 Hz, respectively). The 1H–1H COSY experiment revealed the presence of four partial structures (Figure 2). The HMBC correlations of 1 (Figure 2) displayed from H3-20 (δH 1.71) to C-1, 5, 9, and 10 (δC 209.2, 82.1, 41.7, and 58.1); both H3-18 and 19 (δH 1.24 and 1.21) to C-3, 4 (δC 65.9 and 45.2), and 5; H3-17 (δH 1.66) to C-7, 8 (δC 75.3 and 76.2), and 9; H3-16 (δH 1.49) to C-12, 13, and 14 (δC 68.9, 77.4, and 139.9) suggested that the five methyls were positioned at C-4, 8, 10, and 13. Furthermore, the HMBC correlations were observed from both H3-20 and H2-2 (δH 3.34 and 2.64, each 1H) to a ketone carbon (δC 209.2); H-3 (δH 4.50) to C-2 (δC 45.1), 4, 18 (δC 24.0), and 19 (δC 20.1); 5-OH (δH 5.72) to C-4, 5 and 6; H-7 (δH 3.67) to C-6 (δC 27.7), 8, 9, and 17 (δC 25.9); 7-OH (δH 3.59) to C-6 and 7; H-9 (δH 2.72) to C-7, 8, 10, 11, 12 and 20; H-11 (δH 5.32) to C-12 and 13; 11-OH (δH 2.25) to C-9 and 11; H-12 (δH 4.06) to C-13 and 16 (δC 28.7); both H-14 (δH 6.78) and H2-15 (δH 5.35 and 5.21, each 1H) to C-13, suggesting that a ketone, three hydroxyls, and one terminal vinyl functionalities were located on C-1, 5, 7, 11, and 14, respectively. The remaining two chlorines were positioned at C-3 and 12 (δC 65.9 and 68.9, respectively). As described above, 1 elucidated a new polyoxygenated chlorinated labdane diterpenoid, spongianol.

Table 1.
13C and 1H NMR data for compounds 1–3.

Figure 2.
The selected COSY (▬), HMBC (→), and key NOESY (↔) correlations of 1.
In the NOESY spectrum of 1, the NOE correlations (Figure 2) of H3-20 with 11-OH, H3-17, and H3-19; 11-OH and H3-19 with H3-17; and H-14 with 11-OH and H3-17 suggested that these protons were positioned at the same orientation. By contrast, the correlations of H-3 with H3-18; 5-OH with H-9, H3-18, and 7-OH; and H-12 with H-9 and H3-16 revealed that these protons were on the same side. Furthermore, the stereochemistry of 1 was evidenced by the experimental CD (circular dichroism) and calculated ECD (electronic circular dichroism) spectra (Figure 3). The theoretical ECD curves of 3R,5R,7S,8R,9R,10S,11S,12S,13S-1 (1a) and its enantiomer 3S,5S,7R,8S,9S,10R,11R,12R,13R-1 (1b) were calculated at the B3LYP/6-311+G(d,p) (including a IEFPCM solvent model for MeOH) level of theory by the Gaussian 9.0 program [27,28]. The CD spectrum of 1 (Figure 3) showed the negative Cotton effect at 293 nm, which was found to be consistent with the calculated ECD of 1a (296 nm, Figure 3), and the absolute configuration of 1 was thus identified as 3R,5R,7S,8R,9R,10S,11S,12S and 13S. Furthermore, the absolute configurations of 1 were consistent with that of the structural analogs (3R,5S,6S,8S,9S,10R,13R)-3-bromo-6-hydroxy-8,13-epoxylabd-14-en-1-one (10), (1S,3R,5S, 6S,8S,9S,10R,13R)-1-acetoxy-3-bromo-6-hydroxy-8,13-epoxy-labd-14-ene (11) and paniculatol (12) (Figure 4) isolated from the red alga Laurencia sp. [29,30].
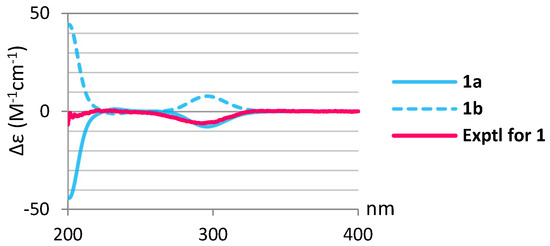
Figure 3.
Calculated ECD curves of 3R,5R,7S,8R,9R,10S,11S,12S,13S-1 (1a) and 3S,5S,7R,8S,9S,10R,11R,12R,13R-1 (1b) and the experimental CD curve of 1.
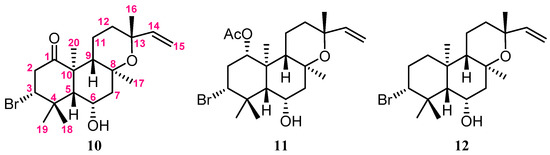
Figure 4.
The structural analogs of 10–12 [29,30].
The HRESIMS of metabolite 2 showed a molecular ion peak [M + Na]+ at 483.3446 m/z, which established the molecular formula C29H48O4, implying six degrees of unsaturation. The IR absorption bands at νmax 3458–3291, 1682, and 1654 cm−1 revealed the presence of the hydroxyl, ketone, and olefin, respectively. The NMR spectroscopic data of 2 (Table 1) displayed six methyls (δC 20.5, 19.6, 19.0, 18.9, 12.3, and 12.0; δH 1.02, 3H, s; 0.84, 3H, d, J = 6.6 Hz; 0.81, 3H, d, J = 6.6 Hz; 0.94, 3H, d, J = 6.0 Hz; 0.85, 3H, t, J = 7.8 Hz and 0.61, 3H, s); six methylenes; five methines (including one oxygenated methane δC 67.2 and δH 4.07, m; and one olefinic methane δC 119.9 and δH 5.66, br s), and five quaternary carbons (including one ketone carbon δC 197.7; one olefinic non-protonated carbon δC 164.3, and two oxygenated quaternary carbons δC 79.7 and 74.6, respectively). The detailed analyses of 1H–1H COSY and HMBC correlations (Figure 5) established the molecular skeleton of 2. Furthermore, a comparison of the NMR data of 2 with the similar structures 3β,5α,9α-trihydroxy-(22E,24R)-23-methylergosta-7,22-dien-6-one (13) and 3β,5α,9α-trihydroxy-(24S)-ergost-7-en-6-one (14) [31] (Figure 6, Supplementary Table S1) confirmed that 2 is a new polyoxygenated sterol.
