Abstract
Brevenal is a ladder frame polyether produced by the dinoflagellate Karenia brevis. This organism is also responsible for the production of the neurotoxic compounds known as brevetoxins. Ingestion or inhalation of the brevetoxins leads to adverse effects such as gastrointestinal maladies and bronchoconstriction. Brevenal shows antagonistic behavior to the brevetoxins and shows beneficial attributes when administered alone. For example, in an asthmatic sheep model, brevenal has been shown to increase tracheal mucosal velocity, an attribute which has led to its development as a potential treatment for Cystic Fibrosis. The mechanism of action of brevenal is poorly understood and the exact binding site has not been elucidated. In an attempt to further understand the mechanism of action of brevenal and potentially develop a second generation drug candidate, a series of brevenal derivatives were prepared through modification of the aldehyde moiety. These derivatives include aliphatic, aromatic and heteroaromatic hydrazide derivatives. The brevenal derivatives were tested using in vitro synaptosome binding assays to determine the ability of the compounds to displace brevetoxin and brevenal from their native receptors. A sheep inhalation model was used to determine if instillation of the brevenal derivatives resulted in bronchoconstriction. Only small modifications were tolerated, with larger moieties leading to loss of affinity for the brevenal receptor and bronchoconstriction in the sheep model.
1. Introduction
The marine dinoflagellate Karenia brevis is associated with the phenomenon known as Florida Red Tide and produces a variety of ladder frame polyether compounds (LFPs), including the highly neurotoxic brevetoxins (1, 2) (Figure 1) [1]. It has long been established that the brevetoxins elicit their effects through activation of site five of voltage gated sodium channels [2,3]. Activation of these sodium channels leads to neurological effects including respiratory difficulty, gastrointestinal maladies and sensation of temperature reversal.
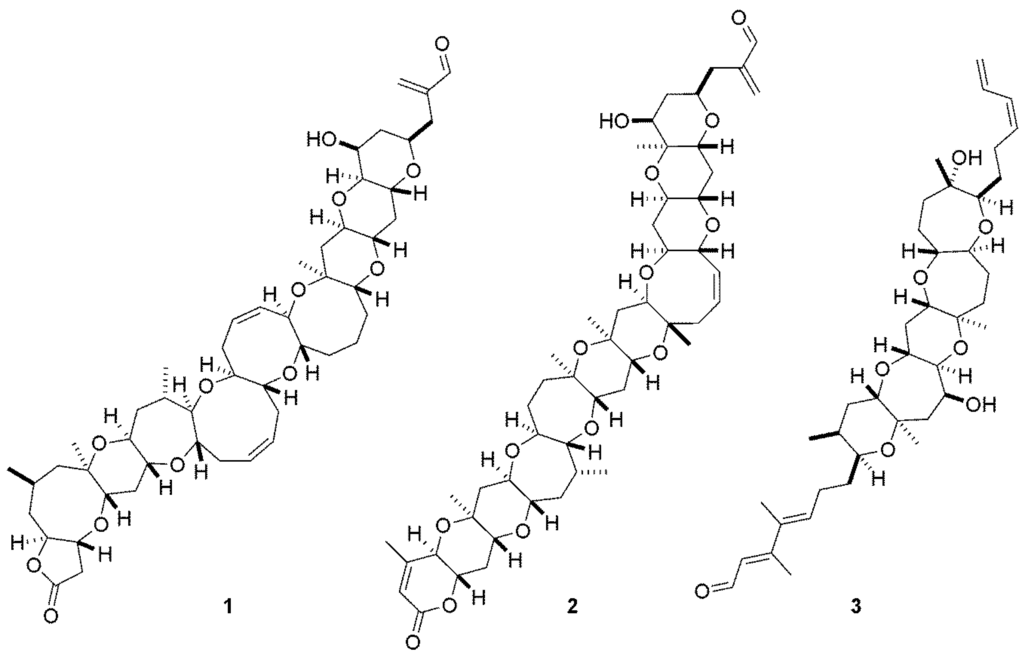
Figure 1.
Chemical structures of brevetoxins PbTx-1 (1) and PbTx-2 (2) and the antagonist, brevenal (3).
In addition to these toxins, K. brevis produces brevenal (3) (Figure 1), a compound shown to possess antagonistic activity to the brevetoxins and other site five activators such as ciguatoxin [4,5,6,7]. In the sheep model of asthma, brevenal was able to both inhibit and reverse brevetoxin induced bronchoconstriction [8].
Furthermore, when administered alone, brevenal was found to increase tracheal mucosal velocity. This finding mirrored that of clinical drugs used in the treatment of cystic fibrosis and spurred the development of brevenal as a potential treatment for this disease. Recently, work completed in our laboratory has shown that brevenal binds to a site that is distinctly different, but linked to site five of voltage gated sodium channels (VGSCs) [9]. In this work, it was shown that brevenal was able to displace brevetoxin (2) from the site five on VSGCs, but brevetoxin was unable to displace brevenal from its binding site in reciprocal studies, leading to the conclusion that brevenal is an allostere for brevetoxin.
In an attempt to further understand the mechanism of action of brevenal and potentially develop a second generation drug candidate, a series of brevenal derivatives were prepared through modification of the aldehyde moiety. These derivatives include aliphatic, aromatic and heteroaromatic derivatives. The brevenal derivatives were tested in in vitro synaptosome binding assays to determine the ability of the semi-synthetic compounds to displace brevetoxins and brevenal from their native receptors.
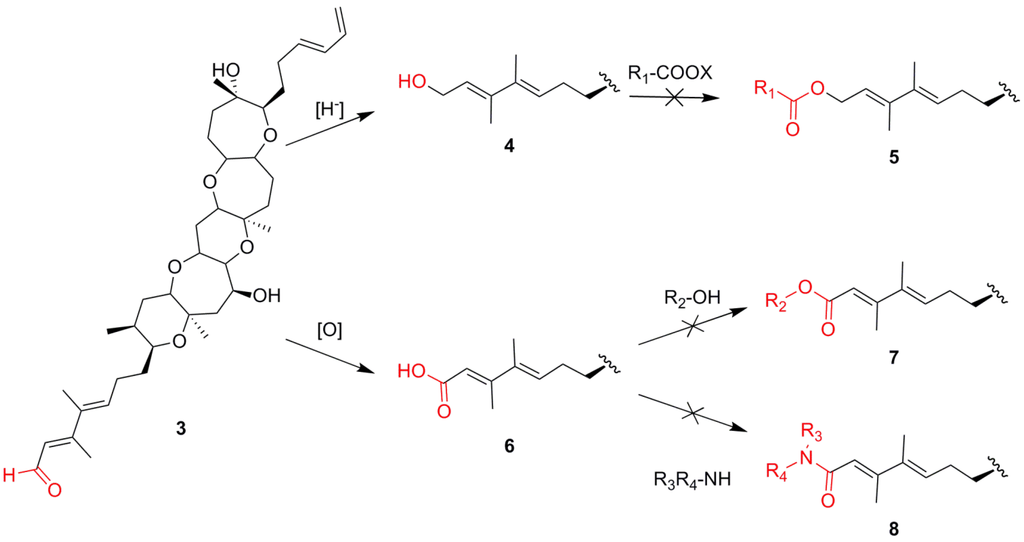
Scheme 1.
Synthetic strategy in an attempt to prepare ester and amide derivatives of brevenal.
Brevenal is more or less devoid of functional “handles” for SAR study, with the exception of the aldehyde moiety, which is the only functional group that can be readily modified and utilized from an SAR point of view. Various transformations can be performed on aldehyde functionalities including reduction, oxidation and reductive aminations to produce the alcohol, acid and amine derivatives, respectively (Scheme 1). All three of these aldehyde products can, potentially, be further derivatized to generate a wide range of compounds for SAR studies, and all three of these transformations were attempted. Reduction of the brevenal to give the alcohol (4) was achieved in high yield. However, attempts to prepare ester derivatives (5) were disappointing, as it was found that the alcohol (4) was unreactive towards any acids or acid derivatives including acid chlorides and anhydrides, even under extended reaction times and elevated temperatures. Oxidation of the aldehyde to the acid (6) required prolonged reaction times and was poor yielding. Even more frustrating, attempts to synthesize esters (7) or amides (8) were unsuccessful as the acid also proved unreactive and was not useful for SAR studies. Perhaps the most utilized modification of an aldehyde group is to perform reductive amination reactions to create amines. Previous attempts within our laboratory to perform this transformation with brevenal had proven unsuccessful.
However, during reactions to prepare fluorescently labeled LFPs for development of a fluorescence based receptor binding assay [10], attachment of fluorescent hydrazides was found to be achievable in a rapid and high yielding manner (Scheme 2). Somewhat unexpectedly, the Schiff base intermediate (9) produced by the reaction of the aldehyde with the hydrazide was highly stable and was not susceptible to reduction with various borohydride reducing reagents such as sodium borohydride, cyanoborohydride, etc. to give the hydrazide derivatives (10). This stability likely arises from conjugation with the butadiene moiety. Indeed, cell uptake studies of fluorescent hydrazone brevenal conjugates indicate that the complexes stay intact intracellularly for at least 48 hours. Due to brevenal’s limited reactivity, a series of substituted hydrazide “Schiff base” derivatives (9) were prepared and studied for their ability to displace brevenal and brevetoxin from their respective receptors (Table 1, Table 2 and Table 3).
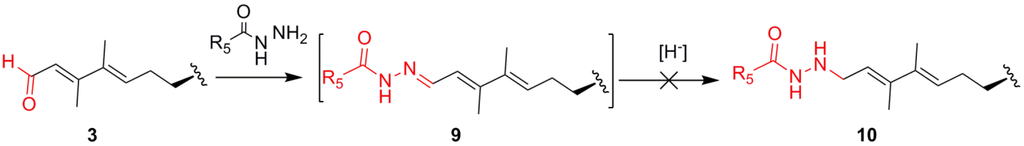
Scheme 2.
