Abstract
Three new sulfated steroidal glycosides (3–5), along with known cholesterol derivatives (1,2), were isolated from the visceral extract of the cone snail Conus pulicarius. The structure of each new compound was elucidated by nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry. The three new compounds exhibited significant in vitro cytotoxicity (GI50 values down to 0.49 μM) against the K562 human leukemia cell line.
1. Introduction
Cone snails are predatory marine molluscs that secrete venom to capture prey, such as marine worms, small fish, molluscs, and other cone snails. The venom that produced by cone snails is composed of various modified peptides, such as conotoxin, which is produced by the Conus species. Conotoxins have generated tremendous interest in the fields of biology and pharmacology, as these peptides cause neurophysiological responses by modulating the activity of ion channels. Over 100,000 neuroactive conotoxins have been discovered from cone snails, which now comprise over 1000 species in the tropical and subtropical areas [1], since the first conotoxins were isolated and characterized [2,3]. During these investigations, ziconotide, the conotoxin peptide that is derived from Conus magus, has gained approval from the Food and Drugs Administration (FDA) as an analgesic for severe and chronic pain, and several other conotoxins are now under investigation in clinical trials as medical agents for the same purpose [4,5].
Small molecules that are produced by the Conus species have gained much less attention as compared to conotoxins, presumably based upon the assumption that the predatory or protective mechanism of the Conus species is mainly dependent on the production and secretion of conotoxins. Despite a thorough literature search, only two publications on the small molecule metabolite that is isolated from the Conus species could be found. One publication describes the isolation of cholesterol and the epidioxysterol 1 from the three species of Conus cone snails (Conus ebraeus, C. leopardus, and C. tessulatus) [6], and the other describes the isolation of a guanine derivative that causes paralysis in mice [7]. Additionally, there is one publication that reported the isolation of thiazoline metabolites (pulicatins) from the cultivation of bacteria Streptomyces sp. CP32 associated with Conus pulicarius [8]. These findings led us to investigate the metabolites that contained in the Conus species, as marine invertebrates are considered to have diverse and complex symbiotic relationships and chemical defense mechanisms. In this work, we investigated the visceral extract of Conus pulicarius collected off the coast of Kosrae, the Federated States of Micronesia (Figure 1), in search for novel toxic metabolites. Herein, five cholesterol derivatives (1–5, Figure 2), which were isolated from this organism, have been described. While compounds 1 and 2 were the previously identified epidioxysterol and cholesterol sulfate, respectively, compounds 3–5 were new steroidal glycosides. In vitro cytotoxicity tests revealed that these new steroidal glycosides were extremely cytotoxic against the human leukemia cell line K562.

Figure 1.
Specimen of Conus pulicarius collected in Kosrae.
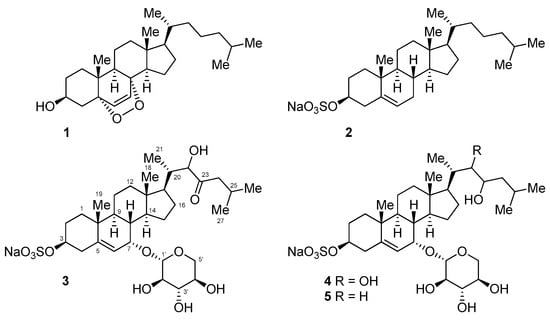
Figure 2.
Structures of the isolated cholesterol derivatives.
