Abstract
In this paper, we reviewed natural compounds isolated from octocorals belonging to the genus Pachyclavularia, including 20 cembrane-, 39 briarane-, and eight briarellin-related diterpenoids, and one secosterol. The chemical constituents of these 68 secondary metabolites, and their names, structures, and bioactivities, along with studies of their biological activities, are summarized in this review. Based on the literature, many of these compounds possess bioactivities, including anti-inflammation properties, cytotoxicity, and ichthyotoxicity, suggesting that they may have the potential to be developed into biomedical agents for treatment.
1. Introduction
Octocorals of the genus Pachyclavularia (phylum Cnidaria, class Anthozoa, order Alcyonacea, family Briareidae) [1], which originally belonged to the family Tubiporidae [2], distributed in the subtropical and tropical waters of the Indo-Pacific Ocean, have been investigated. Since the initial study in 1979 discovered a cembranoid diterpene, pachyclavulariadiol (1), from the Australian octocoral P. violacea [3], subsequent studies over the past few decades have yielded a series of interesting secondary metabolites, particularly diterpenoids originally synthesized from cyclized cembranoids, including briarane- and briarellin-related analogues (Scheme 1) [4]. In this article, we have listed the different types of compounds isolated from two Indo-Pacific octocorals, Pachyclavularia violacea (Scheme 1) and Pachyclavularia sp., and summarized their chemical constituents and research into the biological activities of these metabolites.
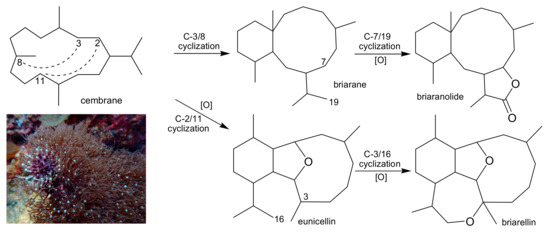
Scheme 1.
Octocoral P. violacea and the proposed biosynthetic pathways of the cembrane, briarane, and briarellin carbon skeletons [4].
2. Cembranoids from Pachyclavularia violacea (Quoy and Gaimard, 1833) (=Clavularia violacea, Briareum violaceum)
The Pachyclavularia genus includes one very common species, Pachyclavularia violacea [2,3]. Bowden and colleagues [3] isolated several compounds, including three new cembrane-type diterpenes, pachyclavulariadiol (1), and its naturally-derived mono- and di-acetylated derivatives (2 and 3), from octocoral P. violacea collected in Australia (Figure 1). The study also established the structures, including the relative configurations, of new furanocembranoids 1–3 and determined the configuration of 1 by spectroscopic/chemical methods and X-ray diffraction, respectively.
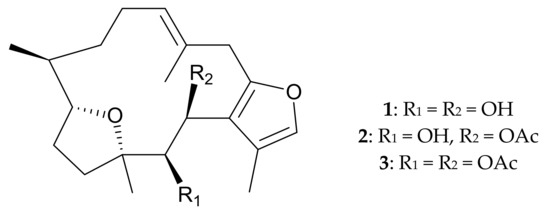
Figure 1.
Structures of pachyclavulariadiol (1) and its natural mono- and di-acetylated derivatives 2 and 3.
In 1989, Inman and Crews [5] reported the isolation of a new cembranolide from P. violacea collected from Vanuatu waters, and named the compound pachyclavularolide (4) (Figure 2). Later, the structure of 4, including the relative configuration, was determined using spectroscopic methods and molecular mechanics calculations, and further confirmed by single-crystal X-ray analyses in a study by Sheu et al. [6]. The former study was the first to present a detailed conformational analysis of a 14-membered cembrane ring [5].
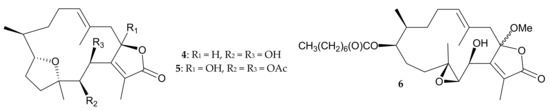
Figure 2.
Structures of pachyclavularolide (4) and pachyclavulariolides E (5) and F (6).
Two new cembranes, pachyclavulariolides E (5) and F (6) (Figure 2), were isolated from P. violacea samples collected from waters off Madang in Papua New Guinea in 2000 [7]. The structures of 5 and 6, including the relative configurations, which were elucidated from spectroscopic data [7], and the structure, including the relative configuration of 5, was further confirmed from single-crystal X-ray analysis by Sheu et al. [6]. A cytotoxic assay showed that compound 6 had an IC50 value of 1.0 μg/mL in the treatment of murine leukemia P388 cells [7], though this compound was claimed to be an artifact due to methanol used in the extraction procedure [7].