Attempted reductive amination type synthesis of hydrazide derivatives (10) led to stable Schiff base intermediates (9).

Table 1.
Aliphatic and small unsubstituted cyclic brevenal derivatives.
| Compound | R | Ki(brevenal) (nM) a | Ki(brevetoxin) (nM) a | Compound | R | Ki(brevenal) (nM) a | Ki(brevetoxin) (nM) a | |
|---|---|---|---|---|---|---|---|---|
| 3 | Brevenal | 137 ± 16 | 1133 ± 153 | 14 |  | 486 ± 85 | 300 ± 67 | |
| 11 | 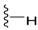 | 114 ± 61 | DNB b | 15 | 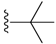 | 1195 ± 263 | 162 ± 22 | |
| 12 | 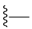 | 995 ± 292 | 496 ± 229 | 16 | 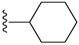 | 534 ± 31 | DNB b | |
| 13 | 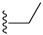 | 328 ± 59 | 163 ± 48 |
a Mean and SEM of at least 3 runs; b Does not bind.

Table 2.
Aromatic and heteroaromatic derivatives of brevenal.
| Compound | R | Ki(brevenal) (nM) a | Ki(brevetoxin) (nM) a | Compound | R | Ki(brevenal) (nM) a | Ki(brevetoxin) (nM) a | |
|---|---|---|---|---|---|---|---|---|
| 3 | Brevenal | 137 ± 16 | 1133 ± 153 | 20 | 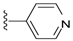 | 620 ± 239 | 75 ± 38 | |
| 17 | 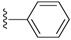 | 584 ± 104 | DNB b | 21 | 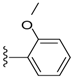 | 570 ± 33 | DNB b | |
| 18 | 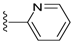 | 727 ± 149 | DNB b | 22 | 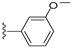 | 1037 ± 391 | 8 ± 3 | |
| 19 | 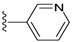 | 412 ± 148 | >5000 | 23 | 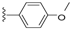 | 837 ± 163 | DNB b |
a Mean and SEM of at least 3 runs; b Does not bind.

Table 3.
Meta-substituted phenyl derivatives of brevenal.
| Compound | R | Ki(brevenal) (nM) a | Ki(brevetoxin) (nM) a | Compound | R | Ki(brevenal) (nM) a | Ki(brevetoxin) (nM) a | |
|---|---|---|---|---|---|---|---|---|
| 3 | Brevenal | 137 ± 16 | 1133 ± 153 | 29 | 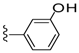 | 692 ± 135 | >5000 | |
| 22 | 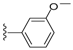 | 1037 ± 391 | 8 ± 3 | 30 | 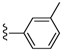 | 1018 ± 79 | 732 ± 287 | |
| 24 | 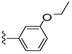 | 639 ± 131 | DNB b | 31 | 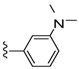 | 1745 ± 421 | DNB b | |
| 25 | 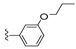 | 525 ± 133 | DNBb | 32 | 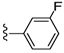 | 455 ± 155 | 440 ± 108 | |
| 26 | 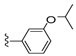 | 1081 ± 167 | 479 ± 140 | 33 | 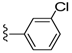 | 884 ± 445 | 109 ± 79 | |
| 27 | 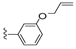 | 500 ± 142 | 253 ± 98 | 34 | 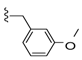 | 331 ± 97 | 23 ± 9 | |
| 28 | 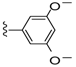 | 551 ± 224 | >5000 | 35 | 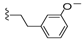 | 231 ± 37 | 66 ± 39 |
a Mean and SEM of at least 3 runs; b Does not bind.
2. Results and Discussion
The first set of derivatives comprised of small aliphatic and unsubstituted cyclic substituents on the hydrazide (11–16) (Table 1). As can be seen in Table 1, addition of a formyl hydrazide moiety (11) is well tolerated and appears to be equipotent in affinity for the brevenal receptor compared to brevenal, while concomitantly being deleterious to brevetoxin displacement. Interestingly, introduction of a methyl substituent (12) led to an almost seven-fold decrease in affinity for the brevenal receptor. Increasing the size of the substituent (12–16) did not appear to regain any of the affinity for the brevenal receptor. However, small aliphatic substituents such as ethyl (13) or t-butyl groups (15) allow displacement of brevetoxin from its receptor in almost seven-fold lower concentrations compared to brevenal.
The second set of derivatives looked at the inclusion of heterocyclic and electron rich aromatic substituents (17–23) (Table 2). From this data it can be seen that all modifications lead to decreased affinity for the brevenal receptor. However, when tested in the brevetoxin assay, the 3-methoxyphenyl analog (22) showed a more than 140-fold increase in potency for displacing the toxin compared to brevenal. This was somewhat confounding, given that the compound also suffered a seven-fold decrease in affinity for the brevenal receptor.
Based on the results obtained for the series of compounds described in Table 2, a series of compounds with various substituents at the 3-position of the phenyl group was prepared (Table 3). Deviation from the methoxy substituted compound (22) with other ether compounds (24–27) led to compounds with unchanged or marginally better affinity for the brevenal receptor. Similarly, removal of the methyl group to give the phenol (29) also led to marginally higher affinity for the brevenal receptor. The effects on brevetoxin displacement were much more impacted by these modifications. None of the oxygen substituted compounds were able to displace brevetoxin at concentrations comparable to the lead compound 22. Similarly, replacement of the methoxy substituent with a methyl (30), dimethylamino (31) or fluorine (32) group again led to little to no improvement in affinity for the brevenal receptor and was detrimental to brevetoxin displacement compared to 22. Substitution of the methoxy group with a chloro moiety (33) led to a slight increase in affinity for the brevenal receptor, while retaining a modest ability to displace brevetoxin. Extension of the linker between the hydrazide carbonyl and the 3-methoxyphenyl substituent from 0 carbons (22) to 1 carbon (34) led to a significant increase in affinity for the brevenal receptor, while simultaneously reducing the potency for brevetoxin displacement. Interestingly, further extension of the linker from 1 carbon (34) to 2 carbons (35) continued this trend with improved binding to the brevenal receptor with concomitant loss of ability to displace brevetoxin.
From the SAR generated for the brevenal receptor, it appears that most modifications of the aldehyde portion of brevenal are not well tolerated. Indeed, any substitution larger than a formyl hydrazide substituent leads to decreased affinity for the brevenal receptor compared to brevenal. This indicates that the receptor for brevenal is highly specialized to this ladder frame polyether. Interestingly, inclusion of “remote” substituents such as the 3-methoxyphenylethyl hydrazide (35) led to a compound with minor loss in affinity for the brevenal receptor. Possible explanations for this retention in affinity for the brevenal receptor could be that either the methoxyphenyl group is extended beyond areas of the brevenal receptor that lead to negative interactions seen with the shorter hydrazides or that any adverse effects are being negated by creation of additional interactions.
The SAR for displacement of brevetoxin appears to be very different in nature to that exhibited for the brevenal receptor. For instance, the data for a compound such as the 3-methoxyphenylhydrazide 22 is confounding in that it is capable of displacing brevetoxin at very low concentrations (8 nM), but exhibits low affinity for the brevenal receptor (1 μM). If the displacement of brevetoxin is through allosteric interactions, then the compound should exhibit higher affinity for its native receptor, as in the case of brevenal. One explanation for this might be that the modifications made to brevenal enable the new molecule to bind at site five of voltage gated sodium channels, thereby directly competing with brevetoxin for its binding site. This hypothesis is somewhat supported by bronchoconstriction studies in sheep. This assay is used to determine if compounds have pulmonary irritant effects when inhaled [9]. The irritant effects, as evidenced by an increase in pulmonary resistance (RL), are for the most part short-lived, but are important because if a compound causes bronchoconstriction it is unlikely that it could be developed as an inhaled drug. To evaluate the current compounds, sheep were given 20 breaths of compounds 11, 19, 22 or 35 at doses of 10, 30 or 100 pg/mL. Compound 11, with similar binding characteristics as brevenal, produced only a minimal 26% ± 2% (mean ± SD, n = 3) increase in RL at 100 pg/mL. Further increases in concentration up to 50 μg /mL did not alter this response (22% ± 3%, n = 3). These results are consistent with those seen for brevenal. This finding suggests that the formyl hydrazide derivative is binding to the receptor in a similar manner as brevenal. However, for compounds 19, 22 and 35, significant increases in RL were observed compared to compound 11 at the 100 pg/mL dose (RL = 81% ± 4%, 96% ± 7% and 83% ± 4% respectively, mean ± SD, n = 3). While not as severe as results observed for brevetoxin (RL = 221% ± 21% at 10 pg/mL) [9], these compounds are significantly more bronchoconstrictive than brevenal (no effects observed up to 50 μg/mL) [11]. Thus, based on these results, only compound 11 would be a candidate for development into an inhaled drug. The bronchoconstriction findings are highly consistent with the receptor affinity data generated for compound 22. The results for compound 35 are less clear, as this compound retains fairly good affinity (231 nM) for the brevenal receptor compared to brevenal (137 nM). However, the fact that it still has a roughly two-fold higher affinity for the brevetoxin receptor (66 nM) may lead to the negative effects associated with brevetoxin receptor activation overriding any positive effects elicited by brevenal receptor activation. Even more puzzling though is the bronchoconstriction caused by compound 19. Since this compound appears to have higher affinity for the brevenal receptor (412 nM) than brevetoxin displacement (1001 nM), it would be expected to have an opposite result to that observed for compound 35. At this time, we can offer no explanation for this result.
