Chitosan Inhibits Helicobacter pylori Growth and Urease Production and Prevents Its Infection of Human Gastric Carcinoma Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of DD95 Chitosan on Growth and Urease Production of H. pylori
2.2. Combination of DD95 Chitosan and Antibiotics against H. pylori
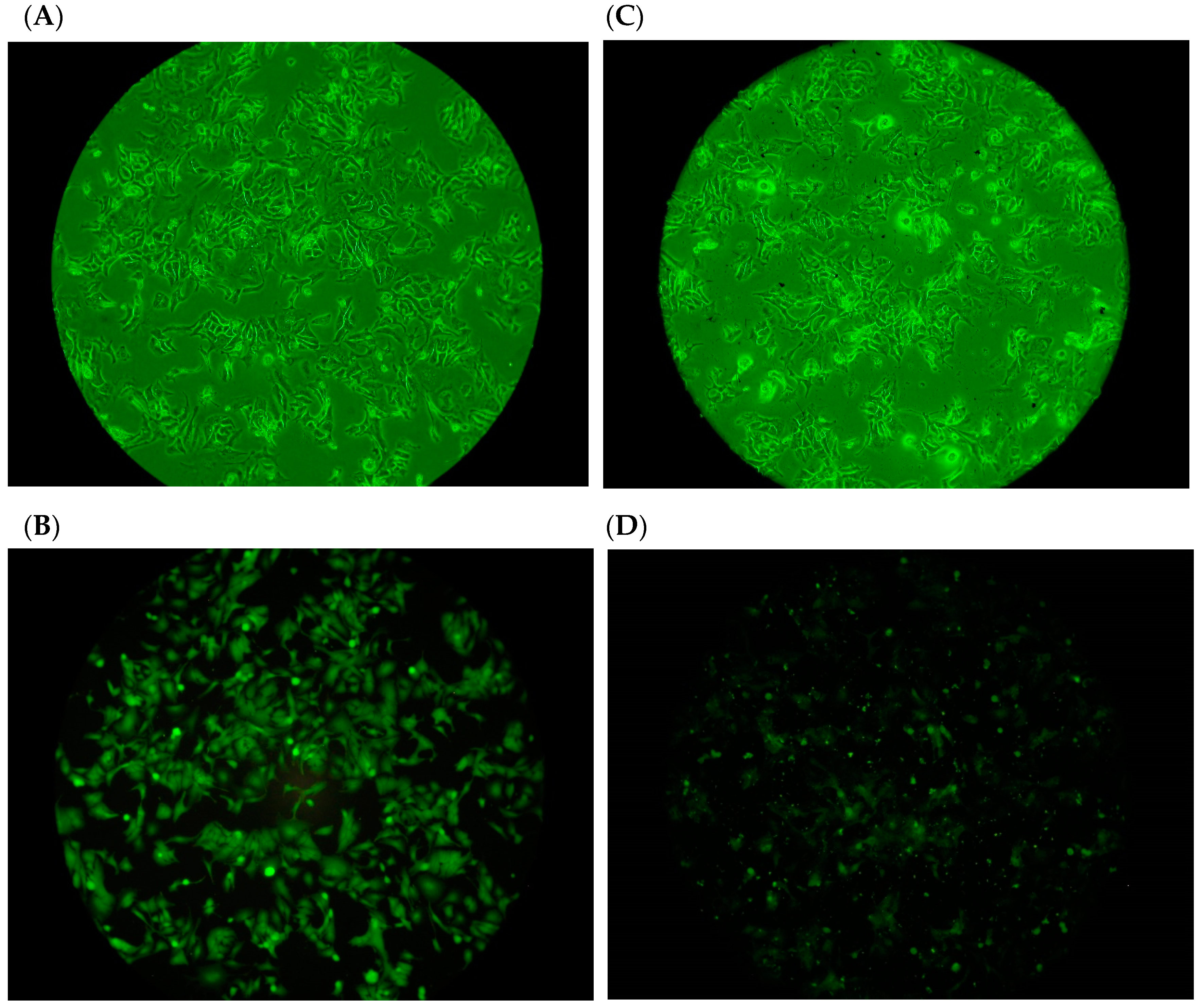
2.3. Adhesion of H. pylori to Human Gastric Carcinoma Cells (TSGH9201) in the Presence of DD95 Chitosan
3. Material and Methods
3.1. Bacterial Strains, Cell Line, and Chemicals
3.2. DD95 Chitosan Preparation
3.3. Culture Conditions
3.4. Antibacterial Test
3.5. Combination Effects of Antibiotic and DD95 Chitosan against H. pylori
3.6. Urease Activity Assay
3.7. Adherence of Fluorescein-Labeled H. pylori to Human Gastric Carcinoma Cells in the Presence of DD95 Chitosan
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moss, S.F. The clinical evidence linking Helicobacter pylori to gastric cancer. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.; Crowe, S.; Valasek, M. the global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef] [PubMed]
- Papastergiou, V.; Georgopoulos, S.D.; Karatapanis, S. Treatment of Helicobacter pylori infection: Meeting the challenge of antimicrobial resistance. World J. Gastroenterol. WJG 2014, 20, 9898. [Google Scholar] [CrossRef]
- Harb, A.H.; El Reda, Z.D.; Sarkis, F.S.; Chaar, H.F.; Sharara, A.I. Efficacy of Reduced-Dose Regimen of a Capsule Containing Bismuth Subcitrate, Metronidazole, and Tetracycline Given with Amoxicillin and Esomeprazole in the Treatment of Helicobacter Pylori Infection; SAGE Publications Sage UK: London, UK, 2015. [Google Scholar]
- Perri, F.; Festa, V.; Merla, A.; Quitadamo, M.; Clemente, R.; Andriulli, A. Amoxicillin-Tetracycline Combinations are Inadequate as Alternative Therapies for Helicobacter pylori Infection. Helicobacter 2002, 7, 99–104. [Google Scholar] [CrossRef]