2. Results
2.1. Isolation of Cholesterol Derivatives from the Conus Pulicarius Cone Snails
The grinded and lyophilized whole-body tissue of Conus pulicarius specimens were extracted with methanol and dichloromethane. The combined extracts were partitioned between n-butanol and water, and the n-butanol fraction was subsequently partitioned between 15% aqueous methanol and n-hexane. The 15% aqueous methanol fraction was subjected to reverse-phase flash column chromatography using the ODS resin with aqueous methanol gradient elution (50% aqueous methanol to 100% methanol) to afford six fractions. The growth inhibitory activity of each fraction against the human leukemia cell line K562 was evaluated to trace the cytotoxic metabolites in the extract. The second and third fractions eluted with 40% (GI50 3.4 μg/mL) and 30% aqueous methanol (GI50 0.9 μg/mL), respectively, demonstrated high levels of cytotoxicity. These fractions were further purified by size-exclusion column chromatography on the Sephadex LH-20 column, and reverse-phase HPLC, using the YMC-Pack Pro C18 ODS column. Compounds 3 and 4 were obtained from the 40% aqueous methanol fraction, and 5 was obtained from the 30% aqueous methanol fraction. Known compounds 1 and 2 were obtained from the fractions that did not exhibit cytotoxicity at the concentration of 100 μg/mL (100% methanol and 10% aqueous methanol fractions, respectively).
2.2. Structure Elucidation of the Isolated Compounds
A comparison of nuclear magnetic resonance (NMR), mass spectrometry (MS), and optical rotation data with those reported previously confirmed that compounds 1 and 2 were 5α,8α-epidioxysterol and cholesterol sulfate [6,9,10,11] (Figure 2).
Compounds 3–5 were found to share the same tetracyclic carbon framework, with the only difference between these compounds being the substitution at C-17, as judged by 1H and 13C NMR (Table 1) and high-resolution mass spectrometry data.

Table 1.
1H and 13C NMR data (500 and 125 MHz) for compounds 3–5 a,b.
Compound 3 was obtained as a colorless oil. Its molecular formula was determined as C32H51NaO11S by HRFABMS and ESIMS, which showed pseudomolecular ion peaks that were corresponding to [M − Na]− and [M + Na]+, respectively. The tetracyclic carbon framework was analogous to that of cholesterol sulfate 2, except for the presence of the oxymethine group at the C-7 position (δH 3.98, δC 70.1), as judged by the COSY correlation between the proton NMR signals at δH 5.74 (H-6), and 3.98 (H-7), in addition to the HMBC correlations between H-7 and the carbon signals at δC 148.2 (C-5), 122.0 (C-6), and 43.0 (C-9) (Table 1, Figure 3).
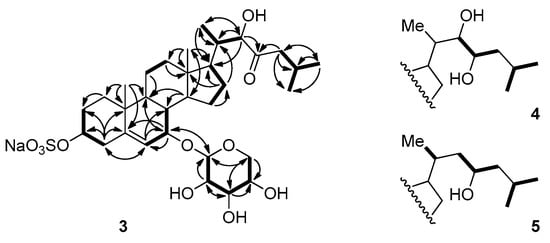
Figure 3.
Selected COSY and HMBC correlations for compounds 3–5.
The relative configuration of the tetracyclic core was confirmed on the basis of coupling constants and correlations observed in the NOESY spectrum (Figure 4). Especially, the NOESY correlation between the proton signals at δH 4.21 (H-3) and 1.23 (H-1α), and the trans diaxial coupling (J = 13.0 Hz) of H-3 with an axial proton at C-4 (H-4β, δ 2.40), both supported the α orientation of H-3. The relative stereochemistry at C-7, where the additional oxygen is attached, was confirmed by a NOESY correlation between H-7 and H-15β (δ 1.10), which suggested that H-7 had the β-pseudoequatorial orientation. This assignment is in accordance with the previous reports, which describe compounds with similar structures as that of 1. In the case of 3β,7β-dihydroxy-5-chole-24-oic acid, which was sulfated at C-3 and N-acetylglucosaminidated at C-7, the H-7 of the α-orientation appeared at δ 3.80 ppm, with a coupling constant of 7.8 to 8.4 Hz [12]. In other reports, comparative data are provided for the C-7 epimers of the synthesized 5-androstene-3,7,17-triol derivative. In the NMR spectra, the H-7 of the 7β-derivative oriented to the α-face appeared as a doublet with J = 7.9 Hz, whereas that of the 7α-derivative appeared as a broad singlet [13]. The same tendency has been observed in the case of 24-methylene- cholest-5-ene-3β,7α-diol and its C-7 epimer [14]. As the H-7 of compound 3 appeared as a broad singlet, this proton was confirmed to have an β-orientation.
