Mugilid Fish Are Sentinels of Exposure to Endocrine Disrupting Compounds in Coastal and Estuarine Environments
Abstract
:1. Introduction
2. Mugilids as Sentinel Species of EDC Pollution
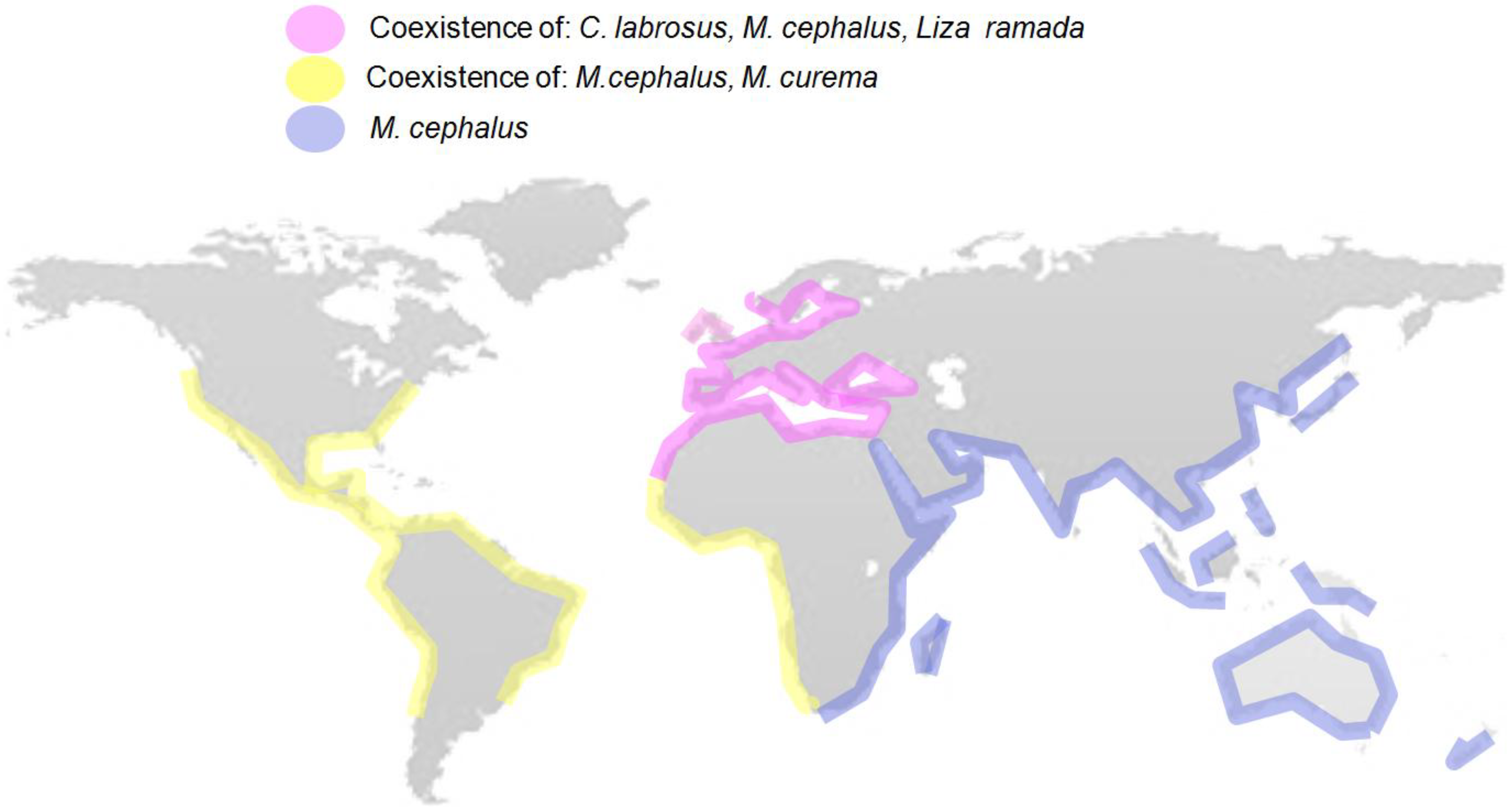
| Species | Common Name | Location | Xenoestrogenicity Endpoints Measured | References |
|---|---|---|---|---|
| Chelon haematocheilus | Redlip mullet | East coast of China (East China Sea) | Intersex condition; VTG protein levels | [100] |
| Chelon labrosus | Thicklip grey mullet | Basque coast (Bay of Biscay) | Intersex condition; VTG protein and mRNA levels; cyp19a1a and cyp19a1b aromatases mRNA levels; oocyte molecular markers; chemical metabolite levels in bile. | [38,101,102] |
| Liza ramada | Thinlip grey mullet | Homa Lagoon (Izmir Bay-Aegean Sea) | Hermaphrodite (intersex) gonads | [103] |
| Mugil cephalus | Flathead grey mullet | Douro estuary (Portugal, East Atlantic coast) | Intersex condition | [48,56] |
| Orbetello Lagoon-West Italy (Mediterranean Sea) | VTG mRNA levels; oocyte development and atresia | [104,105,106] | ||
| South coast of Korea (East China Sea) | Intersex condition; VTG protein levels | [94,100] | ||
| South coast of Japan (East China Sea) | Intersex condition; VTG protein levels | [94,100] | ||
| East coast of China (East China Sea) | Intersex condition; VTG protein levels | [100] | ||
| Mugil soiuy | So-iuy mullet/Far Eastern mullet | North East coast of China (Bo Sea) | VTG mRNA levels | [98] |
3. Effects Mediated by EDCs in Mugilids
| Species | Common Name | Treatment | Effect Endpoints Measured | References |
|---|---|---|---|---|
