Abstract
Manzamine-A is a marine-derived alkaloid which has anti-viral and anti-proliferative properties and is currently being investigated for its efficacy in the treatment of certain viruses (malaria, herpes, HIV-1) and cancers (breast, cervical, colorectal). Manzamine-A has been found to exert effects via modulation of SIX1 gene expression, a gene critical to craniofacial development via the WNT, NOTCH, and PI3K/AKT pathways. To date little work has focused on Manzamine-A and how its use may affect bone. We hypothesize that Manzamine-A, through SIX1, alters bone cell activity. Here, we assessed the effects of Manzamine-A on cells that are responsible for the generation of bone, pre-osteoblasts and osteoblasts. PCR, qrtPCR, MTS cell viability, Caspase 3/7, and functional assays were used to test the effects of Manzamine-A on these cells. Our data suggests Six1 is highly expressed in osteoblasts and their progenitors. Further, osteoblast progenitors and osteoblasts exhibit great sensitivity to Manzamine-A treatment exhibited by a significant decrease in cell viability, increase in cellular apoptosis, and decrease in alkaline phosphatase activity. In silico binding experiment showed that manzamine A potential as an inhibitor of cell proliferation and survival proteins, i.e., Iκb, JAK2, AKT, PKC, FAK, and Bcl-2. Overall, our data suggests Manzamine-A may have great effects on bone health overall and may disrupt skeletal development, homeostasis, and repair.
1. Introduction
Appropriate preclinical and clinical screening for pharmacological therapies is necessary to inform the patient populations of interest which may benefit from these therapies but could incur risks associated with exposure to their health [1,2,3]. The United States Food and Drug Administration and the Center for Drug Evaluation and Research is the clearing house for this information garnered from a variety of sources (academic, pharmaceutical companies, NIH) using label approaches that inform specific de novo effects and those associated with existing comorbidities [1,2,4,5,6]. Note many drugs authorized for emergency usage, currently in clinical trials, or used in off-label contexts may not have adequate risk/benefit assessment for those patients who are being treated. This is especially poignant today as the current COVID crisis has altered our focus on emerging disease and has driven an increase in emergency authorizations and off-label use of many drugs as tools to contain emerging viruses but can also adversely affect vulnerable populations [7,8,9,10,11,12,13,14,15,16,17,18].
Marine natural products remain a highly important source for emerging drugs, especially in the treatments of cancers and viral infections [19,20,21,22,23,24,25,26]. One such potential drug is manzamine-A, a marine sponge derived alkaloid that emerged in the field of infectious disease as a promising control for malaria [27,28,29,30,31,32,33,34,35]. The understanding of manzamine and derived analogs utility in medicine is nascent, but is proposed to include treatment for malaria [27,28,29,30,31,32,33], herpes [29,36,37,38], HIV [29,32,39,40,41,42,43,44], cancer [6,10,13,35,37,38,39,44,45], as well as generally having anti-bacterial, antifungal, and anti-inflammatory activities [15,17,19,20,24,32,34,36,37,38,43,46,47,48,49,50,51,52,53,54,55]. Thus, this emerging drug may show great potential in the treatment of many diseases.
One mechanism of action by which manzamine-A acts is as a novel small molecule inhibitor targeting cells that express a critical homeobox gene SIX1 [45]. SIX1 expression has been linked to organogenesis, DNA specificity, protein–protein interactions, and proliferation and survival of cells [45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]. Thus, from a cellular development and maintenance perspective the use of manzamine-A and related alkaloid analogs may be of concern. Strengthening this argument are data from the murine knockout model that present with multiple anomalies including those related to the musculoskeletal system [61,66]. SIX1 has also been shown to segregate in birth defects related to bone development leading to excess bone formation [86]. Importantly, if manzamine-A use is suspected of affecting bone health, additional patient populations may be implicated including those susceptible to bone loss diseases. The US-CDC reports the age-adjusted prevalence of osteoporosis has increased in the last decade 9.5% in 2008 to 12.6% in 2018 [87]. Furthermore, the prevalence of osteoporosis leads to a significant increased risk of related bone fractures leading to morbidity and mortality and even death (33% of hip fractures in patients over 50 die within a year) [88,89,90,91,92]. Thus, a better understanding of “if and how” manzamine-A interacts with the cell population responsible for bone development and remodeling, the osteoblast, and the predicted target Six-1 is now necessary.
Here, we have taken that first step to characterize if manzamine-A affects osteoblasts, the cells responsible for mineralizing the skeleton, using in vitro exposures and assays. Using both the pre-osteoblast and osteoblast we hypothesized that manzamine-A would affect cell viability and function of these important cells. Further, we hypothesized that as Six1 is a predicted mRNA target of the emerging drug manzamine-A, this target would have altered expression in our osteoblast lineage cells.
2. Results
To determine if Six1 mRNA is expressed in pre-osteoblasts and osteoblasts, agarose gel electrophoresis compared presence of Six1. β-actin was used as an endogenous mRNA control and we utilized cells of a different lineage (monocyte/macrophage) cells, raw 264.7 cells as control cells for study purposes. Six1 was found to be expressed in pre-osteoblasts and particularly enriched expression in osteoblasts as shown in Figure 1. These data further supported our focus on the osteoblast as a potential target of the manzamine-A drug.
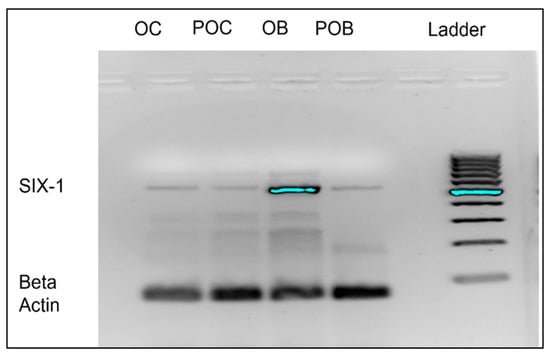
Figure 1.
Osteoblast highly expresses Six1 mRNA. Representative (of n = 3) agarose gel demonstrating great expression of Six1 target in mature differentiated osteoblasts. OC = Osteoclast; POC = Macrophage; OB = Osteoblasts; POB = Pre-Osteoblasts.
We next determined IC50 values of manzamine-A in pre-osteoblasts after 24-, 48-, and 72-h of treatment using standard MTS viability assay, Figure 2. The estimated IC50 values were 3.6447 μmol at 24 h, 2.0358 μmol at 48 h, and 5.4699 μmol at 72 h. These assays were repeated in mature osteoblasts after differentiation, Figure 2. Resulting estimated IC50 values were 4.3678 μmol at 24 h, 4.161 μmol at 48 h, and 3.6573 μmol at 72 h. To interrogate additional cellular effects of manzamine A on the osteoblast cell lineage doses of 2.5 μmol and 5 μmol were chosen for further experimentation.