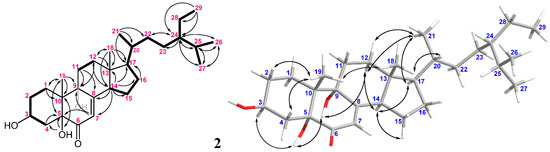
Figure 5.
The selected COSY (▬), HMBC (→), and key NOESY (↔) correlations of 2.
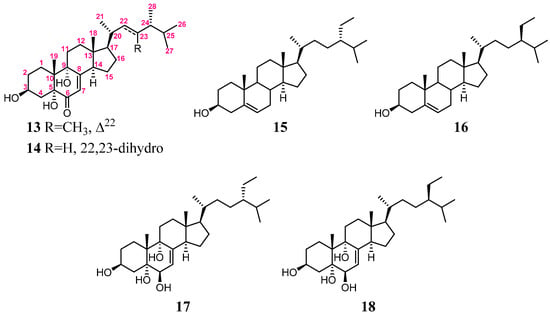
Figure 6.
Structures of compounds 13–18.
The relative configuration of 2 was deduced by the analysis of NOE correlations (Figure 5). The observation of the NOE correlations of 5-OH (δH 3.34) with H-3 and 9-OH (δH 4.07 and 4.12, respectively); 9-OH with H-12α (δH 1.72); H-14 (δH 2.72) with H-12α and H-17 (δH 1.39); H-17 with H3-21 (δH 0.94); and H3-21 with H2-12 elucidated that these protons were cofacial. Furthermore, a comparison of the NMR data of 2 with those of similar analogs, 13 and 14 (Figure 6), confirmed the configuration of the steroidal nucleus ([31], Supplementary Table S1). The chemical shift differences of C-26 and C-27 (ΔδC26,27, useful for assignment of the absolute configuration of C-24 of this side chain [32,33,34]) for compound 2 and the related known 24S and 24R analogs are summarized in Supplementary Table S2. In 24S analogues 15 (CDCl3) and 17 (C5D5N), the ΔδC26,27 values were found to be 0.55 and 0.62, respectively, while those for 24R analogues 16 (CDCl3) and 18 (C5D5N) were 0.77 and 0.73, respectively (Figure 6) [32,35]. The ΔδC26,27 values for 2 were found to be 0.59 and 0.62 in CDCl3 and C5D5N, respectively, indicating that C-24 of 2 had an S-configuration (Figure 7a). In addition, the δC values for C-20, C-24, to C-27 were shown to be much more similar to those of compound 15 than those of 16. Thus, the 24S configuration of 2 was established. Furthermore, the stereochemistry of 2 was evidenced by the experimental CD and calculated ECD spectra (Figure 7b). The theoretical ECD curves of 3S,5R,9R,10R,13R,14R,17R,20R,24S-2 (2a) and its enantiomer 3R,5S,9S,10S,13S,14S,17S,20S,24R-2 (2b) were calculated at the CAM-B3LYP/6-311+G(d,p) (including a IEFPCM solvent model for MeOH) level of theory by the Gaussian 9.0 program [27,28]. The tendency of the experimental CD spectrum of 2 (Figure 7b) was similar to that of the calculated ECD of 2a (Figure 7b); furthermore, the absolute configuration of 2 was identified. On the basis of the above observations, the molecular structure of 2 was determined as 3β,5α,9α-trihydroxy-24S-ethylcholest-7-en-6-one.

Figure 7.
(a) The ΔδC26,27 values of compounds 2 and 15–18; (b) the calculated ECD curves of 3S,5R,9R,10R,13R,14R,17R,20R,24S-2 (2a) and 3R,5S,9S,10S,13S,14S,17S,20S,24R-2 (2b), and the experimental CD curve of 2.
For compound 3, the IR absorption bands at νmax 3393 and 1670 cm−1 revealed the presence of the hydroxy and α,β-unsaturated ester groups, respectively, and the HRESIMS m/z 445.3316 [M + H]+ established the molecular formula of 3 to be C28H44O4. The structure of 3 was established by analyses of 1H–1H COSY and HMBC experiments (Figure 8), and the planar structure of 3 was found to be as same as that of (22E,24R)-ergosta-7, 22-dien-3β,5α-diol-6,5-olide (19) (Figure 9) [36]. Compound 3 exhibited almost the same NMR data as those of 19 except for the chemical shifts of C-16 and C-26 to C-28 (Supplementary Table S3). The chemical shifts of C-16 and C-24 to C-28 for 3 and the related known 24S and 24R analogs 19–21 [32,36] are summarized in Table 2. It was found that C-16 of 3 resonated at a higher chemical shift (δC 28.0) than that of 19 (δC 27.7). The 0.3 ppm difference for carbon shifts of C-16 between both 3 and 19 was similar to those of 20 (δC 28.86) and 21 (δC 28.58). In addition, the chemical shifts of C-24 and C-26−28 of 3 showed closer chemical shift values to compound 20 than to 21. On the basis of the above analysis, compound 3 was determined as (22E,24S)-ergosta-7,22- dien-3β,5α-diol-6,5-olide. Compound 3 is the fourth member of the group of steroids with a rare seven-membered lactone B ring, and the other three members, astersterol A, fortisterol, and (22E,24R)-ergosta-7,22-dien-3β,5α-diol-6,5-olide, were isolated previously from the starfish [37], the marine sponge [38], and the fungus Ganoderma resinaceum [36], respectively.
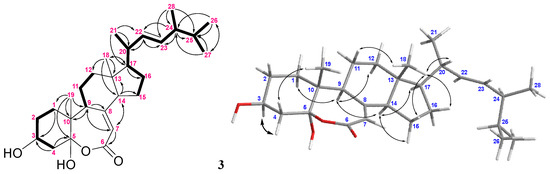
Figure 8.
