Abstract
A series of oxy-polybrominated diphenyl ethers (O-PBDEs) has been isolated from the extracts of Miamira magnifica and Miamira miamirana collected from Queensland, Australia. M. magnifica sequesters the new OH-PBDE 1 and six known OH-PBDEs containing four to six bromines (2–7). M. miamirana also accumulates known tribromo- and tetrabromo OMe-PBDEs 8–10 in both mantle and viscera tissues. To date, Miamira is the only genus of the family Chromodorididae that is known to incorporate O-PBDEs, rather than terpenes, in the mantle where the metabolites may play a putative role in chemical defense. The extract of M. magnifica was tested in a brine shrimp lethality assay and exhibited an LD50 of 58 μg/mL.
1. Introduction
Oxy-polybrominated diphenyl ethers (O-PBDEs) are prolific in nature with extensive biological activities. OH- and OMe-PBDEs have been typically reported from sponges of the genus Lamellodysidea [1], Dysidea [2,3] and Phyllospongia [4,5,6] and occasionally from molluscs [7,8,9]. The bioaccumulation of OMe-PBDEs at ppm levels has also been reported in marine mammals, suggesting the persistent nature of these compounds in the environment [10]. The origin of OH- and OMe-PBDEs has been much debated due to their structural similarities to industrial flame retardants [11]. Biosynthetic studies via 14C measurements have indicated that OMe-PBDEs [12] as well as OH-PBDEs [13] can be of natural origin. OH-PBDEs have been indicated as the biosynthetic product of sponge-associated cyanobacteria, but the definite genetic basis for algal biosynthesis of OH-PBDEs has not been established [14]. Recent studies, however, have revealed that O-PBDEs are biosynthesized by marine bacteria [15].
Nudibranchs of the family Chromodorididae have been known to sequester defensive terpenes from sponges. As part of our ongoing chemoecological studies, we have reported the terpene chemistry of Chromodorid nudibranchs from various species of Glossodoris [16], Chromodoris [17], Goniobranchus [18] and Ardeadoris [19]. To the best of our knowledge, chemical analysis of Chromodorid nudibranchs of the genus Miamira has not been reported elsewhere. In this paper, investigation of brominated secondary metabolites of Miamira magnifica and Miamira miamirana will be described for the first time.
2. Results and Discussion
A new OH-PBDE 1, together with known OH-PBDEs 2–7 [20,21,22,23], has been isolated from the mantle and viscera extracts of M. magnifica (specimen #955). Three known OMe-PBDEs 8–10 [4,5,6] have also been obtained from the extracts of M. miamirana (#1500) (Figure 1). The diethyl ether extracts of each species were purified by means of normal phase (NP) flash chromatography, followed by reverse phase (RP) or normal phase (NP) High Performance Liquid Chromatography (HPLC).
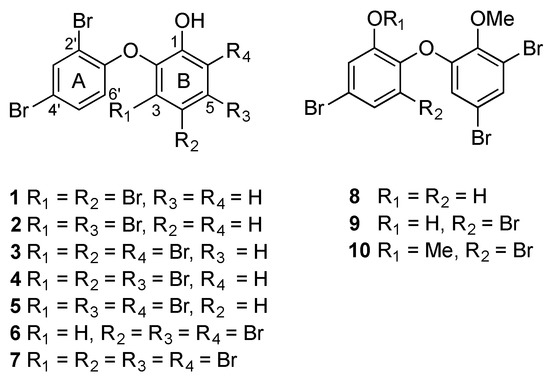
Figure 1.
Structure of O-PBDEs 1–10.
Compound 1 was obtained as a colorless oil from NP flash chromatography of the M. magnifica mantle extract. A molecular formula of C12H579Br4O2 was deduced from the ion peak at m/z 496.7028 (calcd. for m/z 496.7042) in high resolution electrospray ionization mass spectrometry (HRESIMS) (Figure S1). There were five aromatic protons (δH 6.40, 6.97, 7.29, 7.47 and 7.79) and a hydroxy signal (δH 5.70) in the 1H nuclear magnetic resonance (NMR) data (Figure S2). The most upfield proton at δH 6.40 (1H, d, 8.8) was diagnostic for H-6′ in ring A due to the presence of a bromine substituent at C-3 of ring B [24]. In the correlation spectroscopy (COSY) data (Figure S3), the signal for H-6′ was coupled to the signal at δH 7.29 (1H, dd, 8.8, 2.2, H-5′) that was further meta-coupled to the signal at δH 7.79 (1H, d, 2.2, H-3′). The remaining two ortho-coupled aromatic protons at δH 7.47 (1H, d, 8.8) and δH 6.97 (1H, d, 8.8) plus two bromine substituents therefore belonged to ring B.
