Research Progress in the Biosynthetic Mechanisms of Marine Polyether Toxins
Abstract
1. Introduction
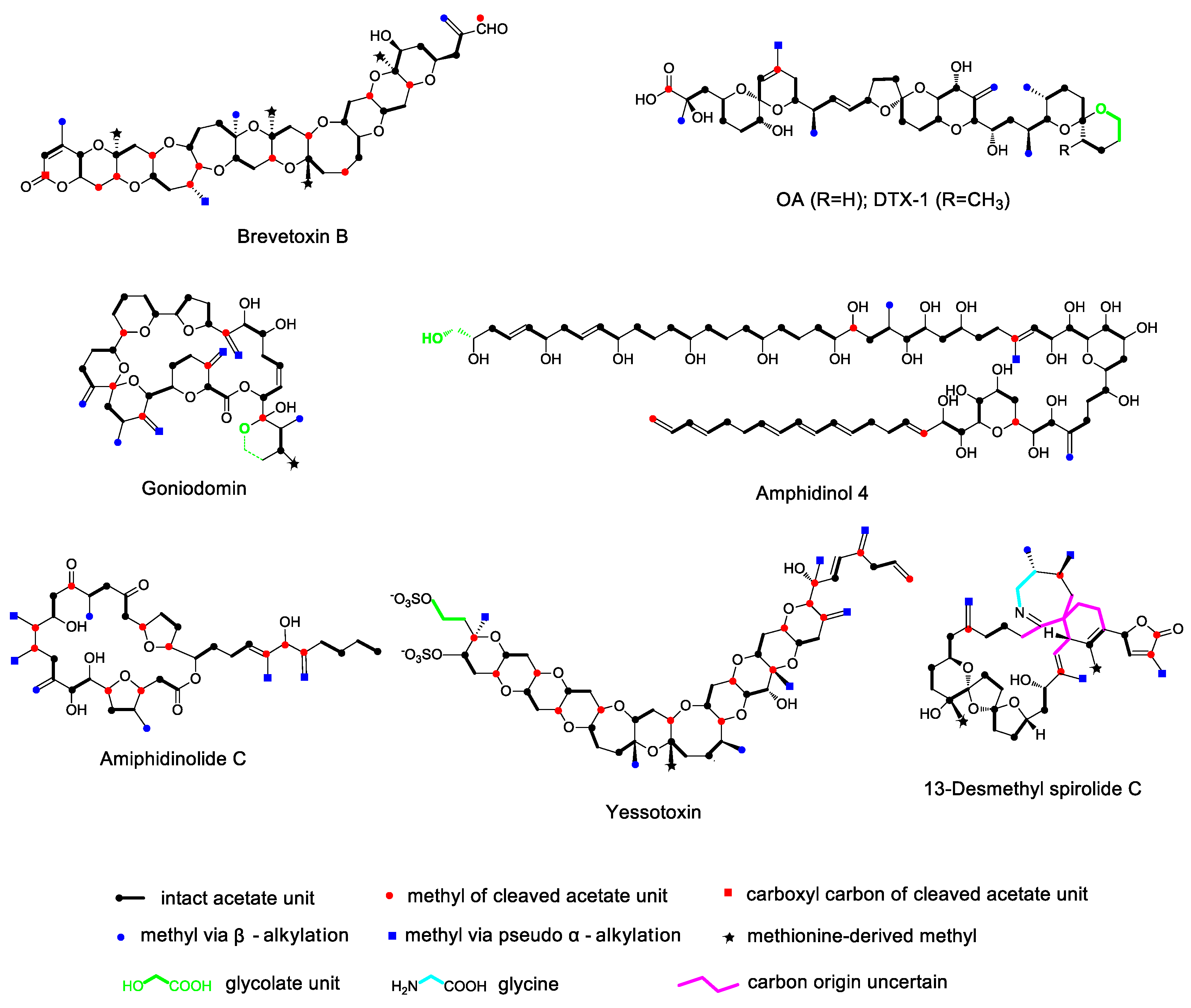
2. Carbon Skeleton Deletion
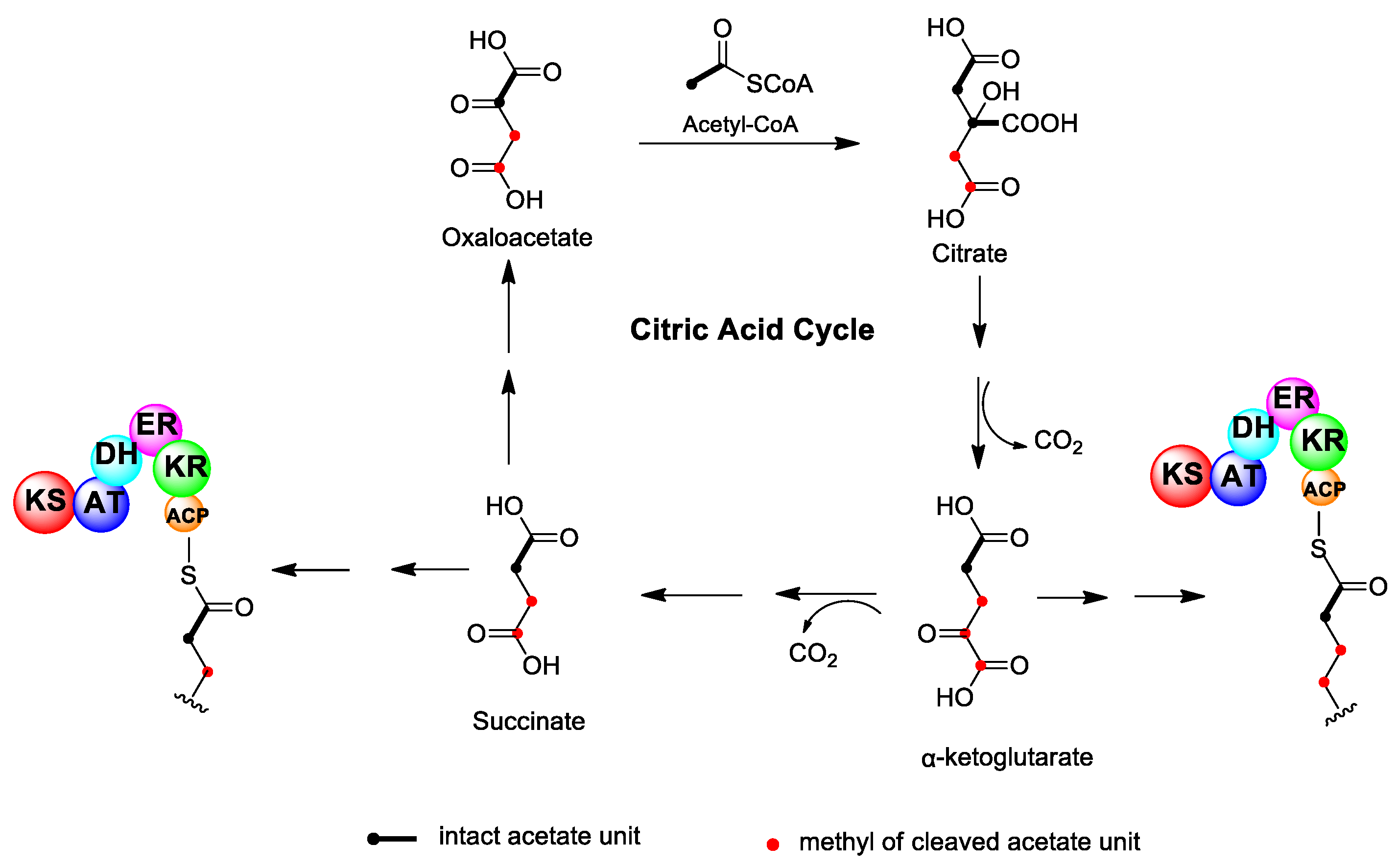
2.1. Carbon Deletion Mediated by Intermediate Metabolites from the Tricarboxylic Acid (TCA) Cycle
2.2. Carbon Deletion Through Favorskii-Type Rearrangement
2.3. Carbon Deletion via Specific Functional Modules within PKS
3. Pendant Alkylation
3.1. α-Alkylation
3.2. β-Alkylation
3.3. Pseudo α-Alkylation
4. Polyether Ring Formation
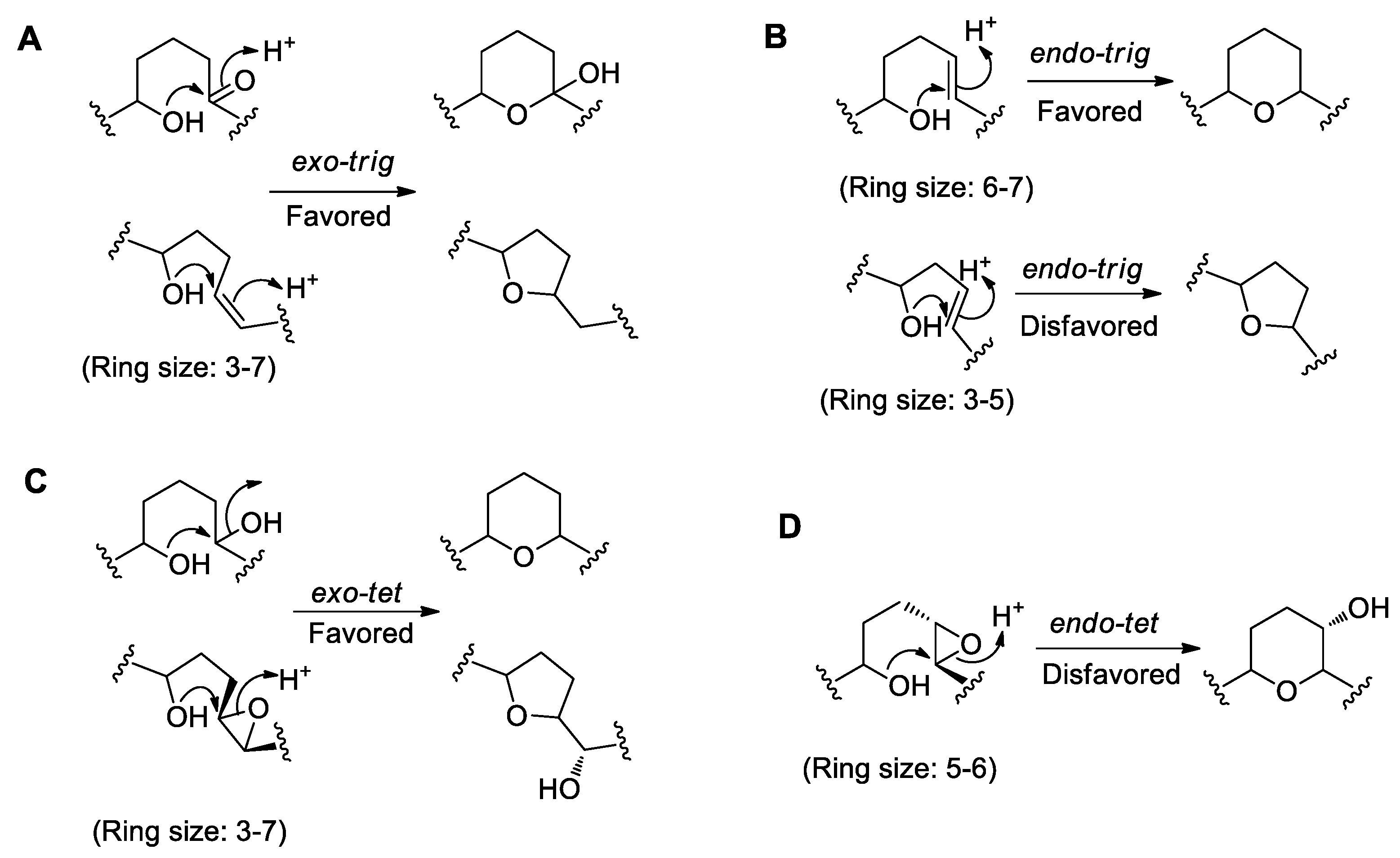
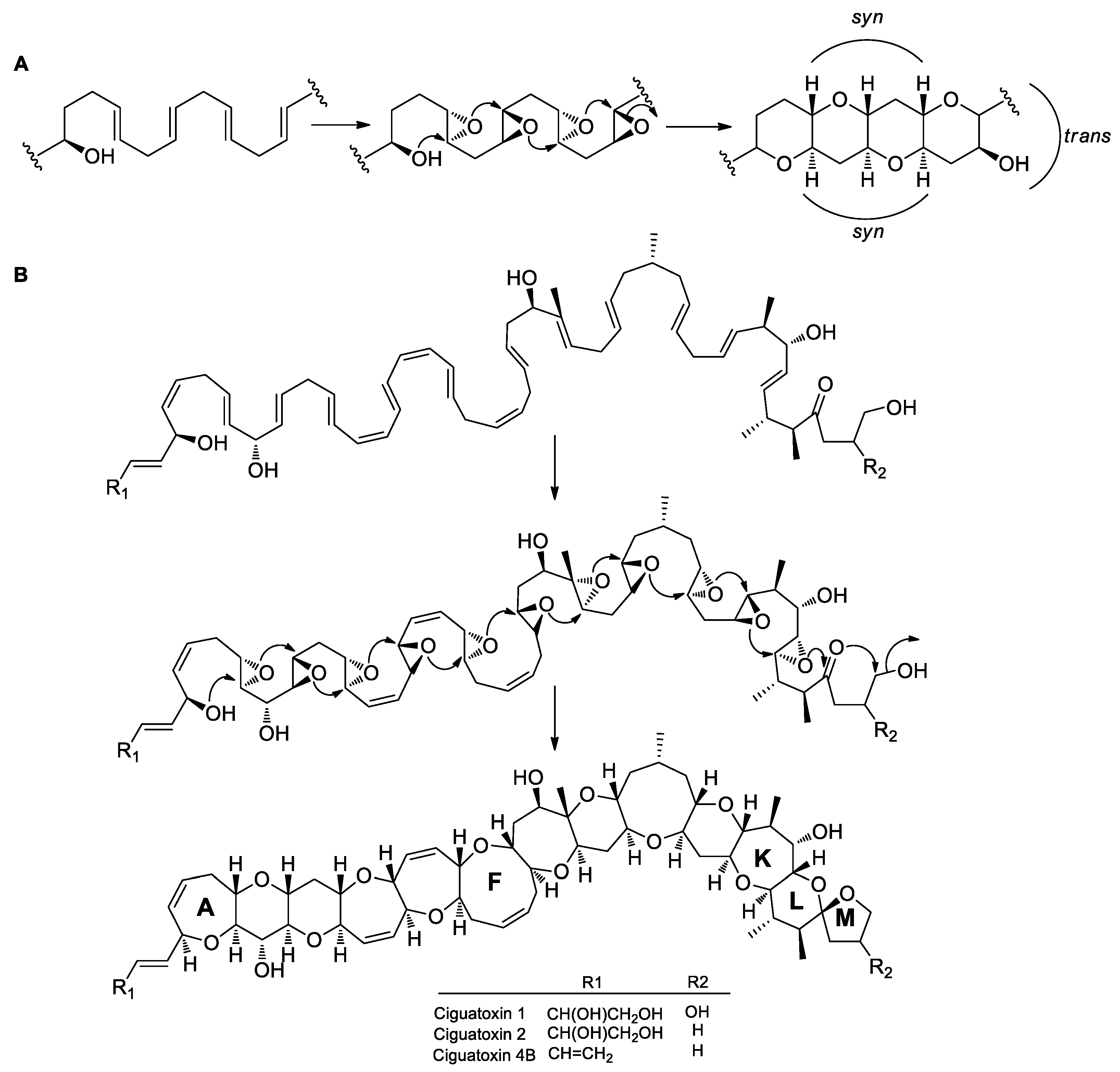
4.1. Baldwin’s Rules for Ether Ring Formation
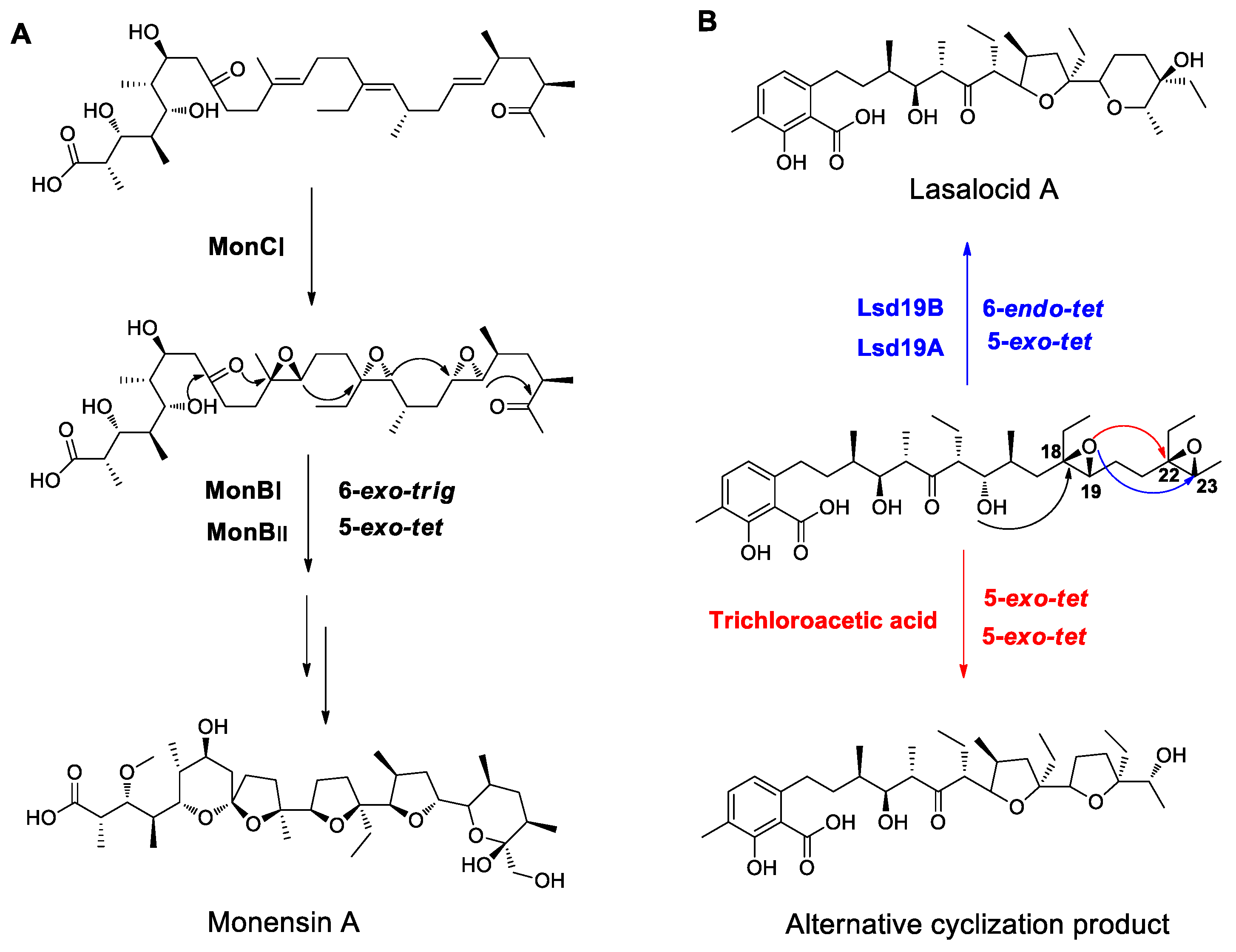
4.2. Examples from Polyether Antibiotics Relevant to Polyether Formation
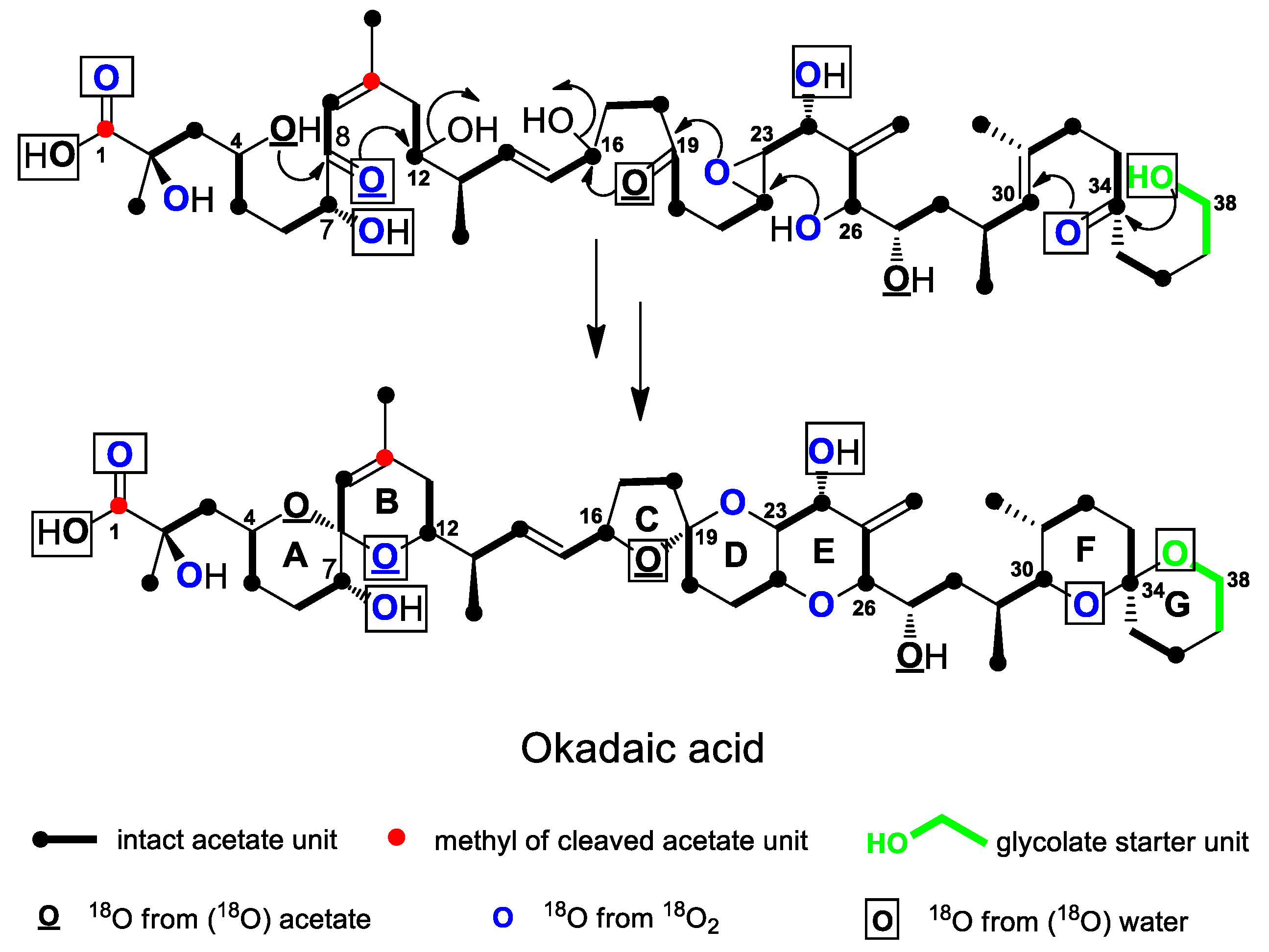
4.3. Ether Ring Formation in OA
4.4. Ether Ring Formation in Fused Polyethers
5. Gene Mining
5.1. New Single-Domain Type I PKS
5.2. Typical Multi-Domain Type I PKS
6. Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicolaou, K.C.; Frederick, M.O.; Aversa, R.J. The continuing saga of the marine polyether biotoxins. Angew. Chem. Int. Ed. Engl. 2008, 47, 7182–7225. [Google Scholar] [CrossRef] [PubMed]
- Assunção, J.; Guedes, A.; Malcata, F. Biotechnological and Pharmacological Applications of Biotoxins and Other Bioactive Molecules from Dinoflagellates. Mar. Drugs 2017, 15, 393. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Andersen, A.J.C.; Andersen, N.G.; Nielsen, K.F.; Hansen, P.J.; Larsen, T.O. Chemical Diversity, Origin, and Analysis of Phycotoxins. J. Nat. Prod. 2016, 79, 662–673. [Google Scholar] [CrossRef]
- Ajani, P.; Harwood, D.; Murray, S. Recent Trends in Marine Phycotoxins from Australian Coastal Waters. Mar. Drugs 2017, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Tamele, I.; Silva, M.; Vasconcelos, V. The Incidence of Marine Toxins and the Associated Seafood Poisoning Episodes in the African Countries of the Indian Ocean and the Red Sea. Toxins 2019, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Morabito, S.; Silvestro, S.; Faggio, C. How the marine biotoxins affect human health. Nat. Prod. Res. 2017, 32, 621–631. [Google Scholar] [CrossRef]
- Farabegoli, F.; Blanco, L.; Rodríguez, L.; Vieites, J.; Cabado, A. Phycotoxins in Marine Shellfish: Origin, Occurrence and Effects on Humans. Mar. Drugs 2018, 16, 188. [Google Scholar] [CrossRef]
- Landsberg, J.; Flewelling, L.; Naar, J. Karenia brevis red tides, brevetoxins in the food web, and impacts on natural resources: Decadal advancements. Harmful Algae 2009, 8, 598–607. [Google Scholar] [CrossRef]
- Fleming, L.E.; Kirkpatrick, B.; Backer, L.C.; Walsh, C.J.; Nierenberg, K.; Clark, J.; Reich, A.; Hollenbeck, J.; Benson, J.; Cheng, Y.S.; et al. Review of Florida red tide and human health effects. Harmful Algae 2011, 10, 224–233. [Google Scholar] [CrossRef]
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and Derivative Brevetoxins: Historical Background, Multiplicity, and Effects. Environ. Health. Perspect. 2005, 113, 621–625. [Google Scholar] [CrossRef]
- Poli, M.; Mende, T.J.; Baden, D.G. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol. Pharmacol. 1986, 30, 129–135. [Google Scholar] [PubMed]
- Holmes, M. The origin of ciguatera—An update. Ciguatera Inf. Bull. Noumea 1992, 2, 8–9. [Google Scholar]