The selected COSY (▬), HMBC (→), and key NOESY (↔) correlations of 3.
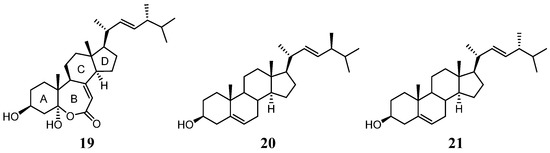
Figure 9.
Structures of compounds 19–21.

Table 2.
The 13C NMR data at C-16 and C-24–C-28 of 3 and related compounds 19−21.
Metabolite 4 was isolated as a colorless oil. Its molecular formula was determined to be C11H18O3 from the HRESIMS (m/z 221.1149 [M + Na]+), indicating three degrees of unsaturation. The IR spectrum displayed the absorptions of the hydroxyl, ketone, and carboxylic acid groups (3445, 1700, and 1683 cm–1, respectively). The NMR data (Table 3) showed the presence of two methyls (δC 29.9 and 25.4; δH 2.14 and 1.91, each 3H, s); five methylenes; one alkene methane (δC 115.3 and δH 5.69, br s); and three quaternary carbons (included one ketone carbon δC 209.4, one carbonyl carbon δC 169.5, and one alkene quaternary carbon δC 163.5). The detailed analysis of 1H–1H COSY and HMBC correlations of 4 (Figure 10) assigned the positions of a carboxylic acid group, an olefinic double bond, and ketone functionalities to be at C-1, C-2, and C-9, respectively. Moreover, the NOE correlation observed for H-2 (δH 5.69) with H3-11 (δH 1.91) in 4 suggested the Z geometry of this double bond and consequently established the structure of 4 to be (Z)-3-methyl- 9-oxodec-2-enoic acid.

Table 3.
13C and 1H NMR data for compound 4.
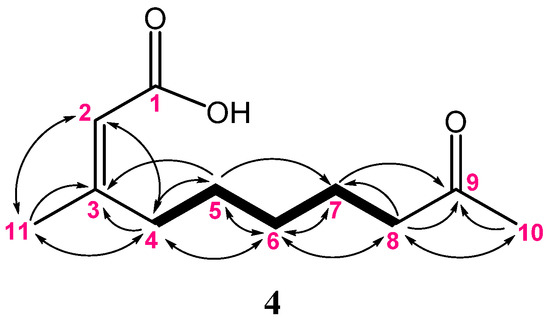
Figure 10.
The selected COSY (▬) and HMBC (→) correlations of 4.
10-Hydroxykahukuene B (5) [23] is a brominated diterpene with a rare prenylated chamigrane skeleton. To the best of our knowledge, two examples of this skeleton have been reported in the marine red alga Laurencia sp. [23,39,40], and 5 represent the first example of a metabolite with a prenylated chamigrane skeleton that has been isolated from the sponge. Pacifenol (6), the first trihalogenated compound with a chamigrane skeleton, was isolated by Sims and associates from the Californian red alga Laurencia pacifica [41]. After that, pacifenol was also isolated from other marine red algae, including L. caduciramulosa [42] and L. marianensis [24], among others. Mollusks of the genus Aplysia are known to be animals that do not biosynthesize the halogenated sesquiterpenes by themselves but obtain and accumulate these metabolites by ingesting alga and, in some cases, transform the alga metabolites into other compounds in the digestive gland [43,44]. Our present study is the first report to discover pacifenol in the sponge. Metabolites 7 and 8 have also been isolated from the Red Sea sponges Dysidea herbacea [45] and Lamellodysidea herbacea [25], but they were discovered for the first time in sponges of the genus Spongia in the present study.
It was previously known that sponges could take in and accumulate organohalides from environmental seawater and that these compounds might be transformed into chemical defense substances [46]. Macroalgae are important primary producers in coral reefs, and many species inhabit areas near sponges [47,48]. Algae synthesize secondary metabolites for competition and survival [49,50,51], and the red alga Laurencia sp. is known for producing diverse halides, many of which have been shown to have antibacterial activity [52,53]. The sponges could inhale these halides or even transform them chemically into compounds such as 1, 5, and 6 for their own use [46].
Compounds 1–9 were tested for cytotoxicity using a resazurin assay in the HCC Huh7 cell line. Among them, compounds 5 and 8 showed weak cytotoxicity against the Huh7 cell line, with 17% and 32% inhibition toward the proliferation of Huh7 cells at 50 μM, respectively. Furthermore, 5 and 8 could inhibit the 43% and 53% proliferation of Huh7 cells at 200 μM, respectively. The growth inhibition assay of Staphylococcus aureus (S. aureus) was subsequently applied for compounds 1–9. The results showed that 9 displayed 31%, 37%, and 89% inhibition on the growth of S. aureus at 50, 100, and 200 µM, respectively.
The anti-inflammatory activities of compounds 1–9 inhibiting superoxide anion (O2−) generation and elastase release in fMLF/CB-stimulated human neutrophils [54,55,56] were also evaluated (Table 4). Compounds 7 and 8 exhibited medium inhibitory activity against elastase release (55.96 ± 3.88 and 60.80 ± 6.49%, respectively) at 20 μM, with IC50 values of 17.23 ± 2.45 and 14.60 ± 2.24 μM, respectively. However, compounds 7 and 8 showed weak inhibition of superoxide anion generation (25.24 ± 4.68% and 22.38 ± 3.95%, respectively) at 20 μM. Furthermore, compounds 6 and 9 were found to display inhibitory activity to some extent (20.00 ± 4.87% and 21.22 ± 4.71%, respectively) against elastase release at 20 μM.

Table 4.
Effects of compounds on superoxide anion generation and elastase release in fMLF/CB-induced human neutrophils.