| Chelon haematocheilus | Redlip mullet | Nonylphenol (0.01, 0.1, 1, 10 & 100 ng/mL) | Steroid levels in cultured oocytes | [115] |
| Chelon labrosus | Thicklip grey mullet | Perfluorooctane sulfonate (2 mg/L) | VTG and cyp19a1b aromatase mRNA | [116] |
| Liza aurata | Golden grey mullet | 17β-estradiol (2 μg/L & 0.07 mg/kg body weight) | VTG mRNA levels | [117] |
| Mugil cephalus | Flathead grey mullet | 17β-estradiol (1, 8, 15 & 120 mg/kg feed) | Oocyte development; GSI | [111] |
| 17α-ethinylestradiol (20 mg/kg feed) | Gonad development; GSI; steroid plasma levels; gonad aromatase activity. | |||
| 17α-ethinylestradiol (0.04 & 4 μg/kg body weight) | Gonad development; VTG protein levels. | |||
| 17α-methyltestosterone (20 mg/kg feed) | Gonad development; GSI; steroid plasma levels; gonad aromatase activity. | |||
| 17α-methyltestosterone (5, 10 & 15 mg/kg feed) | Gonad development; VTG protein levels | |||
| 17α-methyltestosterone (4 mg/kg body weight) | Gonad development, steroid plasma levels, | |||
| Domperidone (5 mg/kg body weight) | ||||
| GnRH (10 μg/kg body weight) | ||||
| Domperidone (5 mg/kg body weight) + GnRH (10 μg/kg) |
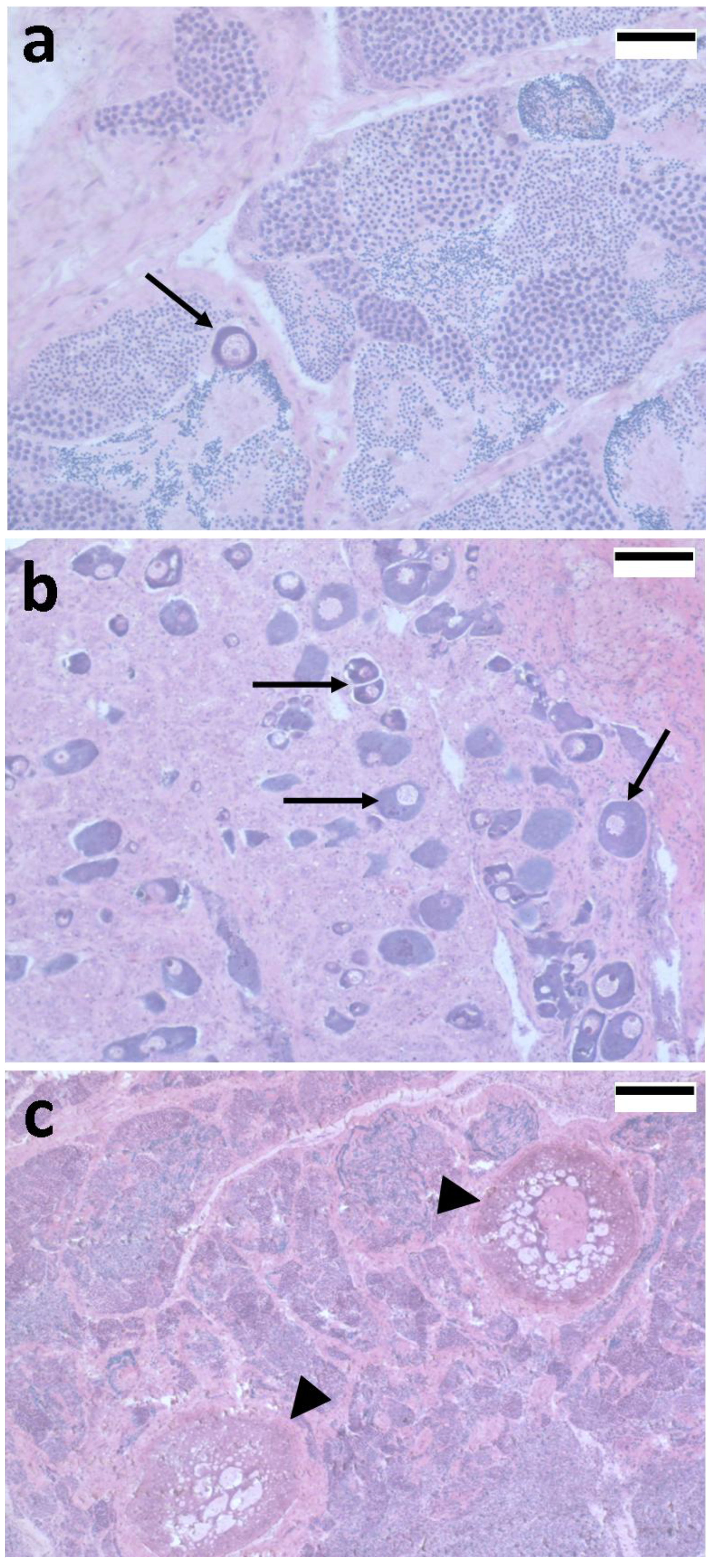
4. Novel Intersex Markers in Mullets
4.1. 5S rRNA as a Sex Marker in Fish Gonads: A Crucial Molecule in Oocyte Differentiation
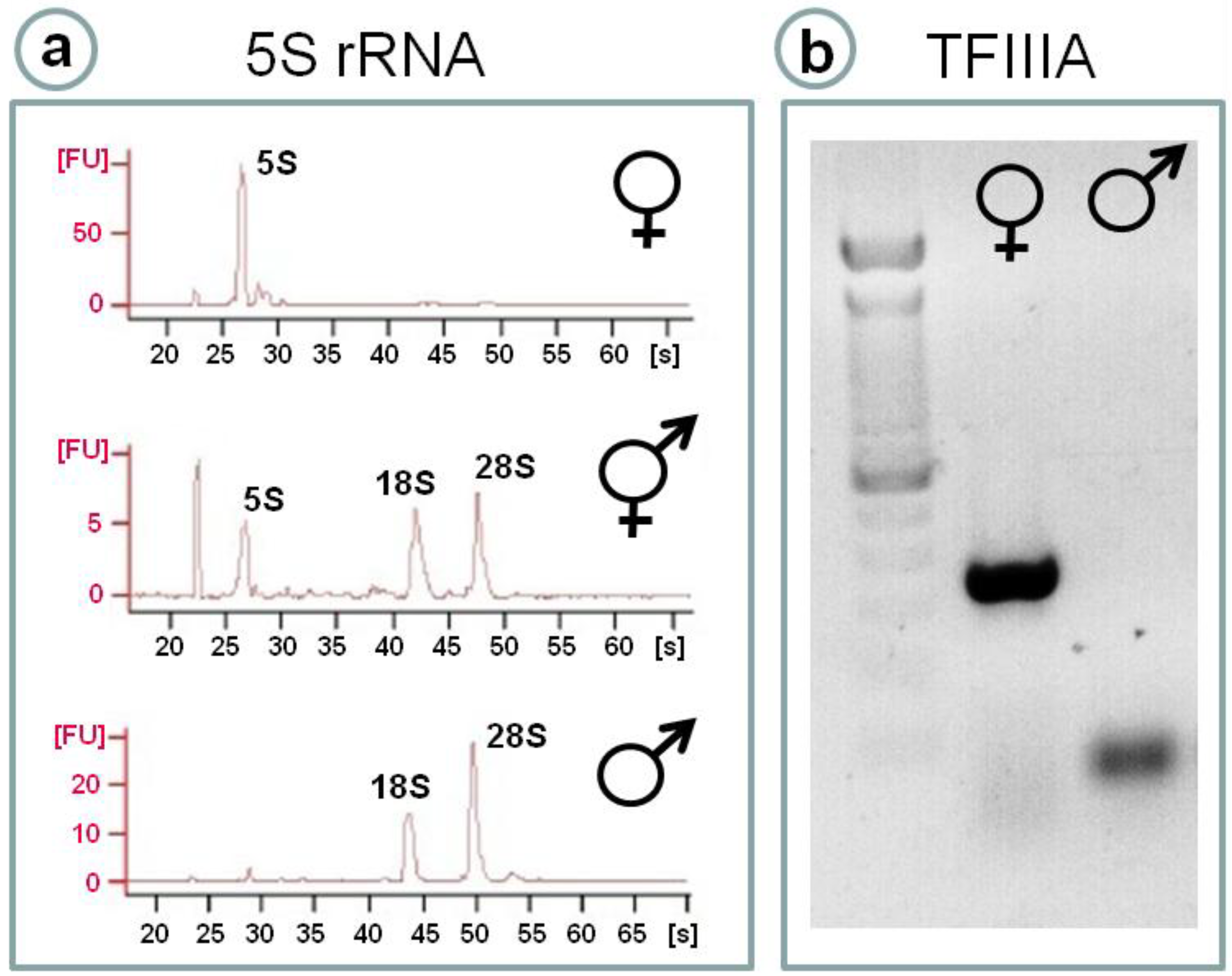
4.2. Transcriptional Regulation of Ovarian 5S rRNA and Storage of Ribosomal Subunits

4.3. Nucleo-Cytoplasmic Transport of Proteins and Ribonucleoproteins during Oocyte Differentiation
4.4. How Is 5S rRNA Related Gene Expression Regulated in Fish Oocytes?
- -
- Which are the dynamics of activation of these oocyte markers during normal female sexual determination/differentiation?