- Lombet, A.; Bidard, J.N.; Lazdunski, M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS. Lett. 1987, 219, 355–359. [Google Scholar] [CrossRef]
- Crump, J.A.; McLay, C.L.; Chambers, S.T. Ciguatera fish poisoning. Postgrad. Med. J. 1999, 75, 678–679. [Google Scholar] [CrossRef]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food. Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Friedman, M.; Fernandez, M.; Backer, L.; Dickey, R.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.; Bienfang, P. An updated review of ciguatera fish poisoning: Clinical, epidemiological, environmental, and public health management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- Alexander, J.; Audunsson, G.; Benford, D.; Cockburn, A.; Cradevi, J.; Dogliotti, E.; Domenico, A.; Fernandez-Cruz, M.; Fink-Gremmels, J.; Furst, P. Marine biotoxins in shellfish-okadaic acid and analogues. EFSA J. 2008, 589, 1–62. [Google Scholar]
- Cetinkaya, F.; Mus, T.E. Shellfish Poisoning and Toxins. J. Biol. Environ. Sci. 2012, 6, 115–119. [Google Scholar]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.; Pizarro, G.; Paz, B.; Franco, J.; Blanco, J. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef]
- George, J.; Baden, D.G.; Gerwick, W.H.; Murray, T.F. Bidirectional influence of sodium channel activation on NMDA receptor–dependent cerebrocortical neuron structural plasticity. Proc. Natl. Acad. Sci. USA 2012, 109, 19840–19845. [Google Scholar] [CrossRef]
- Kamat, P.K.; Rai, S.; Swarnkar, S.; Shukla, R.; Nath, C. Molecular and cellular mechanism of okadaic acid (OKA)-induced neurotoxicity: A novel tool for Alzheimer’s disease therapeutic application. Mol. Neurobiol. 2014, 50, 852–865. [Google Scholar] [CrossRef] [PubMed]
- Koehler, D.; Shah, Z.A.; Williams, F.E. The GSK3β inhibitor, TDZD-8, rescues cognition in a zebrafish model of okadaic acid-induced Alzheimer’s disease. Neurochem. Int. 2019, 122, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Koehler, D.; Williams, F.E. Utilizing zebrafish and okadaic acid to study Alzheimer’s disease. Neural Regen. Res. 2018, 13, 1538. [Google Scholar] [PubMed]
- Wang, R.; Lv, L.; Zhao, Y.; Yang, N. Okadaic acid inhibits cell multiplication and induces apoptosis in a549 cells, a human lung adenocarcinoma cell line. Int. J. Clin. Exp. Med. 2014, 7, 2025. [Google Scholar] [PubMed]
- Ferron, P.J.; Hogeveen, K.; Fessard, V.; Hégarat, L. Comparative analysis of the cytotoxic effects of okadaic acid-group toxins on human intestinal cell lines. Mar. Drugs 2014, 12, 4616–4634. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.D.; Choi, T.S.; Kim, B.M.; Jung, J.H.; Bang, Y.J.; Shin, D.Y. Oocyte-based screening of cytokinesis inhibitors and identification of pectenotoxin-2 that induces Bim/Bax-mediated apoptosis in p53-deficient tumors. Oncogene 2005, 24, 4813. [Google Scholar] [CrossRef]
- Park, S.S.; Park, S.K.; Lim, J.H.; Choi, Y.H.; Kim, W.J.; Moon, S.K. Esculetin inhibits cell proliferation through the Ras/ERK1/2 pathway in human colon cancer cells. Oncol. Rep. 2011, 25, 223–230. [Google Scholar]
- Kim, G.Y.; Kim, W.J.; Choi, Y.H. Pectenotoxin-2 from marine sponges: A potential anti-cancer agent—A review. Mar. Drugs 2011, 9, 2176–2187. [Google Scholar] [CrossRef]
- Wisecaver, J.H.; Hackett, J.D. Dinoflagellate genome evolution. Annu. Rev. Microbiol. 2011, 65, 369–387. [Google Scholar] [CrossRef]