Twelve new cembranolides, pachyclavulariolides G–R (7–18) (Figure 3), and two known analogues, pachyclavulariolide (4) and pachyclavulariolide E (5) [5,7] (Figure 2), were obtained from P. violacea specimens collected from Kenting Coast, Taiwan [6,8]. The structures, including the relative configurations, of cembranolides 7–18 were established by spectroscopic/chemical methods [6,8], and the structures of 4, 5, 7, and 8 were also verified by single-crystal analyses [6]. However, the hydroxy group at C-2 in briarane 11 was still unconfirmed at that stage [6], and information regarding the reasonable biosynthetic pathway suggested that briarane 12 could be the precursor of briarane 16 [8]. Cytotoxic assays revealed that pachyclavulariolide K (11) had ED50 values of 2.8, 3.3, 6.7, and 7.6 μg/mL against P388 (mouse lymphoma), HT-29 (human colorectal adenocarcinoma), A549 (human epithelial carcinoma), and KB cells (human nasopharynx carcinoma), respectvively, while pachyclavulariolides E (5), I (9), J (10), and M (13) had ED50 values against P388 cells of 4.0, 1.3, 2.5, and 3.2 μg/mL, respectively [6,8].
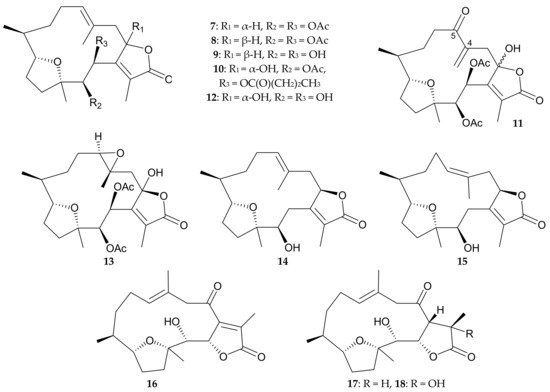
Figure 3.
Structures of pachyclavulariolides G–R (7–18).
A new cytotoxic cembranoid, claviolide (19) (Figure 4), was isolated from the dichloromethane extract of Clavularia violacea, collected at a depth of 5–6 m off Green Island, Taiwan. The structure, including the relative configuration, of 19 was established using a spectroscopic approach, and this compound was found to exhibit significant cytotoxicity towards P388 and HT-29 tumor cells, with ED50 values of 0.84 and 0.38 μg/mL, respectively [9].
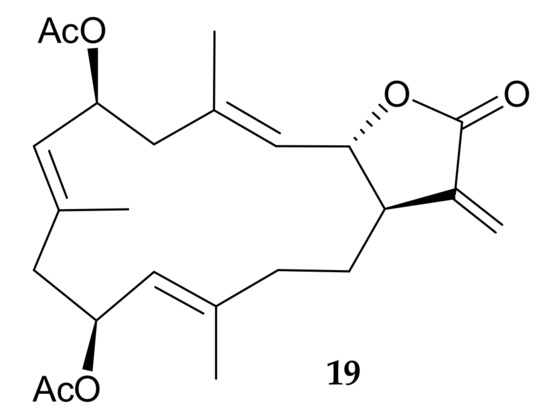
Figure 4.
Structure of claviolide (19).
Chang and coworkers isolated a new cembranoid, briaviodiol A (20) (Figure 5), from the organic extract of Briareum violaceum [2] collected off Pingtung Coast, Southern Taiwan, and confirmed its structure, including the relative configuration, by single-crystal X-ray diffraction analysis [10].
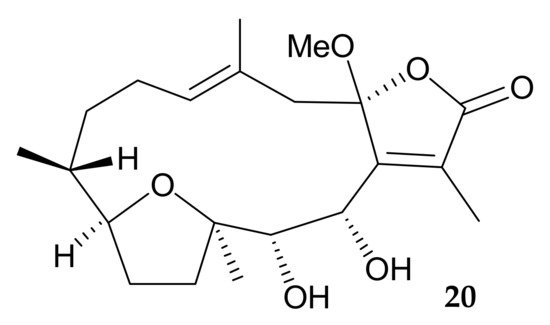
Figure 5.