- -
- Which are the environmental (EDC exposure among them) and molecular clues, epigenetic mediators and pathways that regulate activation of 5S rRNA related genes so as to mask any other RNA production in PGCs that initiate differentiation into oogonia instead of spermatogonia?
- -
- Could the levels of 5S rRNA accumulated into oocytes give information about the effects of EDCs on oogenesis and oocyte quality?
- -
- Would these molecular markers function as intersex markers in other mugilid fish species, or in fish species with asynchronous gamete development?
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Conflicts of Interest
References
- Boehm, A.B.; Bischel, H.N. Oceans and Human Health. In Encyclopedia of Environmental Health; Nriagu, J., Ed.; Elsevier B.V.: Burlington, MA, USA, 2011; Volume 4, pp. 223–230. [Google Scholar]
- DellaSala, D.A. Oceans and Global Change: One Blue Planet. Ref. Module Earth Syst. Environ. Sci. 2013. [Google Scholar] [CrossRef]
- Matthiessen, P. Endocrine disruption in marine fish. Pure Appl. Chem. 2003, 75, 2249–2261. [Google Scholar]
- WHO/UNEP (World Health Organization/United Nations Environment Programme). State-of-the-Science of Endocrine Disrupting Chemicals 2012; Bergman, A., Heindel, J.J., Jobling, S., Kidd, K.A., Zoeller, R.T., Eds.; WHO Press: Geneva, Switzerland, 2013; ISBN: 978-92-807-3274-0 (UNEP) and 978-92-4-150503-1 (WHO). [Google Scholar]
- EEA (European Environmental Agency). Environmental Agreements: Environmental Effectiveness; Environmental Issue Report No. 3; European Environmental Agency: Copenhagen, Denmark, 1997; Volume 1–2. [Google Scholar]
- Tyler, C.R.; Jobling, S. Roach, sex, and gender-bending chemicals: The feminization of wild fish in English Rivers. Bioscience 2008, 58, 1051–1059. [Google Scholar] [CrossRef]
- Söffker, M.; Tyler, C.R. Endocrine disrupting chemicals and sexual behaviors in fish—A critical review on effects and possible consequences. Crit. Rev. Toxicol. 2012, 42, 653–668. [Google Scholar]
- Tyler, C.R.; Jobling, S.; Sumpter, J.P. Endocrine disruption in wildlife: A critical review of the evidence. Crit. Rev. Toxicol. 1998, 28, 319–361. [Google Scholar] [CrossRef] [PubMed]
- Goksøyr, A.; Arukwe, A.; Larsson, J.; Cajaraville, M.P.; Hauser, L.; Nilsen, B.M.; Lowe, D.; Matthiessen, P. Molecular/cellular processes and the impact on reproduction. In Effects of Pollution on Fish; Lawrence, A.J., Hemingway, K.L., Eds.; Blackwell Science Ltd.: Oxford, UK, 2003; Chapter 5; pp. 179–220. [Google Scholar]
- Goksøyr, A. Endocrine disruptors in the marine environment: Mechanisms of toxicity and their influence on reproductive processes in fish. J. Toxicol. Environ. Health Part A 2006, 69, 175–184. [Google Scholar]
- Bahamonde, P.A.; Munkittrick, K.R.; Martyniuk, C.J. Intersex in teleost fish: Are we distinguishing endocrine disruption from natural phenomena? Gen. Comp. Endocrinol. 2013, 192, 25–35. [Google Scholar] [CrossRef]
- Bateman, K.S.; Stentiford, G.D.; Feist, S.W. A ranking system for the evaluation of intersex condition in European flounder (Platichthys flesus). Environ. Toxicol. Chem. 2004, 23, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Williams, R.; Johnson, A.; Taylor, A.; Gross-Sorokin, M.; Nolan, M.; Tyler, C.R.; van Aerle, R.; Santos, E.; Brighty, G. Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environ. Health Perspect. 2006, 114, 32–39. [Google Scholar]
- Harris, C.; Hamilton, P.B.; Runnalls, T.J.; Vinciotti, V.; Henshaw, A.; Hodgson, D.; Coe, T.S.; Jobling, S.; Tyler, C.R.; Sumpter, J.P. The consequences of feminization in breeding groups of wild fish. Environ. Health Perspect. 2011, 119, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.; Paull, G.C.; Coe, T.S.; Katsu, Y.; Urushitani, H.; Iguchi, T.; Tyler, C.R. Sexual reprogramming and estrogenic sensitization in wild fish exposed to ethinylestradiol. Environ. Sci. Technol. 2009, 43, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Kidd, K.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Nicol, E.; de-Bastos, E.S.; Williams, R.J.; Sumpter, J.P.; Jobling, S.; Stevens, J.R.; Tyler, C.R. Populations of a cyprinid fish are self-sustaining despite widespread feminization of males. BMC Biol. 2014, 12, 1:1–1:13. [Google Scholar]
- Mills, L.J.; Chichester, C. Review of evidence: Are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Sci. Total Environ. 2005, 343, 1–34. [Google Scholar] [CrossRef]
- Arukwe, A.; Goksøyr, A. Eggshell and egg yolk proteins in fish: Hepatic protein for the next generation: Oogenetic, population, and evolutionary implications of endocrine disruption. Comp. Hepatol. 2003, 2, 4:1–4:21. [Google Scholar]
- Hyllner, S.J.; Oppen-Berntsen, D.O.; Helvik, J.V.; Walther, B.T.; Haux, C. Oestradiol-17β induces major vitelline envelope proteins in both sexes in teleosts. J. Endocrinol. 1991, 131, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish? An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Piferrer, F. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 2001, 197, 229–281. [Google Scholar] [CrossRef]
- Diotel, N.; Le Page, Y.; Mouriec, K.; Tong, S.-K.; Pellegrini, E.; Vaillant, C.; Anglade, I.; Brion, F.; Pakdel, F.; Chung, B.-C.; et al. Aromatase in the brain of teleost fish: Expression, regulation and putative functions. Front. Neuroendocrinol. 2010, 31, 172–192. [Google Scholar] [CrossRef] [PubMed]
- Callard, G.V.; Tchoudakova, A. Evolutionary and functional significance of two CYP19 genes differentially expressed in brain and ovary of goldfish. J. Steroid Biochem. Mol. Biol. 1997, 61, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Cheshenko, K.; Pakdel, F.; Segner, H.; Kah, O.; Eggen, R.I.L. Interference of endocrine disrupting chemicals with aromatase CYP19 expression or activity, and consequences for reproduction of teleost fish. Gen. Comp. Endocrinol. 2008, 155, 31–62. [Google Scholar] [CrossRef] [PubMed]