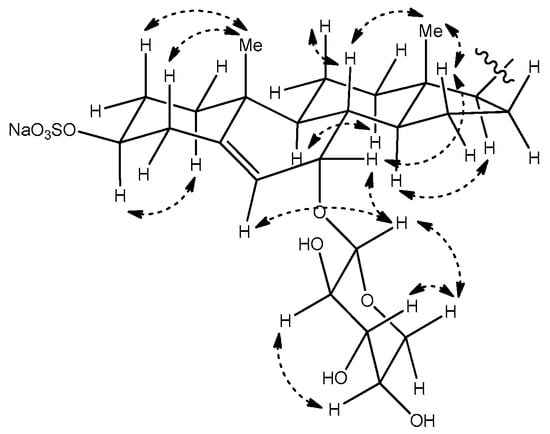
Figure 4.
Selected NOESY correlations for compound 3.
The following proton signals were common in the 1H NMR spectra of all three compounds—a dioxymethine signal at δ 4.40 (H-1′), three hydroxymethine signals at δ 3.10 (H-2′), 3.31 (H-3′), and 3.46 (H-4′), and the signals of the two protons that were attached to the same oxymethylene carbon (C-5′, δC 66.8) at δ 3.83 (H-5′α) and 3.19 (H-5′β). This consistency in signals indicated the presence of the sugar moiety, which was identified as β-d-xylose based upon the interpretation of the coupling constants and NOESY correlations between the signals in this region. In detail, a proton signal at the anomeric position (H-1′) was coupled to a signal of H-2′ with J = 7.4 Hz, and H-2′ was again coupled to H-3′ with J = 8.9 Hz. NOESY correlations were also observed between H-1′, H-3′, and H-5′β (Figure 3 and Figure 4). These observations hinted at a diaxial relationship existing between these protons, and thus the sugar moiety was identified as β-d-xylose. This assignment was in accordance with the result that was obtained by the treatment of compound 3 with 2N HCl, which showed that the 1H NMR data and optical rotation value of the hydrolysis product coincided with those of d-xylose. The xylose moiety was connected to C-7 of the tetracyclic carbon framework, as judged by the HMBC correlations between the anomeric proton and the C-7 oxymethine carbon.
The remaining oxymethine proton signal appearing at δH 4.12 (H-22) showed HMBC correlations with the carbon signals at δC 53.3 (C-17), 40.1 (C-20), and 13.3 (C-21), and the signal of the ketone carbon at δC 215.0 (C-23). The two signals of the protons attached to the α-carbon with the chemical shift of δC 48.5 (C-24) appeared at δH 2.38 and 1.96 (H-24), and these signals showed COSY and HMBC correlations with the proton and carbon signals of isopropyl methine (C-25, δC 25.4). Based on these observations, the side branch attached at C-17 could be established, as depicted in Figure 2 and Figure 3.
Compound 4 was obtained as a pale-yellow amorphous solid. The 1H NMR data for 4 was quite similar to that of 3, except for the additional oxymethine proton signal at δH 3.55 (H-23). In the 13C NMR spectra, the corresponding carbon signal at δC 72.2 (C-23) was observed and the signal corresponding to the carbonyl carbon was absent. Based upon these observations, 4 was identified as the 22,23-dihydroxy derivative, i.e., the reduced form of 3. This assignment is in accordance with the molecular formula of C32H53NaO11S that were derived from (–)-HRFABMS, the COSY, and HMBC correlations (Figure 3).
Compound 5 had one less oxymethine signal in its 1H and 13C NMR spectra as compared to 2. Instead, a carbon signal was observed at δC 46.3 (C-22), which was correlated to the two protons that appeared at δH 1.44 and 1.54 (H-22) in the HSQC spectra. The molecular formula of C32H53NaO10S obtained by (–)-HRFABMS also suggests that 5 has one less hydroxy group as compared to 4. Consequently, the structure of 5 established from the COSY and HMBC correlations was as depicted in Figure 2 and Figure 3.