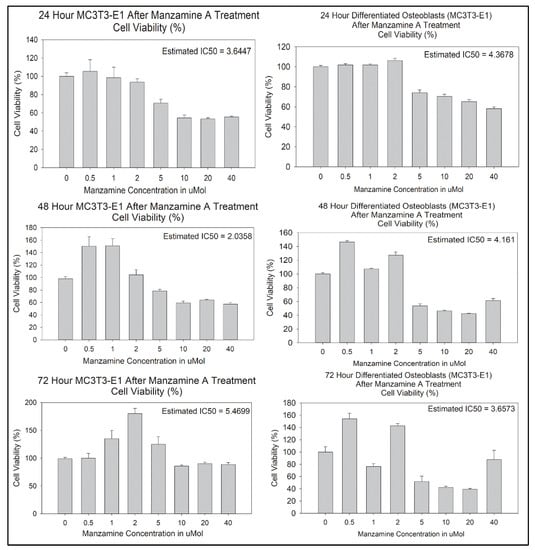
Figure 2.
Manzamine treatment alters osteoblast lineage cell viability. MTS assay was used to determine IC50 values for downstream experimental studies. Note alteration to viability at all post-treatment timepoints. (n = 3 replicates per treatment).
Caspase 3/7 assay was utilized to determine how manzamine A affected programmed cell death, apoptosis, in our osteoblast cell lines. In our pre-osteoblasts, there was a statistically significant increase in apoptosis at 24 h, 48 h, and 72 h in both the 2.5 μmol and 5 μmol doses groups, Figure 3. This effect was mirrored in our mature osteoblast cells at 24 and 48 h where statistically significant increases in apoptosis were observed, Figure 3. However, at 72 h, there was a statistically significant decrease in cellular apoptosis in the 5 μmol manzamine dosed group. We interpret this as the result of most cellular apoptosis having already robustly occurred in this dosed group. This is supported by our observation of the cells in wells at that time point (Supplemental Figure S1).
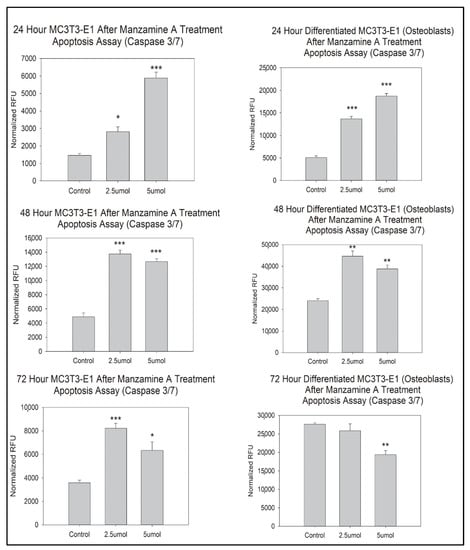
Figure 3.
Manzamine treatment drives apoptosis in osteoblast lineage cells. Note increases in apoptosis for most comparisons with the exception of 72 h mature osteoblasts. n = 3 replicates per treatment. * p < 0.05, ** p < 0.01, *** p < 0.001. (RFU = Relative Fluorescent Unit).
To determine the effects of manzamine A on osteoblast lineage function a quantitative alkaline phosphatase assay was used. Our data suggest great and statistically significant decreases in alkaline phosphatase by enzymatic reaction detection in our pre-osteoblasts after 72 h. This effect was not observed at the 7-day timepoint, Figure 4. We attribute the lack of change at 7-days due to the length of the assay in which cells were likely able to renew or stabilize after the dose treatment. Experimentation was repeated in our differentiated osteoblast blast and significant decreases in alkaline phosphatase were observed at both 72 h and at 7 days for both delivered doses. These data support a scenario where manzamine-A is likely to disrupt the function of the osteoblast to produce bone matrix.
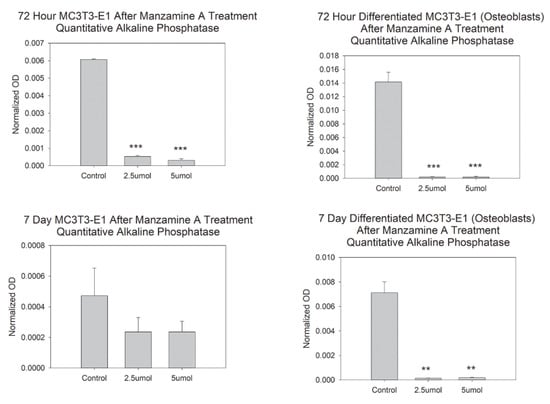
Figure 4.
Manzamine decreases osteoblast function. Quantitative alkaline phosphatase activity was decreased by manzamine treatments. n = 3 replicates per treatment. ** p < 0.01, *** p < 0.001. (OD = Optical Density).
We sought to interrogate how manzamine-A treatment would alter Six1 mRNA expression in our cells of the osteoblast lineage. Our data demonstrated marked and significant decreases in mRNA expression after 24 h in our pre-osteoblasts and after 24 and 48 h in our differentiated osteoblasts for both the doses utilized here, Figure 5. These support the interaction between manzamine-A and Six1 in resulting cell function.
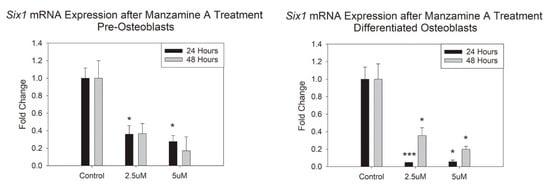
Figure 5.
Manzamine treatment results in decreased Six1 mRNA expression. mRNA expression changes represented as fold change. Note significant decreases especially for the mature osteoblast cells. n = 3 replicates per treatment. * p < 0.05, *** p < 0.001. (OD = Optical Density).
Finally, we sought to apply in silico binding experiments to predict manzamine A activity as an inhibitor of cell viability. Protein kinase is an important signaling protein in many biological pathways including cell proliferation and apoptosis. Most of the protein kinase inhibitors bind competitively at the highly conserved ATP-binding domain [93,94,95]. Protein kinase inhibitors have been shown to be active against multiple protein kinases due to the conservation nature of the ATP-binding domain. Manzamine A has been previously shown as an inhibitor of three protein kinases, i.e., GSK3b, CDK5, and RSK1 [96,97]. It is safe to assume that manzamine A may have activity against more protein kinases due to its reported activities for multiple targets and diseases. In this study, manzamine A is docked at the ATP-competitive domain of seven protein kinases (TGF-β, Iκb, JAK2, PI3K, AKT, PKC, and FAK) involved in cell proliferation, survival, and apoptosis pathways. The binding affinities of manzamine A to these proteins were compared with ATP. The results showed that manzamine A has a higher affinity than ATP for Iκb, JAK2, AKT, PKC, and FAK, suggesting its potential as an inhibitor for these proteins (Figure 6 and Table 1). Inhibition of these proteins will result in the downregulation of cell proliferation and survival proteins, and promote expression of caspase 3/7 leading to apoptosis. Manzamine A was also docked to Bcl-2 protein, an important regulator of apoptosis. Inhibition of Bcl-2 will lead to higher expression of caspase 3/7 and apoptosis. Manzamine A showed good binding affinity to Bcl-2 (−10.1 kcal/mol). This indicated manzamine A potential as a Bcl-2 inhibitor although it may be less active than the known Bcl-2 inhibitor venotoclax [98] (binding affinity = −12.2 kcal/mol).