Two plausible structures of 1 with different substitution patterns in ring B were considered (Figure 2). Since one of the two bromine substituents had already been assigned to C-3 based on the chemical shift of H-6′, the other bromine was positioned at either C-4 or C-6. Both proton signals at δH 7.47 (1H, d, 8.8) and at δH 6.97 (1H, d, 8.8) showed heteronuclear multiple bond correlation (HMBC) to the carbons at δC 149.1, 120.0 and 116.0 (Figure S4). Hence, these HMBC data did not differentiate the two candidate structures.

Figure 2.
Candidate structures of 1.
To solve the data discrepancies, the 13C NMR data of ring B in 1 (Figures S4 and S5) were compared to the calculated values [25] for 1a and 1b (Table 1). Notably, the experimental 13C NMR data matched closely to the expected values for 1a, suggesting that the metabolite had a 3,4-disubstitution pattern rather than the alternative 3,6-disubstitution pattern. A 1D nuclear Overhauser effect (NOE) experiment was then undertaken to confirm this hypothesis (Figure S6). Irradiation of the hydroxy group at C-1 (δH 5.70) enhanced the H-6 signal (δH 6.97), indicating their proximity in the molecule (Figure 3).

Table 1.
Comparison of experimental and calculated 13C NMR data of ring B in 1.
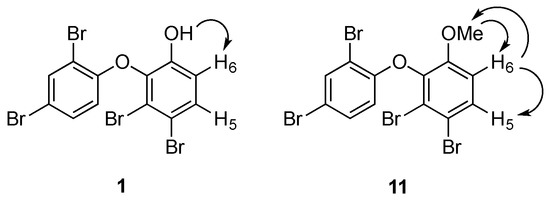
Figure 3.
Selected 1D NOE correlations in 1 and 11.
For the purpose of confirmation, the phenol group was reacted with methyl iodide to yield a methyl ether analogue (11). Two separate 1D NOE experiments were carried out (Figure 3). Irradiation of the methoxy group enhanced the signal of H-6 (Figure S7), while irradiation of H-6 sharpened the signals of H-5 and of the methoxy group (Figure S8). These NOE data confirmed the bromination pattern at C-3 and C-4 in 1. Thus, compound 1 was proposed as 2-(2′,4′-dibromophenoxy)-3,4-dibromophenol.
The isolated OH-PBDEs 1–7 from M. magnifica displayed an identical 2,4-dibromination pattern in ring A relative to the diphenyl ether bond. Moreover, they also showed identical ortho-hydroxylation in ring B relative to the ether linkage with a varying bromination pattern at C-3, C-4, C-5, and C-6. Similarly, OMe-PBDEs 8–10 from M. miamirana showed an ortho-hydroxylation and ortho-methoxylation in ring A and/or ring B relative to the ether linkage with an identical 4,6-dibromination pattern in ring B.
The first observation of OH-PBDEs in nudibranchs was reported from Chromodoris funerea (=lineolata) collected in Iwayama Bay, Palau [7]. Our group reported the isolation of OH- and OMe-PBDEs as well as sesquiterpenes from the digestive tissues of three specimens of the Discodorid nudibranch, Asteronotus cespitosus, collected from the Great Barrier Reef and the Phillipines. Since only sesquiterpenes were found in the mantle extracts, it was suggested that A. cespitosus selectively incorporated these secondary metabolites from the food source as defensive chemicals. It was also speculated that OH- and OMe-PBDEs were eliminated as they were either too toxic to be integrated into the body tissue, or not as effective as defensive weapons [8]. Unfortunately, the bioactivity of these compounds was not tested.