- Trant, J.M.; Gavasso, S.; Ackers, J.; Chung, B.; Place, A.R. Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Danio rerio). J. Exp. Zool. 2001, 290, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Hinfray, N.; Palluel, O.; Piccini, B.; Sanchez, W.; Aït-Aïssa, S.; Noury, P.; Gomez, E.; Geraudie, P.; Minier, C.; Brion, F.; et al. Endocrine disruption in wild populations of chub (Leuciscus cephalus) in contaminated French streams. Sci. Total Environ. 2010, 408, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Kishida, M.; Callard, G.V. Distinct cytochrome P450 aromatase isoforms in zebrafish (Danio rerio) brain and ovary are differentially programmed and estrogen regulated during early development. Endocrinology 2001, 142, 740–750. [Google Scholar] [PubMed]
- Kirby, M.F.; Allen, Y.T.; Dyer, R.A.; Feist, S.W.; Katsiadaki, I.; Matthiessen, P.; Scott, A.P.; Smith, A.; Stentiford, G.D.; Thain, J.E.; et al. Surveys of plasma vitellogenin and intersex in male flounder (Platichthys flesus) as measures of endocrine disruption by estrogenic contamination in United Kingdom estuaries: temporal trends, 1996 to 2001. Environ. Toxicol. Chem. 1996, 23, 748–758. [Google Scholar]
- Matthiessen, P.; Allen, Y.; Bamber, S.; Craft, J.; Hurst, M.; Hutchinson, T.; Feist, S.; Katsiadaki, I.; Kirby, M.; Robinson, C.; et al. The impact of oestrogenic and androgenic contamination on marine organisms in the United Kingdom—Summary of the EDMAR programme. Endocrine Disruption in the Marine Environment. Mar. Environ. Res. 2002, 54, 645–649. [Google Scholar] [CrossRef]
- Allen, Y.; Matthiessen, P.; Scott, A.P.; Haworth, S.; Feist, S.; Thain, J.E. The extent of oestrogenic contamination in the UK estuarine and marine environments-further surveys of flounder. Sci. Total Environ. 1999, 233, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Lahr, J.; Kuiper, R.V.; Grinwis, G.C.; Rankouhi, T.R.; Giesy, J.P.; Gerritsen, A. Estrogenic effects in fish in The Netherlands: Some preliminary results. Toxicology 2002, 181–182, 147–150. [Google Scholar]
- Vethaak, A.D.; Lahr, J.; Schrap, S.M.; Belfroid, A.C.; Rijs, G.B.; Gerritsen, A.; de Boer, J.; Bulder, A.S.; Grinwis, G.C.; Kuiper, R.V.; et al. An integrated assessment of estrogenic contamination and biological effects in the aquatic environment of The Netherlands. Chemosphere 2005, 59, 511–524. [Google Scholar]
- Mills, L.J.; Gutjahr-Gobell, R.E.; Horowitz, D.B.; Denslow, N.D.; Chow, M.C.; Zaroogian, G.E. Relationship between reproductive success and male plasma vitellogenin concentrations in cunner, Tautogolabrus adspersus. Environ. Health Perspect. 2003, 111, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.A.; Armstrong, J.L.; Sakamoto, K.; Steinert, S.; Perkins, E.; Lomax, D.P.; Johnson, L.L.; Schlenk, D. The relationships of biochemical endpoints to histopathology and population metrics in feral flatfish species collected near the municipal wastewater outfall of Orange County, California, USA. Environ. Toxicol. Chem. 2003, 22, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Bessho, H.; Hara, A.; Nakamura, M.; Iguchi, T.; Fujita, K. Elevated serum vitellogenin levels and gonadal abnormalities in wild male flounder (Pleuronectes yokohamae) from Tokyo Bay, Japan. Mar. Environ. Res. 2000, 49, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, N.; Mochida, K.; Adachi, S.; Hara, A.; Hotta, K.; Nakamura, Y.; Matsubara, T. Development of enzyme-linked immunosorbent assays for two forms of vitellogenin in Japanese common goby (Acanthogobius flavimanus). Gen. Comp. Endocrinol. 2003, 131, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Puy-Azurmendi, E.; Ortiz-Zarragoitia, M.; Villagrasa, M.; Kuster, M.; Aragón, P.; Atienza, J.; Puchades, R.; Maquieira, A.; Domínguez, C.; López de Alda, M.; et al. Endocrine disruption in thicklip grey mullet (Chelon labrosus) from the Urdaibai Biosphere Reserve (Bay of Biscay, Southwestern Europe). Sci. Total Environ. 2013, 443, 233–244. [Google Scholar]
- De Metrio, G.; Corriero, A.; Desantis, S.; Zubani, D.; Cirillo, F.; Deflorio, M.; Bridges, C.R.; Eicker, J.; de la Serna, J.M.; Megalofonou, P.; et al. Evidence of a high percentage of intersex in the Mediterranean swordfish (Xiphias gladius L.). Mar. Pollut. Bull. 2003, 46, 358–361. [Google Scholar]
- Desantis, S.; Corriero, A.; Cirillo, F.; Deflorio, M.; Brill, R.; Griffiths, M.; Lopata, A.L.; de la Serna, J.M.; Bridges, C.R.; Kime, D.E.; et al. Immunohistochemical localization of CYP1A, vitellogenin and Zona radiata proteins in the liver of swordfish (Xiphias gladius L.) taken from the Mediterranean Sea, South Atlantic, South Western Indian and Central North Pacific Oceans. Aquat. Toxicol. 2005, 71, 1–12. [Google Scholar]
- Fossi, M.C.; Casini, S.; Marsili, L.; Neri, G.; Mori, G.; Ancora, S.; Moscatelli, A.; Ausili, A.; Notarbartolo-di-Sciara, G. Biomarkers for endocrine disruptors in three species of Mediterranean large pelagic fish. Mar. Environ. Res. 2002, 54, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Macías, D.; Saber, S.; Osuna, A.M.; Cruz-Castán, R.M.; Gómez-Vives, M.J.; Báez, J.C. First record of intersexuality in Euthynnus alletteratus in the Mediterranean Sea: Histological description. Mar. Biodivers. Rec. 2014, 7, e3. [Google Scholar] [CrossRef]
- Martin-Skilton, R.; Lavado, R.; Thibaut, R.; Minier, C.; Porte, C. Evidence of endocrine alteration in the red mullet, Mullus barbatus from the NW Mediterranean. Environ. Pollut. 2006, 141, 60–68. [Google Scholar]
- Scott, A.P.; Katsiadaki, I.; Witthames, P.R.; Hylland, K.; Davies, I.M.; McIntosh, A.D.; Thain, J. Vitellogenin in the blood plasma of male cod (Gadus morhua): A sign of oestrogenic endocrine disruption in the open sea? Mar. Environ. Res. 2006, 61, 149–170. [Google Scholar]
- Scott, A.P.; Sanders, M.; Stentiford, G.D.; Reese, R.A.; Katsiadaki, I. Evidence for estrogenic endocrine disruption in an offshore flatfish, the dab (Limanda limanda L.). Mar. Environ. Res. 2007, 64, 128–148. [Google Scholar]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar]
- Suter, G.W. Ecological Risk Assessment; Lewis Publishers: Boca Raton, FL, USA, 1993; p. 538. [Google Scholar]
- Ferreira, M.; Antunes, P.; Gil, O.; Vale, C.; Reis-Henriques, M.A. Organochlorine contaminants in flounder (Platichthys flesus) and mullet (Mugil cephalus) from Douro estuary, and their use as sentinel species for environmental monitoring. Aquat. Toxicol. 2004, 69, 347–357. [Google Scholar] [CrossRef] [PubMed]