- Weissman, K.J. Introduction to polyketide biosynthesis. Methods Enzymol. 2009, 459, 3–16. [Google Scholar]
- Kohli, G.S.; John, U.; Van Dolah, F.M.; Murray, S.A. Evolutionary distinctiveness of fatty acid and polyketide synthesis in eukaryotes. ISME J. 2016, 10, 1877. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Repeta, D.J.; Nakanishi, K.; Zagorski, M.G. Biosynthetic origins and assignments of carbon 13 NMR peaks of brevetoxin B. J. Am. Chem. Soc. 1986, 108, 7855–7856. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.N.; Shimizu, Y. Biosynthesis of brevetoxins. Evidence for the mixed origin of the backbone carbon chain and possible involvement of dicarboxylic acids. J. Am. Chem. Soc. 1987, 109, 2184–2185. [Google Scholar] [CrossRef]
- Lee, M.S.; Qin, G.; Nakanishi, K.; Zagorski, M.G. Biosynthetic studies of brevetoxins, potent neurotoxins produced by the dinoflagellate Gymnodinium breve. J. Am. Chem. Soc. 1989, 111, 6234–6241. [Google Scholar] [CrossRef]
- Murata, M.; Izumikawa, M.; Tachibana, K.; Fujita, T.; Naoki, H. Labeling pattern of okadaic acid from 18O2 and [18O2] acetate elucidated by collision-induced dissociation tandem mass spectrometry. J. Am. Chem. Soc. 1998, 120, 147–151. [Google Scholar] [CrossRef]
- Norte, M.; Padilla, A.; Fernández, J.J. Studies on the biosynthesis of the polyether marine toxin dinophysistoxin-1 (DTX-1). Tetrahedron Lett. 1994, 35, 1441–1444. [Google Scholar] [CrossRef]
- Needham, J.; McLachlan, J.L.; Walter, J.A.; Wright, J.L.C. Biosynthetic origin of C-37 and C-38 in the polyether toxins okadaic acid and dinophysistoxin-1. J. Chem. Soc. Chem. Commun. 1994, 22, 2599–2600. [Google Scholar] [CrossRef]
- Norte, M.; Padilla, A.; Fernández, J.J.; Souto, M.L. Structural determination and biosynthetic origin of two ester derivatives of okadaic acid isolated from Prorocentrum lima. Tetrahedron 1994, 50, 9175–9180. [Google Scholar] [CrossRef]
- Needham, J.; Hu, T.; McLachlan, J.L.; Walter, J.A.; Wright, J.L. Biosynthetic studies of the DSP toxin DTX-4 and an okadaic acid diol ester. J. Chem. Soc. Chem. Commun. 1995, 1623–1624. [Google Scholar] [CrossRef]
- Vilches, T.S.; Norte, M.; Daranas, A.H.; Fernández, J.J. Biosynthetic studies on water-soluble derivative 5c (DTX5c). Mar. Drugs 2012, 10, 2234–2245. [Google Scholar] [CrossRef]
- Murakami, M.; Okita, Y.; Matsuda, H.; Okino, T.; Yamaguchi, K. From the dinoflagellate Alexandrium hiranoi. Phytochemistry 1998, 48, 85–88. [Google Scholar] [CrossRef]
- Houdai, T.; Matsuoka, S.; Murata, M.; Satake, M.; Ota, S.; Oshima, Y.; Rhodes, L.L. Acetate labeling patterns of dinoflagellate polyketides, amphidinols 2, 3 and 4. Tetrahedron 2001, 57, 5551–5555. [Google Scholar] [CrossRef]
- Meng, Y.; Van Wagoner, R.M.; Misner, I.; Tomas, C.; Wright, J.L. Structure and biosynthesis of amphidinol 17, a hemolytic compound from Amphidinium carterae. J. Nat. Prod. 2010, 73, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Tsuda, M. Amphidinolides, bioactive macrolides from symbiotic marine dinoflagellates. Nat. Prod. Rep. 2004, 21, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J. Amphidinolides and its related macrolides from marine dinoflagellates. J. Antibiot. 2008, 61, 271. [Google Scholar] [CrossRef]
- Yamazaki, M.; Tachibana, K.; Satake, M. Complete 13C-labeling pattern of yessotoxin a marine ladder-frame polyether. Tetrahedron 2011, 67, 877–880. [Google Scholar] [CrossRef]