The Paul group also reported the sequestration of OH-PBDEs in the gastropterid molluscs (Order Cephalaspidea), Sagaminopteron psychedelicum and Sagaminopteron nigropunctatum that were found feeding on the sponge Dysidea granulosa in Guam. The difference between the two molluscs lies in their defense strategies; S. psychedelicum has a bright coloration, whereas S. nigropunctatum is cryptic. Chemical analysis of the extracts of both species showed the sequestration of 2 as the major component in all body parts. Compound 2 was accumulated in the mantle of S. psychedelicum (4.03%) and S. nigropunctatum (2.37%) at approximately the same concentration found in the sponge extract. The same metabolite was present at twice the concentration in the parapodia of S. psychedelicum (7.97%) and S. nigropunctatum (10.10%). In the mucus of S. psychedelicum, 2 was detected in trace amounts; whereas in the mucus (1.84%) and egg masses (2.22%) of S. nigropunctatum, the level of 2 was quite significant [9].
In contrast to the anatomical distribution of metabolites in A. cespitosus [8], OH-PBDEs were found in the mantle and dorsal horn of M. magnifica as well as in the digestive tissues (Table 2). Metabolites 1–3 were found in all three tissue types, whereas the more highly brominated 4–7 were only found in the gut tissues. Compounds 3–6 have been reported from the sponge Lamellodysidea herbacea [22,23]; whereas compounds 2 and 7 were initially isolated from an unidentified Australian marine sponge [20] and the sponge Dysidea sp. [21], respectively. The identification of three known O-PBDEs in M. magnifica that were also detected from the sponge L. herbacea, leads to speculation that M. magnifica may sequester the O-PBDEs from this sponge. Likewise, tri- and tetrabrominated OMe-PBDEs 8–10 were found in the mantle extracts of M. miamirana, as well as in the digestive tissues. Each of compounds 8–10 has been reported individually from various genera of sponges but all three compounds have been found in Phyllospongia sp. [4,5,6]. It is thus reasonable to propose that M. miamirana may feed on this sponge. Our data suggest a preference for sequestration of O-PBDEs with fewer number of bromine substituents into the mantle of these two species.

Table 2.
Anatomical distribution and percentage weight of O-PBDEs in M. magnifica and M. miamirana.
OH-tetraBDE 2 has been reported to show antifeedant activity against tropical reef fish [26], the gastropod Stylocheilus longicauda [27], the pufferfish Canthigaster solandri and two species of crab (Leptodius spp.) [9,28], highlighting its significant predator deterrent properties. Meanwhile, Handayani and co-workers (1997) reported that the toxicity level of OH-PBDEs against the brine shrimp Artemia salina is directly proportional to the number of bromine substituents. Accordingly, OH-hexaBDE 7 displayed the strongest toxicity in the assay with an LC50 of less than 1 μg/mL, compared to those of OH-tetraBDEs (LC50 3.30–8.66 μg/mL). Moreover, methylation of the hydroxy group in OH-tetraBDEs significantly reduced the activity in the brine shrimp assay (LC50 26.25 μg/mL) [29]. An extract prepared from two specimens of M. magnifica collected near Mooloolaba (#1252-3) was screened and found to exhibit an LD50 value of 58 μg/mL against brine shrimp (Figure S9). The extract of M. miamirana could not be tested in the same assay due to the limited amount of material. Consequently, direct comparison of the toxicity between the two species could not be drawn. We propose, however, that the sequestration of O-PBDEs with fewer number of bromines in M. magnifica and M. miamirana may be due to the significant deterrent properties and low toxicity level of the selected O-PBDEs.
To date, Miamira is the only known genus of Chromodorid nudibranch that sequester O-PBDEs, rather than terpenes, in the mantle where the metabolites may play a putative role in chemical defense. Some closely-related Chromodoris spp. have been shown to selectively accumulate a 16-membered macrolide, latrunculin A, in the mantle parts, leaving other secondary metabolites in the viscera [30]. A recent study has reported the exclusive incorporation of (−)-furodysinin in the dorsal horn of Ceratosoma trilobatum and Ceratosoma gracilimum, emphasizing the protective function of the dorsal horn in Ceratosoma nudibranchs [31]. Compared to C. funerea (=lineolata) and A. cespitosus, M. magnifica and M. miamirana may also have developed adaptive digestive systems that enable them to consume O-PBDEs without damaging their internal organs. However, further research would need to be undertaken to test this hypothesis.