- Huang, Y.-Q.; Huang, G.-R.; Wu, M.-H.; Tang, H.-Y.; Huang, Z.-S.; Zhou, X.-H.; Yu, W.-Q.; Su, J.-W.; Mo, X.-Q.; Chen, B.-P. Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: Treatment of Helicobacter pylori-induced multidrug resistance. World J. Gastroenterol. WJG 2015, 21, 4225. [Google Scholar] [CrossRef]
- Cardoso, O.; Donato, M.M.; Luxo, C.; Almeida, N.; Liberal, J.; Figueirinha, A.; Batista, M.T. Anti-Helicobacter pylori potential of Agrimonia eupatoria L. and Fragaria vesca. J. Funct. Foods 2018, 44, 299–303. [Google Scholar] [CrossRef]
- Sharifi, A.; Azizi, M.; Moradi-Choghakabodi, P.; Aghaei, S.; Azizi, A. In vitro anti-Helicobacter pylori activity of aqueous extract from Persian Oak testa. Chin. Herb. Med. 2019, 11, 394–399. [Google Scholar] [CrossRef]
- Spósito, L.; Oda, F.B.; Vieira, J.H.; Carvalho, F.A.; dos Santos Ramos, M.A.; de Castro, R.C.; Crevelin, E.J.; Crotti, A.E.M.; Santos, A.G.; da Silva, P.B. In vitro and in vivo anti-Helicobacter pylori activity of Casearia sylvestris leaf derivatives. J. Ethnopharmacol. 2019, 233, 1–12. [Google Scholar] [CrossRef]
- Mendes, J.; Paschoalin, R.; Carmona, V.; Neto, A.R.S.; Marques, A.; Marconcini, J.; Mattoso, L.; Medeiros, E.; Oliveira, J. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.-M.; Tamayo, A.; Rubio, J.; Veiga, M.-D. Influence of chitosan swelling behaviour on controlled release of tenofovir from mucoadhesive vaginal systems for prevention of sexual transmission of HIV. Mar. Drugs 2017, 15, 50. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.-Y.; Lu, S.-T.; Li, P.-W.; Li, S.-D. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef]
- Gong, Y.; Tao, L.; Wang, F.; Liu, W.; Jing, L.; Liu, D.; Hu, S.; Xie, Y.; Zhou, N. Chitosan as an adjuvant for a Helicobacter pylori therapeutic vaccine. Mol. Med. Rep. 2015, 12, 4123–4132. [Google Scholar] [CrossRef] [PubMed]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-H.; Lin, H.-T.V.; Wu, G.-J.; Tsai, G.J. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Singh, D.Y.; Prasad, N.K. Double liposomes mediated dual drug targeting for treatment of Helicobacter pylori infections. Die Pharm.-Int. J. Pharm. Sci. 2011, 66, 368–373. [Google Scholar]