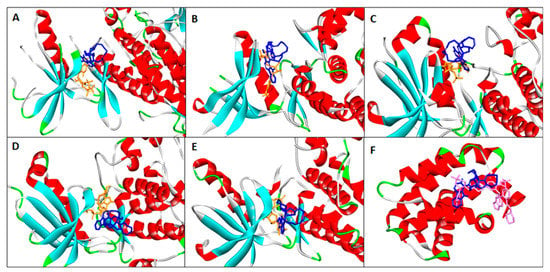
Figure 6.
In silico molecular docking of manzamine A to apoptosis regulating proteins. (A) Iκb; (B) JAK2; (C) AKT; (D) PKC; (E) FAK; and (F) Bcl-2. Manzamine A, ATP, and ventoclax are shown as blue, orange, and pink structures, respectively.

Table 1.
Binding affinity (kcal/mol) and coordinates.
3. Discussion
This is the first step into understanding how manzamine-A may affect organ systems that are not the intended target of this emerging drug. Further these experiments provide a platform by which other cell types, and more importantly other emerging natural products can be studied for potential health effects. Overall, our data paints a picture of effects that manzamine-A would have after short term treatment on a bone cell line. Namely, decreases in osteoblast function should be expected and further that bone as an organ is a potential target of these drugs. This implication takes on many forms that necessitate further study as manzamine-A has shown great promise in targeting of disease processes such as cancers. Population indications for manzamine A are widespread as indicated above. Thus, individuals at risk of bone effects due to manzamine A use could include the fetus and or offspring of a dosed expectant mother, an adolescent or young adult in a positive curve for the building of bone density, and more directly those patients at risk of bone wasting diseases such as osteopenia or osteoporosis, the elderly including women after menopause. Other natural products have been shown to target bone related cells, specifically altering bone remodeling via osteoclast activity. Interestingly in this context several drugs have been purported to potentially be useful as a therapeutic where there is a bone wasting disease [99,100,101]. Here, our data suggests manzamine-A will also target bone as an organ but will likely result in further bone loss in stark contrast to Thiaplakortone B or Hymenialdisine for example.
Limitations here include the approach. These initial data were collected using an in vitro modeling system only. Future research will necessitate in vivo dosing of manzamine A likely initially in experimental models to determine the widespread and localized effects in may have on organ systems including bone. Further, a single target was chosen to confirm our bio-informatic conclusion that Six1 was targeted by manzamine-A. This hypothesis proved true, but it is likely that manzamine A has pleiotropic effects including other Six1 associated mRNA pathway targets (Gro, Mdfi, Dach, and Eya1) and pathways associated with bone development and health (WNT, NOTCH, PI3K/AKT). Further, although limited in scope, in silico binding experiment has provided a glimpse of possible targets associated with these pathways and supported his hypothesis. Future research will be needed to focus on exploring these and additional molecular targets of the drug to get a clear picture of potential effects in multicellular systems. Furthermore, if manzamines continue to prove to have undesirable side effects such as bone loss, approaches to mitigate these effects will be explored [102]. The next logical target are other bone cells (progenitor cells, osteoclast, osteocytes) as well as re-interrogating the effects of manzamine A on bone remodeling and homeostasis [103] Overall, this initial study did show dramatic effects of manzamine A on the osteoblast, where viability was altered, apoptosis was increased, and function of the cell was diminished.
4. Materials and Methods
MC3T3-E1 cells (Subclone 4 CRL-2593) were obtained from ATCC (Manassas, VA, USA) and maintained as recommended to produce pre-osteoblast cells for studies. Briefly, cells were maintained in a 75 cm2 flask using Alpha Eagle Minimum Essential Medium (Alpha-MEM, Lonza, Walkersville Inc., Walkersville, MD, USA, BE02–002F) with 10% Fetal Bovine Serum (FBS, Atlanta Biologicals, Atlanta, GA, USA, S11150H) and 1% Penicillin/Streptomycin (Lonza, 10k/10k 17–602E) until 85% confluent when they were moved to a 175 cm2 flask after trypsin-EDTA (0.1%, Gibco/Fisher Scientific, Hampton, NH, USA, 15400–054) dissociation. Cells (Pre-osteoblasts) were subcultured as necessary throughout the experimentation. To induce osteoblast differentiation 0.25 mM ascorbic acid (Fisher Scientific, Hampton, NH, USA, A61–25), 0.1 μm dexamethasone (Fisher Scientific, Hampton, NH, USA, AC230300010), and 10 mM β-glycerophosphate (Fisher Scientific, Hampton, NH, USA, L03425) were added to standard Alpha-MEM media to produce osteogenic media (OM, Osteoblasts). Control cells for PCR study of Six1 mRNA expression were inclusive of Raw 264.7 cells (ATCC, Manassas, VA, USA, TIB-71) maintained as macrophage precursors or differentiated to mature osteoclast phenotype. These cells were maintained in a 75 cm2 flask using Dulbecco’s Modified Eagle’s Medium (DMEM, Lonza, Basel, Switzerland, 12604F) with 10% FBS and 1% Penicillin/Streptomycin until 85% confluent, when they were moved to a 175 cm2 flask after cell scraper dissociation. Cells were sub-cultured as necessary throughout the experimentation. To induce mature osteoclast phenotype murine RANK Ligand 50 ng/mL (Peprotech, Secaucus, NJ, USA, 315–11) was added to the standard DMEM media (RANKL, Osteoclasts).
Manzamine-A was produced by co-author Dr. Mark Hamann’s laboratory at Medical University of South Carolina following previously published protocols. Briefly, lipophilic alkaloid extracts were previously prepared from the Indonesian sponge Acanthostrongylophora (collected from Manado Bay, Northern Sulawesi, Indonesia, in 2003). Crude material was separated using vacuum liquid chromatography, and manzamine was purified by crystallization. Purified manzamine was then transformed into its hydrochloride salt and recrystallized to reach high purity (>99%) and optimized aqueous solubility as described previously [41,44,45].
Pre-osteoblasts, mature osteoblasts, macrophages, and osteoclasts, were seeded in triplicate at a density of 300,000 cells per well in triplicate in 6 well culture plates and treated with control un-supplemented media, and appropriate differentiation media as defined above. After 24 h in isolated culture, RNA was isolated using the OMEGA bio-tek E.Z.N.A. Total RNA kit 1 (Omega Bio-tek, Norcross, GA, USA, R6834-02) according to manufacturer’s protocol. Quality and quantity of RNA was assessed using a Synergy Hi Microplate reader and a Take3 Microvolume Plate (BioTek, Winooski, VT, USA). Complimentary DNA synthesis was performed using Quanta qScript cDNA Synthesis reagents following manufacturers protocol (Quanta Biosciences, Beverly, MA, USA, 95047-025). Presence of neurotransmitters and GPCRs was determined via PCR using cDNA, designed primers from Integrated DNA Technologies (Coralville, IA, USA,) (Table 1), Platinum Taq DNA Polymerase (Fisher Scientific, Hampton, NH, USA, 100021273), and separation on 1.5% agarose gels employing primers for Six1 (GCTGTCACC GGGCCTATTTA/Reverse ATGAGCAAGCCAACCCTGTT) β-Actin (Forward GCAGGAGTACGATGAGTCCG/Reverse ACGCAGCTCAGTAACAGTCC) as a control. Annealing temperature was optimized to 53C. Each assay was repeated three independent times.