Structure of briaviodiol A (20).
Interestingly, with the exception of cembranoids 6 (pachyclavulariolide F) and 19 (claviolide), cembranoids (18/20) from P. violacea have been found to possess a tetrahydrofuran moiety, and these furanocembranoids are likely to be chemical markers of cembranoids obtained from P. violacea. Among the furanocembranoids, the α,β-unsaturated ketone moiety in pachyclavulariolide K (11) seems to enhance cytotoxicity toward various tumor cells.
3. Briaranes from Pachyclavularia sp. and Pachyclavularia violacea
Uchio and colleagues [11] identified three new unnamed briaranes, 21–23 (Figure 6), from specimens of the octocoral Pachyclavularia sp. collected off the Great Barrier Reef, Australia. The authors established the structures of 21–23, including the relative configurations, using a spectroscopic approach, and also found that briarane 21 exhibited ichthyotoxicity towards the mosquito fish Gambusia affinis.
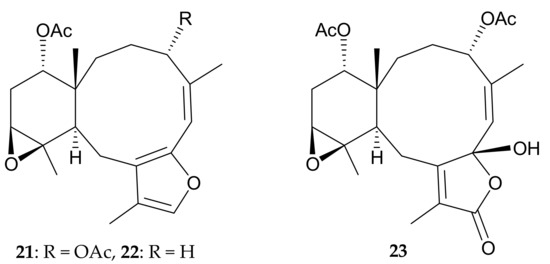
Figure 6.
Structures of briaranes 21–23.
Five briarane diterpenoids, including three new metabolites 2β-acetoxy-2-(debutyryloxy)-stecholide E (24), 9-deacetylstylatulide lactone (25), and 4β-acetoxy-9-deacetylstylatulide lactone (26), along with two known metabolites, brianthein W (27) [12] and 9-deacetylbriareolide H (28) [13,14] (Figure 7), were isolated from a Taiwanese soft coral Briareum sp., which was originally identified as Pachyclavularia violacea collected off the coast of Kenting, Taiwan [15]. The structures of new briaranes 24–26, including the relative configurations, were established by chemical/spectroscopic methods. Cytotoxic assays were performed on briaranes 24–28 to assess their toxicity against P388, HT-29, KB, and A549 tumor cells, and the results showed that all briaranes except 26 exhibited cytotoxicity against P388 cells, with ED50 values of 0.61, 1.12, 0.76 and 0.28 μg/mL, respectively [15], indicating that the acetoxy group at C-4 in 26 redeuce the cytotoxicity. Briaranes 25 and 28 showed cytotoxicity towards HT-29 and KB cells, with ED50 values of 1.79 and 0.27 μg/mL, respectively [15]. The cytotoxicities of 9-hydroxybriaranes 24, 25, and 28 were not significantly different to their corresponding 9-acetyl derivatives, 2β-acetoxy-2-(debutyryloxy)-stecholide E acetate, stylatulide lactone [16], and briareolide H [13], respectively [15].
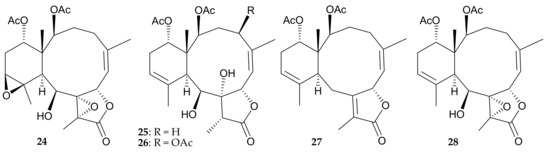
Figure 7.
Structures of 2β-acetoxy-2-(debutyryloxy)stecholide E (24), 9-deacetylstylatulide lactone (25), 4β-acetoxy-9-deacetylstylatulide lactone (26), brianthein W (27), and 9-deacetylbriareolide H (28).
In 2000, Xu et al. [7] isolated four new briarane derivatives, pachyclavulariolides A–D (29–32) (Figure 8), from P. violacea specimens collected from reefs off Madang, Papua New Guinea. The authors used a spectroscopic approach to determine the structures, including the relative configurations, of 29–32, and further employed single-crystal X-ray diffraction analysis to identify the structure, including the relative configuration, of briarane 30 (pachyclavulariolide B). They also found that briaranes 29–32 were the first briaranes of this type to possess an 11,14-ether bridge that resulted in an oxanorbornane structure.