- Yamazaki, M.; Izumikawa, M.; Tachibana, K.; Satake, M.; Itoh, Y.; Hashimoto, M. Origins of oxygen atoms in a marine ladder-frame polyether: Evidence of monooxygenation by 18O-labeling and using tandem mass spectrometry. J. Org. Chem. 2012, 77, 4902–4906. [Google Scholar] [CrossRef]
- MacKinnon, S.L.; Cembella, A.D.; Burton, I.W.; Lewis, N.; LeBlanc, P.; Walter, J.A. Biosynthesis of 13-Desmethyl Spirolide C by the Dinoflagellate Alexandrium ostenfeldii. J. Org. Chem. 2006, 71, 8724–8731. [Google Scholar] [CrossRef]
- Shimizu, Y. Microalgal metabolites. Curr. Opin. Microbiol. 2003, 6, 236–243. [Google Scholar] [CrossRef]
- Wright, J.L.; Hu, T.; McLachlan, J.; Needham, J.; Walter, J. Biosynthesis of DTX-4: Confirmation of a polyketide pathway, proof of a baeyer-villiger oxidation step, and evidence for an unusual carbon deletion process. J. Am. Chem. Soc. 1996, 118, 8757–8758. [Google Scholar] [CrossRef]
- Macpherson, G.R.; Burton, I.W.; LeBlanc, P.; Walter, J.A.; Wright, J.L. Studies of the Biosynthesis of DTX-5a and DTX-5b by the Dinoflagellate Prorocentrum maculosum: Regiospecificity of the Putative Baeyer−Villigerase and Insertion of a Single Amino Acid in a Polyketide Chain. J. Org. Chem. 2003, 68, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Seto, H.; Sato, T.; Urano, S.; Uzawa, J.; Yonehara, H. Utilization of 13C-13C coupling in structural and biosynthetic studies. VII1) the structure and biosynthesis of vulgamycin. Tetrahedron Lett. 1976, 17, 4367–4370. [Google Scholar] [CrossRef]
- Simpson, T.J.; Holker, J.S. The biosynthesis of a pyrone metabolite of Aspergillus melleus an application of long-range 13C-13C coupling constants. Tetrahedron Lett. 1975, 16, 4693–4696. [Google Scholar] [CrossRef]
- Brereton, R.G.; Garson, M.J.; Staunton, J. Biosynthesis of fungal metabolites: Asperlactone and its relationship to other metabolites of Aspergillus melleus. J. Chem. Soc. Perkin Trans. 1984, 1027–1033. [Google Scholar] [CrossRef]
- Reichenbach, H.; Höfle, G. Biologically active secondary metabolites from myxobacteria. Biotechnol. Adv. 1993, 11, 219–277. [Google Scholar] [CrossRef]
- Piel, J.; Hertweck, C.; Shipley, P.R.; Hunt, D.M.; Newman, M.S.; Moore, B.S. Cloning, sequencing and analysis of the enterocin biosynthesis gene cluster from the marine isolate ‘Streptomyces maritimus’: Evidence for the derailment of an aromatic polyketide synthase. Chem. Biol. 2000, 7, 943–955. [Google Scholar] [CrossRef]
- Xiang, L.; Kalaitzis, J.A.; Nilsen, G.; Chen, L.; Moore, B.S. Mutational analysis of the enterocin Favorskii biosynthetic rearrangement. Org. Lett. 2002, 4, 957–960. [Google Scholar] [CrossRef]
- Xiang, L.; Kalaitzis, J.A.; Moore, B.S. EncM, a versatile enterocin biosynthetic enzyme involved in Favorskii oxidative rearrangement, aldol condensation, and heterocycle-forming reactions. Proc. Natl. Acad. Sci. USA 2004, 101, 15609–15614. [Google Scholar] [CrossRef]
- Walsh, C.T.; Wencewicz, T.A. Flavoenzymes: Versatile catalysts in biosynthetic pathways. Nat. Prod. Rep. 2013, 30, 175–200. [Google Scholar] [CrossRef]
- Julien, B.; Tian, Z.Q.; Reid, R.; Reeves, C.D. Analysis of the ambruticin and jerangolid gene clusters of Sorangium cellulosum reveals unusual mechanisms of polyketide biosynthesis. Chem. Biol. 2006, 13, 1277–1286. [Google Scholar] [CrossRef]