Cell viability was measured using a CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS, Promega, Madison, WI, USA). The conversion of MTS tetrazolium into Formazan is directly proportional to the number of viable cells in each well. Osteoblast lineage cells were seeded in 96 well plates at a density of 4000 cells/well and treated with Manzamine at serial concentrations (0.5–40 µMol) compared to a control group (0 umol) without exposure to Manzamine and then assayed at 24, 48, and 72 h (triplicate replicates). Inhibitory Concentration (IC50) values were determined using AAT BioQuest Graph and used for all downstream studies.
Cellular apoptosis levels were measured using Apo-ONE Homogenous Caspase 3/7 Assay (Promega, Madison, WI, USA). This assay measures the amount of Caspase 3 and Caspase 7 activity to quantify apoptosis levels. Cells were seeded in 96 well plates at a density of 4000 cells/well and treated with Manzamine at 2.5 µmol and 5 µmol concentrations compared to a control group without exposure to Manzamine then assayed at 24, 48, and 72 h (triplicate replicates) On assay, cells were incubated with 100 μL/well substrate/buffer solution (1:100). Contents were mixed for 30 s at 300 rpm and incubated at room temperature for one hour. Fluorescence was measured using a 96-well plate reader (Biotek, Winooski, VT, USA) with excitation at 485 and emission at 530 nm.
Functional levels of pre-osteoblasts and osteoblasts were measured using a SIGMAFAST p-nitrophenyl phosphate alkaline phosphatase assay from Sigma-Aldrich (St. Louis, MO, USA). This assay measures the quantification of the reaction of the enzyme, alkaline phosphatase, an important factor that leads to mineralization of bone. Cells were seeded in 96 well plates at a density of 4000 cells/well and treated with Manzamine at 2.5 μmol and 5 μmol concentrations compared to a control group without exposure to Manzamine then assayed at 24, 48, and 72 h (triplicate replicates). On assay medium was removed from cells, and cell lysis was performed using Triton ×100 at 0.01% (Sigma). After 30 min of incubation at 4 °C, deionized water and a p-Nitrophenyl phosphate solution were added to the lysis buffer. Three control wells containing no cells were also treated and served as blank controls to mathematically subtract the effects of the lysis buffer and water on final optical densities. Plates were incubated at room temperature in the dark for 30 min. The absorbance at 405 nm was recorded with a 96-well plate reader (Biotek, Winooski, VT, USA). ALP activity was then calculated using the following formula: ((optical density—the mean optical density of the control wells) × total volume × dilution)/(18.45 × sample volume).
To quantify expression the specific mRNA target SIX1, cDNA was subjected to quantitative PCR using Applied Biosystems TaqMan Gene Expression Master Mix and targeted TaqMan gene expression assay (Mm00808212_m1) and normalized to control endogenous Gapdh activity (Mm99999915_g1). Briefly cells were cultured in 6 well plates with a seeding density of 300,000 cells/well. Pre-osteoblasts and osteoblasts were treated with 2.5 μmol and 5 μmol doses of Manzamine compared to a control group without exposure to Manzamine. Cells were then harvested at 24 and 48 h for RTQPCR experiments (triplicate replicates). RNA was isolated from both cell types using the RNA Isolation protocol from the E.Z.N.A. Total RNA Kit from Omega. After RNA Isolation, the RNA was transcribed into complementary cDNA using the cDNA Synthesis protocol from qScript cDNA Synthesis Kit from QuantaBio. cDNA is then used as the template strand in a quantitative PCR reaction using a TaqMan Fast Advanced Master Mix. Data were normalized to GAPDH RNA expression by ΔCT. Quantitative data were compared for gene expression changes due to treatment with manzamine by ΔΔCT methodology. Previously published statistical analysis methodology was used to determine differences for gene expression after manzamine related the target of interest [104]. Differences were considered significant if p ≤ 0.05.
All statistical analyses were done by comparing the Manzamine dosed groups to the control groups using a standard t test or non-parametric Mann–Whitney U after assessing normality with a Shapiro–Wilk test and homogeneity of variance with a Levene’s test using Welch’s correction for variance when needed. Significance was determined if p values were less than 0.05.
The structures of manzamine A, ATP, and venetoclax were optimized using the MM2 energy-minimized function in the Chem3D Ultra version 16.0. The crystal structures of the receptor proteins (Table 1) were obtained from the Protein Data Bank [105,106]. AutoDockTools version 1.5.6 were used to prepare the receptor proteins and ligands for the molecular docking experiment. The grid box parameters used were: grid box spacing = 1.0 Å; x-dimension = y-dimension = z-dimension = 20. AutoDock Vina program was used to perform the docking and calculate the binding affinity [107,108]. The results were processed and analyzed using the BIOVIA Discovery Studio Visualizer version 17.2.0 (Dassault Systèmes, San Diego, CA, USA). The binding affinities are summarized in Table 1.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md20100647/s1, Figure S1: Phase Images of Cells Treated with Manzamine.
