Abstract
The peer-reviewed marine pharmacology literature from 2009 to 2011 is presented in this review, following the format used in the 1998–2008 reviews of this series. The pharmacology of structurally-characterized compounds isolated from marine animals, algae, fungi and bacteria is discussed in a comprehensive manner. Antibacterial, antifungal, antiprotozoal, antituberculosis, and antiviral pharmacological activities were reported for 102 marine natural products. Additionally, 60 marine compounds were observed to affect the immune and nervous system as well as possess antidiabetic and anti-inflammatory effects. Finally, 68 marine metabolites were shown to interact with a variety of receptors and molecular targets, and thus will probably contribute to multiple pharmacological classes upon further mechanism of action studies. Marine pharmacology during 2009–2011 remained a global enterprise, with researchers from 35 countries, and the United States, contributing to the preclinical pharmacology of 262 marine compounds which are part of the preclinical pharmaceutical pipeline. Continued pharmacological research with marine natural products will contribute to enhance the marine pharmaceutical clinical pipeline, which in 2013 consisted of 17 marine natural products, analogs or derivatives targeting a limited number of disease categories.
Keywords:
drug; marine; chemical; metabolite; natural; product; pharmacology; pharmaceutical; review; toxicology 1. Introduction
The current article presents a systematic review of the preclinical pharmacology of the marine natural products literature in 2009–2011, with a similar format to previous reviews [1,2,3,4,5,6,7], and which resulted from extensive searches of several databases, including Marinlit, PubMed, Current Contents® and Chemical Abstracts®. We have limited this review to the peer-reviewed literature that reported bioactivity or pharmacology of structurally characterized marine chemicals, and have continued to use a modification of Schmitz’s chemical classification [8] to assign marine structures to six major chemical classes, namely, polyketides, terpenes, peptides, alkaloids, shikimates, and sugars. The preclinical antibacterial, antifungal, antiprotozoal, antituberculosis, and antiviral pharmacology of marine chemicals is presented in Table 1, with the corresponding structures shown in Figure 1. Marine compounds that affect the immune and nervous systems, as well as those with antidiabetic and anti-inflammatory effects are shown in Table 2, with their corresponding structures presented in Figure 2. Finally, marine compounds that have been demonstrated to affect a wide variety of cellular and molecular targets are exhibited in Table 3, and their structures presented in Figure 3. Several publications during 2009–2011 described extracts or as yet structurally uncharacterized marine compounds, and although they have been excluded from the current review, they certainly deserve further investigation because they report novel and interesting in vitro or in vivo preclinical pharmacology: antimicrobial and antistaphylococcal biofilm activity of three 5-kDa peptides isolated from coelomocyte effector cells of the sea urchin Paracentrotus lividus that could benefit patients with medical device-associated infections [9]; an antibacterial polyunsaturated fatty acid, eicosapentanoic acid, isolated from extracts of the marine diatom Phaeodactylum tricornutum with activity against a range of Gram-positive and Gram-negative bacteria, including multidrug-resistant Staphyloccus aureus [10]; potent anticoagulant activity of sulfated polysaccharides isolated from the Brazilian brown seaweed Dictyota cervicornis, which was close to that of clinically used low molecular weight heparin [11]; potent anticoagulant activity of a sulfated polysaccharide isolated from the Chinese green seaweed Monostroma latissimum by a mechanism involving thrombin inhibition in the presence of heparin cofactor II [12]; in vitro antileishmanial activity of dichloromethane extracts of a Tunisian sponge Sarcotragus sp., which demonstrated concomitant morphological alterations of Leishmania major promastigotes in vitro [13]; in vivo and in vitro antifilarial activity of the marine sponge Haliclona exigua extracts against adult nematode Brugia malayi, a parasite that may cause lymphatic filariasis [14]; significant nontoxic and anti-herpes simplex virus HSV-1 and HSV-2 activity in sulfated polysaccharide extracts isolated from four species of red and brown marine algae from New Zealand [15]; anti-herpes simplex virus HSV-1 activity in high molecular weight exopolysaccharides purified from the French marine sponge Celtodoryx girardae and its symbiotic bacteria [16]; anti-inflammatory activity of the crude extracts and fractions of the Mediterranean sponge Spongia officinalis in the in vivo rat carrageenan-induced paw edema assay [17]; in vivo anti-inflammatory activity in polyphenolic extracts from the red alga Laurencia undulata resulting in significant inhibition of asthmatic reactions [18]; in vitro anti-inflammatory effect in an ethanolic extract from the brown alga Ishige okamurae via inhibition of NF-κB transcription factor [19]; induction of oxidative death in a human glioma cell line through a caspase-9 apoptotic pathway by extracts from the marine sponge Polymastia janeirensis [20]; apoptotic activity in extracts from the marine diatom Cocconeis scutellum associated with activation of caspases-8 and -3 in human breast cancer lines [21]; human neutrophil anti-elastase activity of purified sulfated polysaccharides from the red alga Delesseria sanguinea [22]; high antioxidant activity in methanolic extracts of the Korean red alga Polysiphonia morrowii that protected against hydroxyl radical-induced DNA damage in vitro [23]; antioxidant activity in phenolic compounds from the marine alga Halimeda monile that protected against chemically induced rat liver injury in vivo [24]; significant antioxidant properties of polysaccharides from a marine fungus Penicillium sp. F23-2 against superoxide and hydroxyl radicals [25]; in vitro antioxidant activities of acetylated, phosphorylated and benzoylated derivatives of the marine red alga Porphyra haitanensis phorphyran [26]; acceleration of skin wound healing by amino acids isolated from the mollusc Rapana venosa suggesting a possible therapeutic use in skin burns [27]; neuroprotective effects in extracts of the South Indian green seaweed Ulva reticulata that inhibited both acetyl-and butyryl-cholinesterases, and was comparable to agents currently approved for Alzheimer’s disease treatment [28].