For the establishment of absolute stereochemistry at C-22 of 3, C-22 and C-23 of 4, and C-23 of 5, we have tried to prepare the MTPA ester derivatives of the aglycon. Disappointingly, attempts to obtain the desulfated aglycon under various conditions, such as using an acid (2N HCl, p-toluenesulfonic acid) or a base (pyridine, potassium carbonate) only resulted in the decomposition of the starting material with the formation of products whose 1H NMR spectrum had few assignable signals. The direct acylation of the obtained compounds using MTPA chloride and the acetonide formation of 2 also failed because of the decomposition of the starting material. No valid method for establishing the absolute configuration of the oxymethine stereogenic center of the side branch in the new compounds could be found up to now.
The relative stereochemistry of C-20 in compounds 3–5 was established to be the same as that of cholesterol sulfate 2 based on the comparison of 1H and 13C NMR data of 2 and 5. In detail, the carbon signal of C-17 appeared at δC 58.2, and the signal of the attached proton appeared at δH 1.15 in the NMR spectra of 5, whereas these signals were observed at δC 57.5 and δH 1.09 in the spectra of 2. The signals of C-20, C-21 and the attached proton were also similar; δc-20 37.1, δc-21 19.2, and δH-21 1.40, δH-21 0.95 in the NMR data of 2, δc-20 35.2, δc-21 20.0, and δH-20 1.44, δH-21 0.98 in those of 5 (see Supplementary Materials).
All of the isolated compounds were tested for in vitro cytotoxicity against the human leukemia cell line K562. While compounds 1 and 2 did not exhibit any cytotoxicity (GI50 > 60.00 μM), the new sulfated steroidal glycosides 3–5 demonstrated potent cytotoxicities with GI50 values of 1.50 ± 0.25 μM, 1.39 ± 0.05 μM, and 0.49 ± 0.03 μM, respectively. Staurosporine, used as a positive control, showed an GI50 value of 2.29 ± 0.02 μM with in the same 96 well-plate.
3. Discussion
Five cholesterol derivatives (1–5), including three new sulfated steroidal glycosides (3–5), were isolated from the Conus pulicarius that were collected in Kosrae, Micronesia. The characteristic structural features of the new compounds include the sulfate group at the C-3 position and the xylose linked to C-7, which is different from those of known steroidal glycosides. Sulfated steroidal glycosides have been primarily isolated from marine organisms, such as algae and invertebrates [15,16]. Especially, the starfishes of various species are frequently found to contain sulfated steroidal glycosides, called asterosaponins, which have a sulfate group at the C-3 position and various sugar moieties linked to C-6. Compounds (3–5) were named as Conusaponin A-C, as these are the first example of steroidal glycosides isolated from Conus species.
The new compounds showed potent growth inhibitory activity against the human leukemia cell line K562. This finding, combined with those regarding the previously reported steroidal glycosides with potent activities against various cancer cell lines [16,17,18,19,20,21,22], would provide new insights into the structure-activity relationships of cytotoxic sulfated steroidal glycosides.
4. Materials and Methods
4.1. General Procedures
The optical rotations were measured using a JASCO digital polarimeter in a 5 cm long cell. Fourier transform infra-red (FTIR) spectra were recorded on a JASCO FT/IR-4100 spectrometer (JASCO, Tokyo, Japan). 1H and 13C NMR spectra were recorded on Varian Unity 500 500 MHz and 125 MHz spectrometers, respectively. The chemical shifts have been reported in ppm and referenced to the solvent resonances, resulting from incomplete deuteration as the internal references (CD3OD: δH 3.31 ppm, δC 49.00 ppm). HPLC was performed with YMC-Pack Pro C18 columns using a Shodex RI-101 detector (Shoko Science, Yokohama, Japan).