Author Contributions
Conceptualization, M.H. and J.C.; Data curation, S.H., Y.-M.C., M.H. and J.C.; Formal analysis, Y.-M.C., M.H. and J.C.; Funding acquisition, J.C.; Investigation, S.H., Y.-M.C., M.H. and J.C.; Methodology, S.H., Y.-M.C., M.H. and J.C.; Project administration, J.C.; Resources, M.H. and J.C.; Supervision, J.C.; Validation, Y.-M.C., M.H. and J.C.; Writing—original draft, S.H., Y.-M.C., M.H. and J.C.; Writing—review and editing, S.H., Y.-M.C., M.H. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ohio State College of Medicine.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Raw data provided by request to corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ALP | Alkaline Phosphatase |
| °C | Degrees Celsius |
| COVID | Corona Virus Disease |
| Dach | Dachshund Homolog 1 |
| DNA | Deoxyribonucleic acid |
| Eya1 | Eyes absent homolog 1 |
| Gro | Groucho |
| HIV | Human Immunodeficiency Virus |
| IC50 | Inhibitory concentration 50% |
| Mdfi | MyoD Family Inhibitor |
| mRNA | Message ribonucleic acid |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| NIH | United States National Institutes of Health |
| Notch | Notch Receptor |
| PCR | Polymerase Chain Reaction |
| qrtPCR | Quantitative Real Time Polymerase Chain Reaction |
| PI3K/AKT | Phosphoinositide 3-kinases/Protein kinase B |
| RNA | Ribonucleic Acid |
| Six1 | Sine Oculis Homeobox |
| US-CDC | United State Center for Disease Control |
| WNT | Wingless Intergrated |
References
- Ren, Z.; Bremer, A.A.; Pawlyk, A.C. Drug development research in pregnant and lactating women. Am. J. Obs. Gynecol 2021, 225, 33–42. [Google Scholar]
- Tsamantioti, E.S.; Hashmi, M.F. Teratogenic Medications. In StatPearls; Statpearl Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wesley, B.D.; Sewell, C.A.; Chang, C.Y.; Hatfield, K.P.; Nguyen, C.P. Prescription medications for use in pregnancy-perspective from the US Food and Drug Administration. Am. J. Obs. Gynecol. 2021, 225, 21–32. [Google Scholar] [CrossRef]
- Barrow, P. Review of embryo-fetal developmental toxicity studies performed for pharmaceuticals approved by FDA in 2016 and 2017. Reprod. Toxicol. 2018, 80, 117–125. [Google Scholar] [CrossRef]
- Brucker, M.C.; King, T.L. The 2015 US Food and Drug Administration Pregnancy and Lactation Labeling Rule. J. Midwifery Womens Health 2017, 62, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, E.; Chai, S.; Kroumpouzos, G. Drug safety: Pregnancy rating classifications and controversies. Clin. Derm. 2016, 34, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, C.; Dever, D.; Stanbery, L.; Edelman, G.; Dworkin, L.; Nemunaitis, J. FDA efficiency for approval process of COVID-19 therapeutics. Infect. Agent Cancer 2020, 15, 73. [Google Scholar] [CrossRef]
- Chary, M.A.; Barbuto, A.F.; Izadmehr, S.; Hayes, B.D.; Burns, M.M. COVID-19: Therapeutics and Their Toxicities. J. Med. Toxicol. 2020, 16, 284–294. [Google Scholar] [CrossRef]
- Dauner, D.G.; Dauner, K.N. Summary of adverse drug events for hydroxychloroquine, azithromycin, and chloroquine during the COVID-19 pandemic. J. Am. Pharm. Assoc. 2021, 61, 293–298. [Google Scholar] [CrossRef]
- Kato, S.; Kurzrock, R. Repurposing Interleukin-6 Inhibitors to Combat COVID-19. J. Immunother. Precis. Oncol. 2020, 3, 52–55. [Google Scholar] [CrossRef]
- Li, D.; Sempowski, G.D.; Saunders, K.O.; Acharya, P.; Haynes, B.F. SARS-CoV-2 Neutralizing Antibodies for COVID-19 Prevention and Treatment. Annu. Rev. Med. 2021, 73, 1–16. [Google Scholar] [CrossRef]
- Manivannan, E.; Karthikeyan, C.; Moorthy, N.; Chaturvedi, S.C. The Rise and Fall of Chloroquine/Hydroxychloroquine as Compassionate Therapy of COVID-19. Front. Pharm. 2021, 12, 584940. [Google Scholar] [CrossRef] [PubMed]
- Mouffak, S.; Shubbar, Q.; Saleh, E.; El-Awady, R. Recent advances in management of COVID-19: A review. Biomed. Pharm. 2021, 143, 112107. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; Akhtar, M.F.; Haris, M.; Abdel-Daim, M.M. Recent updates on immunological, pharmacological, and alternative approaches to combat COVID-19. Inflammopharmacology 2021, 29, 1331–1346. [Google Scholar] [CrossRef]
- Shyr, Z.A.; Gorshkov, K.; Chen, C.Z.; Zheng, W. Drug Discovery Strategies for SARS-CoV-2. J. Pharm. Exp. 2020, 375, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ho, M. Emerging antibody-based therapeutics against SARS-CoV-2 during the global pandemic. Antib. Ther. 2020, 3, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines 2021, 9, 689. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Behrouz, S.; Farjam, M.; Rad, M.N.S. A Mini Review on Discovery and Synthesis of Remdesivir as an Effective and Promising Drug against COVID-19. Russ. J. Bioorg. Chem. 2021, 47, 609–621. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Galvano, F.; D’Orazio, N. Novel Therapeutic Strategies Against Cancer: Marine-derived Drugs May Be the Answer? Anticancer.Agents Med. Chem. 2016, 16, 1549–1557. [Google Scholar] [CrossRef]
- Mioso, R.; Marante, F.J.; Bezerra, R.S.; Borges, F.V.; Santos, B.V.; Laguna, I.H. Cytotoxic Compounds Derived from Marine Sponges. A Review (2010–2012). Molecules 2017, 22, 208. [Google Scholar] [CrossRef]
- Mioso, R.; Marante, F.J.; Laguna, I.H. Chemical constituents of the fermentation broth of the marine-derived fungus Penicillium roqueforti. Rev. Iberoam. Micol. 2015, 32, 147–152. [Google Scholar] [CrossRef]
- Nair, D.G.; Weiskirchen, R.; Al-Musharafi, S.K. The use of marine-derived bioactive compounds as potential hepatoprotective agents. Acta Pharm. Sin. 2015, 36, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M.; Battershill, C.N. Therapeutic agents from the sea: Biodiversity, chemo-evolutionary insight and advances to the end of Darwin’s 200th year. Diving Hyperb. Med. 2009, 39, 216–225. [Google Scholar] [PubMed]
- Newman, D.J.; Cragg, G.M.; Holbeck, S.; Sausville, E.A. Natural products and derivatives as leads to cell cycle pathway targets in cancer chemotherapy. Curr. Cancer Drug Targets 2002, 2, 279–308. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef]