3. Materials and Methods
3.1. General Experimental Procedures
Measurements of circular dichroisms, optical rotations, and IR spectra were carried out on a Jasco J-715 CD spectrometer, JASCO P-1020 polarimeter, and FT/IR-4100 infrared spectrophotometer (JASCO Corporation, Tokyo, Japan), respectively. ESIMS was performed on a Bruker APEX II (Bruker, Bremen, Germany) mass spectrometer, and HRESIMS was performed on a Bruker APEX II and Impact HD Q-TOF mass spectrometers (Bruker, Bremen, Germany). The NMR spectra were recorded on a Varian 400MR FT-NMR at 400 and 100 MHz for 1H and 13C, respectively; a Varian Unity INOVA500 FT-NMR (both Varian Inc., Palo Alto, CA, USA) at 500 and 125 MHz for 1H and 13C, respectively; or a JEOL ECZ600R FT-NMR (Japan) at 600 and 150 MHz for 1H and 13C, respectively. Silica gel and reversed-phase (RP-18, 230–400 mesh) silica gel were used for column chromatography and analytical thin-layer chromatography (TLC) analysis (Kieselgel 60 F-254, 0.2 mm, Merck, Darmstadt, Germany), respectively. The isolation and purification of compounds by high-performance liquid chromatography (HPLC) were achieved using a Hitachi L-2455 HPLC apparatus (Hitachi, Tokyo, Japan) equipped with a Supelco C18 column (250 × 21.2 mm, 5 μm, Supelco, Bellefonte, PA, USA).
3.2. Animal Material
The sponge Spongia sp. was collected in March 2016 off the Red Sea coast of Jeddah, Saudi Arabia (21°22′11.08″ N, 39°06′56.62″ E). A voucher sample (RSS-1) was deposited at the Department of Pharmacognosy, College of Pharmacy, King Saud University, Saudi Arabia.
3.3. Extraction and Separation
The freeze-dried material Spongia sp. (550 g dry wt) was minced and extracted exhaustively with EtOAc/MeOH/CH2Cl2 (1:1:0.5). The solvent-free extract was suspended in water and partitioned with CH2Cl2, EtOAc, and then n-BuOH saturated with water to obtain CH2Cl2 (18.47 g), EtOAc (0.78 g), and n-BuOH (1.0 g) fractions. The CH2Cl2 fraction was chromatographed over a silica gel column using EtOAc in n-hexane (0% to 100%, stepwise) and then MeOH in EtOAc (0% to 100%, stepwise) to yield 12 fractions (F1–F12).
Fraction F3 (0.986 g) eluted with n-hexane/EtOAc (9:1) was re-chromatographed over a RP-18 column using H2O in MeOH (100% to 0%, stepwise) to give six subfractions (F3-1 to F3-6). F3-5 (54.6 mg, eluted with MeOH/H2O 8:2) was isolated using RP-18 HPLC (MeOH/H2O 9:1) to give six subfractions (F3-5-1 to F3-5-6); F3-5-2 (32.5 mg) was further purified on RP-18 HPLC (MeOH/H2O 7.5:2.5) to afford 6 (1.6 mg).
F5 (1.706 g) eluted with n-hexane/EtOAc (6.5:3.5) was re-chromatographed over a RP-18 column using H2O in MeOH (100% to 0%, stepwise) to give eight subfractions (F5-1 to F5-8). F5-4 (32.4 mg, eluted with MeOH/H2O 6:4) was isolated using RP-18 HPLC (MeOH/H2O 7:3) to give eight subfractions (F5-4-1 to F5-4-8). F5-4-1 (29.5 mg) was further purified on RP-18 HPLC (CH3CN /H2O 4:6) to afford 7 (5.8 mg) and 8 (1.7 mg). F5-6 (48.2 mg, eluted with MeOH/H2O 1:0) was separated on RP-18 HPLC (IPA/MeOH 1:19) to give six subfractions (F5-6-1 to F5-6-6), F5-6-1 (27.6 mg) was purified on RP-18 HPLC (MeOH/H2O 8:2) to afford 1 (2.9 mg) and 5 (4.6 mg).
F7 (1.505 g) eluted with n-hexane/EtOAc (2.5:7.5) was re-chromatographed over a RP-18 column using H2O in MeOH (100% to 0%, stepwise) to give eight subfractions (F7-1 to F7-8). F7-3 (146.3 mg, eluted with MeOH/H2O 4:6) was isolated using RP-18 HPLC (MeOH/H2O 1:1) to give 10 subfractions (F7-3-1 to F7-3-10). F7-3-7 (13.2 mg) was purified on RP-18 HPLC (CH3CN/H2O 2.8:7.2) to afford 4 (4.5 mg). F7-6 (67.0 mg, eluted with MeOH/H2O 1:0) was isolated using RP-18 HPLC (isopropanol/MeOH 1:19) to give nine subfractions (F7-6-1 to F7-6-9); F7-6-5 (16.1 mg) was further separated on RP-18 HPLC (MeOH/H2O 93:7) to afford 3 (1.0 mg); F7-6-7 (25.7 mg) was purified on RP-18 HPLC (CH3CN/H2O 8:2) to afford 2 (4.9 mg).
3.3.1. Spongianol (1)
Colorless oil; − 14.5 (c 0.29, CH3OH); IR (neat) vmax 3420, 2979, 2919, 1698, and 1646 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 443 and 445 [M + Na]+; HRESIMS m/z 443.1364 and [M + Na]+ (calcd for C20H3035Cl2O5Na, 443.1363).
3.3.2. 3β,5α,9α-Trihydroxy-24S-ethylcholest-7-en-6-one (2)
White powder; − 12.7 (c 0.49, CH3OH); IR (neat) vmax 3291, 2959, 2872, 1682 and 1654 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 483 [M + Na]+; HRESIMS m/z 483.3446 [M + Na]+ (calcd for C29H48O4Na, 483.3445).
3.3.3. (22E,24S)-Ergosta-7,22-dien-3β,5α-diol-6,5-olide (3)
Colorless crystal; + 38.5 (c 0.28, CH3OH); IR (neat) νmax 3393, 2954, 2917, 2849, and 1670 cm–1; 1H NMR and 13C data, see Table 1; HRESIMS m/z 445.3316 [M + H]+ (calcd for C28H45O4, 445.3312).