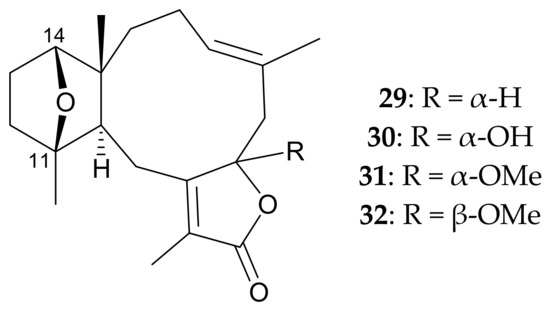
Figure 8.
Structures of pachyclavulariolides A–D (29–32).
Research by a group in Japan identified nine new briaranes, pachyclavulides A–I (33–41) (Figure 9), from P. violacea collected off Okinawa, and determined their structures, including the relative configurations, using spectroscopic methods [17,18]. The absolute configuration of pachyclavulide A (33) was determined by single-crystal X-ray diffraction analysis of its p-bromobenzoyl ester [17]. Pachyclavulides B (34) and E (37) were found to show cytotoxicity against human central neural system (CNS) SNB-75 and A549 (human alveolar epithelial carcinoma) tumor cells, with GI50 values of 5.2 and 5.1 μM, respectively [18] and pachyclavulide C (35) was not cytotoxic toward SNB-75 cells. It seems that the hydroxy group at C-16 in briarane 34 will enhance the cytotoxity. Enantioselective preparation of the six-membered ring of pachyclavulide B (34) was also achieved [19].
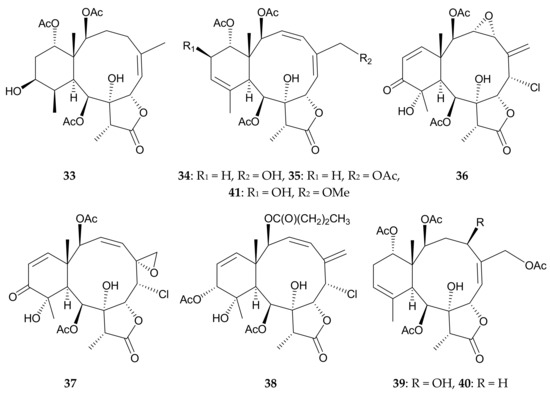
Figure 9.
Structures of pachyclavulides A–I (33–41).
In addition, another group of researchers isolated four new briaranes, brianodins A–D (42–45), from soft coral identified as Pachyclavularia sp. from Okinawa, Japan [20]. They established the structures, including the relative configurations, of briaranes 42–45 by interpretation of spectroscopic data, and determined the absolute configurations of brianodins C (44) and D (45) by the modified Mosher’s method (Figure 10). The 1,2-diol moiety at C-8 and C-17 in 43–45 is rarely found in briarane analogues [21,22,23,24,25,26].
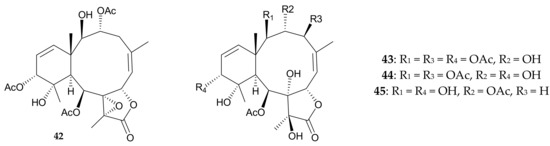
Figure 10.
Structures of brianodins A–D (42–45).
Sixteen briarane analogues, including ten new metabolites briaviolides A–J (46–55), along with six known briaranes, solenolides A (56) and D (57) [27], excavatolide A (58) [28], briaexcavatolide I (59) [29] (Figure 11), 4β-acetoxy-9-deacetylstulatulide lactone (26) and 9-deacetylstylatulide lactone (25) [15] (Figure 7), were isolated from Briareum violaceum [2] collected in the waters of Taiwan [30]. Using spectroscopic/chemical methods, the structures, including the relative configurations, of new briaranes 46–55 were determined, and the absolute configuration of briaviolide A (46) was further confirmed by X-ray crystallographic analysis [30]. In anti-inflammatory activity assays, briaviolides E (50) and I (54) were found to possess inhibitory effects against the production of superoxide anions by human neutrophils in vitro (inhibition rates = 34.17% and 28.66%, respectively), and inhibited the release of elastase (inhibition rates = 26.03% and 28.81%, respectively) at a concentration of 10 μg/mL; while the monobenzoyl derivative of 46 was found to possess selective activity to inhibit elastase release (inhibition rate = 28.60%) at a concentration of 10 μg/mL [30]. By comparison of the structure-activity relationships between briaranes 53 (briaviolide H) and 54 (briaviolide I), indicating that the hydroperoxy group at C-12 in 54 enhance the activity to inhibit the elastase release and the generation of superoxide anions.