3. Experimental Section
3.1. Brevenal Isolation
Brevenal was purified from cultures of Karenia brevis (Wilson strain) in similar fashion as previously described [4,5]. Briefly, K. brevis cultures were homogenized in the presence of chloroform. The chloroform layer was collected and evaporated under reduced pressure. The residue was partitioned between petroleum ether and a methanol-water mixture to remove pigments and lipid debris. The methanol-water fraction was evaporated under reduced pressure. The crude product was fractionated using counter-current chromatography using a water, methanol and acetonitrile mixture as the stationary phase, and a pentane-methylene chloride mixture as the mobile (descending) phase. The brevenal rich fractions were combined and evaporated before being subjected to a second counter current chromatography purification using a mixture of methanol, water and glyme as the stationary phase and xylene and pentane as the mobile (ascending) phase. The brevenal rich fractions were evaporated. The crude product was subjected to HPLC (Phenomonex phenyl-hexyl column: Isocratic elution, 96:4 methanol:water with detection at 215 and 290 nm). A final purification of the brevenal was achieved through HPLC (Dynamax C18; isocratic elution, 89:11 methanol:water with detection at 215 and 290 nm).
3.2. Hydrazide Intermediates
Hydrazides used in the synthesis of the following brevenal analogs were purchased from the following vendors and used as received: Hydrazides for compounds 11, 21, 29, 30 and intermediates used in the synthesis of compounds 28, 31 and 33 were purchased from Alfa Aesar (Ward Hill, MA, USA); hydrazides for compounds 12, 13, 17, 19, 22, 32 and intermediates used in the synthesis of compounds 34 and 35 were purchased from Sigma Aldrich, (St. Louis, MO, USA); hydrazides for compounds 15 and 20 were purchased from Acros, (Geel, Belgium); hydrazides for compounds 14, 16 and 23 were purchased from Oakwood Chemicals, (Columbia, SC, USA); hydrazide for compound 18 and the hydroxybenzoic acid intermediate used in the synthesis of compounds 24–27 were purchased from TCI America (Portland, OR, USA).
Hydrazides used in the synthesis of compounds 24–27 were prepared from 3-hydroxybenzoic acid (36) in three steps as shown in Scheme 3.
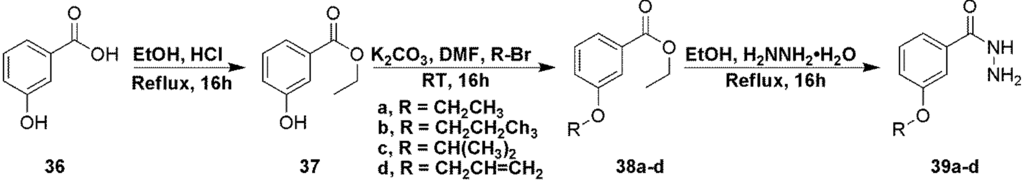
Scheme 3.
Preparation of hydrazide intermediates 39a–d.
3,5-Dimethoxyphenylbenzhydrazide (41), used in the preparation of compound 31 was prepared in a one step process from the methyl ester (40) as described in Scheme 4.
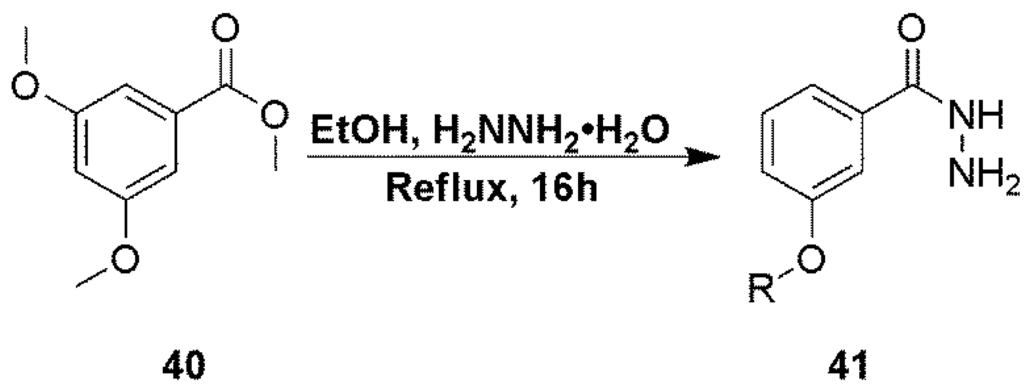
Scheme 4.
Preparation of hydrazide intermediate 41.
The hydrazides used in the synthesis of target compounds 31 and 33–35 were prepared from their acids in two steps as shown in Scheme 5.
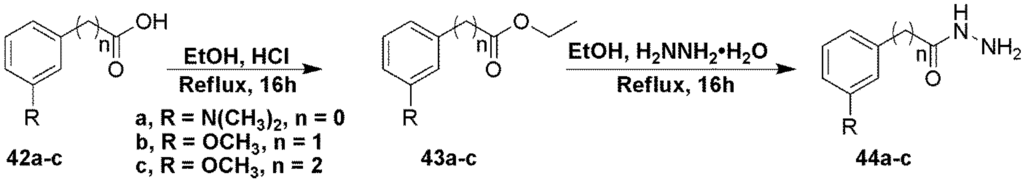
Scheme 5.
Preparation of hydrazide intermediates 44a-d.
3.3. Analytical Techniques
3.3.1. LCMS
A low resolution LC/MS method was used to confirm the mass (M + 1) of all intermediates and products. The compounds were run under acidic conditions with the mobile phase consisting of acetonitrile (Honeywell Burdick and Jackson, Muskegon, MI, USA)–water (Fisher Scientific, Fair Lawn, NJ, USA)–formic acid (Fisher Scientific, Fair Lawn, NJ, USA) (80/20/0.1, v/v/v) at 100 μL/min over 3 min using an Agilent 1100 LC system equipped with a binary pump, autosampler and degasser (Agilent Technologies, Santa Clara, CA, USA) coupled to 2000 QTRAP mass spectrometer via an eletrospray ion (ESI) source (Turbospray) (Applied Biosystems, Foster City, CA, USA). The parameters for MS detection were scan type, Q1 MS scanning from 115 to 1700 amu, positive mode; cycle time, 1.0 s; duration, 2.965 min; cycles, 177; delay time, 0.0 s; scan mode, profile; step size, 0.1 amu; resolution Q1, unit; settling time, 0.0 ms; pause between mass ranges, 5.007 ms; curtain gas, 25 psi; ion spray voltage, 5000 V (positive); temperature, 300 °C; ion source gas 1, 35 psi; ion source gas 2, 50 psi; interface heater, on; declustering potential, 100 V; entrance potential, 11; and collision cell potential range, 8.9–63 V. Analyst software v1.4.1 (AB Sciex, Framingham, MA, USA) was used to run the following for the entire MS method: instrument control, data acquisition, and analysis.
3.3.2. HRMS
Verification of target compound structures was verified using HRMS and 1H NMR. Prior to HRMS, separations were performed using an Agilent Eclipse plus C18 column (1.8 mm, 2.1 × 50 mm) (Agilent Technologies, Santa Clara, CA, USA) protected by an EC C18 guard column (2.7 μm, 2.1 × 5 mm) (Agilent Technologies, Santa Clara, CA, USA). An isocratic mobile phase gradient was used consisting of 80% MPB. Mobile phase A: 100% water (Fisher Scientific, Fair Lawn, NJ, USA) + 0.1% formic acid (Fisher Scientific, Fair Lawn, NJ, USA); mobile phase B: 100% LCMS Acetonitrile (Honeywell Burdick and Jackson, Muskegon, MI, USA) + 0.1% formic acid (Fisher Scientific, Fair Lawn, NJ, USA) attached to an Infinity 1290 UPLC compromising of a binary pump, a degasser, a diode array detector, thermostatted column compartment, and autosampler. HRMS of target compounds was performed on a Bruker micrOTOF-Q II HRMS (Bruker Biospin, Billerica, MA, USA) using an ESI source set in positive mode, using a dry heater setting of 200 °C, capillary voltage of 4000 V, nebulizer pressure of 3.0 Bar, and drying gas flow of 10.0 L/min. The end plate offset was set to −500 V, the collision cell RF was set to 60.0 Vpp and the scan with was set from 50 to 3000 m/z. The whole system was run by Hystar 3.2 (Bruker Biospin, Billerica, MA, USA) and the data were samples were processed and analyzed using Compass (Bruker Biospin, Billerica, MA, USA).
3.3.3. NMR
1H NMR of all compounds was performed using a Bruker AVACE I 500 MHz instrument (Bruker Biospin, Billerica, MA fitted with a 1.7 mm TXI probe-head (Bruker Biospin, Billerica, MA, USA) optimized for 1H observations. The Bruker standard ZG pulse sequence was used for all compounds. Acquisition and processing of NMR data was performed using Topspin 2.1 patch level 6. One dimensional 1H NMR spectra were acquired for all compounds at 298 K and optimized for lock parameters, tune and match parameters, setting of receiver gain and shimming. All NMR solvents were purchased from Sigma Aldrich, St. Louis, MO, USA.
3.4. Receptor Binding Assays
Detailed descriptions of the methods used in this study can be found in [10].
Fluorescent-Ligand Binding Assays
To determine the inhibition of binding of the fluorescent ligand, competition binding experiments were performed as previously described [11]. Serial dilutions (1:10) of various competitors, ranging in concentration from 1 × 10−12 M to 1 × 10−5 M, were assayed for inhibition of binding of the fluorescent ligand (1 nM final concentration). The percent total binding curves were analyzed by non-linear regression analysis by GraphPad Prism v4.03 (GraphPad Software, Inc., La Jolla, CA, USA). Curves were analyzed by non-linear regression analysis, and equilibrium inhibition constants (Ki) were determined using the Kd value for the fluorescent ligands obtained in saturation experiments. Results presented are the mean ± SEM of at least three runs for each compound.
3.5. Sheep Bronchoconstriction Assay
Detailed descriptions of the methods used in this study can be found in [8].
3.5.1. Animals
Adult ewes were used for this study. Animals were conscious, supported in a cart and intubated during the course of the experiments. All instrumentation was performed under local anesthesia. The study was conducted at Mount Sinai Medical Center under the approval of the Mount Sinai Medical Center Animal Research Committee. Pulmonary Resistance: Breath by breath measurements of pulmonary resistance (RL) were measured by the esophageal balloon technique. Analysis of 5–10 breaths was used to determine RL.