- UNEP (United Nations Environmental Programme). Report of the Meeting of Experts to Review the MED POL Biomonitoring Programme; UNEP(OCA)/MED WG.132/7; UNEP: Athens, Greece, 1997. [Google Scholar]
- Waltham, N.J.; Teasdale, P.R.; Connolly, R.M. Use of flathead mullet (Mugil cephalus) in coastal biomonitor studies: Review and recommendations for future studies. Mar. Pollut. Bull. 2013, 69, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, A.K.; Panfili, J.; Durand, J.D. A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Rev. Fish Biol. Fish. 1758, 22, 641–681. [Google Scholar]
- Burgess, P.J.; Matthews, R.A. A standardized method for the in vivo maintenance of Cryptocaryon irritans (Ciliophora) using the grey mullet Chelon labrosus as an experimental host. J. Parasitol. 1994, 80, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Merella, P.; Garippa, G. Metazoan parasites of grey mullets (Teleostea: Mugilidae) from the Mistras Lagoon (Sardinia, western Mediterranean). Sci. Mar. 2001, 65, 201–206. [Google Scholar] [CrossRef]
- Turan, C.; Caliskan, M.; Kucuktas, H. Phylogenetic relationships of nine mullet species (Mugilidae) in the Mediterranean Sea. Hydrobiologia 2005, 532, 45–51. [Google Scholar] [CrossRef]
- Delgado de Carvalho, C.; Marocco Corneta, C.; Sanches Uieda, V. Schooling behavior of Mugil curema (Perciformes: Mugilidae) in an estuary in southeastern Brazil. Neotrop. Ichthyol. 2007, 5, 81–83. [Google Scholar]
- Ferreira, M.; Moradas-Ferreira, P.; Reis-Henriques, M.A. Oxidative stress biomarkers in two resident species, mullet (Mugil cephalus) and flounder (Platichthys flesus), from a polluted site in River Douro Estuary, Portugal. Aquat. Toxicol. 2005, 69, 347–357. [Google Scholar] [CrossRef]
- Fossi, M.C.; Mauceri, A.; Leonzio, C.; Ancora, E.; Minniti, F.; Maisano, M.; Lo Cascio, P.; Ferrando, S.; Fasulo, S. Stress factors in the gills of Liza aurata (Perciformes, Mugilidae) living in polluted environments. Ital. J. Zool. 2005, 72, 285–292. [Google Scholar] [CrossRef]
- Orbea, A.; Ortiz-Zarragoitia, M.; Solé, M.; Porte, C.; Cajaraville, M.P. Antioxidant enzymes and peroxisome proliferation in relation to contaminant body burdens of PAHs and PCBs in bivalve molluscs, crabs, and fish from the Urdaibai and Plentzia estuaries (Bay of Biscay). Aquat. Toxicol. 2002, 58, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Maruya, K.A.; Francendese, L.; Manning, R.O. Residues of toxaphene decrease in estuarine fish after removal of contaminated sediments. Estuaries 2005, 28, 786–793. [Google Scholar] [CrossRef]
- Fishbase. Available online: http://www.fishbase.org (accessed on 25 March 2014).
- Durand, J.D.; Shen, K.N.; Chen, W.J.; Jamandre, B.W.; Blel, H.; Diop, K.; Nirchio, M.; Garcia de León, F.J.; Whitfield, A.K.; Chang, C.W.; et al. Systematics of the grey mullets (Teleostei: Mugiliformes: Mugilidae): Molecular phylogenetic evidence challenges two centuries of morphology-based taxonomy. Mol. Phylogenet. Evol. 2012, 64, 73–92. [Google Scholar]
- Nelson, J.S. Fishes of the World, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2006; p. 601. [Google Scholar]
- McDonough, C.J.; Roumillat, W.A.; Wenner, C.A. Sexual differentiation and gonad development in striped mullet (Mugil cephalus L.) from South Carolina estuaries. Fish. Bul. 2005, 103, 601–619. [Google Scholar]
- Rossi, A.R.; Crosettit, D.; Gornung, E.; Sola, L. Cytogenetic analysis of global populations of Mugil cephalus (striped mullet) by different staining techniques and fluorescent in situ hybridization. Heredity 1996, 76, 77–82. [Google Scholar] [CrossRef]
- Bruslé, J. Sexuality and biology of reproduction in grey mullets. In Aquaculture of the Grey Mullet; Oren, O.H., Ed.; Cambridge University Press: Cambridge, UK, 1981; pp. 99–154. [Google Scholar]
- Greeley, M.S., Jr.; Calder, R.D.; Wallace, A.R. Oocyte growth and development in the striped mullet, M. cephalus, during seasonal ovarian recrudescence: Relationship to fecundity and size at maturity. Fish. Bull. 1987, 85, 187–200. [Google Scholar]
- Render, J.H.; Thompson, B.A.; Allen, R.L. Reproductive development of striped mullet in Louisiana estuarine waters with notes on the applicability of reproductive assessment methods for isochronal species. Trans. Am. Fish. Soc. 1995, 124, 26–36. [Google Scholar] [CrossRef]
- Solomon, F.N.; Rammarine, I.W. Reproductive biology of white mullet, Mugil curema (Valeciennes) in the Southern Caribbean. Fish. Res. 2007, 88, 133–138. [Google Scholar] [CrossRef]
- Mićković, B.; Nikčević, M.; Hegediš, A.; Regner, S.; Gačić, Z.; Krpo-Ćetković, J. Mullet fry (Mugilidae) in coastal waters of Montenegro, their spatial distribution and migration phenology. Arch. Biol. Sci. Belgrade 2010, 62, 107–114. [Google Scholar]
- Yashuov, A.; Berner-Samsonov, E. Contribution to the knowledge of eggs and early larval stages of mullets (Mugilidae) along the Israeli coast. Badmigeh 1970, 22, 72–89. [Google Scholar]
- Crosetti, D.; Cataudella, S. Grey mullet culture. In World Animal Science 34B: Production of Aquatic Animals; Nash, C.E., Ed.; Elsevier B.V.: Burlington, MA, USA, 1995; pp. 271–288. [Google Scholar]
- Koutrakis, E.T.; Kokkinakis, A.K.; Eleftheriadis, E.A.; Argyropoulou, D. Seasonal changes in distribution and abundance of the fish fauna in the two estuarine systems of Strymonikos Gulf (Macedonia, Greece). Belgian J. Zool. 2000, 130, 41–48. [Google Scholar]
- Oliva-Paterna, F.J.; Andreu, A.; Miñano, P.A.; Verdiell, D.; Egea, A.; de Maya, J.A.; Ruiz- Navarro, A.; García-Alonso, J.; Fernández-Delgado, C.; Torralva, M. Y-O-Y fish species richness in the littoral shallows of the meso-saline coastal lagoon (Mar Menor, Mediterranean coast of the Iberian Peninsula). J. Appl. Ichthyol. 2006, 22, 235–237. [Google Scholar]
- Simier, M.; Blanc, L.; Aliaume, C.; Diouf, P.S.; Albaret, J.J. Spatial and temporal structure of fish assemblages in an “inverse estuary”, the Sine Saloum system (Senegal). Estuar. Coast. Shelf Sci. 2004, 59, 69–86. [Google Scholar] [CrossRef]
- Strydom, N.A. Occurrence of larval and early juveniles fishes in the surf zone adjacent to two intermittently open estuaries, South Africa. Environ. Biol. Fish. 2003, 66, 349–359. [Google Scholar] [CrossRef]
- Cardona, L. Non-competitive coexistence between Mediterranean grey mullet: Evidence from seasonal changes in food availability, niche breadth and trophic overlap. J. Fish Biol. 2001, 59, 729–744. [Google Scholar] [CrossRef]