4.2. Biological Material Collection, Extraction, and Isolation
Twenty specimens of Conus pulicarius (3–4 cm) were collected by hand at 1–3 m depth offshore of Kosrae, the Federated States of Micronesia, in January, 2015. The collected specimens (300 g, wet wt.) were immediately freeze-dried and kept at −20 °C until the time of our investigation. The specimens were thawed at room temperature in a fume hood for 3 h, and then the shell was removed from the viscera. The viscera were then grinded in a blender and lyophilized to yield the 17.7 g of a sticky solid. This solid was extracted using methanol (300 mL × 2) and dichloromethane (300 mL × 1) at room temperature. The combined extract (2.8 g) was partitioned between n-butanol and water, and the organic layer (820 mg) was further partitioned between 15% aqueous methanol and n-hexane. Subsequently, the aqueous methanol fraction (430 mg) was subjected to reverse-phase column chromatography (YMC Gel ODS-A, 60 Å, 230 mesh) with a stepped gradient solvent system of 50, 40, 30, 20, and 10% aqueous methanol, and 100% methanol. The fraction eluted with 40% aqueous methanol (34.0 mg) was then subjected to size-exclusion column chromatography (LH-20), followed by further purification by reverse-phase HPLC (YMC-Pack Pro C18) to afford 3 (3.2 mg) and 4 (5.0 mg). The 30% aqueous methanol fraction (59.0 mg) was also subjected to size-exclusion chromatography and reverse-phase HPLC to afford 5 (3.1 mg). The 10% aqueous methanol and 100% methanol fractions were purified by reverse-phase HPLC to afford 1 (2.8 mg) and 2 (0.8 mg), respectively.
Compound 3: pale yellow amorphous solid; −73.1 (c 0.5, CH3OH); UV λmax (log ε) 285 (4.5), 211 (3.4) nm; IR (KBr) νmax 3337, 2921, 2885, 1055, 1033, 1014 cm−1; 1H and 13C NMR (CD3OD, 500 and 125 MHz), see Table 1 and Supplementary Materials; (+)-LRESIMS m/z 689.84 [M + Na]+; (–)-HRFABMS m/z 643.3156 [M − Na]− (calcd. for C32H51O11S, m/z 643.3152).
Compound 4: pale yellow amorphous solid; −59.1 (c 0.5, CH3OH); UV λmax (log ε) 286 (3.2), 235 (3.5), 211 (4.5) nm; IR (KBr) νmax 3726, 2866, 1055, 1032, 1012 cm−1; 1H and 13C NMR (CD3OD, 500 and 125 MHz), see Table 1 and Supplementary Materials; (+)-LRESIMS m/z 691.67 [M + Na]+; (–)-HRFABMS m/z 645.3307 [M − Na]− (calcd. for C32H53O11S, m/z 645.3309).
Compound 5: pale yellow amorphous solid; −40.7 (c 0.5, CH3OH); UV λmax (log ε) 277 (3.9), 236 (4.2), 211 (4.5) nm; IR (KBr) νmax 3725, 2865, 1055, 1033, 1055 cm−1; 1H and 13C NMR (CD3OD, 500 and 125 MHz), see Table 1 and Supplementary Materials; (+)-LRESIMS m/z 675.70 [M + Na]+; (–)-HRFABMS m/z 629.3363 [M − Na]− (calcd. for C32H53O10S, m/z 629.3359).
4.3. Cytotoxicity Assay
The growth inhibition assay against human leukemia cell line K562 was performed according to a published protocol [23,24]. In brief, the cells were added to a 96-well plate containing either a control (staurosprine) or the test compounds. After incubation for 48 h, 10 μL of the WST-1 solution was added to each well of the culture plate (containing 100 μL of the RPMI medium). After incubation for 2 h at 37 °C, the optical density (OD) of the assay solution was measured at 450 nm by using the ELISA plate reader. Cell viability was calculated as a percentage, with the following equation: % cell viability = (ODsample/ODcontrol) × 100. The GI50 values were determined by plotting cell viability versus the concentration of compound. Results were reported as the average values and standard deviations of triplicate samples.