3.3.4. (Z)-3-Methyl-9-oxodec-2-enoic Acid (4)
Colorless oil; IR (neat) νmax 3445, 2923, 2859, 1700, 1683, and 1647 cm−1; 1H and 13C NMR data, see Table 3; HRESIMS m/z 221.1149 [M + Na]+ (calcd for C11H18O3Na, 221.1148).
3.4. DFT and TD-DFT Calculations
The preliminary geometry optimization of conformers was simulated using the DFT approach at the B3LYP/6-31G(d) level of theory [27]. The ECD spectra were simulated by using the time-dependent DFT (TD-DFT) approach at the B3LYP/6-311+G(d,p) or CAM-B3LYP/6-311+G(d,p) level of theory. The range-separated functional CAM-B3LYP is recommended for ECD calculations [27]. The bulk solvent effect of methanol was taken into account with the integral equation formalism polarizable continuum model (IEFPCM solvent model for MeOH). All calculations were performed by the Gaussian 09 program [28]. The calculated ECD curves were converted using GaussSum 2.2.5 and illustrated using Microsoft Excel.
3.5. Cytotoxicity Assay
The cytotoxicity assay was performed using the methods described in a previous paper [57,58]. Huh7 cells were cultured in a 96-well plate containing 100 μL of culture medium in triplicate and treated with indicated concentrations of compounds for 72 h. At the assay time point, resazurin (Cayman Chemical) was added and incubated for 4 h at 37 °C. The DMSO wells was defined as the control and assigned a relative cell viability of 100%. Sorafenib, the positive control, inhibited the 52% proliferation of Huh7 cells at 12.5 µM.
3.6. Antibacterial Assay
The antibacterial assay was performed using the methods described in a previous paper [59]. S. aureus was cultured in Lysogeny broth (LB) in a shaker–incubator at 37 °C for 24 h. The cultures were then diluted to an absorbance at 600 nm of 0.04 using sterile LB. The diluted bacteria aliquots were placed (100 μL per well) into 96-well flat-bottom plates. Tested compounds (cpd) were then added to the final concentration at 50 μM, 100 μM, and 200 μM, respectively. Background controls (1% DMSO in LB solution), positive controls (1% DMSO in the diluted bacteria solution), and known drug controls (tetracyclin; inhibited the 99% growth of bacteria at 50 µM) were run on the same plate. The absorbance at 600 nm (A) was measured right after the testing compounds were added for the basal absorbance and after 16 h incubation at 37 °C. The percentage bacterial growth was calculated as follows: [(Acpd − Acpd_basal) − Abackground control]/[(Apositive control − Apositive control_basal) − Abackground control] × 100.
3.7. Anti-inflammatory Activity
Human neutrophils were isolated from the blood of healthy adult volunteers and enriched by using dextran sedimentation, Ficoll–Hypaque gradient centrifugation, and hypotonic lysis, as described previously [56]. Then, neutrophils were incubated in Ca2+-free HBSS buffer (pH 7.4, ice-cold).
3.7.1. Superoxide Anion Generation
Neutrophils (6 × 105 cells/mL) incubated (with 0.6 mg/mL ferricytochrome c and 1 mM Ca2+) in HBSS at 37 °C were treated with DMSO (as a control) or the tested compound for 5 min. Neutrophils were primed with 1 μg/mL cytochalasin B (CB) for 3 min before being activated by 100 nM fMLF for 10 min. The change in superoxide anion generation was spectrophotometrically measured at 550 nm (U-3010, Hitachi, Tokyo, Japan) [54,55]. LY294002 [2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one] was used as a positive control.
3.7.2. Elastase Release
Neutrophils (6 × 105 cells/mL) incubated (with 100 μM MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide and 1 mM Ca2+) in HBSS at 37 °C were treated with DMSO or the tested compound for 5 min. Neutrophils were, then, activated with fMLF (100 nM)/CB (0.5 μg/mL) for 10 min. The change in elastase release was spectrophotometrically measured at 405 nm (U-3010, Hitachi, Tokyo, Japan) [55].
4. Conclusions
New metabolites (1–4) along with five known compounds (5–9) were isolated from a Red Sea sponge, Spongia sp. Compounds 5 and 8 showed weak cytotoxicity to HCC Huh7 cells, while 9 displayed significant inhibition against S. aureus. Furthermore, compounds 7 and 8 exhibited notable activity to inhibit elastase release and weaker inhibitory activity toward superoxide anion generation. Both compounds 6 and 9 also showed inhibition against elastase release. Although compound 5 was found to be inactive in the present study, its antibacterial activity against S. aureus and E. coli has been reported [23]. It is noteworthy that some of the isolates from this sponge were also found in red algae, which suggests that the specific metabolites of sponges could have originated from alga and would be accumulated and/or transformed into metabolites in sponges.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md20040241/s1, Table S1: Selected 1H and 13C NMR data of 2 and similar compounds 13 and 14 in CDCl3 and C5D5N, Table S2: Selected 13C NMR data at C20–C29 of 2 and related compounds 15–18, Table S3: 13C and 1H NMR data of 3 and related compound 19, Table S4: Cytotoxicity of compounds 1–9, Table S5: The cartesian coordinates of conformer of compound 1 at the B3LYP/6-311+G(d,p) level of theory, Table S6: The cartesian coordinates of conformer of compound 2 at the CAM-B3LYP/6-311+G(d,p) level of theory, Figures S1–S30: 1D and 2D NMR spectra and HRESIMS spectra of compounds 1–4, Figures S31–S45: 1H and 13C NMR spectra and LR- or HR-ESIMS of compounds 5–9.
Author Contributions
Conceptualization and guiding the experiment, J.-H.S.; investigation, C.-J.T., A.F.A. and J.-H.S.; analysis, C.-J.T., A.F.A., C.-H.C. and F.-R.C.; writing—original draft C.-J.T., A.F.A. and J.-H.S.; writing—review and editing, C.-J.T., C.-H.C. and J.-H.S.; biological activity analyses, C.-H.Y. and T.-L.H.; collection of the sponge, A.F.A.; species identification of the sponge, Y.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was mainly supported by grants from the Ministry of Science and Technology (MOST 108-2320-B-110-003-MY2 and 110-2320-B-110-001) and the NSYSU-KMU Joint Research Project, grant numbers NSYSUKMU 109-I002 and 110-P016, awarded to J.-H.S.