3. Materials and Methods
3.1. General Experimental Procedure
NMR spectra were recorded on a Bruker (Karlsruhe, Germany) Avance 500 MHz and 700 MHz spectrometer at ambient probe temperature. All NMR spectra were run in either choloroform-d (CDCl3) or acetone-d6 and referenced to solvent signals at 7.26 ppm (1H) or 2.05 ppm (1H), respectively. The 13C NMR data were acquired from the heteronuclear single quantum correlations (HSQC) and heteronuclear multiple bond correlations (HMBC) experiments. Low resolution electrospray ion mass spectra (LRESIMS) were measured using a Bruker Esquire HCT, whereas high resolution electrospray ion mass spectra (HRESIMS) were measured using a Bruker MicroTof Q instrument, each with a standard ESI source. Normal phase flash chromatography was performed using silica gel 60 (40–63 μm; Scharlau, Barcelona, Spain). Normal phase HPLC was carried out using a Waters 515 (Milford, MA, USA) pump connected to a Gilson (Middleton, WI, USA) 132 series refractive index detector with a Waters μPorasil (10 μm, 7.8 × 300 mm) column. Separations were performed using isocratic elution conditions using premixed, filtered and degassed mobile phases. Reverse phase HPLC was conducted on an Agilent (Santa Clara, CA, USA) 1100 series with in-line vacuum degassing unit, an Agilent D1311A quaternary pump, a variable wavelength UV detector, refractive index detector and a Phenomenex (Lane Cove, Australia) Gemini (5 μm, 110 Å, 10 × 250 mm) column.
3.2. Biological Material
A single specimen of M. magnifica (specimen #955) was collected from North Stradbroke Island, QLD, Australia, in November 2013. Two individuals of M. magnifica (#1252-3) were obtained from Mudjimba, Mooloolaba, QLD, Australia, in March 2014. An individual of M. miamirana (#1500) was supplied by Cairns Marine, QLD, Australia, in June 2016. M. magnifica (#955) was dissected into mantle, viscera and dorsal horn before chemical analysis. M. magnifica (#1252-3) and M. miamirana (#1500) were dissected into mantle and viscera only.
3.3. Extraction and Isolation of O-PBDEs
The mantle, viscera and dorsal horn tissues of M. magnifica (#955) were individually extracted with acetone then partitioned against diethyl ether to yield 28 mg of mantle extract, 17.8 mg of viscera extract and 2.3 mg of dorsal horn extract. The mantle extract of M. magnifica was subjected to NP flash chromatography using a gradient of hexanes-dichloromethane (DCM)-ethyl acetate (EtOAc)-methanol (MeOH) to yield four fractions. Fraction 1 was further separated by a second NP flash column using a gradient of hexanes-EtOAc to give six subfractions. Subfraction 1b was purified with NP HPLC (5% EtOAc/hexanes) to obtain 2 (0.2 mg) and 3 (0.1 mg). Fraction 3 was also subjected to NP HPLC (5% EtOAc/hexanes) to furnish 1 (0.2 mg), 2 (3.0 mg), 3 (1.5 mg) and 5 (0.1 mg). Fraction 4 was collected as a mixture of 1 and sterol (2 mg). The separation of gut extract of M. magnifica was carried out similarly. Fraction 1 from NP flash chromatography was purified by RP HPLC (80% MeCN/water with 0.1% trifluoroacetic acid (TFA)) to afford 2 (2 mg), 3 (0.2 mg), 7 (0.1 mg) as well as fractions containing 3–6 (0.5 mg). Compounds 1–3 were identified from the dorsal horn extract by data comparison with those of purified compounds. Following the same method, the viscera and mantle tissues of M. magnifica (#1252-3) were extracted separately for dereplication analysis. The mantle and viscera extracts of M. magnifica (#1252-3) contained the same metabolites as those of M. magnifica (#955).
Extraction of the mantle and viscera tissues of M. miamirana (#1500) yielded 6 mg of mantle extract and 3 mg of gut extract. Fraction 3 of NP flash chromatography of the mantle extract furnished a 3:1 mixture of 8 and 9 (1 mg). Fraction 2 also contained 10 as a mixture with sterol (1 mg). Compounds 8–10 were also detected in the viscera extract. The IUPAC numbers for O-PBDE congeners isolated in this study is presented in Table S1.