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/15/12/379/s1, 1H and 13C NMR spectra of 1–5, and 2D (HMBC, HSQC, COSY, NOESY) NMR spectra of 3–5.
Acknowledgments
This work was supported by a grant of the Korea Institute of Ocean Science and Technology and the Ministry of Oceans and Fisheries, Republic of Korea. We thank the Kosrae State Government, the Federated States of Micronesia, for allowing marine organism research.
Author Contributions
Collection of the Conus pulicarius, isolation and purification of the secondary metabolites, and structure elucidation of isolated compounds were carried out by Yeon-Ju Lee, Saem Han, Hyi-Seung Lee, Hee Jae Shin, Jong Seok Lee, and Jihoon Lee. Evaluation of the cytotoxicity against human leukemia cell line (K562) of the isolated compounds was carried out Su-Hyun Kim. Yeon-Ju Lee wrote the manuscript and the manuscript was finalized through contributions from all authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Franco, A.; Pisarewicz, K.; Moller, C.; Mora, D.; Fields, G.B.; Mari, F. Hyperhydroxylation: A new strategy for neuronal targeting by venomous marine molluscs. In Molluscs: From Chemo-Ecological Study to Biotechnological Application, 1st ed.; Cimino, G., Gavagnin, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 83–103. [Google Scholar]
- Cruz, L.J.; Gray, W.R.; Oliviera, B.M. Purification and properties of a myotoxin from Conus geographus venom. Arch. Biochem. Biophys. 1978, 190, 539–548. [Google Scholar] [CrossRef]
- Olivera, B.M.; Gray, W.R.; Zeikus, R.; McIntosh, J.M.; Varga, J.; Rivier, J.; de Santos, V.; Cruz, L.J. Peptide neurotoxins from fish-hunting cone snails. Science 1985, 230, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.M.; Baell, J.B.; Norton, R.S. Strategies for the development of conotoxins as new therapeutic leads. Mar. Drugs 2013, 11, 2293–2313. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, N.R.; Christie, M.J. Conotoxins that could provide analgesia through voltage gated sodium channel inhibition. Toxins 2015, 7, 5386–5407. [Google Scholar] [CrossRef] [PubMed]
- Aknin, M.; Faure, I.V.; Gaydou, E.M. 5α,8α-Epidioxycholest-6-en-3-β-ol from three cone snails of the Indian Ocean. J. Am. Oil Chem. Soc. 1998, 75, 1679–1681. [Google Scholar] [CrossRef]
- Neves, J.L.B.; Lin, Z.; Imperial, J.S.; Antunes, A.; Vasconcelos, V.; Oliviera, B.M.; Schmidt, E.W. Small molecules in the cone snail arsenal. Org. Lett. 2015, 17, 4933–4935. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Antemano, R.R.; Hughen, R.W.; Tianero, M.D.B.; Peraud, O.; Haygood, M.G.; Concepcion, G.P.; Oliviera, B.M.; Light, A.; Schmidt, E.W. Pulicatins A–E, neuroactive thiazoline metabolites from cone snail-associated bacteria. J. Nat. Prod. 2010, 73, 1922–1926. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.A.L.; Gopichand, Y.; Schmitz, F.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. 26. Isolation and structure elucidation of nine new 5α,8α-epidoxy sterols from four marine organisms. J. Org. Chem. 1981, 46, 3860–3866. [Google Scholar] [CrossRef]
- Miyamoto, T.; Honda, M.; Sugiyama, S.; Higuchi, R.; Komori, T. Isolation and structure of two 5,8α-epidioxysterols and a cholesteryl ester mixture from the albumen gland of Aplysia juliana. Liebigs Ann. Chem. 1988, 1988, 589–592. [Google Scholar] [CrossRef]