- Hotos, G.N.; Vlahos, N. Salinity tolerance of Mugil cephalus and Chelon labrosus (Pisces: Mugilidae) fry in experimental conditions. Aquaculture 1998, 167, 329–338. [Google Scholar] [CrossRef]
- Almeida, P.R. Feeding ecology of Liza ramada (Risso, 1810) (Pisces, Mugilidae) in a south-western estuary of Portugal. Estuar. Coast. Shelf Sci. 2003, 57, 313–323. [Google Scholar] [CrossRef]
- Laffaille, P.; Feunteun, P.; Lefebvre, C.; Radreau, A.; Sagan, G.; Lefeuvre, J.C. Can thin-lipped mullet directly exploit the primary and detritic production of European macrotidal salt marshes? Estuar. Coast. Shelf Sci. 2002, 54, 729–736. [Google Scholar] [CrossRef]
- Zetina-Rejón, M.J.; Arreguín-Sánchez, F.; Chávez, E.A. Trophic structure and flows of energy in the Huizache-Caimanero lagoon complex on the Pacific coast of Mexico. Estuar. Coast. Shelf Sci. 2003, 57, 803–815. [Google Scholar]
- Cardona, L. Habitat selection by grey mullets (Osteichthyes: Mugilidae) in Mediterranean estuaries: The role of salinity. Sci. Mar. 2006, 70, 443–455. [Google Scholar] [CrossRef]
- Torriceli, P.; Tongiorgi, P.; Almansi, P. Migration of grey mullet fry into the Arno river: Seasonal appearance, daily activity, and feeding rhythms. Fish. Res. 1981, 1, 219–234. [Google Scholar] [CrossRef]
- Oren, O.H. Aquaculture of Grey Mullets; International Biological Programme No. 26; Cambridge University Press: Cambridge, UK, 1981; p. 507. [Google Scholar]
- Saleh, M.A. Cultured Aquatic Species Information Programme (CASIP). Mugil cephalus. FAO Fisheries and Aquaculture Department; FAO: Rome, Italy, 2006. Available online: http://www.fao.org/fishery/culturedspecies/Mugil_cephalus/en (accessed on 10 August 2014).
- Pastor, D.; Boix, J.; Fernández, V.; Albaigés, J. Bioaccumulation of organochlorinated contaminants in three estuarine fish species (Mullus barbatus, Mugil cephalus and Dicentrarcus labrax). Mar. Pollut. Bull. 1996, 32, 257–262. [Google Scholar] [CrossRef]
- Yilmaz, F. The comparison of heavy metal concentrations (Cd, Cu, Mn, Pb, and Zn) in tissues of three economically important fish (Anguilla anguilla, Mugil cephalus and Oreochromis niloticus) inhabiting Köycegiz Lake-Mugla (Turkey). Turk. J. Sci. Technol. 2009, 4, 7–15. [Google Scholar]
- Ben Ameur, W.; Trabelsi, S.; El Megdiche, Y.; Ben Hassine, S.; Barhoumi, B.; Hammami, B.; Eljarrat, E.; Barceló, D.; Ridha Driss, M. Concentration of polychlorinated biphenyls and organochlorine pesticides in mullet (Mugil cephalus) and sea bass (Dicentrarchus labrax) from Bizerte Lagoon (Northern Tunisia). Chemosphere 2013, 90, 2372–2380. [Google Scholar]
- Türkmen, A.; Türkmen, M.; Tepe, Y.; Mazlum, Y.; Oymael, S. Metal concentrations in blue crab (Callinectes sapidus) and mullet (Mugil cephalus) in Iskenderun Bay, Northern East Mediterranean, Turkey. Bull. Environ. Contam. Toxicol. 2006, 77, 186–193. [Google Scholar]
- Ben Ameur, W.; de Lapuente, J.; El Megdiche, Y.; Barhoumi, B.; Trabelsi, S.; Camps, L.; Serret, J.; Ramos-López, D.; Gonzalez-Linares, J.; Ridha Driss, M.; et al. Oxidative stress, genotoxicity and histopathology biomarker responses in mullet (Mugil cephalus) and sea bass (Dicentrarchus labrax) liver from Bizerte Lagoon (Tunisia). Mar. Pollut. Bull. 2012, 64, 241–251. [Google Scholar]
- Boglione, C.; Costa, C.; Giganti, M.; Cecchetti, M.; di Dato, P.; Scardi, M.; Cataudella, S. Biological monitoring of wild thicklip grey mullet (Chelon labrosus), golden grey mullet (Liza aurata), thinlip mullet (Liza ramada) and flathead mullet (Mugil cephalus) (Pisces: Mugilidae) from different Adriatic sites: Meristic counts and skeletal anomalies. Ecol. Indic. 2006, 6, 712–732. [Google Scholar] [CrossRef]
- An, L.; Hu, J.; Yang, M.; Zheng, B.; Wei, A.; Shang, J.; Zhao, X. CYP1A mRNA expression in redeye mullets (Liza haematocheila) from Bohai Bay, China. Mar. Pollut. Bull. 2011, 62, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Ergene, S.; Çavaş, T.; Çelik, A.; Köleli, N.; Kaya, F.; Karahan, A. Monitoring of nuclear abnormalities in peripheral erythrocytes of three fish species from the Goksu Delta (Turkey): Genotoxic damage in relation to water pollution. Ecotoxicology 2007, 16, 385–391. [Google Scholar]
- Hafez, A.M. Mugil cephalus genome: A sensitive monitor for genotoxicity and cytotoxicity in aquatic environment. Austr. J. Basic Appl. Sci. 2009, 3, 2176–2187. [Google Scholar]
- Aoki, J.; Nagae, M.; Takao, J.; Hara, A.; Lee, Y.D.; Yeo, I.K.; Lim, B.S.; Park, C.B.; Soyano, K. Survey of contamination of estrogenic chemicals in Japanese and Korean coastal waters using the wild grey mullet (Mugil cephalus). Sci. Total Environ. 2010, 408, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, N.; Matsubara, T.; Fujita, T.; Sullivan, C.V.; Hara, A. Multiple piscine vitellogenins: Biomarkers of fish exposure to estrogenic endocrine disruptors in aquatic environments. Mar. Biol. 2006, 149, 35–47. [Google Scholar] [CrossRef]
- Asturiano, J.F.; Romaguera, F.; Aragón, P.; Atienza, J.; Puchades, R.; Maquieira, A. Sandwich immunoassay for determination of vitellogenin in golden grey mullet (Liza aurata) serum as a field exposure biomarker. Anal. Bioanal. Chem. 2005, 381, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Fujita, T.; Hiramatsu, N.; Shimizu, M.; Sawaguchi, S.; Matsubara, T.; Kagawa, H.; Nagae, M.; Sullivan, C.V.; Hara, A. Egg yolk proteins in gray mullet (Mugil cephalus): Purification and classification of multiple lipovitellins and other vitellogenin-derived yolk proteins and molecular cloning of the parent vitellogenins genes. J. Exp. Zool. 2007, 307A, 324–341. [Google Scholar] [CrossRef]
- An, L.; Hu, J.; Zhang, Z.; Yang, M. Quantitative real-time RT-PCR for determination of vitellogenin mRNA in so-iuy mullet (Mugil soiuy). Anal. Bioanal. Chem. 2006, 386, 1995–2001. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.-N.; Zhao, Y.-B.; Peng, H.; Huang, C.; Chen, S.-L.; Hu, J.-Y. Comparison of ERα-mediated estrogenic binding activity between So-iuy mullet (Mugil soiuy) and medaka. China Environ. Sci. 2010, 11, 1490–1495. [Google Scholar]