- Adeniyi, B.A.; Anyiam, F.M. In vitro anti-Helicobacter pylori potential of methanol extract of Allium ascalonicum Linn. (Liliaceae) leaf: Susceptibility and effect on urease activity. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 358–361. [Google Scholar]
- Chantarasataporn, P.; Tepkasikul, P.; Kingcha, Y.; Yoksan, R.; Pichyangkura, R.; Visessanguan, W.; Chirachanchai, S. Water-based oligochitosan and nanowhisker chitosan as potential food preservatives for shelf-life extension of minced pork. Food Chem. 2014, 159, 463–470. [Google Scholar] [CrossRef]
- Tsai, G.J.; Su, W.H.; Chen, H.C.; Pan, C.L. Antimicrobial activity of shrimp chitin and chitosan from different treatments and application to fish preservation. Fish. Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef]
- Chang, S.H.; Chen, C.H.; Tsai, G.J. Effects of chitosan on Clostridium perfringens and application in the preservation of pork sausage. Mar. Drugs 2020, 18, 70. [Google Scholar] [CrossRef]
- Hoffman, J. Pharmacological therapy of Helicobacter pylori infection. Semin. Gastrointest. Dis. 1997, 8, 156–163. [Google Scholar]
- Chiba, N. Ulcer disease and Helicobacter pylori infection: Current treatment. Evid.-Based Gastroenterol. Hepatol. 2019, 68. [Google Scholar] [CrossRef]
- Ramteke, S.; Ganesh, N.; Bhattacharya, S.; Jain, N.K. Amoxicillin, clarithromycin, and omeprazole based targeted nanoparticles for the treatment of H. pylori. J. Drug Target. 2009, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Molnar, B.; Szoke, D.; Ruzsovics, A.; Tulassay, Z. Significantly elevated Helicobacter pylori density and different genotype distribution in erosions as compared with normal gastric biopsy specimen detected by quantitative real-time PCR. Eur. J. Gastroenterol. Hepatol. 