3.5.2. Aerosols
Aerosols were generated using a Raindrop medication nebulizer. To control aerosol delivery a dosimetry system activated by a piston respirator was used. Nebulized aerosols were delivered directly into the tracheal tube only during inspiration at a tidal volume of 500 mL and at a frequency of 20 breaths/min.
3.5.3. Agents
Compounds were diluted to final experimental concentrations in 30% ETOH:70% 0.9% NaCl.
3.5.4. Protocol Airway Responses
Baseline RL was measured and then the sheep were challenged with 20 breaths of compound. RL was measured after each delivered concentration to assess the bronchoconstrictor activity. Values in the text are reported as mean ± SD increases in RL over baseline for n = 3/group. A One Way Analysis of Variance followed by Student-Newman-Keuls Method to detect pairwise differences was used to determine if compounds produced a significant increase in RL.
3.6. Synthetic Procedures
3.6.1. Brevenal Derivatives
In a typical reaction, brevenal was dissolved in DMF and the hydrazide (2 eq) was added, followed by addition of a catalytic amount of tungstophosphoric acid. The reaction mixture was heated at 60 °C for 4 h. The solvents were evaporated under vacuum and the residue was taken up in methanol. The mixture was filtered through a 0.2 μm nylon filter and subjected to purification by HPLC. Desired products were positively identified by HRMS mass spectrometry and NMR. Spectroscopic data collected for all compounds can be found in the supplementary data document.
Compound 11 was prepared by reaction of brevenal with formylhydrazide to give the product as a white solid in 71% yield. 1H NMR (CD3OD), δ 0.89 (t, J = 6 Hz, 1H), 0.96 (d, J = 6 Hz, 3H), 1.03 (s, 3H), 1.12 (s, 3H), 1.18 (s, 3H), 1.34 (m, 6H), 1.50 (m, 2H), 1.62 (m, 4H), 1.75 (m, 6H), 1.86 (m, 5H), 2.03 (m, 5H), 2.21 (m, 3H), 2.36 (m, 1H), 3.24 (m, 2H), 3.33 (m, 2H), 3.54 (br s, 1H), 3.71 (m, 1H), 3.96 (s, 1H), 4.06 (dd, J = 11 Hz and 5 Hz, 1H), 5.08 (d, J = 10 Hz, 1H), 5.18 (d, J = 17 Hz, 1H), 5.44 (q, 1H), 5.90 (dt, J = 16 Hz and 7 Hz, 1H), 6.03 (t, J = 11 Hz, 1H), 6.20 (d, J = 9 Hz, 1H), 6.69 (m, 1H), 8.10 (d, J = 10 Hz, 1H), 8.56 (s, 1H); HRMS calculated for C40H63N2O8 (M + H)+, 699.4579; found 699.4580.
Compound 12 was prepared by reaction of brevenal with acetylhydrazide to give the product as a white solid in 52% yield. 1H NMR (CD3OD), δ 0.91 (t, J = 6 Hz, 1H), 0.97 (d, J = 7 Hz, 3H), 1.05 (s, 3H), 1.13 (s, 3H), 1.20 (s, 3H), 1.31 (m, 6H), 1.50 (m, 2H), 1.64 (m, 4H), 1.75 (m, 6H), 1.86 (m, 5H), 2.04 (m, 5H), 2.21 (m, 3H), 2.33 (m, 1H), 3.25 (m, 2H), 3.35 (m, 2H), 3.54 (m, 1H), 3.64 (s, 3H), 3.69 (t, 1H), 3.97 (m, 1H), 4.08 (m, 1H), 4.21 (m, 1H), 5.09 (d, J = 10 Hz, 1H), 5.19 (d, J = 17 Hz, 1H), 5.45 (m, 1H), 5.90 (m, 1H), 6.05 (m, 1H), 6.69 (m, 1H), 8.19 (d, J = 10 Hz, 1H); HRMS calculated for C41H65N2O8 (M + H)+, 713.4735; found 713.4729.
Compound 13 was prepared by reaction of brevenal with propanoylhydrazide to give the product as a white solid in 39% yield. 1H NMR (CD3OD), δ 0.95 (d, J = 7 Hz, 3H), 1.03 (s, 3H), 1.12 (s, 3H), 1.16 (m, 5H), 1.27 (m, 5H), 1.36 (m, 2H), 1.50 (m, 2H), 1.64 (m, 4H), 1.76 (m, 6H), 1.86 (d, 5H), 2.03 (m, 5H), 2.14 (m, 1H), 2.25 (m, 4H), 2.35 (m, 1H), 2.63 (m, 1H), 3.23 (m, 2H), 3.32 (m, 2H), 3.53 (m, 1H), 3.70 (m, 1H), 3.95 (m, 1H), 4.06 (m, 1H), 5.07 (d, J = 10 Hz, 1H), 5.17 (dd, J = 16 Hz and 2 Hz, 1H), 5.43 (m, 1H), 5.86 (m, 1H), 6.02 (m, 1H), 6.32 (d, J = 10 Hz, 1H), 6.68 (m, 1H), 8.19 (d, J = 10 Hz, 1H); HRMS calculated for C42H67N2O8 (M + H)+, 727.4892; found 727.4885.
Compound 14 was prepared by reaction of brevenal with isobutyrohydrazide to give the product as a white solid in 64% yield. 1H NMR (CD3OD), δ 0.69 (s, 1H), 0.89 (t, J = 7 Hz, 3H), 0.95 (d, J = 7 Hz, 3H), 1.02 (s, 3H), 1.10 (s, 3H), 1.14 (s, 3H), 1.16 (s, 3H), 1.17 (s, 3H), 1.62 (m, 4H), 1.74 (m, 6H), 1.86 (m, 5H), 2.02 (m, 5H), 2.24 (m, 3H), 2.31 (m, 3H), 3.24 (m, 2H), 3.31 (m, 4H), 3.52 (m, 1H), 3.67 (m, 3H), 3.95 (m, 1H), 4.18 (dd, J = 10 Hz and 5 Hz, 2H), 5.07 (d, J = 10 Hz, 1H), 5.17 (d, J = 16 Hz, 1H), 5.87 (m, 1H), 6.02 (m, 1H), 6.31 (d, J = 10 Hz, 1H), 6.67 (m, 1H), 8.22 (d, J = 9 Hz, 1H); HRMS calculated for C43H69N2O8 (M + H)+, 741.5048; found 741.5055.
Compound 15 was prepared by reaction of brevenal with pivaloylhydrazide to give the product as a white solid in 84% yield. 1H NMR (CD3OD), δ 0.95 (d, J = 7 Hz, 3H), 1.02 (s, 3H), 1.11 (s, 3H), 1.18 (s, 3H), 1.23 (s, 9H), 1.28 (m, 2H), 1.35 (m, 3H), 1.50 (m, 2H), 1.62 (m, 3H), 1.74 (m, 6H), 1.84 (m, 5H), 2.03 (m, 6H), 2.23 (m, 3H), 3.23 (m, 2H), 3.30 (m, 5H), 3.53 (m, 1H), 3.70 (m, 1H), 3.96 (t, J = 4 Hz, 1H), 4.05 (m, 1H), 5.07 (d, J = 10 Hz, 1H), 5.17 (d, J = 16 Hz, 1H), 5.43 (m, 1H), 5.88 (t, J = 7 Hz, 1H), 6.02 (t, J = 11 Hz, 1H), 6.33 (d, J = 10 Hz, 1H), 6.68 (dt, J = 17 Hz and 10 Hz, 1H), 8.39 (d, J = 10 Hz, 1H); HRMS calculated for C44H71N2O8 (M + H)+, 755.5205; found 755.5226.
Compound 16 was prepared by reaction of brevenal with cyclohexanoylhydrazide to give the product as a white solid in 55% yield. 1H NMR (CD3OD), δ 0.93 (d, 3H), 1.01 (s, 3H), 1.10 (s, 3H), 1.16 (s, 3H), 1.32 (m, 9H), 1.48 (m, 4H), 1.61 (m, 4H), 1.70–1.80 (m, 9H), 1.84 (m, 7H), 2.01 (m, 7H), 2.21 (m, 5H), 3.21 (m, 2H), 3.51 (m, 1H), 3.67 (m, 1H), 3.93 (m, 2H), 4.03 (m, 1H), 5.05 (d, J = 11 Hz, 1H), 5.15 (d, J = 17 Hz, 1H), 5.41 (m, 1H), 5.85 (m, 1H), 6.00 (t, J = 12 Hz, 1H), 6.30 (d, J = 10 Hz, 1H), 6.67 (m, 1H), 8.18 (d, J = 10 Hz, 1H); HRMS calculated for C46H73N2O8 (M + H)+, 781.5361; found 781.5360.
Compound 17 was prepared by reaction of brevenal with benzoylhydrazide to give the product as a white solid in 45% yield. 1H NMR (CD3OD), δ 0.95 (d, J = 7 Hz, 3H), 1.02 (s, 3H), 1.11 (s, 3H), 1.18 (s, 3H), 1.30 (m, 3H), 1.36 (m, 3H), 1.50 (m, 2H), 1.61 (m, 3H), 1.76 (m, 6H), 1.87 (m, 5H), 2.04 (m, 6H), 2.25 (m, 4H), 3.23 (m, 2H), 3.33 (m, 3H), 3.54 (m, 1H), 3.70 (m, 1H), 3.96 (s, 1H), 4.06 (m, 1H), 5.07 (d, J = 11 Hz, 1H), 5.17 (dd, J = 17 Hz and 2 Hz, 1H), 5.42 (m, 1H), 5.92 (m, 1H), 6.01 (t, J = 11 Hz, 1H), 6.40 (d, J = 10 Hz, 1H), 6.67 (m, 1H), 7.49 (t, J = 7 Hz, 2H), 7.59 (t, J = 7 Hz, 1H), 7.87 (d, J = 7 Hz, 2H), 8.47 (d, J = 10 Hz, 1H); HRMS calculated for C46H67N2O8 (M + H)+, 775.4892; found 775.4892.