Institutional Review Board Statement
The research protocol was approved by the Institutional Review Board of Chang Gung Medical Hospital (IRB No: 99-3848B, 26 January 2011). The study was conducted in accordance with the Declaration of Helsinki. Blood samples were provided by healthy volunteers who signed written informed consent.
Informed Consent Statement
All subjects gave their informed consent for inclusion before the blood donation.
Data Availability Statement
Data of the present study are available in the article and supplementary materials.
Acknowledgments
We thank the Instrumentation Center at National Sun Yat-sen University, Kaohsiung, Taiwan for the measurement of NMR and MS data (MOST 110-2731-M-110-001); the Center for Advanced Instrumentation and Department of Applied Chemistry at National Yang Ming Chiao Tung University, Hsinchu, Taiwan for measurement of MS data; and the Natural Product Libraries and High-Throughput Screening (NPS) Core at Kaohsiung Medical University, Kaohsiung, Taiwan, for high-throughput screening and technical support. The NPS Core is funded by the National Core Facility for Biopharmaceuticals (NCFB) (grant numbers MOST 110-2740-B-037-001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413. [Google Scholar] [CrossRef] [PubMed]
- Maximo, P.; Ferreira, L.M.; Branco, P.; Lima, P.; Lourenco, A. The role of Spongia sp. in the discovery of marine lead compounds. Mar. Drugs 2016, 14, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.-Q.; Liao, X.-J.; Zhao, B.-X.; Xu, S.-H. Novel 3,4-seco-3,19-dinorspongian and 5,17-epoxy-19-norspongian diterpenes from the marine sponge Spongia sp. Org. Chem. Front. 2020, 7, 3253–3261. [Google Scholar] [CrossRef]
- Liang, Y.-Q.; Liao, X.-J.; Lin, J.-L.; Xu, W.; Chen, G.-D.; Zhao, B.-X.; Xu, S.-H. Spongiains A−C: Three new spongian diterpenes with ring A rearrangement from the marine sponge Spongia sp. Tetrahedron 2019, 75, 3802–3808. [Google Scholar] [CrossRef]
- Abdjul, D.B.; Yamazaki, H.; Kanno, S.I.; Wewengkang, D.S.; Rotinsulu, H.; Sumilat, D.A.; Ukai, K.; Kapojos, M.M.; Namikoshi, M. Furanoterpenes, new types of protein tyrosine phosphatase 1B inhibitors, from two Indonesian marine sponges, Ircinia and Spongia spp. Bioorg. Med. Chem. Lett. 2017, 27, 1159–1161. [Google Scholar] [CrossRef]
- Bauvais, C.; Bonneau, N.; Blond, A.; Perez, T.; Bourguet-Kondracki, M.L.; Zirah, S. Furanoterpene diversity and variability in the marine sponge Spongia officinalis, from untargeted LC-MS/MS metabolomic profiling to furanolactam derivatives. Metabolites 2017, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- El-Desoky, A.H.; Kato, H.; Tsukamoto, S. Ceylonins G−I: Spongian diterpenes from the marine sponge Spongia ceylonensis. J. Nat. Prod. 2017, 71, 765–769. [Google Scholar] [CrossRef]
- Chen, Q.; Mao, Q.; Bao, M.; Mou, Y.; Fang, C.; Zhao, M.; Jiang, W.; Yu, X.; Wang, C.; Dai, L.; et al. Spongian diterpenes including one with a rearranged skeleton from the marine sponge Spongia officinalis. J. Nat. Prod. 2019, 82, 1714–1718. [Google Scholar] [CrossRef]
- Yang, I.; Lee, J.; Lee, J.; Hahn, D.; Chin, J.; Won, D.H.; Ko, J.; Choi, H.; Hong, A.; Nam, S.-J.; et al. Scalalactams A−D, scalarane sesterterpenes with a γ-lactam moiety from a Korean Spongia sp. marine sponge. Molecules 2018, 23, 3187. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.-J.; Ko, H.; Ju, M.-K.; Hwang, H.; Chin, J.; Ham, J.; Lee, B.; Lee, J.; Won, D.-H.; Choi, H.; et al. Scalarane sesterterpenes from a marine sponge of the genus Spongia and their FXR antagonistic activity. J. Nat. Prod. 2007, 70, 1691–1695. [Google Scholar] [CrossRef]
- Li, J.; Gu, B.-B.; Sun, F.; Xu, J.-R.; Jiao, W.-H.; Yu, H.-B.; Han, B.-N.; Yang, F.; Zhang, X.-C.; Lin, H.-W. Sesquiterpene quinones/hydroquinones from the marine sponge Spongia pertusa Esper. J. Nat. Prod. 2017, 80, 1436–1445. [Google Scholar] [PubMed]
- Ito, T.; Nguyen, H.M.; Win, N.N.; Vo, H.Q.; Nguyen, H.T.; Morita, H. Three new sesquiterpene aminoquinones from a Vietnamese Spongia sp. and their biological activities. J. Nat. Prod. 2018, 72, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-Q.; Liao, X.-J.; Zhao, B.-X.; Xu, S.-H. (+)- and (−)-Spongiterpene, a pair of new valerenane sesquiterpene enantiomers from the marine sponge Spongia sp. Nat. Prod. Res. 2019, 35, 1–6. [Google Scholar] [CrossRef]
- Grassia, A.; Bruno, I.; Debitus, C.; Marzocco, S.; Pinto, A.; Gomez-Paloma, L.; Riccio, R. Spongidepsin, a new cytotoxic macrolide from Spongia sp. Tetrahedron 2001, 57, 6257–6260. [Google Scholar] [CrossRef]
- Migliuolo, A.; Piccialli, V.; Sica, D. Two new 9,11-secosterols from the marine sponge Spongia officinalis. Synthesis of 9,11-seco-3b,6a,11-trihydroxy-5a-cholest-7-en-9-one. Steroids 1992, 57, 344–347. [Google Scholar] [CrossRef]