2008, 20, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ricci, V.; Zarrilli, R.; Romano, M. Voyage of Helicobacter pylori in human stomach: Odyssey of a bacterium. Dig. Liver Dis. 2002, 34, 2–8. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.; Gong, M.; Lin, Y.; Xu, Y.; Ye, L.; Yu, X.; Liu, J.; Liu, J.; He, S. Synergistic effects of nanoparticle heating and amoxicillin on H. pylori inhibition. J. Magn. Magn. Mater. 2019, 485, 95–104. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.M.; Tian, F.; Zhang, Q.; Zhang, H.P.; Zhang, H.; Chen, W. Antagonistic activities of lactobacilli against Helicobacter pylori growth and infection in human gastric epithelial cells. J. Food Sci. 2012, 77, M9–M14. [Google Scholar] [CrossRef]
- Toei, K.; Kohara, T. A conductomeric method for colloid titrations. Anal. Chim. Acta 1976, 83, 59–65. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Huang, L.-F.; Lin, C.-C.; Tsen, H.-Y. Antagonistic activity against Helicobacter pylori infection in vitro by a strain of Enterococcus faecium TM39. Int. J. Food Microbiol. 2004, 96, 1–12. [Google Scholar] [CrossRef]
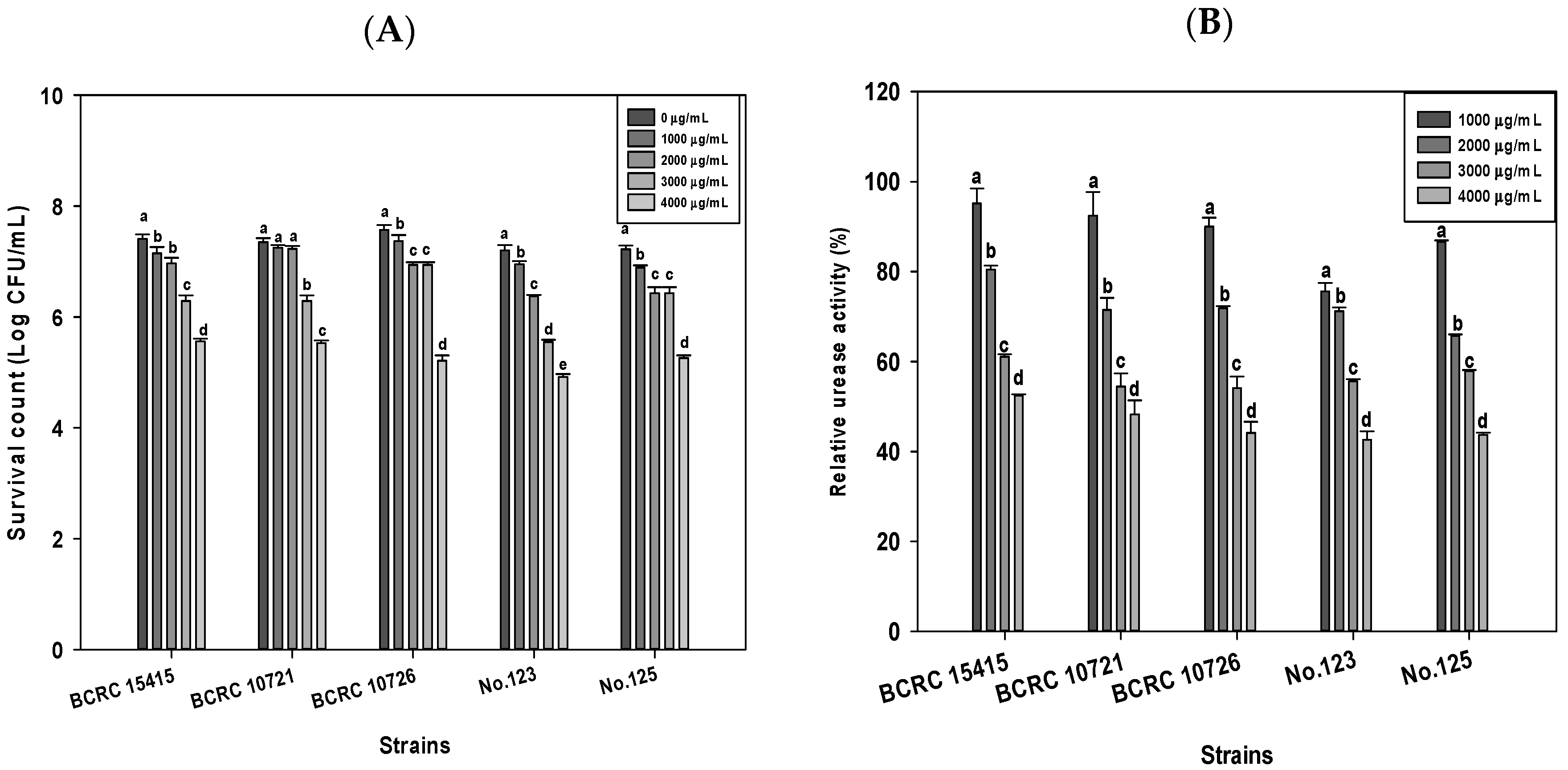
| pH | BCRC 10726 | No.123 | No.125 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial Count 2 (log CFU/mL) | Survival (%) | Bacterial Count (log CFU/mL) | Survival (%) | Bacterial Count (log CFU/mL) | Survival (%) | ||||
| Control | Chitosan | Control | Chitosan | Control | Chitosan | ||||
| 2 | 5.49 ± 0.01 D | 3.22 ± 0.06 | 0.53 D,3 | 5.41 ± 0.02 D | 3.23 ± 0.03 | 0.65 D | 5.20 ± 0.03 E | 3.27 ± 0.03 | 1.17 D |
| 3 | 6.62 ± 0.01 C | 4.47 ± 0.01 | 0.70 D | 6.66 ± 0.05 C | 4.95 ± 0.06 | 1.94 D | 6.59 ± 0.02 D | 4.99 ± 0.05 | 3.51 D |
| 4 | 6.83 ± 0.03 B | 5.50 ± 0.00 | 4.71 C | 6.85 ± 0.05 B | 5.36 ± 0.05 | 3.22 D | 6.79 ± 0.05 C | 5.44 ± 0.03 | 4.51 D |