Compound 18 was prepared by reaction of brevenal with picalinoylhydrazide to give the product as a pale yellow solid in 43% yield. 1H NMR (CD3OD), δ 0.96 (d, J = 6 Hz, 3H), 1.02 (s, 3H), 1.11 (s, 3H), 1.18 (s, 3H), 1.29 (m, 6H), 1.37 (m, 3H), 1.62 (m, 3H), 1.76 (m, 5H), 1.87 (m, 5H), 2.05 (m, 6H), 2.13 (m, 2H), 2.24 (m, 3H), 3.24 (m, 2H), 3.32 (m, 2H), 3.54 (m, 1H), 3.71 (m, 1H), 3.96 (m, 1H), 4.06 (m, 1H), 5.07 (d, J = 10 Hz, 1H), 5.17 (d, J = 18 Hz, 1H), 5.42 (m, 1H), 5.94 (m, 1H), 6.02 (m, 1H), 6.42 (d, J = 9 Hz, 1H), 6.68 (m, 1H), 7.57 (m, 1H), 7.97 (m, 1H), 8.15 (m, 1H), 8.60 (d, J = 10 Hz, 1H), 8.65 (d, J = 5 Hz, 1H); HRMS calculated for C45H66N3O8 (M+H)+, 776.4844; found 776.4830.
Compound 19 was prepared by reaction of brevenal with nicotinoylhydrazide to give the product as a pale yellow solid in 32% yield. 1H NMR (CD3OD), δ 0.95 (d, J = 7 Hz, 3H), 1.02 (s, 3H), 1.11 (s, 3H), 1.18 (s, 3H), 1.37 (m, 2H), 1.50 (m, 2H), 1.62 (m, 3H), 1.75 (m, 7H), 1.85 (m, 5H), 2.02 (m, 6H), 2.15 (m, 1H), 2.25 (m, 3H), 2.35 (m, 1H), 3.24 (m, 2H), 3.32 (m, 5H), 3.53 (m, 1H), 3.70 (m, 1H), 3.96 (s, 1H), 4.05 (m, 1H), 5.07 (d, J = 10 Hz, 1H), 5.17 (d, J = 16 Hz, 1H), 5.43 (m, 1H), 5.94 (t, J = 8 Hz, 1H), 6.02 (t, J = 11 Hz, 1H), 6.40 (d, J = 10 Hz, 1H), 6.68 (m, 1H), 7.57 (dd, J = 8 Hz and 5 Hz, 1H), 8.30 (d, J = 8 Hz, 1H), 8.47 (d, J = 10 Hz, 1H), 8.71 (d, J = 5 Hz, 1H), 9.03 (s, 1H); HRMS calculated for C45H66N3O8 (M + H)+, 776.4844; found 776.4849.
Compound 20 was prepared by reaction of brevenal with isonicatinoylhydrazide to give the product as a pale yellow solid in 84% yield. 1H NMR (CD3OD), δ 0.94 (d, J = 7 Hz, 3H), 1.01 (s, 3H), 1.10 (s, 3H), 1.17 (s, 3H), 1.26 (m, 5H), 1.35 (m, 3H), 1.50 (m, 2H), 1.60 (m, 3H), 1.74 (m, 6H), 1.84 (m, 4H), 2.01 (m, 5H), 2.12 (m, 1H), 2.23 (m, 2H), 2.32 (m, 1H), 3.22 (m, 2H), 3.31 (m, 3H), 3.52 (m, 1H), 3.69 (m, 1H), 3.94 (t, J = 4 Hz, 1H), 4.04 (m, 1H), 5.05 (d, J = 10 Hz, 1H), 5.15 (d, J = 17 Hz, 1H), 5.41 (m, 1H), 5.93 (t, J = 7 Hz, 1H), 6.00 (t, J = 11 Hz, 1H), 6.39 (d, J = 9 Hz, 1H), 6.67 (m, 1H), 7.83 (d, J = 5 Hz, 2H), 8.49 (d, J = 7 Hz, 1H), 8.70 (d, J = 5 Hz, 2H); HRMS calculated for C45H66N3O8 (M + H)+, 776.4844; found 776.4839.
Compound 21 was prepared by reaction of brevenal with 2-methoxybenzoylhydrazide to give the product as a pale yellow solid in 78% yield. 1H NMR (CD3OD), δ 0.92 (d, J = 7 Hz, 3H), 0.99 (s, 3H), 1.08 (s, 3H), 1.15 (s, 3H), 1.27 (m, 6H), 1.35 (m, 2H), 1.46 (m, 2H), 1.59 (m, 4H), 1.72 (m, 5H), 1.84 (m, 5H), 1.98 (m, 5H), 2.12 (m, 1H), 2.21 (m, 2H), 2.31 (m, 1H), 3.20 (m, 1H), 3.29 (m, 3H), 3.50 (m, 1H), 3.68 (m, 1H), 3.93 (m, 4H), 4.02 (m, 1H), 5.04 (d, J = 10 Hz, 1H), 5.13 (d, J = 17 Hz, 1H), 5.40 (m, 1H), 5.88 (t, J = 7 Hz, 1H), 5.99 (t, J = 11 Hz, 1H), 6.37 (d, J = 10 Hz, 1H), 6.64 (m, 1H), 7.03 (t, J = 7 Hz, 1H), 7.11 (d, J = 8 Hz, 1H), 7.48 (m, 1H), 7.82 (dd, J = 8 Hz and 2 Hz, 1H), 8.36 (d, J = 10 Hz, 1H); HRMS calculated for C47H69N2O9 (M + H)+, 805.4998; found 805.5025.
Compound 22 was prepared by reaction of brevenal with 3-methoxybenzoylhydrazide to give the product as a pale yellow solid in 58% yield. 1H NMR (CD3OD), δ 0.94 (d, J = 7 Hz, 3H), 1.01 (s, 3H), 1.10 (s, 3H), 1.17 (s, 3H), 1.25 (s, 8H), 1.49 (m, 2H), 1.61 (m, 4H), 1.73 (m, 5H), 1.84 (m, 3H), 2.01 (m, 5H), 2.13 (m, 1H), 2.23 (m, 3H), 2.33 (m, 1H), 3.21 (m, 2H), 3.31 (m, 3H), 3.52 (m, 1H), 3.70 (m, 1H), 3.83 (s, 3H), 3.94 (m, 1H), 4.03 (m, 1H), 4.55 (m, 1H), 5.06 (d, J = 10 Hz, 1H), 5.15 (d, J = 15 Hz, 1H), 5.41 (m, 1H), 5.90 (t, J = 8 Hz, 1H), 6.00 (t, J = 11 Hz, 1H), 6.39 (d, J = 10 Hz, 1H), 6.67 (m, 1H), 7.11 (dd, J = 8 Hz and 2 Hz, 1H), 7.37 (t, J = 8 Hz, 1H), 7.43 (m, 2H), 8.46 (d, J = 9 Hz, 1H); HRMS calculated for C47H69N2O9 (M + H)+, 805.4998; found 805.5007.
Compound 23 was prepared by reaction of brevenal with 4-methoxybenzoylhydrazide to give the product as a pale yellow solid in 88% yield. 1H NMR (CD3OD), δ 0.92 (d, J = 7 Hz, 3H), 0.99 (s, 3H), 1.08 (s, 3H), 1.15 (s, 3H), 1.23 (m, 7H), 1.33 (m, 2H), 1.48 (m, 2H), 1.59 (m, 3H), 1.73 (m, 5H), 1.82 (m, 4H), 2.00 (m, 5H), 2.11 (m, 1H), 2.20 (m, 2H), 2.30 (m, 1H), 3.21 (m, 2H), 3.29 (m, 3H), 3.50 (m, 1H), 3.67 (m, 1H), 3.81 (s, 3H), 3.92 (t, J = 4 Hz, 1H), 4.02 (m, 1H), 5.04 (d, J = 10 Hz, 1H), 5.13 (dd, J = 17 Hz and 2 Hz, 1H), 5.40 (m, 1H), 5.87 (t, J = 7 Hz, 1H), 5.99 (t, J = 11 Hz, 1H), 6.37 (d, J = 10 Hz, 1H), 6.64 (m, 1H), 6.97 (d, J = 9 Hz, 2H), 7.83 (d, J = 9 Hz, 2H), 8.42 (d, J = 10 Hz, 1H); HRMS calculated for C47H69N2O9 (M + H)+, 805.4998; found 805.5009.
Compound 24 was prepared by reaction of brevenal with 3-ethoxybenzoylhydrazide (39a) to give the product as a white solid in 41% yield. 1H NMR (CD3OD), δ 0.86 (t, J = 7 Hz, 2H), 0.94 (d, J = 7 Hz, 3H), 1.01 (s, 3H), 1.09 (s, 3H), 1.16 (s, 3H), 1.27 (m, 9H), 1.38 (t, J = 7 Hz, 3H), 1.49 (m, 1H), 1.59 (m, 3H), 1.74 (m, 4H), 1.83 (m, 4H), 2.00 (m, 6H), 2.13 (m, 2H), 2.24 (m, 2H), 2.34 (m, 1H), 3.22 (m, 2H), 3.31 (m, 2H), 3.52 (m, 1H), 3.69 (m, 1H), 3.94 (m, 1H), 4.05 (m, 2H), 5.05 (d, J = 10 Hz, 1H), 5.15 (dd, J = 17 Hz and 1 Hz, 1H), 5.41 (m, 1H), 5.90 (m, 1H), 6.01 (m, 1H), 6.39 (d, J = 10 Hz, 1H), 6.66 (m, 1H), 7.08 (d, J = 8 Hz, 1H), 7.36 (t, J = 8 Hz, 1H), 7.41 (m, 2H), 8.45 (d, J = 10 Hz, 1H); HRMS calculated for C48H71N2O9 (M + H)+, 819.5154; found 819.5159.