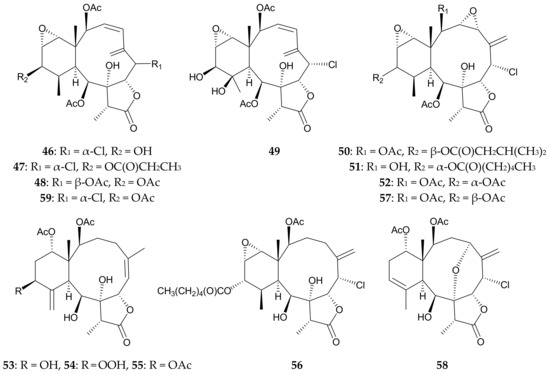
Figure 11.
Structures of briaviolides A–J (46–55), solenolides A (56) and D (57), excavatolide A (58), and briaexcavatolide I (59).
A study performed by Cheng et al. revised the structure of solenolide D (57) [31], and this compound was later found to be a potent anti-inflammatory agent with an efficacy comparable to that of indomethacin [27]. Potency in excess of a 70% reduction of edema was observed at a concentration of approximately 15 μg/per ear (mouse ear assay) [27].
4. Briarellins from Pachyclavularia violacea
Eight novel briarellins, including pachyclavulariaenones A–G (60–66) [32,33] and secopachy-clavulariaenone A (67) [6] (Figure 12), were isolated from P. violacea collected off Kenting Coast, Taiwan. Pachyclavulariaenone B (61) was also isolated from P. violacea specimens collected in the Togian Islands near Sulawesi Island, Indonesia [34]. The structures, including the relative configurations, of briarellins 60–67 were established by spectroscopic methods, and the structures, including the relative configurations, of briarellins 62 (pachyclavulariaenone C) [32] and 65 (pachyclavulariaenone F) [33] were further confirmed by single-crystal X-ray diffraction analyses. Briarellins 60–67 were found to be unprecedented examples of this type of compound, as they possess a six-membered carbocyclic ring cis-fused to both the ten-membered carbocyclic ring and seven-membered ether ring, making three ring-junction protons cis to each other [6,32,33]. Cytotoxic assays revealed that pachyclavulariaenone G (66) exhibited cytotoxicity towards P388 and HT-29 tumor cells, with ED50 values of 0.2 and 3.2 μg/mL, respectively [33]. It is obvious to note that the hydroxy groups at C-4 and C-6 in 66 caused this compound to be more cytotoxic than its analogues 62, 64, and 65.
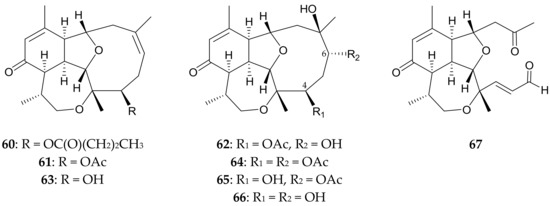
Figure 12.
Structures of pachyclavulariaenones A–G (60–66) and secopachyclavulariaenone A (67).
5. Secosterol from Pachyclavularia violacea
A new 9,11-secosterol, (24S*)-3β,11-dihydroxy-5β,6β-epoxy-24-methyl-9,11-secocholestan-9-one (68) (Figure 13), was isolated from P. violacea specimens collected off the Togian Islands, Indonesia, and its structure, including the relative configurations, was elucidated on the basis of spectroscopic data [34].
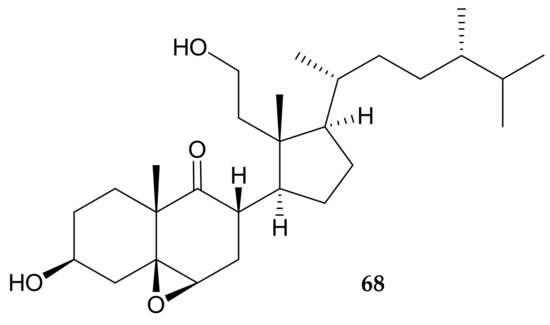
Figure 13.
Structure of secosterol 68.