- Liu, T.; Cane, D.E.; Deng, Z. The enzymology of polyether biosynthesis. Methods Enzymol. 2009, 459, 187–214. [Google Scholar] [PubMed]
- Herbst, D.A.; Townsend, C.A.; Maier, T. The architectures of iterative type I PKS and FAS. Nat. Prod. Rep. 2018, 35, 1046–1069. [Google Scholar] [CrossRef] [PubMed]
- Kohl, W.; Irschik, H.; Reichenbach, H.; Höfle, G. Antibiotika aus gleitenden bakterien, XXII. Die biosynthese des antibiotikums myxopyronin A aus myxococcus fulvus stamm Mx f50. Liebigs. Ann. Chem. 1984, 1984, 1088–1093. [Google Scholar] [CrossRef]
- Simunovic, V.; Zapp, J.; Rachid, S.; Krug, D.; Meiser, P.; Müller, R. Myxovirescin A Biosynthesis is Directed by Hybrid Polyketide Synthases/Nonribosomal Peptide Synthetase, 3-Hydroxy-3-Methylglutaryl-CoA Synthases, and trans-Acting Acyltransferases. ChemBioChem 2006, 7, 1206–1220. [Google Scholar] [CrossRef] [PubMed]
- Calderone, C.T.; Iwig, D.F.; Dorrestein, P.C.; Kelleher, N.L.; Walsh, C.T. Incorporation of nonmethyl branches by isoprenoid-like logic: Multiple β-alkylation events in the biosynthesis of myxovirescin A1. Chem. Biol 2007, 14, 835–846. [Google Scholar] [CrossRef]
- Simunovic, V.; Müller, R. 3-Hydroxy-3-methylglutaryl-CoA-like synthases direct the formation of methyl and ethyl side groups in the biosynthesis of the antibiotic myxovirescin A. ChemBioChem 2007, 8, 497–500. [Google Scholar] [CrossRef]
- Chang, Z.; Sitachitta, N.; Rossi, J.V.; Roberts, M.A.; Flatt, P.M.; Jia, J.; Sherman, D.H.; Gerwick, W.H. Biosynthetic Pathway and Gene Cluster Analysis of Curacin A, an Antitubulin Natural Product from the Tropical Marine Cyanobacterium Lyngbya m ajuscula. J. Nat. Prod. 2004, 67, 1356–1367. [Google Scholar] [CrossRef]
- Edwards, D.J.; Marquez, B.L.; Nogle, L.M.; McPhail, K.; Goeger, D.E.; Roberts, M.A.; Gerwick, W.H. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem. Biol. 2004, 11, 817–833. [Google Scholar] [CrossRef]
- Piel, J. A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc. Natl. Acad. Sci. USA 2002, 99, 14002–14007. [Google Scholar] [CrossRef]
- Calderone, C.T.; Kowtoniuk, W.E.; Kelleher, N.L.; Walsh, C.T.; Dorrestein, P.C. Convergence of isoprene and polyketide biosynthetic machinery: Isoprenyl-S-carrier proteins in the pksX pathway of Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2006, 103, 8977–8982. [Google Scholar] [CrossRef]
- Butcher, R.A.; Schroeder, F.C.; Fischbach, M.A.; Straight, P.D.; Kolter, R.; Walsh, C.T.; Clardy, J. The identification of bacillaene, the product of the PksX megacomplex in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2007, 104, 1506–1509. [Google Scholar] [CrossRef]
- Chen, X.H.; Vater, J.; Piel, J.; Franke, P.; Scholz, R.; Schneider, K.; Koumoutsi, A.; Hitzeroth, G.; Grammel, N.; Strittmatter, A.W. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB 42. J. Bacteriol. 2006, 188, 4024–4036. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Y.; Shen, B. Bifunctional acyltransferase/decarboxylase LnmK as the missing link for β-alkylation in polyketide biosynthesis. J. Am. Chem. Soc. 2009, 131, 6900–6901. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, S.X.; Ju, J.; Tang, G.; Liu, T.; Shen, B. Characterization of the lnmKLM genes unveiling key intermediates for β-alkylation in leinamycin biosynthesis. Org. Lett. 2010, 13, 498–501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Sayed, A.K.; Hothersall, J.; Cooper, S.M.; Stephens, E.; Simpson, T.J.; Thomas, C.M. Characterization of the mupirocin biosynthesis gene cluster from Pseudomonas fluorescens NCIMB 10586. Chem. Biol. 2003, 10, 419–430. [Google Scholar] [CrossRef]