- Ang, K.K.; Holmes, M.J.; Higa, T.; Hamann, M.T.; Kara, U.A. In vivo antimalarial activity of the beta-carboline alkaloid manzamine A. Antimicrob. Agents Chemother. 2000, 44, 1645–1649. [Google Scholar] [CrossRef]
- Ang, K.K.; Holmes, M.J.; Kara, U.A. Immune-mediated parasite clearance in mice infected with Plasmodium berghei following treatment with manzamine A. Parasitol. Res. 2001, 87, 715–721. [Google Scholar] [CrossRef]
- Laport, M.S.; Santos, O.C.; Muricy, G. Marine sponges: Potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef]
- Peng, J.; Kudrimoti, S.; Prasanna, S.; Odde, S.; Doerksen, R.J.; Pennaka, H.K.; Choo, Y.M.; Rao, K.V.; Tekwani, B.L.; Madgula, V.; et al. Structure-activity relationship and mechanism of action studies of manzamine analogues for the control of neuroinflammation and cerebral infections. J. Med. Chem. 2010, 53, 61–76. [Google Scholar] [CrossRef]
- Radwan, M.; Hanora, A.; Khalifa, S.; Abou-El-Ela, S.H. Manzamines: A potential for novel cures. Cell Cycle 2012, 11, 1765–1772. [Google Scholar] [CrossRef]
- Rao, K.V.; Santarsiero, B.D.; Mesecar, A.D.; Schinazi, R.F.; Tekwani, B.L.; Hamann, M.T. New manzamine alkaloids with activity against infectious and tropical parasitic diseases from an Indonesian sponge. J. Nat. Prod. 2003, 66, 823–828. [Google Scholar] [CrossRef]
- Thompson, M.J.; Louth, J.C.; Little, S.M.; Jackson, M.P.; Boursereau, Y.; Chen, B.; Coldham, I. Synthesis and evaluation of 1-amino-6-halo-beta-carbolines as antimalarial and antiprion agents. ChemMedChem 2012, 7, 578–586. [Google Scholar] [CrossRef]
- Hu, J.F.; Hamann, M.T.; Hill, R.; Kelly, M. The manzamine alkaloids. Alkaloids. Chem. Biol. 2003, 60, 207–285. [Google Scholar]
- Hanna, G.S.; Choo, Y.M.; Harbit, R.; Paeth, H.; Wilde, S.; Mackle, J.; Verga, J.U.; Wolf, B.J.; Thomas, O.P.; Croot, P.; et al. Contemporary Approaches to the Discovery and Development of Broad-Spectrum Natural Product Prototypes for the Control of Coronaviruses. J. Nat. Prod. 2021, 84, 3001–3007. [Google Scholar] [CrossRef]
- Ichiba, T.; Corgiat, J.M.; Scheuer, P.J.; Kelly-Borges, M. 8-Hydroxymanzamine A, a beta-carboline alkaloid from a sponge, Pachypellina sp. J. Nat. Prod. 1994, 57, 168–170. [Google Scholar] [CrossRef]
- Palem, J.R.; Bedadala, G.R.; El Sayed, K.A.; Hsia, S.C. Manzamine A as a novel inhibitor of herpes simplex virus type-1 replication in cultured corneal cells. Planta Med. 2011, 77, 46–51. [Google Scholar] [CrossRef]
- Palem, J.R.; Mudit, M.; Hsia, S.V.; Sayed, K.A.E. Discovery and preliminary structure-activity relationship of the marine natural product manzamines as herpes simplex virus type-1 inhibitors. Zeitschrift Nat. C J. Biosci. 2017, 72, 49–54. [Google Scholar] [CrossRef]
- Indraningrat, A.A.; Smidt, H.; Sipkema, D. Bioprospecting Sponge-Associated Microbes for Antimicrobial Compounds. Mar. Drugs 2016, 14, 87. [Google Scholar] [CrossRef]
- Peng, J.; Hu, J.F.; Kazi, A.B.; Li, Z.; Avery, M.; Peraud, O.; Hill, R.T.; Franzblau, S.G.; Zhang, F.; Schinazi, R.F.; et al. Manadomanzamines A and B: A novel alkaloid ring system with potent activity against mycobacteria and HIV-1. J. Am. Chem. Soc. 2003, 125, 13382–13386. [Google Scholar] [CrossRef]
- Rao, K.V.; Donia, M.S.; Peng, J.; Garcia-Palomero, E.; Alonso, D.; Martinez, A.; Medina, M.; Franzblau, S.G.; Tekwani, B.L.; Khan, S.I.; et al. Manzamine B and E and ircinal A related alkaloids from an Indonesian Acanthostrongylophora sponge and their activity against infectious, tropical parasitic, and Alzheimer’s diseases. J. Nat. Prod. 2006, 69, 1034–1040. [Google Scholar] [CrossRef]
- Rao, K.V.; Kasanah, N.; Wahyuono, S.; Tekwani, B.L.; Schinazi, R.F.; Hamann, M.T. Three new manzamine alkaloids from a common Indonesian sponge and their activity against infectious and tropical parasitic diseases. J. Nat. Prod. 2004, 67, 1314–1318. [Google Scholar] [CrossRef]
- Sayed, K.A.; Khalil, A.A.; Yousaf, M.; Labadie, G.; Kumar, G.M.; Franzblau, S.G.; Mayer, A.M.; Avery, M.A.; Hamann, M.T. Semisynthetic studies on the manzamine alkaloids. J. Nat. Prod. 2008, 71, 300–308. [Google Scholar] [CrossRef]
- Yousaf, M.; Hammond, N.L.; Peng, J.; Wahyuono, S.; McIntosh, K.A.; Charman, W.N.; Mayer, A.M.; Hamann, M.T. New manzamine alkaloids from an Indo-Pacific sponge. Pharmacokinetics, oral availability, and the significant activity of several manzamines against HIV-I, AIDS opportunistic infections, and inflammatory diseases. J. Med. Chem. 2004, 47, 3512–3517. [Google Scholar] [CrossRef]
- Karan, D.; Dubey, S.; Pirisi, L.; Nagel, A.; Pina, I.; Choo, Y.M.; Hamann, M.T. The Marine Natural Product Manzamine A Inhibits Cervical Cancer by Targeting the SIX1 Protein. J. Nat. Prod. 2020, 83, 286–295. [Google Scholar] [CrossRef]
- Adrados, I.; Larrasa-Alonso, J.; Galarreta, A.; Lopez-Antona, I.; Menendez, C.; Abad, M.; Gil, J.; Moreno-Bueno, G.; Palmero, I. The homeoprotein SIX1 controls cellular senescence through the regulation of p16INK4A and differentiation-related genes. Oncogene 2016, 35, 3485–3494. [Google Scholar] [CrossRef]
- Bessarab, D.A.; Chong, S.W.; Korzh, V. Expression of zebrafish six1 during sensory organ development and myogenesis. Dev. Dyn. 2004, 230, 781–786. [Google Scholar] [CrossRef]
- Bonnet, A.; Dai, F.; Brand-Saberi, B.; Duprez, D. Vestigial-like 2 acts downstream of MyoD activation and is associated with skeletal muscle differentiation in chick myogenesis. Mech. Dev. 2010, 127, 120–136. [Google Scholar] [CrossRef]
- Bonnin, M.A.; Laclef, C.; Blaise, R.; Eloy-Trinquet, S.; Relaix, F.; Maire, P.; Duprez, D. Six1 is not involved in limb tendon development, but is expressed in limb connective tissue under Shh regulation. Mech. Dev. 2005, 122, 573–585. [Google Scholar] [CrossRef]
- Cheng, Q.; Ning, D.; Chen, J.; Li, X.; Chen, X.P.; Jiang, L. SIX1 and DACH1 influence the proliferation and apoptosis of hepatocellular carcinoma through regulating p53. Cancer Biol. Ther. 2018, 19, 381–390. [Google Scholar] [CrossRef]
- Coletta, R.D.; Christensen, K.; Reichenberger, K.J.; Lamb, J.; Micomonaco, D.; Huang, L.; Wolf, D.M.; Muller-Tidow, C.; Golub, T.R.; Kawakami, K.; et al. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc. Natl. Acad. Sci. USA 2004, 101, 6478–6483. [Google Scholar] [CrossRef]