6. Conclusions
Ever since pachyclavulariadiol (1) was obtained from a specimen of the octocoral P. violacea collected in Australia [4], 68 interesting secondary metabolites, including 20 cembranes (29.41%), 39 briaranes (57.35%), eight briarellins (11.76%), and one secosterol (1.47%), have been isolated from octocorals belonging to the genus Pachyclavularia. Many studies have reported that compounds obtained from Pachyclavularia spp. possess various biologic activities, such as cytotoxicity (seven cembranes, six briaranes, and one briarellin), anti-inflammatory activity (three briaranes), and ichthyotoxicity (one briarane). It is interesting to note that all compounds from Pachyclavularia spp. reported as having been isolated between 1979 and 2014 were collected from octocorals distributed in the Indo-Pacific Ocean. The briarane-type natural products act as a chemical marker of octocorals belonging to the family Briareidae. Up to date, 379 briaranes [35,36,37,38,39,40], 13 eunicellins, 34 asbestinane, one cembrane, and one pyranone [4], have been isolated from the octocorals belonging to the family Briareidae, including the genera Briareum and Erythropodium. As more than 50% of the compounds obtained from the Pachyclavularia genus are briarane-type diterpenoids, we support that the taxonomic position of the Pachyclavularia genus should be readjusted and placed under the family Briareidae. Based on above findings, these results suggest that continuing investigation of new terpenoid analogues with the potential bioactivities from this marine organism are worthwhile for further development. The octocoral Pachyclavularia violacea had been transplanted to culturing tanks located in the National Museum of Marine Biology and Aquarium, Taiwan, for extraction of additional natural products to establish a stable supply of bioactive material.
Acknowledgments
This research was supported by grants from the National Museum of Marine Biology and Aquarium; the National Dong Hwa University; the National Sun Yat-sen University; and the Ministry of Science and Technology (grant nos. MOST 106-2320-B-291-001-MY3 and 104-2320-B-291-001-MY3), Taiwan, awarded to Ping-Jyun Sung.
Author Contributions
Yu-Chia Chang contributed in terms of writing the manuscript. Jyh-Horng Sheu, Yang-Chang Wu, and Ping-Jyun Sung conceived and designed the format of the manuscript. All the authors contributed in terms of critical reading and discussion of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Samimi-Namin, K.; van Ofwegen, L.P. Overview of the genus Briareum (Cnidaria, Octocorallia, Briareidae) in the Indo-Pacific, with the description of a new species. ZooKeys 2016, 557, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Bayer, F.M. Key to the genera of octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981, 94, 902–947. [Google Scholar]
- Bowden, B.F.; Coll, J.C.; Mitchell, S.J.; Raston, C.L.; Stokie, G.J.; White, A.H. Studies of Australian soft corals. XV. The structure of pachyclavulariadiol, a novel furano-diterpene from Pachyclavularia violacea. Aust. J. Chem. 1979, 32, 2265–2274. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chen, M.-C. The heterocyclic natural products of gorgonian corals of genus Briareum exclusive of briarane-type diterpenoids. Heterocycles 2002, 57, 1705–1715. [Google Scholar] [CrossRef]
- Inman, W.; Crews, P. The structure and conformational properties of a cembranolide diterpene from Clavularia violacea. J. Org. Chem. 1989, 54, 2526–2529. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Wang, G.-H.; Sung, P.-J.; Duh, C.-Y.; Chiang, M.Y. Pachyclavulariolides G–L and secopachyclavulariaenone A, seven novel diterpenoids from the soft coral Pachyclavularia violacea. Tetrahedron 2001, 57, 7639–7648. [Google Scholar] [CrossRef]