- Gurney, R.; Thomas, C.M. Mupirocin: Biosynthesis, special features and applications of an antibiotic from a Gram-negative bacterium. Appl. Microbiol. Biotechnol. 2011, 90, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.E. Rules for ring closure. J. Chem. Soc. Chem. Commun. 1976, 734–736. [Google Scholar] [CrossRef]
- Day, L.; Chamberlin, J.; Gordee, E.; Chen, S.; Gorman, M.; Hamill, R.; Ness, T.; Weeks, R.; Stroshane, R. Biosynthesis of monensin. Antimicrob. Agents Chemother. 1973, 4, 410–414. [Google Scholar] [CrossRef]
- Cane, D.E.; Hubbard, B.R. Polyether biosynthesis. 3. Origin of the carbon skeleton and oxygen atoms of lenoremycin. J. Am. Chem. Soc. 1987, 109, 6533–6535. [Google Scholar] [CrossRef]
- Cane, D.E.; Liang, T.C.; Hasler, H. Polyether biosynthesis. Origin of the oxygen atoms of monensin A. J. Am. Chem. Soc. 1981, 103, 5962–5965. [Google Scholar] [CrossRef]
- Cane, D.E.; Liang, T.C.; Hasler, H. Polyether biosynthesis. 2. Origin of the oxygen atoms of monensin A. J. Am. Chem. Soc. 1982, 104, 7274–7281. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, X.; Dong, H.; Tu, G.; Wang, M.; Wang, B.; Deng, Z. A complete gene cluster from Streptomyces nanchangensis NS3226 encoding biosynthesis of the polyether ionophore nanchangmycin. Chem. Biol. 2003, 10, 431–441. [Google Scholar] [CrossRef]
- Leadlay, P.; Staunton, J.; Oliynyk, M.; Bisang, C.; Cortes, J.; Frost, E.; Hughes-Thomas, Z.; Jones, M.; Kendrew, S.; Lester, J. Engineering of complex polyketide biosynthesis—Insights from sequencing of the monensin biosynthetic gene cluster. J. Ind. Microbiol. Biotechnol. 2001, 27, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.M.; Mironenko, T.; Sun, Y.; Hong, H.; Deng, Z.; Leadlay, P.F.; Weissman, K.J.; Haydock, S.F. Insights into polyether biosynthesis from analysis of the nigericin biosynthetic gene cluster in Streptomyces sp. DSM4137. Chem. Biol. 2007, 14, 703–714. [Google Scholar] [CrossRef]
- Demydchuk, Y.; Sun, Y.; Hong, H.; Staunton, J.; Spencer, J.B.; Leadlay, P.F. Analysis of the tetronomycin gene cluster: Insights into the biosynthesis of a polyether tetronate antibiotic. ChemBioChem 2008, 9, 1136–1145. [Google Scholar] [CrossRef]
- Smith, L.; Hong, H.; Spencer, J.B.; Leadlay, P.F. Analysis of specific mutants in the lasalocid gene cluster: Evidence for enzymatic catalysis of a disfavoured polyether ring closure. ChemBioChem 2008, 9, 2967–2975. [Google Scholar] [CrossRef]
- Migita, A.; Watanabe, M.; Hirose, Y.; Watanabe, K.; Tokiwano, T.; Kinashi, H.; Oikawa, H. Identification of a gene cluster of polyether antibiotic lasalocid from Streptomyces lasaliensis. Biosci. Biotechnol. Biochem. 2009, 73, 169–176. [Google Scholar] [CrossRef]
- Oliynyk, M.; Stark, C.B.; Bhatt, A.; Jones, M.A.; Hughes-Thomas, Z.A.; Wilkinson, C.; Oliynyk, Z.; Demydchuk, Y.; Staunton, J.; Leadlay, P.F. Analysis of the biosynthetic gene cluster for the polyether antibiotic monensin in Streptomyces cinnamonensis and evidence for the role of monB and monC genes in oxidative cyclization. Mol. Microbiol. 2003, 49, 1179–1190. [Google Scholar] [CrossRef]