| 5 | 6.92 ± 0.03 B | 5.76 ± 0.01 | 6.93 B | 6.90 ± 0.06 B | 5.77 ± 0.03 | 7.46 C | 6.92 ± 0.04 B | 5.85 ± 0.03 | 8.62 C |
| 6 | 6.97 ± 0.02 A | 5.89 ± 0.00 | 8.29 B | 7.10 ± 0.07 A | 6.04 ± 0.08 | 8.74 C | 7.09 ± 0.02 A | 6.09 ± 0.03 | 10.02 C |
| 7 | 6.97 ± 0.01 A | 6.93 ± 0.02 | 89.90 A | 7.06 ± 0.06 A | 7.02 ± 0.06 | 90.72 B | 7.06 ± 0.02 A | 7.02 ± 0.04 | 89.89 B |
| 8 | 6.97 ± 0.00 A | 6.93 ± 0.01 | 91.63 A | 7.03 ± 0.01 A | 7.02 ± 0.01 | 96.63 A | 7.06 ± 0.02 A | 7.04 ± 0.03 | 94.30 A |
| pH | BCRC 10726 | No. 123 | No. 125 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OD550 | Relative Urease Activity (%) | OD550 | Relative Urease Activity (%) | OD550 | Relative Urease Activity (%) | ||||
| Control | Chitosan | Control | Chitosan | Control | Chitosan | ||||
| 2 | 0.40 ± 0.00 C | 0.15 ± 0.00 2 | 37.84 ± 0.35 D,3 | 0.39 ± 0.01 C | 0.18 ± 0.00 | 45.58 ± 1.52 F | 0.41 ± 0.00 C | 0.19 ± 0.01 | 46.58 ± 0.37 F |
| 3 | 0.41 ± 0.00 C | 0.18 ± 0.01 | 43.72 ± 0.35 C | 0.40 ± 0.01 C | 0.19 ± 0.01 | 48.63 ± 1.93 E | 0.42 ± 0.00 C | 0.22 ± 0.00 | 51.93 ± 0.64 E |
| 4 | 0.44 ± 0.01 A | 0.21 ± 0.00 | 47.82 ± 0.98 B | 0.44 ± 0.01 A | 0.22 ± 0.00 | 49.25 ± 0.58 D,E | 0.45 ± 0.00 A | 0.25 ± 0.01 | 54.84 ± 0.54 D |
| 5 | 0.42 ± 0.00 B | 0.20 ± 0.01 | 48.61 ± 1.08 B | 0.43 ± 0.01 A,B | 0.22 ± 0.00 | 50.51 ± 0.69 C,D | 0.44 ± 0.00 B | 0.24 ± 0.00 | 56.02 ± 0.41 C,D |
| 6 | 0.41 ± 0.00 C | 0.20 ± 0.01 | 49.51 ± 0.25 B | 0.42 ± 0.00 B | 0.22 ± 0.01 | 51.34 ± 0.51 C | 0.43 ± 0.01 B | 0.25 ± 0.00 | 47.39 ± 1.09 C |
| 7 | 0.20 ± 0.00 D | 0.20 ± 0.00 | 97.54 ± 0.50 A | 0.21 ± 0.01 D | 0.20 ± 0.01 | 93.45 ± 1.06 B | 0.21 ± 0.01 D | 0.20 ± 0.01 | 94.85 ± 0.61 B |
| 8 | 0.20 ± 0.01 D | 0.20 ± 0.01 | 97.35 ± 0.71 A | 0.21 ± 0.01 D | 0.20 ± 0.01 | 97.89 ± 0.24 A | 0.21 ± 0.01 D | 0.20 ± 0.01 | 97.89 ± 0.63 A |
| Antibiotic (μg/mL) | BCRC 10726 | No. 123 | No. 125 | ||||
|---|---|---|---|---|---|---|---|