2-(2′,4′-Dibromophenoxy)-3,4-dibromophenol (1): colorless oil (0.5 mg); 1H NMR (CDCl3, 500 MHz) δ 7.79 (1H, d, J = 2.2, H-3′), 7.47 (1H, d, J = 8.8, H-5), 7.29 (1H, dd, J = 8.8, 2.2, H-5′), 6.97 (1H, d, J = 8.8, H-6), 6.40 (1H, d, J = 8.8, H-6′), 5.70 (1H, s, OH); 1H NMR (acetone-d6, 500 MHz) δ 7.83 (1H, d, J = 2.4, H-3′), 7.53 (1H, d, J = 8.8, H-5), 7.42 (1H, dd, J = 8.8, 2.4, H-5′), 7.08 (1H, d, J = 8.8, H-6), 6.52 (1H, d, J = 8.8, H-6′), 5.61 (1H, s, OH) (Figure S10); 13C NMR (CDCl3, 125 MHz) 152.2 (C-1′), 149.1 (C-1), 140.3 (C-2), 136.4 (C3′), 131.6 (C-5′), 131.2 (C-5), 120.0 (C-3), 117.1 (C-6), 116.0 (C-2′, C-4), 115.8 (C-6′), 112.8 (C-4′); HRESIMS m/z 496.7028 (calcd. for C12H679Br4O2, 496.7042).
3.4. Methylation of 1
The methylation of 1 was carried out using the method of Dexter et al. (1993) [32] to yield 11. The 1H NMR data of 11 (Figure S11) were consistent with those reported in the literature [33].
3.5. Brine Shrimp Lethality Assay
For bioassay purpose, the mantle and viscera extracts of M. magnifica (#1252-3) were combined. The brine shrimp lethality assay was conducted based on literature methods [30].
4. Conclusions
A new OH-PBDE (1), along with nine known OH- and OMe-PBDEs (2–10), has been isolated from the extract of M. magnifica and M. miamirana collected from North Stradbroke Island, Queensland. Our study suggested that Miamira spp. selectively sequestered dietary derived O-PBDEs in the mantle as putative defense metabolites. Our finding highlighted the chemical diversity in Chromodorididae, as it demonstrated the first report of O-PBDEs from this family of nudibranchs.
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/14/11/198/s1, Figure S1: High Resolution ESIMS of 1, Figure S2: 1H NMR (CDCl3, 500 MHz) of 1, Figure S3: COSY spectrum (CDCl3, 500 MHz) of 1, Figure S4: HMBC spectrum (CDCl3, 500 MHz) of 1, Figure S5: HSQC spectrum (CDCl3, 500 MHz) of 1, Figure S6: 1D NOE spectrum (acetone-d6, 700 MHz) of 1 by irradiating the –OH signal, Figure S7: 1D NOE spectrum (CDCl3, 500 MHz) of 11 by irradiating the –OMe signal, Figure S8: 1D NOE spectrum (CDCl3, 500 MHz) of 11 by irradiating the H-6 signal, Figure S9: Mortality to brine shrimp (LD50) for extract of M. magnifica (#1252-3), Figure S10: 1H NMR (acetone-d6, 500 MHz) of 1, Figure S11: 1H NMR (CDCl3, 500 MHz) of 11, Table S1: IUPAC numbers for O-PBDE congeners isolated in this study.
Acknowledgments
The authors thank AusAID for an Australian Leadership Award (ALA) Fellowship and Allison Sudradjat Prize (to A.S.D.) as well as the Australian Pacific Science Foundation (APSF) and the University of Queensland for financial support (to K.L.C. and M.J.G.). The nudibranch collections in South East Queensland were made under permits from the Queensland Government (General Fisheries Permit #161624) and the Moreton Bay Marine Park #QS2012/MAN185. The assistance of Tri Le (NMR), Gregory Pierens (NMR) and Graham McFarlane is gratefully acknowledged.