- Migliuolo, A.; Piccialli, V.; Sica, D.; Giordano, F. New Δ8- and Δ8(14)-5α-6α-epoxysterols from the marine sponge Spongia officinalis. Steroids 1993, 58, 134–140. [Google Scholar] [CrossRef]
- Pettit, G.R.; Cichacz, Z.A.; Gao, F.; Herald, C.L.; Boyd, M.R.; Schmidt, J.M.; Hooper, J.N.A. Antineoplastic agents. 257. Isolation and structure of spongistatin 1. J. Org. Chem. 1993, 58, 1302–1304. [Google Scholar] [CrossRef]
- Salim, A.A.; Rae, J.; Fontaine, F.; Conte, M.M.; Khalil, Z.; Martin, S.; Parton, R.G.; Capon, R.J. Heterofibrins: Inhibitors of lipid droplet formation from a deep-water southern Australian marine sponge, Spongia (Heterofibria) sp. Org. Biomol. Chem. 2010, 8, 3188–3194. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Villani, G.; Varcamonti, M.; Sayem, S.M.A.; van Soest, R.; Gavagnin, M. Bioactive terpenes from Spongia officinalis. J. Nat. Prod. 2011, 74, 1241–1247. [Google Scholar] [CrossRef]
- Guella, G.; Mancini, I.; Pietra, F. C-15 acetogenins and terpenes of the dictyoceratid sponge Spongia zimocca of IL-Rogiolo: A case of seaweed-metabolite transfer to, and elaboration within, a sponge? Comp. Biochem. Physiol. B. 1992, 103, 1019–1023. [Google Scholar] [CrossRef]
- Xu, S.-H.; Cen, Y.-Z.; Zeng, L.-M.; Su, J.-Y. Isolation and structural determination of heterocyclic alkaloidal compounds. Chin. J. Org. Chem. 2000, 20, 248–250. [Google Scholar]
- Tai, C.-J.; Huang, C.-Y.; Ahmed, A.-F.; Orfali, R.-S.; Alarif, W.-M.; Huang, Y.M.; Wang, Y.-H.; Hwang, T.-L.; Sheu, J.-H. An anti-inflammatory 2,4-cyclized-3,4-secospongian diterpenoid and furanoterpene-related metabolites of a marine sponge Spongia sp. from the Red Sea. Mar. Drugs 2021, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.-Y.; Li, X.-M.; Li, K.; Ding, L.-P.; Gloer, J.B.; Wang, B.-G. Diterpenes, sesquiterpenes, and a C15-acetogenin from the marine red alga Laurencia mariannensis. J. Nat. Prod. 2007, 70, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Denys, R.; Coll, J.C.; Bowden, B.F. Tropical marine algae. IX. A new sesquiterpenoid metabolite from the red alga Laurencia marianensis. Aust. J. Chem. 1993, 46, 933–937. [Google Scholar] [CrossRef]
- Sauleau, P.; Retailleau, P.; Vacelet, J.; Bourguet-Kondracki, M.L. New polychlorinated pyrrolidinones from the Red Sea marine sponge Lamellodysidea herbacea. Tetrahedron 2005, 61, 955–963. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Badr, J.M.; Shaala, L.A.; Mohamed, G.A.; Bamanie, F.H. Ehrenasterol and biemnic acid; new bioactive compounds from the Red Sea sponge Biemna ehrenbergi. Phytochem. Lett. 2015, 12, 296–301. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Briand, A.; Kornprobst, J.-M.; Al-Easa, H.S.; Rizk, A.F.M.; Toupet, L. (−)-Paniculatol, a new ent-labdane bromoditerpene from Laurencia paniculata. Tetrahedron Lett. 1997, 38, 3399–3400. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakano, S.; Takahashi, Y.; Abe, T.; Masuda, M.; Takahashi, H.; Kobayashi, K. Brominated labdane-type diterpenoids from an Okinawan Laurencia sp. J. Nat. Prod. 2002, 65, 801–804. [Google Scholar] [CrossRef]
- Yaoita, Y.; Amemiya, K.; Ohnuma, H.; Furumura, K.; Masaki, A.; Matsuki, T.; Kikuchi, M. Sterol constituents from five edible mushrooms. Chem. Pharm. Bull. 1998, 46, 944–950. [Google Scholar] [CrossRef] [Green Version]
- Sright, J.L.C.; McInnes, A.G.; Shimizu, S.; Smith, D.G.; Walter, J.A.; Idler, D.; Khalil, W. Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonance spectroscopy. Can. J. Chem. 1978, 56, 1898–1903. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, I.; Goad, L.J.; Clague, A.D.H.; Mulheirn, L.J. The 220 MHz NMR spectra of phytosterols. Phytochemistry 1976, 15, 195–200. [Google Scholar] [CrossRef]
- Ioannou, E.; Abdel-Razik, A.F.; Zervou, M.; Christofidis, D.; Alexi, X.; Vagias, C.; Alexis, M.N.; Roussis, V. 5α,8α-Epidioxysterols from the gorgonian Eunicella cavolini and the ascidian Trididemnum inarmatum: Isolation and evaluation of their antiproliferative activity. Steroids 2009, 74, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Migliuolo, A.; Notaro, G.; Piccialli, V.; Sica, D. New tetrahydroxylated sterols from the marine sponge Spongia officinalis. J. Nat. Prod. 1990, 53, 1414–1424. [Google Scholar] [CrossRef]
- Shi, Q.; Huang, Y.; Su, H.; Gao, Y.; Peng, X.; Zhou, L.; Li, X.; Qiu, M. C28 steroids from the fruiting bodies of Ganoderma resinaceum with potential anti-inflammatory activity. Phytochemistry 2019, 168, 112109. [Google Scholar] [CrossRef]
- De Marino, S.; Palagiano, E.; Zollo, F.; Minale, L.; Iorizzi, M. A novel sulphated steroid with a 7-membered 5-oxalactone B-ring from an Antarctic starfish of the family Asteriidae. Tetrahedron 1997, 53, 8625–8628. [Google Scholar] [CrossRef]