- Xu, L.; Patrick, B.O.; Roberge, M.; Allen, T.; van Ofwegen, L.; Andersen, R.J. New diterpenoids from the octocoral Pachyclavularia violacea collected in Papua New Guinea. Tetrahedron 2000, 56, 9031–9037. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Wang, G.-H.; Duh, C.-Y.; Soong, K. Pachyclavulariolides M–R, six novel diterpenoids from a Taiwanese soft coral Pachyclavularia violacea. J. Nat. Prod. 2003, 66, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Duh, C.-Y.; El-Gamal, A.A.H.; Chu, C.-J.; Wang, S.-K.; Dai, C.-F. New cytotoxic constituents from the Formosan soft corals Clavularia viridis and Clavularia violacea. J. Nat. Prod. 2002, 65, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Huang, I.-C.; Chiang, M.Y.-N.; Hwang, T.-L.; Kung, T.-H.; Lin, C.-S.; Sheu, J.-H.; Sung, P.-J. Briaviodiol A, a new cembranoid from a soft coral Briareum violacea. Chem. Pharm. Bull. 2010, 58, 1666–1668. [Google Scholar] [CrossRef] [PubMed]
- Uchio, Y.; Fukazawa, Y.; Bowden, B.F.; Coll, J.C. New diterpenes from an Australian Pachyclavularia species (Coelenterata, Anthozoa, Octocorallia). Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 1989, 31, 548–553. [Google Scholar]
- Cardellina, J.H., II; James, T.R., Jr.; Chen, M.H.M.; Clardy, J. Structure of brianthein W, from the soft coral Briareum polyanthes. J. Org. Chem. 1984, 49, 3398–3399. [Google Scholar] [CrossRef]
- Pordesimo, E.O.; Schmitz, F.J.; Ciereszko, L.S.; Hossain, M.B.; van der Helm, D. New briarein diterpenes from the Caribbean gorgonians Erythropodium caribaeorum and Briareum sp. J. Org. Chem. 1991, 56, 2344–2357. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; König, G.M. Studies of Australian soft corals. XLVIII. New briaran diterpenoids from the gorgonian coral Junceella gemmacea. Aust. J. Chem. 1990, 43, 151–159. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Sung, P.-J.; Huang, L.-H.; Lee, S.-F.; Wu, T.; Chang, B.-Y.; Duh, C.-Y.; Fang, L.-S.; Soong, K.; Lee, T.-J. New cytotoxic briaran diterpenes from the Formosan gorgonian Briareum sp. J. Nat. Prod. 1996, 59, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Wratten, S.J.; Faulkner, D.J. Some diterpenes from the sea pen Stylatula sp. Tetrahedron 1979, 35, 1907–1912. [Google Scholar] [CrossRef]
- Iwasaki, J.; Ito, H.; Aoyagi, M.; Sato, Y.; Iguchi, K. Briarane-type diterpenoids from the Okinawan soft coral Pachyclavularia violacea. J. Nat. Prod. 2006, 69, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Iwasaki, J.; Sato, Y.; Aoyagi, M.; Iguchi, K.; Yamori, T. Marine diterpenoids with a briarane skeleton from the Okinawan soft coral Pachyclavularia violacea. Chem. Pharm. Bull. 2007, 55, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, J.; Ito, H.; Nakamura, M.; Iguchi, K. A synthetic study of briarane-type marine diterpenoid, pachyclavulide B. Tetrahedron Lett. 2006, 47, 1483–1486. [Google Scholar] [CrossRef]
- Ishiyama, H.; Okubo, T.; Yasuda, T.; Takahashi, Y.; Iguchi, K.; Kobayashi, J. Brianodins A–D, briarane-type diterpenoids from soft coral Pachyclavularia sp. J. Nat. Prod. 2008, 71, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Iwagawa, T.; Takayama, K.; Okamura, H.; Nakatani, M.; Doe, M.; Takemura, K.; Shiro, M. Cytotoxic briarane diterpenes from a gorgonacean Briareum sp. Heterocycles 1999, 51, 2619–2625. [Google Scholar] [CrossRef]
- Iwagawa, T.; Hirose, T.; Takayama, K.; Okamura, H.; Nakatani, M.; Doe, M.; Takemura, K. Violides N–P, new briarane diterpenes from a gorgonacean Briareum sp. Heterocycles 2000, 53, 1789–1792. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, J.-H.; Duh, C.-Y.; Chiang, M.Y.; Sheu, J.-H. Briaexcavatolides K–N, new briarane diterpenes from the gorgonian Briareum excavatum. J. Nat. Prod. 2001, 64, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Hu, W.-P.; Wu, S.-L.; Su, J.-H.; Fang, L.-S.; Wang, J.-J.; Sheu, J.-H. Briaexcavatolides X–Z, three new briarane-related derivatives from the gorgonian coral Briareum excavatum. Tetrahedron 2004, 60, 8975–8979. [Google Scholar] [CrossRef]