Compound 25 was prepared by reaction of brevenal with 3-propoxybenzoylhydrazide (39b) to give the product as a white solid in 64% yield. 1H NMR (CD3OD), δ 0.86 (t, J = 7 Hz, 2H), 0.94 (d, J = 7 Hz, 3H), 1.02 (m, 5H), 1.09 (s, 3H), 1.17 (s, 3H), 1.27 (m, 9H), 1.48 (m, 1H), 1.59 (m, 3H), 1.75 (m, 6H), 1.84 (m, 4H), 2.01 (m, 5H), 2.13 (m, 2H), 2.22 (m, 2H), 2.33 (m, 1H), 3.22 (m, 2H), 3.31 (m, 2H), 3.52 (m, 1H), 3.70 (m, 1H), 3.96 (m, 3H), 4.03 (m, 1H), 5.05 (d, J = 10 Hz, 1H), 5.15 (d, J = 16 Hz, 1H), 5.30 (t, J = 5 Hz, 1H), 5.40 (m, 1H), 5.90 (m, 1H), 6.00 (t, J = 11 Hz, 1H), 6.39 (d, J = 9 Hz, 1H), 6.67 (m, 1H), 7.09 (d, J = 8 Hz, 1H), 7.36 (t, J = 8 Hz, 1H), 7.41 (m, 2H), 8.46 (d, J = 10 Hz, 1H); HRMS calculated for C49H73N2O9 (M + H)+, 833.5311; found 833.5321.
Compound 26 was prepared by reaction of brevenal with 3-isopropoxybenzoylhydrazide (39c) to give the product as a white solid in 41% yield. 1H NMR (CD3OD), δ 0.86 (t, J = 7 Hz, 2H), 0.94 (m, 3H), 1.01 (s, 3H), 1.10 (s, 3H), 1.16 (s, 3H), 1.25 (m, 7H), 1.30 (m, 8H), 1.49 (m, 1H), 1.59 (m, 3H), 1.72 (m, 4H), 1.83 (m, 4H), 2.00 (m, 5H), 2.13 (m, 2H), 2.22 (m, 2H), 3.22 (m, 2H), 3.30 (m, 2H), 3.52 (m, 1H), 3.68 (m, 1H), 3.94 (m, 1H), 4.04 (m, 1H), 4.64 (m, 1H), 5.05 (d, J = 10 Hz, 1H), 5.15 (d, J = 17 Hz, 1H), 5.31 (t, J = 5 Hz, 1H), 5.42 (m, 1H), 5.90 (t, J = 7 Hz, 1H), 6.00 (t, J = 10 Hz, 1H), 6.38 (d, J = 11 Hz, 1H), 6.66 (m, 1H), 7.08 (dd, J = 7 Hz and 2 Hz, 1H), 7.35 (t, J = 9 Hz, 1H), 7.40 (m, 2H), 8.45 (d, J = 10 Hz, 1H); HRMS calculated for C49H73N2O9 (M + H)+, 833.5311; found 833.5294.
Compound 27 was prepared by reaction of brevenal with 3-(allyloxy)benzoylhydrazide (39d) to give the product as a white solid in 77% yield. 1H NMR (CD3OD), δ 0.94 (d, J = 6 Hz, 3H), 1.01 (s, 3H), 1.09 (s, 3H), 1.16 (s, 3H), 1.27 (m, 3H), 1.34 (m, 2H), 1.49 (m, 2H), 1.60 (m, 4H), 1.73 (m, 6H), 1.83 (m, 4H), 2.00 (m, 6H), 2.12 (m, 2H), 2.23 (m, 3H), 2.33 (m, 1H), 3.22 (m, 2H), 3.30 (m, 2H), 3.52 (m, 1H), 3.69 (m, 1H), 3.94 (m, 1H), 4.04 (m, 1H), 4.58 (d, J = 4 Hz, 2H), 5.05 (d, J = 9 Hz, 1H), 5.15 (d, J = 17 Hz, 1H), 5.23 (d, J = 11 Hz, 1H), 5.41 (m, 2H), 5.90 (t, J = 7 Hz, 1H), 6.04 (m, 2H), 6.38 (d, J = 10 Hz, 1H), 6.67 (m, 1H), 7.12 (d, J = 7 Hz, 1H), 7.37 (t, J = 6 Hz, 1H), 7.42 (br s, 2H), 8.45 (d, J = 10 Hz, 1H); HRMS calculated for C49H71N2O9 (M + H)+, 831.5154; found 831.5169.
Compound 28 was prepared by reaction of brevenal with 3,5-dimethoxybenzoylhydrazide (41) to give the product as a pale yellow solid in 78% yield. 1H NMR (CD3OD), δ 0.92 (d, J = 7 Hz, 3H), 0.99 (s, 3H), 1.08 (s, 3H), 1.15 (s, 3H), 1.25 (m, 4H), 1.32 (m, 2H), 1.48 (m, 2H), 1.58 (m, 4H), 1.71 (m, 6H), 1.82 (m, 4H), 1.99 (m, 6H), 2.13 (m, 2H), 2.22 (m, 2H), 2.32 (m, 1H), 3.20 (m, 2H), 3.29 (m, 2H), 3.50 (m, 1H), 3.65 (m, 1H), 3.79 (s, 6H), 3.92 (br s, 1H), 4.02 (m, 1H), 5.04 (d, J = 10 Hz, 1H), 5.14 (d, 1H), 5.40 (m, 1H), 5.89 (m, 1H), 5.99 (t, J = 11 Hz, 1H), 6.36 (d, J = 10 Hz, 1H), 6.64 (m, 2H), 7.00 (s, 2H), 8.44 (d, J = 9 Hz, 1H); HRMS calculated for C48H71N2O10 (M + H)+, 835.5103; found 835.5109.
Compound 29 was prepared by reaction of brevenal with 3-hydroxybenzoylhydrazide to give the product as a white solid in 36% yield. 1H NMR (CD3OD), δ 0.98 (d, J = 7 Hz, 3H), 1.05 (s, 3H), 1.14 (s, 3H), 1.21 (s, 3H), 1.32 (m, 9H), 1.39 (m, 2H), 1.54 (m, 2H), 1.65 (m, 4H), 1.77 (m, 5H), 1.88 (m, 4H), 2.05 (m, 5H), 2.29 (m, 4H), 3.26 (m, 2H), 3.35 (m, 2H), 3.56 (m, 1H), 3.72 (m, 1H), 3.98 (t, J = 4 Hz, 1H), 4.08 (m, 1H), 5.10 (d, J = 10 Hz, 1H), 5.19 (d, J = 18 Hz, 1H), 5.45 (m, 1H), 5.94 (m, 1H), 6.04 (t, J = 10 Hz, 1H), 6.42 (d, J = 10 Hz, 1H), 6.70 (m, 1H), 7.00 (m, 1H), 7.32 (m, 2H), 8.48 (t, J = 10 Hz, 1H); HRMS calculated for C46H67N2O9 (M + H)+, 791.4841; found 791.4818.
Compound 30 was prepared by reaction of brevenal with 3-methylbenzoylhydrazide to give the product as a white solid in 78% yield. 1H NMR (CD3OD), δ 0.98 (d, J = 7 Hz, 3H), 1.05 (s, 3H), 1.14 (s, 3H), 1.21 (s, 3H), 1.30 (m, 3H), 1.39 (m, 2H), 1.54 (m, 2H), 1.64 (m, 4H), 1.78 (m, 6H), 1.87 (m, 5H), 2.05 (m, 6H), 2.18 (m, 1H), 2.28 (m, 3H), 2.36 (m, 1H), 2.43 (s, 3H), 3.26 (m, 2H), 3.35 (m, 2H), 3.57 (m, 1H), 3.73 (m, 1H), 3.98 (t, J = 4 Hz, 1H), 4.08 (m, 1H), 5.10 (d, J = 10 Hz, 1H), 5.19 (dd, J = 17 Hz and 2 Hz, 1H), 5.47 (m, 1H), 5.94 (t, J = 7 Hz, 1H), 6.05 (t, J = 11 Hz, 1H), 6.43 (d, J = 10 Hz, 1H), 6.71 (m, 1H), 7.40 (m, 2H), 7.69 (d, J = 7 Hz, 1H), 7.73 (s, 1H), 8.50 (d, J = 10 Hz, 1H); HRMS calculated for C47H69N2O8 (M + H)+, 789.5048; found 789.5065.
Compound 31 was prepared by reaction of brevenal with 3-dimethylaminobenzoylhydrazide (44a) to give the product as a white solid in 60% yield. 1H NMR (CD3OD), δ 0.98 (d, J = 7 Hz, 3H), 1.05 (s, 3H), 1.14 (s, 3H), 1.21 (s, 3H), 1.29 (m, 3H), 1.39 (m, 2H), 1.53 (m, 2H), 1.64 (m, 4H), 1.77 (m, 6H), 1.88 (m, 5H), 2.04 (m, 6H), 2.16 (m, 1H), 2.28 (m, 3H), 2.38 (m, 1H), 3.01 (s, 6H), 3.26 (m, 2H), 3.34 (m, 2H), 3.57 (m, 1H), 3.74 (m, 1H), 3.98 (t, J = 4 Hz, 1H), 4.08 (m, 1H), 5.10 (d, J = 10 Hz, 1H), 5.19 (dd, J = 17 Hz and 2 Hz, 1H), 5.46 (m, 1H), 5.94 (t, J = 7 Hz, 1H), 6.05 (t, J = 11 Hz, 1H), 6.43 (dd, J = 10 Hz and 3 Hz, 1H), 6.70 (m, 1H), 6.98 (dd, J = 8 Hz and 2 Hz, 1H), 7.19 (d, J = 8 Hz, 1H), 7.27 (br s, 1H), 7.31 (t, J = 8 Hz, 1H), 8.51 (br s, 1H); HRMS calculated for C48H72N3O8 (M + H)+, 818.5314; found 818.5334.