- Huang, X.-C.; Guo, Y.-W.; Song, G.-Q. Fortisterol, a novel steroid with an unusual seven-membered lactone ring B from the Chinese marine sponge Biemna fortis Topsent. J. Asian Nat. Prod. Res. 2006, 8, 485–489. [Google Scholar] [CrossRef]
- Angawi, R.F.; Alarif, W.M.; Hamza, R.I.; Badria, F.A.; Ayyad, S.E.N. New cytotoxic laurene-, cuparene-, and laurokamurene-type sesquiterpenes from the red alga Laurencia obtusa. Helv. Chim. Acta 2014, 97, 1388–1395. [Google Scholar] [CrossRef]
- Brennan, M.R.; Kim, I.K.; Erickson, K.L. Kahukuenes, new diterpenoids from the marine alga Laurencia majuscula. J. Nat. Prod. 1993, 56, 76–84. [Google Scholar] [CrossRef]
- Sims, J.J.; Fenical, W.; Wing, R.M.; Radlick, P. Marine natural products. I. Pacifenol, a rare sesquiterpene containing bromine and chlorine from the red alga, Laurencia pacifica. J. Am. Chem. Soc. 1971, 93, 3774–3775. [Google Scholar] [CrossRef]
- Cassano, V.; De-Paula, J.C.; Fujii, M.; Gama, B.A.P.; Teixeira, V. Sesquiterpenes from the introduced red seaweed Laurencia caduciramulosa (Rhodomelaceae, Ceramiales). Biochem. Syst. Ecol. 2008, 36, 223–226. [Google Scholar] [CrossRef]
- Stallard, M.O.; Faulkner, D.J. Chemical constituents of the digestive gland of the sea hare Aplysia californica. II. Chemical transformations. Comp. Biochem. Physiol. 1974, 49, 37–41. [Google Scholar]
- Palaniveloo, K.; Vairappan, C.S. Chemical relationship between red algae genus Laurencia and sea hare (Aplysia dactylomela Rang) in the North Borneo Island. J. Appl. Phycol. 2014, 26, 1199–1205. [Google Scholar] [CrossRef]
- Carmely, S.; Gebreyesus, T.; Kashman, Y.; Skelton, B.W.; White, A.H.; Yosief, T. Dysidamide, a novel metabolite from a Red Sea sponge Dysidea herbacea. Aust. J. Chem. 1990, 43, 1881–1888. [Google Scholar] [CrossRef]
- Olinger, L.K.; Strangman, W.K.; McMurray, S.E.; Pawlik, J.R. Sponges with microbial symbionts transform dissolved organic matter and take up organohalides. Front. Mar. Sci. 2021, 8, 665789. [Google Scholar] [CrossRef]
- Berumen, M.L.; Hoey, A.S.; Bass, W.H.; Bouwmeester, J.; Catania, D.; Cochran, J.E.M.; Khalil, M.T.; Miyake, S.; Mughal, M.R.; Spaet, J.L.Y.; et al. The status of coral reef ecology research in the Red Sea. Coral Reefs 2013, 32, 737–748. [Google Scholar] [CrossRef]
- Trautman, D.A.; Hinde, R.; Borowitzka, M.A. Population dynamics of an association between a coral reef sponge and a red macroalga. J. Exp. Mar. Biol. Ecol. 2000, 244, 87–105. [Google Scholar] [CrossRef]
- Lubarsky, H.V.; Hubas, C.; Chocholek, M.; Larson, F.; Manz, W.; Paterson, D.M.; Gerbersdorf, S.U. The stabilisation potential of individual and mixed assemblages of natural bacteria and microalgae. PLoS ONE 2010, 5, e13794. [Google Scholar] [CrossRef] [Green Version]
- Wiese, J.; Thiel, V.; Nagel, K.; Staufenberger, T.; Imhoff, J.F. Diversity of antibiotic-active bacteria associated with the brown alga Laminaria saccharina from the Baltic Sea. Mar. Biotechnol. 2009, 11, 287–300. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, P.D.; De Nys, R. Chemical mediation of colonization of seaweed surfaces. J. Phycol. 2002, 38, 621–629. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Suzuki, M.; Abe, T.; Masuda, M. Halogenated metabolites with antibacterial activity from the Okinawan Laurencia species. Phytochemistry 2001, 58, 517–523. [Google Scholar] [CrossRef]
- Vairappan, C.S. Potent antibacterial activity of halogenated metabolites from Malaysian red algae, Laurencia majuscula (Rhodomelaceae, Ceramiales). Biomol. Eng. 2003, 20, 255–259. [Google Scholar] [CrossRef]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Chung, P.-J.; Ho, C.-M.; Kuo, C.-Y.; Hung, M.-F.; Huang, Y.-T.; Chang, W.-Y.; Chang, Y.-W.; Chan, K.-H.; Hwang, T.-L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, T.-L.; Su, Y.-C.; Chang, H.-L.; Leu, Y.-L.; Chung, P.-J.; Kuo, L.-M.; Chang, Y.-J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res. 2009, 50, 1395–1408. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-S.; Chang, H.-S.; Hsiao, H.-H.; Chen, Y.-F.; Kuo, Y.-P.; Yen, F.-L.; Yen, C.-H. Identification of beilschmiedia tsangii root extract as a liver cancer cell–normal keratinocyte dual-selective NRF2 regulator. Antioxidants 2021, 10, 544. [Google Scholar] [CrossRef]
- Kao, Y.-T.; Chen, Y.-S.; Tang, K.-W.; Lee, J.-C.; Tseng, C.-H.; Tzeng, C.-C.; Yen, C.-H.; Chen, Y.-L. Discovery of 4-anilinoquinolinylchalcone derivatives as potential NRF2 activators. Molecules 2020, 25, 3133. [Google Scholar] [CrossRef]
- Jiang, L.W.; Watkins, D.; Jin, Y.; Gong, C.; King, A.; Washington, A.Z.; Green, K.D.; Garneau-Tsodikova, S.; Oyelere, A.K.; Arya, D.P. Rapid synthesis, RNA binding, and antibacterial screening of a peptidic-aminosugar (PA) library. ACS Chem. Biol. 2015, 10, 1278–1289. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).