- Freyer, L.; Morrow, B.E. Canonical Wnt signaling modulates Tbx1, Eya1, and Six1 expression, restricting neurogenesis in the otic vesicle. Dev. Dyn. 2010, 239, 1708–1722. [Google Scholar] [CrossRef]
- Funato, N. New Insights Into Cranial Synchondrosis Development: A Mini Review. Front. Cell Dev. Biol. 2020, 8, 706. [Google Scholar] [CrossRef]
- Grifone, R.; Demignon, J.; Giordani, J.; Niro, C.; Souil, E.; Bertin, F.; Laclef, C.; Xu, P.X.; Maire, P. Eya1 and Eya2 proteins are required for hypaxial somitic myogenesis in the mouse embryo. Dev. Biol. 2007, 302, 602–616. [Google Scholar] [CrossRef]
- Grifone, R.; Laclef, C.; Spitz, F.; Lopez, S.; Demignon, J.; Guidotti, J.E.; Kawakami, K.; Xu, P.X.; Kelly, R.; Petrof, B.J.; et al. Six1 and Eya1 expression can reprogram adult muscle from the slow-twitch phenotype into the fast-twitch phenotype. Mol. Cell. Biol. 2004, 24, 6253–6267. [Google Scholar] [CrossRef]
- He, Z.; Li, G.; Tang, L.; Li, Y. SIX1 overexpression predicts poor prognosis and induces radioresistance through AKT signaling in esophageal squamous cell carcinoma. Onco Targets Ther. 2017, 10, 1071–1079. [Google Scholar] [CrossRef]
- Hosseinipour, M.; Wan, F.; Altomare, D.; Creek, K.E.; Pirisi, L. HPV16-transformed human keratinocytes depend on SIX1 expression for proliferation and HPV E6/E7 gene expression. Virology 2019, 537, 20–30. [Google Scholar] [CrossRef]
- Ikeda, K.; Kageyama, R.; Suzuki, Y.; Kawakami, K. Six1 is indispensable for production of functional progenitor cells during olfactory epithelial development. Int. J. Dev. Biol. 2010, 54, 1453–1464. [Google Scholar] [CrossRef]
- Ji, Q.; Zhu, J.; Fang, C.L.; Jin, H.; Zhan, D.P.; Huang, J. Down-regulation of MIAT suppresses osteosarcoma progression by acting as a ceRNA for miR-141-3p to regulate SIX1-mediated PI3K/AKT pathway. Eur. Rev. Med. Pharm. Sci. 2020, 24, 2218–2228. [Google Scholar]
- Jin, H.; Cui, M.; Kong, J.; Cui, X.; Lin, Z.; Wu, Q.; Liu, S. Sineoculis homeobox homolog 1 protein is associated with breast cancer progression and survival outcome. Exp. Mol. Pathol. 2014, 97, 247–252. [Google Scholar] [CrossRef]
- Laclef, C.; Hamard, G.; Demignon, J.; Souil, E.; Houbron, C.; Maire, P. Altered myogenesis in Six1-deficient mice. Development 2003, 130, 2239–2252. [Google Scholar] [CrossRef]
- Laclef, C.; Souil, E.; Demignon, J.; Maire, P. Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech. Dev. 2003, 120, 669–679. [Google Scholar] [CrossRef]
- Li, B.; Kuriyama, S.; Moreno, M.; Mayor, R. The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development 2009, 136, 3267–3278. [Google Scholar] [CrossRef]
- Li, C.M.; Guo, M.; Borczuk, A.; Powell, C.A.; Wei, M.; Thaker, H.M.; Friedman, R.; Klein, U.; Tycko, B. Gene expression in Wilms’ tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. Am. J. Pathol. 2002, 160, 2181–2190. [Google Scholar] [CrossRef]
- Li, W.; Qin, Y.; Zhou, R.; Liu, Y.; Zhang, G. High expression of SIX1 is an independent predictor of poor prognosis in endometrial cancer. Am. J. Transl. Res. 2021, 13, 2840–2848. [Google Scholar]
- Li, X.; Oghi, K.A.; Zhang, J.; Krones, A.; Bush, K.T.; Glass, C.K.; Nigam, S.K.; Aggarwal, A.K.; Maas, R.; Rose, D.W.; et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature 2003, 426, 247–254. [Google Scholar] [CrossRef]
- Li, Y.M.; Li, X.J.; Yang, H.L.; Zhang, Y.B.; Li, J.C. MicroRNA-23b suppresses cervical cancer biological progression by directly targeting six1 and affecting epithelial-to-mesenchymal transition and AKT/mTOR signaling pathway. Eur. Rev. Med. Pharm. Sci. 2019, 23, 4688–4697. [Google Scholar]
- Nagel, S.; Meyer, C.; Kaufmann, M.; Drexler, H.G.; MacLeod, R.A. Aberrant expression of homeobox gene SIX1 in Hodgkin lymphoma. Oncotarget 2015, 6, 40112–40126. [Google Scholar] [CrossRef]
- Nie, X.; Xu, J.; El-Hashash, A.; Xu, P.X. Six1 regulates Grem1 expression in the metanephric mesenchyme to initiate branching morphogenesis. Dev. Biol. 2011, 352, 141–151. [Google Scholar] [CrossRef]
- Petropoulos, H.; Skerjanc, I.S. Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J. Biol. Chem. 2002, 277, 15393–15399. [Google Scholar] [CrossRef]
- Relaix, F.; Demignon, J.; Laclef, C.; Pujol, J.; Santolini, M.; Niro, C.; Lagha, M.; Rocancourt, D.; Buckingham, M.; Maire, P. Six homeoproteins directly activate Myod expression in the gene regulatory networks that control early myogenesis. PLoS Genet. 2013, 9, e1003425. [Google Scholar] [CrossRef]
- Riddiford, N.; Schlosser, G. Six1 and Eya1 both promote and arrest neuronal differentiation by activating multiple Notch pathway genes. Dev. Biol. 2017, 431, 152–167. [Google Scholar] [CrossRef]
- Rodriguez, S.; Sickles, H.M.; Deleonardis, C.; Alcaraz, A.; Gridley, T.; Lin, D.M. Notch2 is required for maintaining sustentacular cell function in the adult mouse main olfactory epithelium. Dev. Biol. 2008, 314, 40–58. [Google Scholar] [CrossRef]
- Sato, S.; Ikeda, K.; Shioi, G.; Nakao, K.; Yajima, H.; Kawakami, K. Regulation of Six1 expression by evolutionarily conserved enhancers in tetrapods. Dev. Biol. 2012, 368, 95–108. [Google Scholar] [CrossRef]
- Song, W.; Ma, J.; Lei, B.; Yuan, X.; Cheng, B.; Yang, H.; Wang, M.; Feng, Z.; Wang, L. Sine oculis homeobox 1 promotes proliferation and migration of human colorectal cancer cells through activation of Wnt/beta-catenin signaling. Cancer Sci. 2019, 110, 608–616. [Google Scholar] [CrossRef]
- Tavares, A.L.P.; Cox, T.C.; Maxson, R.M.; Ford, H.L.; Clouthier, D.E. Negative regulation of endothelin signaling by SIX1 is required for proper maxillary development. Development 2017, 144, 2021–2031. [Google Scholar] [CrossRef]
- Towers, C.G.; Guarnieri, A.L.; Micalizzi, D.S.; Harrell, J.C.; Gillen, A.E.; Kim, J.; Wang, C.A.; Oliphant, M.U.J.; Drasin, D.J.; Guney, M.A.; et al. The Six1 oncoprotein downregulates p53 via concomitant regulation of RPL26 and microRNA-27a-3p. Nat. Commun. 2015, 6, 10077. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Liu, H.; Sun, L.; Zhang, R.; Li, L.; Wangding, M.; Wang, J. Six1 induces protein synthesis signaling expression in duck myoblasts mainly via up-regulation of mTOR. Genet. Mol. Biol. 2016, 39, 151–161. [Google Scholar] [CrossRef]