- Minn, C.V.; Kiem, P.V.; Huong, L.M.; Kim, Y.H. Cytotoxic constituents of Diadema setosum. Arch. Pharm. Res. 2004, 27, 734–737. [Google Scholar] [CrossRef]
- Kakiyama, G.; Ogawa, S.; Iida, T.; Fujimoto, Y.; Mushiake, K.; Goto, T.; Mano, N.; Goto, J.; Nambara, T. Nuclear magnetic resonance spectroscopy of 3β,7β-dihydroxy-5-cholen-24-oic acid multi-conjugates: Unusual bile acid metabolites in human urine. Chem. Phys. Lipids 2006, 140, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-P.; Yu, P.; Zhang, H.-J.; Liu, H.-M. Synthesis of 5-androstene-3β,7α,17β-triol and 5-androstene-3β,7β,17β-triol. Chin. J. Chem. 2008, 26, 1666–1668. [Google Scholar] [CrossRef]
- De Riccardis, F.; Minale, L.; Iorizzi, M.; Debitus, C.; Lévi, C. Marine Sterols. Side-chain-oxygenated sterols, possibly of abiotic origin, from the New Caledonian sponge Stelodoryx chlorophylla. J. Nat. Prod. 1993, 56, 282–287. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Minale, L.; Riccio, R. Polyoxygenated steroids of marine origin. Chem. Rev. 1993, 93, 1839–1895. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, T.V.; Kicha, A.A.; Ivanchina, N.V.; Kalinovsky, A.I.; Popov, R.S.; Vishchuk, O.S.; Stonik, V.A. Asterosaponins from the Far Eastern starfish Leptasterias ochotensis and their anticancer activity. Steroids 2014, 87, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Luyen, B.T.T.; Kim, E.-J.; Kang, H.-K.; Kim, S.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Minh, C.V.; Kim, Y.H. Asterosaponins from the starfish Astropecten monacanthus suppress growth and induce apoptosis in HL-60, PC-3, and SNU-C5 human cancer cell lines. Biol. Pharm. Bull. 2014, 37, 315–321. [Google Scholar]
- Ngoan, B.T.; Hanh, T.T.H.; Vien, L.T.; Diep, C.N.; Thao, N.P.; Thao, D.T.; Thanh, N.V.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; et al. Asterosaponins and glycosylated polyhydroxysteroids from the starfish Culcita novaeguineae and their cytotoxic activities. J. Asian Nat. Prod. Res. 2015, 17, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-X.; Kang, Y.-F.; Han, H. Three new cytotoxic polyhydroxysteroidal glycosides from starfish Craspidaster hesperus. Mar. Drugs 2016, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Calabro, K.; Kalahroodi, E.L.; Rodrigues, D.; Díaz, C.; de la Cruz, M.; Cautain, B.; Laville, R.; Reyes, F.; Pérez, T.; Soussi, B.; et al. Poecillastrosides, steroidal saponins from the Mediterranean deep-sea sponge Poecillastra compressa (Bowerbank, 1866). Mar. Drugs 2017, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Kicha, A.A.; Kalinovsky, A.I.; Ivanchina, N.V.; Malyarenko, T.V.; Dmitrenok, P.S.; Kuzmich, A.S.; Sokolova, E.V.; Stonik, V.A. Furostane series asterosaponins and other unusual steroid oligoglycosides from the tropical starfish Pentaceraster regulus. J. Nat. Prod. 2017, 80, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, M.; Shiga, M.; Sasamoto, K.; Mizoguchi, M.; He, P.-G. A new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem. Pharm. Bull. 1993, 41, 1118–1122. [Google Scholar] [CrossRef]
- Wang, X.-D.; Li, C.-Y.; Jiang, M.-M.; Li, D.; Wen, P.; Song, X.; Chen, J.-D.; Guo, L.-X.; Hu, X.-P.; Li, G.-Q.; et al. Induction of apoptosis in human leukemia cells through an intrinsic pathway by cathachunine, a unique alkaloid isolated from Catharanthus roseus. Phytomedicine 2016, 23, 641–653. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).