Author Contributions
Ariyanti S. Dewi isolated and identified the compounds; Karen L. Cheney collected and identified the specimens; Holly H. Urquhart conducted the brine shrimp lethality assay; Karen L. Cheney, Joanne T. Blanchfield, Mary J. Garson designed the experiments. Ariyanti S. Dewi, Joanne T. Blanchfield and Mary J. Garson wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hanif, N.; Tanaka, J.; Setiawan, A.; Trianto, A.; de Voogd, N.J.; Murni, A.; Tanaka, C.; Higa, T. Polybrominated diphenyl ethers from the Indonesian sponge Lamellodysidea herbacea. J. Nat. Prod. 2007, 70, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Utkina, N.K.; Kazantseva, M.V.; Denisenko, V.A. Brominated diphenyl ethers from the marine sponge Dysidea fragilis. Chem. Nat. Compd. 1987, 23, 508–509. [Google Scholar] [CrossRef]
- Il’in, S.G.; Utkina, N.K.; Veselova, M.V.; Struchkov, Y.T. Crystal structure of the complex of brominated diphenyl ethers from the marine sponge Dysidea fragilis. Russ. Chem. Bull. 1996, 756–758. [Google Scholar]
- Carté, B.; Faulkner, D.J. Polybrominated diphenyl ethers from Dysidea herbacea, Dysidea chlorea and Phyllospongia foliascens. Tetrahedron 1981, 37, 2335–2339. [Google Scholar] [CrossRef]
- Hattori, T.; Konno, A.; Adachi, K.; Shizuri, Y. Four new bioactive bromophenols from the Palauan sponge Phyllospongia dendyi. Fish. Sci. 2001, 67, 899–903. [Google Scholar] [CrossRef]
- Liu, H.; Namikoshi, M.; Meguro, S.; Nagai, H.; Kobayashi, H.; Yao, X. Isolation and characterization of polybrominated diphenyl ethers as inhibitors of microtubule assembly from the marine sponge Phyllospongia dendyi collected at Palau. J. Nat. Prod. 2004, 67, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Carte, B.; Kernan, M.R.; Barrabee, E.B.; Faulkner, D.J.; Matsumoto, G.K.; Clardy, J. Metabolites of the nudibranch Chromodoris funerea and the singlet oxygen oxidation products of furodysin and furodysinin. J. Org. Chem. 1986, 51, 3528–3532. [Google Scholar] [CrossRef]
- Fahey, S.J.; Garson, M.J. Geographic variation of natural products of tropical nudibranch Asteronotus cespitosus. J. Chem. Ecol. 2002, 28, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Becerro, M.A.; Starmer, J.A.; Paul, V.J. Chemical defenses of cryptic and aposematic gastropterid molluscs feeding on their host sponge Dysidea granulosa. J. Chem. Ecol. 2006, 32, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Vetter, W.; Stoll, E.; Garson, M.J.; Fahey, S.J.; Gaus, C.; Müller, J.F. Sponge halogenated natural products found at parts-per-million levels in marine mammals. Environ. Toxicol. Chem. 2002, 21, 2014–2019. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wiseman, S.; Chang, H.; Zhang, X.; Jones, P.D.; Hecker, M.; Kannan, K.; Tanabe, S.; Hu, J.; Lam, M.H.W. Origin of hydroxylated brominated diphenyl ethers: Natural compounds or man-made flame retardants? Environ. Sci. Technol. 2009, 43, 7536–7542. [Google Scholar] [CrossRef] [PubMed]
- Teuten, E.L.; Xu, L.; Reddy, C.M. Two abundant bioaccumulated halogenated compounds are natural products. Science 2005, 307, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Guitart, C.; Slattery, M.; Ankisetty, S.; Radwan, M.; Ross, S.J.; Letcher, R.J.; Reddy, C.M. Contemporary 14C radiocarbon levels of oxygenated polybrominated diphenyl ethers (O-PBDEs) isolated in sponge-cyanobacteria associations. Mar. Pollut. Bull. 2011, 62, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Unson, M.D.; Holland, N.D.; Faulkner, D.J. A brominated secondary metabolite synthesized by the cyanobacterial symbiont of a marine sponge and accumulation of the crystalline metabolite in the sponge tissue. Mar. Biol. 1994, 119, 1–11. [Google Scholar] [CrossRef]