- Wu, W.; Huang, R.; Wu, Q.; Li, P.; Chen, J.; Li, B.; Liu, H. The role of Six1 in the genesis of muscle cell and skeletal muscle development. Int. J. Biol. Sci. 2014, 10, 983–989. [Google Scholar] [CrossRef]
- Wu, W.; Ren, Z.; Li, P.; Yu, D.; Chen, J.; Huang, R.; Liu, H. Six1: A critical transcription factor in tumorigenesis. Int. J. Cancer 2015, 136, 1245–1253. [Google Scholar] [CrossRef]
- Xin, X.; Li, Y.; Yang, X. SIX1 is overexpressed in endometrial carcinoma and promotes the malignant behavior of cancer cells through ERK and AKT signaling. Oncol. Lett. 2016, 12, 3435–3440. [Google Scholar] [CrossRef]
- Xu, P.X.; Zheng, W.; Huang, L.; Maire, P.; Laclef, C.; Silvius, D. Six1 is required for the early organogenesis of mammalian kidney. Development 2003, 130, 3085–3094. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, B.; Li, Y.L.; Yu, X.R. SIX1 reduces the expression of PTEN via activating PI3K/AKT signal to promote cell proliferation and tumorigenesis in osteosarcoma. Biomed. Pharm. 2018, 105, 10–17. [Google Scholar] [CrossRef]
- Yu, J.; McMahon, A.P.; Valerius, M.T. Recent genetic studies of mouse kidney development. Curr. Opin. Genet. Dev. 2004, 14, 550–557. [Google Scholar] [CrossRef]
- Zou, D.; Silvius, D.; Davenport, J.; Grifone, R.; Maire, P.; Xu, P.X. Patterning of the third pharyngeal pouch into thymus/parathyroid by Six and Eya1. Dev. Biol. 2006, 293, 499–512. [Google Scholar] [CrossRef]
- Calpena, E.; Wurmser, M.; McGowan, S.J.; Atique, R.; Bertola, D.R.; Cunningham, M.L.; Gustafson, J.A.; Johnson, D.; Morton, J.E.V.; Passos-Bueno, M.R.; et al. Unexpected role of SIX1 variants in craniosynostosis: Expanding the phenotype of SIX1-related disorders. J. Med. Genet. 2021, 59, 165–169. [Google Scholar] [CrossRef]
- National Center for Health Statistics (Ed.) Osteoporosis or low bone mass in older adults: United States, 2017–2018. In NCHS Data Briefs; US Center for Disease Control: Hyattsville, MD, USA, 2021. [Google Scholar]
- Guzon-Illescas, O.; Perez Fernandez, E.; Crespi Villarias, N.; Quiros Donate, F.J.; Pena, M.; Alonso-Blas, C.; Garcia-Vadillo, A.; Mazzucchelli, R. Mortality after osteoporotic hip fracture: Incidence, trends, and associated factors. J. Orthop. Surg. Res. 2019, 14, 203. [Google Scholar] [CrossRef]
- Lisk, R.; Yeong, K. Reducing mortality from hip fractures: A systematic quality improvement programme. BMJ Open Qual. 2014, 3. [Google Scholar] [CrossRef]
- Morri, M.; Ambrosi, E.; Chiari, P.; Orlandi Magli, A.; Gazineo, D.; D’Alessandro, A.; Forni, C. One-year mortality after hip fracture surgery and prognostic factors: A prospective cohort study. Sci. Rep. 2019, 9, 18718. [Google Scholar] [CrossRef]
- Panula, J.; Pihlajamaki, H.; Mattila, V.M.; Jaatinen, P.; Vahlberg, T.; Aarnio, P.; Kivela, S.L. Mortality and cause of death in hip fracture patients aged 65 or older: A population-based study. BMC Musculoskelet. Disord. 2011, 12, 105. [Google Scholar] [CrossRef]
- Schnell, S.; Friedman, S.M.; Mendelson, D.A.; Bingham, K.W.; Kates, S.L. The 1-year mortality of patients treated in a hip fracture program for elders. Geriatr. Orthop. Surg. Rehabil. 2010, 1, 6–14. [Google Scholar] [CrossRef]
- Fabbro, D.; Cowan-Jacob, S.W.; Moebitz, H. Ten things you should know about protein kinases: IUPHAR Review 14. Br. J. Pharmacol. 2015, 172, 2675–2700. [Google Scholar] [CrossRef]
- Jänne, P.A.; Gray, N.; Settleman, J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat. Rev. Drug Discov. 2009, 8, 709–723. [Google Scholar] [CrossRef]
- Knight, J.D.; Qian, B.; Baker, D.; Kothary, R. Conservation, variability and the modeling of active protein kinases. PLoS ONE 2007, 2, e982. [Google Scholar] [CrossRef]
- Hamann, M.; Alonso, D.; Martín-Aparicio, E.; Fuertes, A.; Pérez-Puerto, M.J.; Castro, A.; Morales, S.; Navarro, M.L.; Del Monte-Millán, M.; Medina, M.; et al. Glycogen synthase kinase-3 (GSK-3) inhibitory activity and structure-activity relationship (SAR) studies of the manzamine alkaloids. Potential for Alzheimer’s disease. J. Nat. Prod. 2007, 70, 1397–1405. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Hall, M.L.; Lach, J.; Clifford, J.; Chandrasena, K.; Canton, C.; Kontoyianni, M.; Choo, Y.-M.; Karan, D.; Hamann, M.T. RSK1 vs. RSK2 Inhibitory Activity of the Marine β-Carboline Alkaloid Manzamine A: A Biochemical, Cervical Cancer Protein Expression, and Computational Study. Mar. Drugs 2021, 19, 506. [Google Scholar] [CrossRef]
- Birkinshaw, R.W.; Gong, J.N.; Luo, C.S.; Lio, D.; White, C.A.; Anderson, M.A.; Blombery, P.; Lessene, G.; Majewski, I.J.; Thijssen, R.; et al. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat. Commun. 2019, 10, 2385. [Google Scholar] [CrossRef]
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J. An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 2012, 45, 863–873. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, D.; Jin, H.; Ye, Z.; Wang, C.; Chen, K.; Kuek, V.; Xu, K.; Qiu, H.; Chen, P.; et al. Hymenialdisine: A Marine Natural Product That Acts on Both Osteoblasts and Osteoclasts and Prevents Estrogen-Dependent Bone Loss in Mice. J. Bone Miner. Res. 2020, 35, 1582–1596. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, D.; Wang, Y.; Dong, C.; Liu, J.; Chen, K.; Song, F.; Wang, C.; Yuan, J.; Davis, R.A.; et al. Thiaplakortone B attenuates RANKL-induced NF-kappaB and MAPK signaling and dampens OVX-induced bone loss in mice. Biomed. Pharmacother. 2022, 154, 113622. [Google Scholar] [CrossRef]
- Shilabin, A.G.; Kasanah, N.; Tekwani, B.L.; Hamann, M.T. Kinetic studies and bioactivity of potential manzamine prodrugs. J Nat. Prod. 2008, 71, 1218–1221. [Google Scholar] [CrossRef]
- Holliday, L.S.; Patel, S.S.; Rody, W.J., Jr. RANKL and RANK in extracellular vesicles: Surprising new players in bone remodeling. Extracell. Vesicles Circ. Nucl. Acids 2021, 2, 18–28. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Jr. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019, 47, D464–D474. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J Mol Graph Model 1999, 17, 57–61. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).