- Soyano, K.; Aoki, J.-Y.; Itashiki, Y.; Park, C.-B.; Nagae, M.; Takao, Y.; Lee, Y.-D.; Yeo, I.-K.; Zhong, J. Contaminations by endocrine disrupting chemicals in coastal waters of the East China Sea. In Coastal Environmental and Ecosystem Issues of the East China Sea; Ishimatsu, A., Lie, H.-J., Eds.; TERRAPUB and Nagasaki University: Nagasaki, Japan, 2010; pp. 215–226. [Google Scholar]
- Bizarro, C.; Ros, O.; Vallejo, A.; Prieto, A.; Etxebarria, N.; Cajaraville, M.P.; Ortiz-Zarragoitia, M. Intersex condition and molecular markers of endocrine disruption in relation with burdens of emerging pollutants in thicklip grey mullets (Chelon labrosus) from Basque estuaries (South-East Bay of Biscay). Mar. Environ. Res. 2014, 96, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Diaz de Cerio, O.; Rojo-Bartolomé, I.; Bizarro, C.; Ortiz-Zarragoitia, M.; Cancio, I. 5S rRNA and accompanying proteins in gonads: Powerful markers to identify sex and reproductive endocrine disruption in fish. Environ. Sci. Technol. 2012, 46, 7763–7771. [Google Scholar]
- Bayhan, B.; Acarli, D. Hermaphrodite thinlip mullet Liza ramada (Risso, 1810) (Teleostei: Mugilidae) from Homa Lagoon (Izmir Bay-Aegean Sea). Aquac. Res. 2006, 37, 1050–1052. [Google Scholar] [CrossRef]
- Corsi, I.; Mariottini, M.; Sensini, C.; Lancini, N.; Focardi, S. Cytochrome P450, acetylcholinesterase and gonadal histology for evaluating contaminant exposure levels in fishes from a highly eutrophic brackish ecosystem: The Orbetello Lagoon, Italy. Mar. Pollut. Bull. 2003, 46, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Barucca, M.; Canapa, A.; Olmo, E.; Regoli, F. Analysis of vitellogenin gene induction as a valuable biomarker of estrogenic exposure in various Mediterranean fish species. Environ. Res. 2006, 101, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Canapa, A.; Barucca, M.; Celeste, A.; Olmo, E.; Regoli, F. Preliminary investigations on vitellogenin m-RNA induction in some bioindicator Mediterranean fish species. Mar. Environ. Res. 2002, 54, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Moe, M.A., Jr. Hermaphroditism in Mullet, Mugil cephalus Linnaeus. Q. J. Florida Acad. Sci. 1966, 29, 111–116. [Google Scholar]
- Bizarro, C.; Aragón, P.; Maquieira, A.; Cajaraville, M.P.; Ortiz-Zarragoitia, M. Characterization by histological and molecular tools of the reproductive cycle and endocrine disruption effects on a thicklip grey mullet (Chelon labrosus) population from the South Bay of Biscay. Comp. Biochem. Physiol. Part A 2012, 163, S33. [Google Scholar] [CrossRef]
- Jobling, S.; Nolan, M.; Tyler, C.R.; Brighty, G.; Sumpter, J.P. Widespread sexual disruption in wild fish. Environ. Sci. Technol. 1998, 32, 2498–2506. [Google Scholar] [CrossRef]
- Nocillado, J.N.; Elizur, A.; Avitan, A.; Carrick, F.; Levavi-Sivan, B. Cytochrome P450 aromatase in grey mullet: cDNA and promoter isolation; brain, pituitary and ovarian expression during puberty. Mol. Cell. Endocrinol. 2007, 263, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Lan, S.C.; Pan, B.S. Feed administration of estradiol-17-beta stimulates female differentiation in juvenile grey mullet Mugil cephalus. Zool. Stud. 1995, 34, 257–264. [Google Scholar]
- Aoki, J.-Y.; Hatsuyama, A.; Hiramatsu, N.; Soyano, K. Effects of ethynylestradiol on vitellogenin synthesis and sex differentiation in juvenile grey mullet (Mugil cephalus) persist after long-term exposure to a clean environment. Comp. Biochem. Physiol. Part C 2011, 154, 346–352. [Google Scholar]
- Chang, C.F.; Hung, C.Y.; Chiang, M.C.; Lan, S.C. The concentrations of plasma sex steroids and gonadal aromatase during controlled sex differentiation in grey mullet, Mugil cephalus. Aquaculture 1999, 177, 37–45. [Google Scholar] [CrossRef]
- Meiri-Ashkenazi, I.; Solomonovich, R.; Rosenfeld, H. Long term effects of masculinizing treatments on the reproductive characteristics of grey mullet (Mugil cephalus). Indian J. Sci. Technol. 2011, 4, 300–301. [Google Scholar]
- Baek, H.J.; Hwang, I.J.; Lee, Y.D.; Kim, H.B. Effects of nonylphenol and 3,3′,4,4′,5-pentachlorobiphenil on in vitro oocyte steroidogenesis in redlip mullet, Chelon haematochelius. Anim. Cells Syst. 2011, 15, 189–196. [Google Scholar] [CrossRef]
- Bilbao, E.; Raingeard, D.; Diaz de Cerio, O.; Ortiz-Zarragoitia, M.; Ruiz, P.; Izagirre, U.; Orbea, A.; Marigómez, I.; Cajaraville, M.P.; Cancio, I. Effects of exposure to Prestige-like heavy fuel oil and to perfluorooctane sulfonate on conventional biomarkers and target gene transcription in the thicklip grey mullet Chelon labrosus. Aquat. Toxicol. 2010, 98, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Cionna, C.; Maradonna, F.; Olivotto, I.; Pizzonia, G.; Carnevali, O. Effects of nonylphenol on juveniles and adults in the grey mullet, Liza aurata. Reprod. Toxicol. 2006, 22, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Aizen, J.; Meiri, I.; Tzchori, I.; Levavi-Sivan, B.; Rosenfeld, H. Enhancing spawning in the grey mullet (Mugil cephalus) by removal of dopaminergic inhibition. Gen. Comp. Endocrinol. 2005, 142, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kobayashi, K.; Nishimura, T.; Higashijima, S.; Tanaka, M. Identification of germline stem cells in the ovary of the teleost medaka. Science 2010, 328, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G.; Ichikawa, M.; Hayashi, M.; Iwasaki, Y.; Miwa, M.; Shikina, S.; Okutsu, T. Sexual plasticity of ovarian germ cells in rainbow trout. Development 2010, 137, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G.; Okutsu, T.; Morita, T.; Terasawa, M.; Yazawa, R.; Takeuchi, Y. Biological characteristics of fish germ cells and their application to developmental biotechnology. Reprod. Domest. Anim. 2012, 47, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Selman, K.; Wallace, R.A.; Sarka, A.; Qi, X. Stages of oocyte development in the zebrafish Brachydanio rerio. J. Morphol. 1993, 218, 203–224. [Google Scholar] [CrossRef]
- Murua, H.; Kraus, G.; Saborido-Rey, F.; Witthames, P.R.; Thorsen, A.; Junquera, S. Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. J. Northw. Atl. Fish. Sci. 2003, 33, 33–54. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdá, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol. 2010, 16, 367–389. [Google Scholar] [CrossRef]