- Agarwal, V.; El Gamal, A.A.; Yamanaka, K.; Poth, D.; Kersten, R.D.; Schorn, M.; Allen, E.E.; Moore, B.S. Biosynthesis of polybrominated aromatic organic compounds by marine bacteria. Nat. Chem. Biol. 2014, 10, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.W.L.; Mudianta, I.W.; Cheney, K.L.; Mollo, E.; Blanchfield, J.T.; Garson, M.J. Isolation of norsesterterpenes and spongian diterpenes from Dorisprismatica (=Glossodoris) atromarginata. J. Nat. Prod. 2015, 78, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Katavic, P.L.; Jumaryatno, P.; Hooper, J.N.A.; Blanchfield, J.T.; Garson, M.J. Oxygenated terpenoids from the Australian sponges Coscinoderma matthewsi and Dysidea sp., and the nudibranch Chromodoris albopunctata. Aust. J. Chem. 2012, 65, 531–538. [Google Scholar] [CrossRef]
- White, A.M.; Pierens, G.K.; Forster, L.C.; Winters, A.E.; Cheney, K.L.; Garson, M.J. Rearranged diterpenes and norditerpenes from three Australian Goniobranchus mollusks. J. Nat. Prod. 2016, 79, 477–483. [Google Scholar] [CrossRef] [PubMed]
- White, A.M.; Dewi, A.S.; Cheney, K.L.; Winters, A.E.; Blanchfield, J.T.; Garson, M.J. Oxygenated diterpenes from the Indo-Pacific nudibranchs Goniobranchus splendidus and Ardeadoris egretta. Nat. Prod. Commun. 2016, 11, 921–924. [Google Scholar]
- Capon, R.; Ghisalberti, E.L.; Jefferies, P.R.; Skelton, B.W.; White, A.H. Structural studies of halogenated diphenyl ethers from a marine sponge. J. Chem. Soc. Perk. Tran. 1981, 39, 2464–2467. [Google Scholar] [CrossRef]
- Salvá, J.; Faulkner, D.J. A new brominated diphenyl ether from a Philippine Dysidea species. J. Nat. Prod. 1990, 53, 757–760. [Google Scholar] [CrossRef]
- Bowden, B.F.; Towerzey, L.; Junk, P.C. A new brominated diphenyl ether from the marine sponge Dysidea herbacea. Aust. J. Chem. 2000, 53, 299–301. [Google Scholar] [CrossRef]
- Agrawal, M.S.; Bowden, B.F. Marine sponge Dysidea herbacea revisited: Another brominated diphenyl ether. Mar. Drugs 2005, 3, 9–14. [Google Scholar] [CrossRef]
- Calcul, L.; Chow, R.; Oliver, A.G.; Tenney, K.; White, K.N.; Wood, A.W.; Fiorilla, C.; Crews, P. NMR Strategy for unraveling structures of bioactive sponge-derived oxy-polyhalogenated diphenyl ethers. J. Nat. Prod. 2009, 72, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Bühlmann, P.; Affolter, C.; Pretsch, E.; Bhuhlmann, P.; Affolter, C. Structure Determination of Organic Compounds; Springer: Berlin, Germany, 2009; Volume 13. [Google Scholar]
- Duffy, J.E.; Paul, V.J. Prey nutritional quality and the effectiveness of chemical defenses against tropical reef fishes. Oecologia 1992, 90, 333–339. [Google Scholar] [CrossRef]
- Pennings, S.C.; Paul, V.J. Secondary chemistry does not limit dietary range of the specialist sea hare Stylocheilus longicauda (Quoy et Gaimard 1824). J. Exp. Mar. Biol. Ecol. 1993, 174, 97–113. [Google Scholar] [CrossRef]
- Pennings, S.C.; Pablo, S.R.; Paul, V.J.; Duffy, J.E. Effects of sponge secondary metabolites in different diets on feeding by three groups of consumers. J. Exp. Mar. Biol. Ecol. 1994, 180, 137–149. [Google Scholar] [CrossRef]
- Handayani, D.; Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; Soest, R.W.M.V.; Kunzmann, A.; Soedarsono. Four new bioactive polybrominated diphenyl ethers of the sponge Dysidea herbacea from West Sumatra, Indonesia. J. Nat. Prod. 1997, 60, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Cheney, K.L.; White, A.; Mudianta, I.W.; Winters, A.E.; Quezada, M.; Capon, R.J.; Mollo, E.; Garson, M.J. Choose your weaponry: Selective storage of a single toxic compound, Latrunculin A, by closely related nudibranch molluscs. PLoS ONE 2016, 11, e0145134. [Google Scholar] [CrossRef] [PubMed]
- Mollo, E.; Gavagnin, M.; Carbone, M.; Guo, Y.W.; Cimino, G. Chemical studies on Indopacific Ceratosoma nudibranchs illuminate the protective role of their dorsal horn. Chemoecology 2005, 15, 31–36. [Google Scholar] [CrossRef]
- Dexter, A.F.; Garson, M.J.; Hemling, M.E. Isolation of a novel bastadin from the temperate marine sponge Ianthella sp. J. Nat. Prod. 1993, 56, 782–786. [Google Scholar] [CrossRef]
- Vetter, W.; Kirres, J.; Bendig, P. Bromination of 2-methoxydiphenyl ether to an average of tetrabrominated 2-methoxydiphenyl ethers. Chemosphere 2011, 84, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).