| Bacterial Count (Log CFU/mL) | Bacterial Count (Log CFU/mL) | Bacterial Count (Log CFU/mL) | |||||
| Chitosan conc. (μg/mL) | Chitosan conc. (μg/mL) | Chitosan conc. (μg/mL) | |||||
| 0 | 4000 | 0 | 4000 | 0 | 4000 | ||
| Control | 7.20 ± 0.03 1 | 5.32 ± 0.10 | 7.61 ± 0.02 | 5.13 ±0.05 | 7.59 ± 0.01 | 5.39 ± 0.07 | |
| Amoxicillin | 50 | 4.36 ± 0.01 | 0.00 ± 0.00 | 4.42 ± 0.01 | 0.00 ± 0.00 | 4.56 ± 0.01 | 0.00 ± 0.00 |
| 100 | 3.26 ± 0.01 | 0.00 ± 0.00 | 4.31 ± 0.01 | 0.00 ± 0.00 | 4.33 ± 0.01 | 0.00 ± 0.00 | |
| 200 | 2.25 ± 0.05 | 0.00 ± 0.00 | 3.74 ± 0.04 | 0.00 ± 0.00 | 4.04 ± 0.02 | 0.00 ± 0.00 | |
| 4000 | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | |
| Tetracycline | 50 | 5.69 ± 0.02 | 3.24 ± 0.02 | 5.37 ± 0.05 | 3.84 ± 0.03 | 5.39 ± 0.01 | 3.92 ± 0.02 |
| 100 | 5.02 ± 0.03 | 0.00 ± 0.00 | 4.75 ± 0.01 | 0.00 ± 0.00 | 4.78 ± 0.01 | 0.00 ± 0.00 | |
| 200 | 3.98 ± 0.02 | 0.00 ± 0.00 | 2.80 ± 0.04 | 0.00 ± 0.00 | 3.22 ± 0.01 | 0.00 ± 0.00 | |
| 4000 | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | |
| Metronidazole | 50 | 4.69 ± 0.01 | 2.46 ± 0.02 | 5.07 ± 0.01 | 3.38 ± 0.02 | 5.11 ± 0.02 | 3.64 ± 0.02 |
| 100 | 4.55 ± 0.02 | 0.00 ± 0.00 | 3.89 ± 0.00 | 0.00 ± 0.00 | 3.95 ± 0.00 | 0.00 ± 0.00 | |
| 200 | 4.28 ± 0.02 | 0.00 ± 0.00 | 3.10 ± 0.02 | 0.00 ± 0.00 | 3.34 ± 0.02 | 0.00 ± 0.00 | |
| 4000 | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | |
| Antibiotic (μg/mL) | BCRC 10726 | No. 123 | No. 125 | ||||
|---|---|---|---|---|---|---|---|
| Bacterial Count (Log CFU/mL) | Bacterial Count (Log CFU/mL) | Bacterial Count (Log CFU/mL) | |||||
| Chitosan (μg/mL) | Chitosan (μg/mL) | Chitosan (μg/mL) | |||||
| 0 | 4000 | 0 | 4000 | 0 | 4000 | ||
| Control | 6.70 ± 0.03 1 | 3.97 ± 0.06 | 6.61 ± 0.02 | 4.28 ± 0.02 | 6.66 ± 0.04 | 4.52 ± 0.01 | |
| Amoxicillin | 50 | 4.08 ± 0.03 | 0.00 ± 0.00 | 3.83 ± 0.01 | 0.00 ± 0.00 | 3.84 ± 0.00 | 0.00 ± 0.00 |
| 100 | 3.44 ± 0.02 | 0.00 ± 0.00 | 3.60 ± 0.01 | 0.00 ± 0.00 | 3.63 ± 0.01 | 0.00 ± 0.00 | |