Compound 32 was prepared by reaction of brevenal with 3-fluorobenzoylhydrazide to give the product as a pale yellow solid in 63% yield. 1H NMR (CD3OD), δ 0.94 (d, J = 7 Hz, 3H), 1.01 (s, 3H), 1.10 (s, 3H), 1.17 (s, 3H), 1.27 (m, 3H), 1.36 (m, 2H), 1.49 (m, 2H), 1.61 (m, 4H), 1.73 (m, 6H), 1.86 (m, 5H), 2.01 (m, 6H), 2.12 (m, 1H), 2.24 (m, 3H), 2.34 (m, 1H), 3.23 (m, 2H), 3.31 (m, 2H), 3.52 (m, 1H), 3.69 (m, 1H), 3.94 (t, J = 4 Hz, 1H), 4.04 (m, 1H), 5.06 (d, J = 10 Hz, 1H), 5.15 (dd, J = 17 Hz and 2 Hz, 1H), 5.41 (m, 1H), 5.91 (t, J = 7 Hz, 1H), 6.01 (t, J = 11 Hz, 1H), 6.39 (d, J = 10 Hz, 1H), 6.66 (dt, J = 17 Hz and 11 Hz, 1H), 7.30 (m, 1H), 7.50 (m, 1H), 7.61 (d, J = 8 Hz, 1H), 7.70 (d, J = 7 Hz, 1H), 8.46 (d, J = 10 Hz, 1H); HRMS calculated for C46H66FN2O8 (M + H)+, 793.4798; found 793.4807.
Compound 33 was prepared by reaction of brevenal with 3-chlorobenzoylhydrazide (44b) to give the product as a white solid in 35% yield. 1H NMR (CD3OD), δ 0.98 (d, J = 7 Hz, 3H), 1.05 (s, 3H), 1.14 (s, 3H), 1.21 (s, 3H), 1.30 (m, 3H), 1.38 (m, 2H), 1.54 (m, 2H), 1.65 (m, 4H), 1.77 (m, 6H), 1.89 (m, 5H), 2.05 (m, 6H), 2.17 (m, 1H), 2.28 (m, 3H), 2.38 (m, 1H), 3.27 (m, 2H), 3.34 (m, 2H), 3.56 (m, 1H), 3.73 (m, 1H), 3.99 (t, J = 4 Hz, 1H), 4.08 (m, 1H), 5.09 (d, J = 10 Hz, 1H), 5.19 (d, J = 17 Hz, 1H), 5.45 (m, 1H), 5.96 (t, J = 7 Hz, 1H), 6.05 (t, J = 11 Hz, 1H), 6.43 (d, J = 9 Hz, 1H), 6.70 (m, 1H), 7.51 (t, J = 8 Hz, 1H), 7.61 (d, J = 7 Hz, 1H), 7.84 (d, J = 8 Hz, 1H), 7.93 (t, J = 2 Hz, 1H), 8.50 (d, J = 10 Hz, 1H); HRMS calculated for C46H66ClN2O8 (M + H)+, 809.4502; found 809.4497.
Compound 34 was prepared by reaction of brevenal with 2-(3-methoxyphenyl)acetohydrazide (44c) to give the product as a white solid in 68% yield. 1H NMR (CD3OD), δ 0.97 (d, J = 7 Hz, 3H), 1.05 (s, 3H), 1.14 (s, 3H), 1.20 (s, 3H), 1.33 (m, 3H), 1.38 (m, 2H), 1.53 (m, 2H), 1.64 (m, 4H), 1.77 (m, 6H), 1.88 (m, 5H), 2.05 (m, 6H), 2.15 (m, 1H), 2.25 (m, 3H), 2.38 (m, 1H), 3.26 (m, 2H), 3.33 (m, 2H), 3.54 (m, 3H), 3.72 (m, 1H), 3.79 (s, 3H), 3.98 (d, J = 8 Hz, 1H), 4.08 (m, 1H), 5.10 (d, J = 10 Hz, 1H), 5.19 (dd, J = 17 Hz and 1 Hz, 1H), 5.45 (m, 1H), 5.91 (m, 1H), 6.05 (t, J = 11 Hz, 1H), 6.34 (d, J = 10 Hz, 1H), 6.70 (m, 1H), 6.81 (m, 1H), 6.90 (m, 2H), 7.21 (m, 1H), 8.27 (d, J = 10 Hz, 1H); HRMS calculated for C48H71N2O9 (M + H)+, 819.5154; found 819.5140.
Compound 35 was prepared by reaction of brevenal with 3-(3-methoxyphenyl)propanehydrazide (44d) to give the product as a white solid in 50% yield. 1H NMR (CD3OD), δ 0.97 (d, J = 7 Hz, 3H), 1.05 (s, 3H), 1.14 (s, 3H), 1.20 (s, 3H), 1.27 (m, 2H), 1.39 (m, 3H), 1.52 (m, 2H), 1.65 (m, 4H), 1.76 (m, 6H), 1.87 (m, 5H), 2.04 (m, 6H), 2.16 (m, 1H), 2.26 (m, 3H), 2.39 (m, 1H), 2.54 (t, J = 8 Hz, 2H), 2.95 (m, 3H), 3.22 (m, 2H), 3.34 (m, 2H), 3.55 (m, 1H), 3.72 (m, 1H), 3.77 (s, 3H), 3.98 (dd, J = 5 Hz and 1 Hz, 1H), 4.08 (m, 1H), 5.10 (d, J = 10 Hz, 1H), 5.19 (dd, J = 17 Hz and 2 Hz, 1H), 5.47 (m, 1H), 5.89 (m, 1H), 6.05 (t, J = 11 Hz, 1H), 6.33 (d, J = 10 Hz, 1H), 6.72 (m, 1H), 6.80 (m, 2H), 7.18 (m, 1H), 8.16 (m, 1H); HRMS calculated for C49H73N2O9 (M + H)+, 833.5311; found 833.5289.
3.6.2. Hydrazide Intermediates
Compound 37. To a solution of 3-hydroxybenzoic acid (5 g, 36.2 mmol) in ethanol (30 mL) was added cHCl (5 drops) and the mixture heated at reflux overnight. The solvents were evaporated to give the target compound as a white solid (6.02 g, 100%). 1H NMR (CD3OD), δ 1.37 (t, J = 7 Hz, 2H), 4.33 (q, J = 7 Hz, 3H), 7.00 (dt, J = 8 Hz and 3 Hz, 1H), 7.26 (dt, J = 8 Hz and 2 Hz, 1H), 7.42 (dt, J = 6 Hz and 2 Hz, 1H), 7.48 (dd, J = 6 Hz and 1 Hz, 1H).
Compound 38a. To a solution of compound 37 (1 g, 6.02 mmol) in DMF (10 mL) was added potassium carbonate (2.5 g, 18.05 mmol) and ethylbromide (2.24 mL, 30.09 mmol) and the mixture was stirred at room temperature overnight. The crude mixture was then poured into water and extracted with hexanes. The organic extracts were washed with water, brine, dried over anhydrous sodium sulfate, filtered and evaporated to give the desired product as a colorless oil (1.121 g, 95%). Product was used without further purification. 1H NMR (CDCl3), δ 1.40 (t, J = 7 Hz, 3H), 1.44 (t, J = 7 Hz, 3H), 4.08 (q, J = 7 Hz, 2H), 4.38 (q, J = 7 Hz, 2H), 7.09 (dd, J = 8 Hz and 2 Hz, 1H), 7.33 (t, J = 8 Hz, 1H), 7.56 (br s, 1H), 7.63 (d, J = 8 Hz, 1H).
Compound 38b was prepared in a similar manner as compound 38a using 1-bromopropane to give the product as a colorless oil in 93% yield. 1H NMR (CDCl3), δ 1.02 (m, 6H), 1.80 (m, 4H), 4.35 (q, J = 7 Hz, 2H), 7.07 (dd, J = 8 Hz and 2 Hz, 1H), 7.31 (t, J = 8 Hz, 1H), 7.55 (d, J = 2 Hz, 1H), 7.61 (d, J = 8 Hz, 1H).
Compound 38c was prepared in a similar manner as compound 38a using 2-bromopropane to give the product as a colorless oil in 86% yield. 1H NMR (CDCl3), δ 1.34 (t, J = 7 Hz, 6H), 1.37 (t, J = 7 Hz, 3H), 4.35 (q, J = 7 Hz, 2H), 4.59 (m, 1H), 7.05 (dd, J = 8 Hz and 3 Hz, 1H), 7.30 (m, 1H), 7.54 (br s, 1H), 7.59 (dd, J = 8 Hz and 4 Hz, 1H).
Compound 38d was prepared in a similar manner as compound 38a using allylbromide to give the product as a pale yellow oil in 63% yield. 1H NMR (CDCl3), δ 1.38 (t, J = 7 Hz, 3H), 4.36 (q, J = 7 Hz, 2H), 4.57 (d, J = 5 Hz, 1H), 5.29 (m, 1H), 5.41 (m, 1H), 6.04 (m, 1H), 7.11 (dt, J = 9 Hz and 3 Hz, 1H), 7.33 (m, 1H), 7.58 (m, 1H), 7.64 (dd, J = 8 Hz and 3 Hz, 1H).
Compound 39a. To a solution of compound 38a (500 mg, 2.57 mmol) in ethanol (5 mL) was added hydrazine hydrate (1.25 mL, 25.74 mmol) and the mixture was heated at reflux overnight. The solvents were evaporated and the crude mixture was partitioned between water and chloroform. The aqueous was extracted with chloroform and the combined organic extracts were washed with water and brine, dried over anhydrous sodium sulfate, filtered and evaporated to give the desired product as a white solid (310 mg, 66%). Product was used without any further purification. 1H NMR (CD3OD), δ 1.40 (t, J = 7 Hz, 3H), 4.08 (q, J = 7 Hz, 2H), 7.07 (dt, J = 7 Hz and 2 Hz, 1H), 7.34 (m, 3H); MS m/z = 181.9 (M + H)+.