- Bhatt, A.; Stark, C.B.; Harvey, B.M.; Gallimore, A.R.; Demydchuk, Y.A.; Spencer, J.B.; Staunton, J.; Leadlay, P.F. Accumulation of an E, E, E-triene by the monensin-producing polyketide synthase when oxidative cyclization is blocked. Angew. Chem. Int. Ed. Engl. 2005, 44, 7075–7078. [Google Scholar] [CrossRef]
- Minami, A.; Shimaya, M.; Suzuki, G.; Migita, A.; Shinde, S.S.; Sato, K.; Watanabe, K.; Tamura, T.; Oguri, H.; Oikawa, H. Sequential enzymatic epoxidation involved in polyether lasalocid biosynthesis. J. Am. Chem. Soc. 2012, 134, 7246–7249. [Google Scholar] [CrossRef]
- Gallimore, A.R.; Stark, C.B.; Bhatt, A.; Harvey, B.M.; Demydchuk, Y.; Bolanos-Garcia, V.; Fowler, D.J.; Staunton, J.; Leadlay, P.F.; Spencer, J.B. Evidence for the role of the monB genes in polyether ring formation during monensin biosynthesis. Chem. Biol. 2006, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, Y.; Migita, A.; Oguri, H.; Watanabe, M.; Tokiwano, T.; Watanabe, K.; Oikawa, H. Epoxide hydrolase Lsd19 for polyether formation in the biosynthesis of lasalocid A: Direct experimental evidence on polyene-polyepoxide hypothesis in polyether biosynthesis. J. Am. Chem. Soc. 2008, 130, 12230–12231. [Google Scholar] [CrossRef] [PubMed]
- Minami, A.; Migita, A.; Inada, D.; Hotta, K.; Watanabe, K.; Oguri, H.; Oikawa, H. Enzymatic epoxide-opening cascades catalyzed by a pair of epoxide hydrolases in the ionophore polyether biosynthesis. Org. Lett. 2011, 13, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Chen, X.; Paton, R.S.; Minami, A.; Li, H.; Swaminathan, K.; Mathews, I.I.; Watanabe, K.; Oikawa, H.; Houk, K.N. Enzymatic catalysis of anti-Baldwin ring closure in polyether biosynthesis. Nature 2012, 483, 355. [Google Scholar] [CrossRef]
- Izumikawa, M.; Murata, M.; Tachibana, K.; Fujita, T.; Naoki, H. 18O-Labelling pattern of okadaic acid from H218O in dinoflagellate Prorocentrum lima elucidated by tandem mass spectrometry. Eur. J. Biochem. 2000, 267, 5179–5183. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Satake, M.; Wright, J.L. Polyketide biosynthesis in dinoflagellates: What makes it different? Nat. Prod. Rep. 2014, 31, 1101–1137. [Google Scholar] [CrossRef]
- Gallimore, A.R.; Spencer, J.B. Stereochemical uniformity in marine polyether ladders - Implications for the biosynthesis and structure of maitotoxin. Angew. Chem. Int. Ed. Engl. 2006, 45, 4406–4413. [Google Scholar] [CrossRef]
- Nakanishi, K. The chemistry of brevetoxins: A review. Toxicon 1985, 23, 473–479. [Google Scholar] [CrossRef]
- Vilotijevic, I.; Jamison, T.F. Epoxide-opening cascades promoted by water. Science. 2007, 317, 1189–1192. [Google Scholar] [CrossRef]
- Snyder, R.; Gibbs, P.; Palacios, A.; Abiy, L.; Dickey, R.; Lopez, J.V.; Rein, K. Polyketide synthase genes from marine dinoflagellates. Mar. Biotechnol. 2003, 5, 1–12. [Google Scholar]
- Snyder, R.V.; Guerrero, M.A.; Sinigalliano, C.D.; Winshell, J.; Perez, R.; Lopez, J.V.; Rein, K.S. Localization of polyketide synthase encoding genes to the toxic dinoflagellate Karenia brevis. Phytochemistry 2005, 66, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Monroe, E.A.; Van Dolah, F.M. The toxic dinoflagellate Karenia brevis encodes novel type I-like polyketide synthases containing discrete catalytic domains. Protist 2008, 159, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; John, U.; Figueroa, R.I.; Rhodes, L.L.; Harwood, D.T.; Groth, M.; Bolch, C.J.; Murray, S.A. Polyketide synthesis genes associated with toxin production in two species of Gambierdiscus (Dinophyceae). BMC Genom. 2015, 16, 410. [Google Scholar] [CrossRef] [PubMed]