- Mazabraud, A.; Wegnez, M.; Denis, H. Biochemical research on oogenesis: RNA accumulation in the oocytes of teleosts. Dev. Biol. 1975, 44, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Denis, H.; Wegnez, M. Biochemical research on oogenesis: Oocytes and liver cells of the teleost fish Tinca tinca contain different kinds of 5S RNA. Dev. Biol. 1977, 59, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Mittelholzer, C.; Andersson, E.; Consten, D.; Hirai, T.; Nagahama, Y.; Norberg, B. 20 beta-hydroxysteroid dehydrogenase and CYP19A1 are differentially expressed during maturation in Atlantic cod (Gadus morhua). J. Mol. Endocrinol. 2007, 39, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Kroupova, H.; Trubiroha, A.; Wuertz, S.; Kloas, W. Stage-dependent differences in RNA composition and content affect the outcome of expression profiling in roach (Rutilus rutilus) ovary. Comp. Biochem. Physiol. Part A 2011, 159, 141–149. [Google Scholar] [CrossRef]
- Penberthy, W.T.; Griffin, D.; Hall, R.K.; Taylor, W.L. The Xenopus B2 factor involved in TFIIIA gene regulation is closely related to Sp1 and interacts in a complex with USF. Gene 2003, 305, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Thiry, M.; Poncin, P. Morphological changes of the nucleolus during oogenesis in oviparous teleost fish, Barbus barbus (L.). J. Struct. Biol. 2005, 152, 1–13. [Google Scholar]
- Campo, D.; Machado-Schiaffino, G.; Horreo, J.L.; Garcia-Vazquez, E. Molecular organization and evolution of 5S rDNA in the genus Merluccius and their phylogenetic implications. J. Mol. Evol. 2009, 68, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Gornung, E.; Colangelo, P.; Annesi, F. 5S ribosomal RNA genes in six species of Mediterranean grey mullets: Genomic organization and phylogenetic inference. Genome 2007, 50, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Szymański, M.; Barciszewska, M.Z.; Erdmann, V.A.; Barciszewski, J. 5S rRNA: Structure and interactions. Biochem. J. 2003, 371, 641–651. [Google Scholar]
- Allison, L.A.; North, M.T.; Neville, L.A. Differential binding of oocyte-type and somatic-type 5S rRNA to TFIIIA and ribosomal protein L5 in Xenopus oocytes: Specialization for storage versus mobilization. Dev. Biol. 1995, 168, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Galetti, P.M., Jr. Two 5S rDNA arrays in neotropical fish species: Is it a general rule for fishes? Genetica 2001, 111, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Pinhal, D.; Yoshimura, T.S.; Araki, C.S.; Martins, C. The 5S rDNA family evolves through concerted and birth-and-death evolution in fish genomes: An example from freshwater stingrays. BMC Evol. Biol. 2011, 11, 151:1–151:14. [Google Scholar]
- He, W.; Qin, Q.; Liu, S.; Li, T.; Wang, J.; Xiao, J.; Xie, L.; Zhang, C.; Liu, Y. Organization and variation analysis of 5S rDNA in different ploidy-level hybrids of red crucian carp × topmouth culter. PLoS One 2012, 7, e38976:1–e38976:12. [Google Scholar]
- Sreenivasan, R.; Cai, M.; Bartfai, R.; Wang, X.; Christoffels, A.; Orban, L. Transcriptomic analyses reveal novel genes with sexually dimorphic expression in the zebrafish gonad and brain. PLoS One 2008, 3, e1791:1–e1791:16. [Google Scholar]
- Layat, E.; Probst, A.V.; Tourmente, S. Structure, function and regulation of Transcription Factor IIIA: From Xenopus to Arabidopsis. Biochim. Biophys. Acta 2013, 1829, 274–282. [Google Scholar] [CrossRef]
- Ogilvie, M.K.; Hanas, J.S. Molecular biology of vertebrate transcription factor IIIA: Cloning and characterization of TFIIIA from channel catfish oocytes. Gene 1997, 203, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, A. Systematic identification of genes expressed during early oogenesis in medaka. Mol. Reprod. Dev. 2000, 55, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Okamoto, G.; Hirata, T.; Shinomiya, A.; Kobayashi, T.; Kubo, Y.; Hori, H.; Kanamori, A. Transgenic medaka enables easy oocytes detection in live fish. Mol. Reprod. Dev. 2009, 76, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Gen, K.; Yamaguchi, S.; Okuzawa, K.; Kagawa, H.; Alam, M.S. Novel expression of importin alpha homologue in marine teleost, Pagrus major. Comp. Biochem. Physiol. Part B 2008, 151, 420–427. [Google Scholar] [CrossRef]
- Holt, J.E.; Ly-Huynh, J.D.; Efthymiadis, A.; Hime, G.R.; Loveland, K.L.; Jans, D.A. Regulation of nuclear import during differentiation; the IMP alpha gene family and spermatogenesis. Curr. Genomics 2007, 8, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Wischnewski, J.; Rudt, F.; Pieler, T. Signals and receptors for the nuclear transport of TFIIIA in Xenopus oocytes. Eur. J. Cell Biol. 2004, 83, 55–66. [Google Scholar] [CrossRef] [PubMed]
- DeJong, J. Basic mechanisms for the control of germ cell gene expression. Gene 2006, 366, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.; Penberthy, W.T.; Lum, H.; Stein, R.W.; Taylor, W.L. Isolation of the B3 transcription factor of the Xenopus TFIIIA gene. Gene 2003, 313, 179–188. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ortiz-Zarragoitia, M.; Bizarro, C.; Rojo-Bartolomé, I.; De Cerio, O.D.; Cajaraville, M.P.; Cancio, I. Mugilid Fish Are Sentinels of Exposure to Endocrine Disrupting Compounds in Coastal and Estuarine Environments. Mar. Drugs 2014, 12, 4756-4782. https://doi.org/10.3390/md12094756
Ortiz-Zarragoitia M, Bizarro C, Rojo-Bartolomé I, De Cerio OD, Cajaraville MP, Cancio I. Mugilid Fish Are Sentinels of Exposure to Endocrine Disrupting Compounds in Coastal and Estuarine Environments. Marine Drugs. 2014; 12(9):4756-4782. https://doi.org/10.3390/md12094756
Chicago/Turabian StyleOrtiz-Zarragoitia, Maren, Cristina Bizarro, Iratxe Rojo-Bartolomé, Oihane Diaz De Cerio, Miren P. Cajaraville, and Ibon Cancio. 2014. "Mugilid Fish Are Sentinels of Exposure to Endocrine Disrupting Compounds in Coastal and Estuarine Environments" Marine Drugs 12, no. 9: 4756-4782. https://doi.org/10.3390/md12094756
APA StyleOrtiz-Zarragoitia, M., Bizarro, C., Rojo-Bartolomé, I., De Cerio, O. D., Cajaraville, M. P., & Cancio, I. (2014). Mugilid Fish Are Sentinels of Exposure to Endocrine Disrupting Compounds in Coastal and Estuarine Environments. Marine Drugs, 12(9), 4756-4782. https://doi.org/10.3390/md12094756