Compound 39b was prepared in similar fashion to compound 39a, using compound 38b and was isolated as a white solid in 85% yield. 1H NMR (CD3OD), δ 1.06 (t, J = 8 Hz, 2H), 1.84 (m, 2H), 3.98 (q, J = 6 Hz, 2H), 7.06 (d, J = 7 Hz, 1H), 7.37 (m, 1H), 7.41 (m, 1H), 7.45 (m, 1H); MS m/z = 195.8 (M + H)+.
Compound 39c was prepared in similar fashion to compound 39a, using compound 38c and was isolated as a white solid in 75% yield. 1H NMR (CD3OD), δ 1.17 (br s, 6H), 4.60 (m, 1H), 6.83 (d, J = 5 Hz, 1H), 7.04 (m, 1H), 7.29 (m, 1H), 7.37 (m, 1H); MS m/z = 195.9 (M + H)+.
Compound 39d was prepared in similar fashion to compound 39a, using compound 38d and was isolated as a white solid in 88% yield. 1H NMR (CD3OD), δ 4.58 (m, 2H), 5.31 (m, 1H), 5.42 (m, 1H), 6.04 (m, 1H), 7.06 (m, 1H), 7.36 (m, 2H), 7.43 (m, 1H). ); MS m/z = 193.9 (M + H)+.
Compound 41. To a solution of compound 40 (1 g, 5.10 mmol) in methanol (10 mL) was added hydrazine hydrate (5 mL, 102 mmol) and the mixture was heated at reflux overnight. The solvents were evaporated and the crude mixture by flash chromatography using a gradient mixture of methanol in dichloromethane to give the desired product as a pale yellow solid (310 mg, 66%). Product was used without any further purification. 1H NMR (CDCl3), δ 3.81 (s, 6H), 6.58 (s, 1H), 6.88 (m, 2H); MS m/z = 197.5 (M + H)+.
Compound 44a. To a solution of 3-(dimethylamino)benzoic acid (1 g, 6.05 mmol) in ethanol (10 mL) was added cHCl (5 drops) and the mixture heated at reflux overnight. The solvents were evaporated to give the target compound as a white solid. This compound was then dissolved in ethanol (10 mL) and hydrazine hydrate (5.89 mL, 121 mmol) was added and the mixture heated at reflux overnight. Solvents were evaporated and the product was purified by flash chromatography using a gradient mixture of ethyl acetate and hexanes to give the desire compound as a white solid (1.02 g, 93% over 2 steps). 1H NMR (CDCl3), δ 2.95 (s, 6H), 6.84 (d, J = 8 Hz, 1H), 6.99 (d, J = 7 Hz, 1H), 7.19 (m, J = 20 Hz, 1H), 7.26 (m, J = 6 Hz, 1H); MS m/z = 180.6 (M + H)+.
Compound 44b. To a solution of 3-chlorobenzoic acid (1 g, 6.39 mmol) in ethanol (10 mL) was added cHCl (5 drops) and the mixture heated at reflux overnight. The solvents were evaporated to give the target compound as a white solid. This compound was then dissolved in ethanol (10 mL) and hydrazine hydrate (6.21 mL, 128 mmol) was added and the mixture heated at reflux overnight. Solvents were evaporated and the product was purified by flash chromatography using a gradient mixture of ethyl acetate and hexanes to give the desire compound as a yellow solid (890 mg, 81% over 2 steps). 1H NMR (CDCl3), δ 7.41 (t, J = 7 Hz, 1H), 7.49 (d, J = 7 Hz, 1H), 7.73 (m, 1H), 7.86 (m, J = 4 Hz, 1H); MS m/z = 171.5, 173.5 (M + H)+.
Compound 44c. To a solution of 3-methoxyphenylacetic acid (1 g, 6.02 mmol) in ethanol (10 mL) was added cHCl (5 drops) and the mixture heated at reflux overnight. The solvents were evaporated to give the target compound as a white solid. This compound was then dissolved in ethanol (10 mL) and hydrazine hydrate (5.86 mL, 120 mmol) was added and the mixture heated at reflux overnight. Solvents were evaporated and the product was purified by flash chromatography using a gradient mixture of ethyl acetate and hexanes to give the desire compound as a white solid (1.03 g, 94% over 2 steps). 1H NMR (CDCl3), δ 3.79 (s, 3H), 3.83 (s, 2H), 6.83 (m, 4H); MS m/z = 181.5 (M + H)+.
Compound 44d. To a solution of 3-methoxyphenylpropanoic acid (1 g, 5.55 mmol) in ethanol (10 mL) was added cHCl (5 drops) and the mixture heated at reflux overnight. The solvents were evaporated to give the target compound as a white solid. This compound was then dissolved in ethanol (10 mL) and hydrazine hydrate (5.83 mL, 119 mmol) was added and the mixture heated at reflux overnight. Solvents were evaporated and the product was purified by flash chromatography using a gradient mixture of ethyl acetate and hexanes to give the desire compound as a white solid (1.10 g, 94% over 2 steps). 1H NMR (CDCl3), δ 2.55 (m, 1H), 2.86 (m, 1H), 2.97 (m, 2H), 3.78 (s, 3H), 6.73 (d, J = 2 Hz, 1H), 6.77 (m, 2H), 7.21 (m, 1H); MS m/z = 195.6 (M + H)+.
4. Conclusions
We report the preparation of a series of brevenal hydrazide derivatives in an attempt to explore structure-activity within the brevenal receptor. From this series, it appears that only small modifications are tolerated, with only the formyl hydrazide retaining good affinity for the brevenal receptor and exhibiting low adverse effects in the sheep bronchoconstriction assay. Additionally, with the in-house findings that the hydrazide derivatives of brevenal appear to have good stability using in vitro testing, this compound is a strong candidate as a back-up or second generation analog for brevenal in development as a treatment for pulmonary disorders. Increasing the size of the substituent leads to a dramatic reduction in affinity for the brevenal receptor. Compounds capable of displacing brevetoxin at concentrations much lower than those needed for brevenal have also been prepared. However, these highly potent compounds cause bronchoconstriction in the sheep model at low concentrations, suggesting that they may act directly at the site of action for brevetoxin and not through previously described allosteric modulation of the brevetoxin receptor. Further studies are required to fully understand the findings reported here, and will be presented in due course.
Acknowledgments
Funding for this research was provided by NIH—1R21NS067503 and NIH P01 ES 10594. Thanks go to Susan Niven and Tanya Hogue for isolating the brevenal used to prepare the analogs and to Emily Probst in the Mass Spectroscopy Facility in the Chemistry Department at UNCW for running the HRMS samples.
Author Contributions
A.G. conceived and synthesized all brevenal derivatives, D.B. provided cultures from which brevenal was isolated, J.R.M., H.M.J. and A.T. ran receptor binding assays, W.M.A. ran sheep inhalation studies, A. B. prepared the samples for high resolution mass spectroscopy and supervised the whole project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baden, D.G. Brevetoxins: Unique polyether dinoflagellate toxins. FASEB J. 1989, 3, 1807–1817. [Google Scholar]
- Purkerson, S.L.; Baden, D.G.; Fieber, L.A. Brevetoxin modulates neuronal sodium channels in two cell lines derived from rat brain. Neurotoxicology 1999, 20, 909–920. [Google Scholar]
- Whitney, P.L.; Baden, D.G. Complex association and dissociation kinetics of brevetoxin binding to voltage-sensitive rat brain sodium channels. Nat. Toxins 1996, 4, 261–270. [Google Scholar] [CrossRef]
- Bourdelais, A.J.; Jacocks, H.M.; Wright, J.L.C.; Bigwarfe, P.M., Jr.; Baden, D.G. A new polyether ladder compound produced by the dinoflagellate Karenia brevis. J. Nat. Prod. 2005, 68, 2–6. [Google Scholar] [CrossRef]
- Bourdelais, A.J.; Campbell, S.; Jacocks, H.; Naar, J.; Wright, J.L.C.; Carsi, J.; Baden, D.G. Brevenal is a natural inhibitor of brevetoxin action in sodium channel receptor binding assays. Cell Mol. Neurobiol. 2004, 24, 553–563. [Google Scholar] [CrossRef]
- Mattei, C.; Wen, P.J.; Nguyen-Huu, T.D.; Alvarez, M.; Benoit, E.; Bourdelais, A.J.; Lewis, R.J.; Baden, D.G.; Molgo, J.; Meunier, F.A. Brevenal Inhibits Pacific ciguatoxin-1B-induced neurosecretion from bovine chromaffin cells. PLoS One 2008, 3, e3448. [Google Scholar] [CrossRef]
- Nguyen-Huu, T.D.; Mattei, C.; Wen, P.J.; Bourdelais, A.J.; Lewis, R.J.; Benoit, E.; Baden, D.G.; Molgo, J.; Meunier, F.A. Ciguatoxin-induced catecholamine secretion in bovine chromaffin cells: Mechanism of action and reversible inhibition by brevenal. Toxicon 2010, 56, 792–796. [Google Scholar] [CrossRef]
- Abraham, W.M.; Bourdelais, A.J.; Sabater, J.R.; Ahmed, A.; Lee, T.A.; Serebriakov, I.; Baden, D.G. Airway Responses to Aerosolized Brevetoxins in an Animal Model of Asthma. Am. J. Respir. Crit. Care Med. 2005, 171, 26–34. [Google Scholar] [CrossRef]
- Gold, E.P.; Jacocks, H.M.; Bourdelais, A.J.; Baden, D.G. Brevenal, a brevetoxin antagonist from Karenia brevis, binds to a previously unreported site on mammalian sodium channels. Harmful Algae 2013, 26, 12–19. [Google Scholar] [CrossRef]
- McCall, J.R.; Jacocks, H.M.; Baden, D.G.; Bourdelais, A.J. Development of a competitive fluorescence-based synaptosome binding assay for Brevetoxins. Harmful Algae 2012, 19, 85–91. [Google Scholar] [CrossRef]
- Abraham, W.M.; Baden, D.G. Novel pharmacological actions of natural antagonists derived from K. brevis (Red tide). Toxicologist 2011, 120, 476, Abstract PS 2219. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).