- Eichholz, K.; Beszteri, B.; John, U. Putative monofunctional type I polyketide synthase units: A dinoflagellate-specific feature? PLoS ONE 2012, 7, e48624. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, T.; Upadhyay, R.J.; Nagasaki, K.; Bhattacharya, D. Dozens of toxin-related genes are expressed in a nontoxic strain of the dinoflagellate Heterocapsa circularisquama. Mol. Biol. Evol. 2012, 29, 1503–1506. [Google Scholar] [CrossRef]
- Pawlowiez, R.; Morey, J.; Darius, H.; Chinain, M.; Van Dolah, F. Transcriptome sequencing reveals single domain Type I-like polyketide synthases in the toxic dinoflagellate Gambierdiscus polynesiensis. Harmful Algae 2014, 36, 29–37. [Google Scholar] [CrossRef]
- Kimura, K.; Okuda, S.; Nakayama, K.; Shikata, T.; Takahashi, F.; Yamaguchi, H.; Skamoto, S.; Yamaguchi, M.; Tomaru, Y. RNA sequencing revealed numerous polyketide synthase genes in the harmful dinoflagellate Karenia mikimotoi. PLoS ONE 2015, 10, e0142731. [Google Scholar] [CrossRef]
- Meyer, J.M.; Rödelsperger, C.; Eichholz, K.; Tillmann, U.; Cembella, A.; McGaughran, A.; John, U. Transcriptomic characterisation and genomic glimps into the toxigenic dinoflagellate Azadinium spinosum, with emphasis on polykeitde synthase genes. BMC Genom. 2015, 16, 27. [Google Scholar] [CrossRef]
- López-Legentil, S.; Song, B.; DeTure, M.; Baden, D.G. Characterization and localization of a hybrid non-ribosomal peptide synthetase and polyketide synthase gene from the toxic dinoflagellate Karenia brevis. Mar. Biotechnol. 2010, 12, 32–41. [Google Scholar] [CrossRef]
- Beedessee, G.; Hisata, K.; Roy, M.C.; Satoh, N.; Shoguchi, E. Multifunctional polyketide synthase genes identified by genomic survey of the symbiotic dinoflagellate, Symbiodinium minutum. BMC Genom. 2015, 16, 941. [Google Scholar] [CrossRef]
- Van Dolah, F.M.; Kohli, G.S.; Morey, J.S.; Murray, S.A. Both modular and single-domain Type I polyketide synthases are expressed in the brevetoxin-producing dinoflagellate, Karenia brevis (Dinophyceae). J. Phycol. 2017, 53, 1325–1339. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; Campbell, K.; John, U.; Smith, K.F.; Fraga, S.; Rhodes, L.L.; Murray, S.A. Role of Modular Polyketide Synthases in the Production of Polyether Ladder Compounds in Ciguatoxin-Producing Gambierdiscus polynesiensis and G. excentricus (Dinophyceae). J. Eukaryot. Microbiol. 2017, 64, 691–706. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Yao, G.; Liu, Y.; Chen, J.; Jiang, H. Research Progress in the Biosynthetic Mechanisms of Marine Polyether Toxins. Mar. Drugs 2019, 17, 594. https://doi.org/10.3390/md17100594
Wan X, Yao G, Liu Y, Chen J, Jiang H. Research Progress in the Biosynthetic Mechanisms of Marine Polyether Toxins. Marine Drugs. 2019; 17(10):594. https://doi.org/10.3390/md17100594
Chicago/Turabian StyleWan, Xiukun, Ge Yao, Yanli Liu, Jisheng Chen, and Hui Jiang. 2019. "Research Progress in the Biosynthetic Mechanisms of Marine Polyether Toxins" Marine Drugs 17, no. 10: 594. https://doi.org/10.3390/md17100594
APA StyleWan, X., Yao, G., Liu, Y., Chen, J., & Jiang, H. (2019). Research Progress in the Biosynthetic Mechanisms of Marine Polyether Toxins. Marine Drugs, 17(10), 594. https://doi.org/10.3390/md17100594