- Iwagawa, T.; Babazono, K.; Nakatani, M.; Doe, M.; Morimoto, Y.; Takemura, K. Briarane diterpenes from a gorgonian Briareum sp. Heterocycles 2005, 65, 607–617. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, Y.-D.; Li, G.-Y.; Chiang, M.Y.; Lin, M.-R.; Huang, I.-C.; Li, J.-J.; Fang, L.-S.; Wang, W.-H. Excavatoids A–D, new polyoxygenated briaranes from the octocoral Briareum excavatum. Tetrahedron 2009, 65, 6918–6924. [Google Scholar] [CrossRef]
- Groweiss, A.; Look, S.A.; Fenical, W. Solenolides, new antiinflammatory and antiviral diterpenoids from a marine octocoral of the genus Solenopodium. J. Org. Chem. 1988, 53, 2401–2406. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Sung, P.-J.; Cheng, M.-C.; Liu, H.-Y.; Fang, L.-S.; Duh, C.-Y.; Chiang, M.Y. Novel cytotoxic diterpenes, excavatolides A–E, isolated from the Formosan gorgonian Briareum excavatum. J. Nat. Prod. 1998, 61, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.-H.; Sung, P.-J.; Su, J.-H.; Liu, H.-Y.; Duh, C.-Y.; Chiang, M.Y. Briaexcavatolides A–J, new diterpenes from the gorgonian Briareum excavatum. Tetrahedron 1999, 55, 14555–14564. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Cheng, Y.-B.; Lin, Y.-S.; Kuo, Y.-H.; Hwang, T.-L.; Shen, Y.-C. New briarane diterpenoids from Taiwanese soft coral Briareum violacea. Mar. Drugs 2014, 12, 4677–4692. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-F.; Yamamura, S.; Terada, Y. Stereochemistry of the brianolide acetate (=solenolide D) by the molecular mechanics calculations. Tetrahedron Lett. 1992, 33, 101–104. [Google Scholar] [CrossRef]
- Wang, G.-H.; Sheu, J.-H.; Chiang, M.Y.; Lee, T.-J. Pachyclavulariaenones A–C, three novel diterpenoids from the soft coral Pachyclavularia violacea. Tetrahedron Lett. 2001, 42, 2333–2336. [Google Scholar] [CrossRef]
- Wang, G.-H.; Sheu, J.-H.; Duh, C.-Y.; Chiang, M.Y. Pachyclavulariaenones D–G, new diterpenoids from the soft coral Pachyclavularia violacea. J. Nat. Prod. 2002, 65, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Anta, C.; González, N.; Rodríguez, J.; Jiménez, C. A new secosterol from the Indonesian octocoral Pachyclavularia violacea. J. Nat. Prod. 2002, 65, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Sheu, J.-H.; Xu, J.-P. Survey of briarane-type diterpenoids of marine origin. Heterocycles 2002, 57, 535–579. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chang, P.-J.; Fang, L.-S.; Sheu, J.-H.; Chen, W.-C.; Chen, Y.-P.; Lin, M.-R. Survey of briarane-type diterpenoids-Part II. Heterocycles 2005, 65, 195–204. [Google Scholar] [CrossRef]
- Sung, P.-J.; Sheu, J.-H.; Wang, W.-H.; Fang, L.-S.; Chung, H.-M.; Pai, C.-H.; Su, Y.-D.; Tsai, W.-T.; Chen, B.-Y.; Lin, M.-R.; et al. Survey of briarane-type diterpenoids-Part III. Heterocycles 2008, 75, 2627–2648. [Google Scholar] [CrossRef]
- Sung, P.-J.; Su, J.-H.; Wang, W.-H.; Sheu, J.-H.; Fang, L.-S.; Wu, Y.-C.; Chen, Y.-H.; Chung, H.-M.; Su, Y.-D.; Chang, Y.-C. Survey of briarane-type diterpenoids-Part IV. Heterocycles 2011, 83, 1241–1258. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Chen, Y.-H.; Chen, Y.-H.; Su, Y.-D.; Chang, Y.-C.; Su, J.-H.; Weng, C.-F.; Lee, C.-H.; Fang, L.-S.; Wang, W.-H.; et al. Briarane diterpenoids isolated from gorgonian corals between 2011 and 2013. Mar. Drugs 2014, 12, 2164–2181. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-D.; Su, J.-H.; Hwang, T.-L.; Wen, Z.-H.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Briarane diterpenoids isolated from octocorals between 2014 and 2016. Mar. Drugs 2017, 15, 44. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).