| 200 | 3.08 ± 0.03 | 0.00 ± 0.00 | 3.30 ± 0.01 | 0.00 ± 0.00 | 3.40 ± 0.00 | 0.00 ± 0.00 | |
| 4000 | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | |
| Tetracycline | 50 | 5.23 ± 0.03 | 0.00 ± 0.00 | 4.39 ± 0.02 | 0.00 ± 0.00 | 4.18 ± 0.02 | 0.00 ± 0.00 |
| 100 | 4.58 ± 0.01 | 0.00 ± 0.00 | 3.75 ± 0.01 | 0.00 ± 0.00 | 3.02 ± 0.02 | 0.00 ± 0.00 | |
| 200 | 3.39 ± 0.01 | 0.00 ± 0.00 | 1.79 ± 0.01 | 0.00 ± 0.00 | 2.47 ± 0.02 | 0.00 ± 0.00 | |
| 4000 | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | |
| Metronidazole | 50 | 4.23 ± 0.00 | 0.00 ± 0.00 | 4.08 ± 0.01 | 0.00 ± 0.00 | 4.46 ± 0.00 | 0.00 ± 0.00 |
| 100 | 4.10 ± 0.05 | 0.00 ± 0.00 | 2.91 ± 0.00 | 0.00 ± 0.00 | 3.87 ± 0.03 | 0.00 ± 0.00 | |
| 200 | 3.84 ± 0.02 | 0.00 ± 0.00 | 2.10 ± 0.02 | 0.00 ± 0.00 | 2.49 ± 0.02 | 0.00 ± 0.00 | |
| 4000 | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | 0.00 ± 0.00 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-H.; Hsieh, P.-L.; Tsai, G.-J. Chitosan Inhibits Helicobacter pylori Growth and Urease Production and Prevents Its Infection of Human Gastric Carcinoma Cells. Mar. Drugs 2020, 18, 542. https://doi.org/10.3390/md18110542
Chang S-H, Hsieh P-L, Tsai G-J. Chitosan Inhibits Helicobacter pylori Growth and Urease Production and Prevents Its Infection of Human Gastric Carcinoma Cells. Marine Drugs. 2020; 18(11):542. https://doi.org/10.3390/md18110542
Chicago/Turabian StyleChang, Shun-Hsien, Pei-Ling Hsieh, and Guo-Jane Tsai. 2020. "Chitosan Inhibits Helicobacter pylori Growth and Urease Production and Prevents Its Infection of Human Gastric Carcinoma Cells" Marine Drugs 18, no. 11: 542. https://doi.org/10.3390/md18110542
APA StyleChang, S.-H., Hsieh, P.-L., & Tsai, G.-J. (2020). Chitosan Inhibits Helicobacter pylori Growth and Urease Production and Prevents Its Infection of Human Gastric Carcinoma Cells. Marine Drugs, 18(11), 542. https://doi.org/10.3390/md18110542