3. Marine Compounds with Antidiabetic and Anti-Inflammatory Activity, and Affecting the Immune and Nervous System
Table 2 presents the preclinical pharmacology of marine chemicals (103–162) which demonstrated antidiabetic and anti-inflammatory activity, as well as affected the immune and nervous system, and whose structures are shown in Figure 2.

Table 2.
Marine pharmacology in 2009–2011: Marine compounds with antidiabetic and anti-inflammatory activity; and affecting the immune and nervous system.
| Drug Class | Compound/organism a+ | Chemistry | Pharmacological activity | IC50 b | MMOA c | Country d | References |
|---|---|---|---|---|---|---|---|
| Antidiabetic | DPHC (103)/alga | Polyketide e | Postprandial hyperglycemia inhibition | 100 mg/kg * | α-glucosidase and α-amylase inhibition | S. KOR | [104] |
| Antidiabetic | dysidine (104)/sponge | Terpene f | Insulin signaling and glucose uptake | 6.7 μM | hPTP1b inhibition | CHN | [105,106] |
| Anti-inflammatory | arenamides A & B (105,106)/bacterium | Peptide g | Modulation of LPS-activated murine macrophages in vitro | 3–10 μM * | Nitric oxide and PGE2 inhibition | USA | [107] |
| Anti-inflammatory | callysterol (107)/sponge | Steroid f | Murine hind paw oedema inhibition | ND | TXB2 inhibition | EGY, NLD, USA | [108] |
| Anti-inflammatory | capnellene (108)/soft coral | Terpene f | In vivo inhibition of microglia activation | 10 mg/kg * | iNOS and COX-2 inhibition | TWN | [109] |
| Anti-inflammatory | elisabethin H (109)/soft coral | Terpene f | Modulation of LPS-activated microglia in vitro | 7.0 μM | TXB2 inhibition | USA | [110] |
| Anti-inflammatory | floridosides (110,111)/alga | Glycolipid | Free-radical oxidative stress inhibition | 22–43 μM * | Myeloperoxidase & MMP inhibition | S.KOR & CHN | [111] |
| Anti-inflammatory | malyngamide 2 (112)/bacterium | PKS/NRPS | LPS-activated macrophage in vitro inhibition | 8.0 μM | NO inhibition | PNG, USA | [112] |
| Anti-inflammatory | malyngamide F (113)/bacterium | PKS/NRPS | Macrophages NO release & iNOS expression inhibition | 7.1 μM | MyD88-dependent pathway inhibition | USA | [113] |
| Anti-inflammatory | PFF-A (114)/alga | Polyketide e | LPS-activated macrophage in vitro inhibition | 4.7 μM | iNOS and COX-2 inhibition | S. KOR | [114] |
| Anti-inflammatory | S. plicata dermatan sulfate (115)/ascidian | Polysaccharide h | Colonic inflammation inhibition | 8 mg/kg * | TNF-α, TGF-β, VEGF inhibition | BRA | [115] |
| Anti-inflammatory | symbiopolyol (116)/dinoflagellate | Polyketide e | Lymphocyte adhesion inhibition | 6.6 μM | VCAM-1 expression inhibition | JPN | [116] |
| Anti-inflammatory | tedanol (117)/sponge | Terpene f | Murine hind paw oedema inhibition | 1 mg/kg * | iNOS, COX-1 and COX-2 inhibition | ITA | [117] |
| Anti-inflammatory | carijoside A (118)/coral | Steroid glycoside f | Neutrophil superoxide and elastase inhibition | 1.8–6.8 μg/mL | Undetermined | TWN | [118] |
| Anti-inflammatory | chabrosterol (119)/soft coral | Steroid f | Macrophage COX-2 & iNOS expression inhibition | 10 μM * | Undetermined | TWN | [119] |
| Anti-inflammatory | coscinolactams (120–122)/sponge | Terpene f | Macrophage PGE2 & nitric oxide inhibition | 10 μM * | Undetermined | ITA, ESP, FRA | [120] |
| Anti-inflammatory | durumhemiketalolide C (123)/soft coral | Terpene f | Macrophage COX-2 & iNOS expression inhibition | 10 μM * | Undetermined | TWN | [121] |
| Anti-inflammatory | durumolide F (124)/soft coral | Terpene f | Macrophage COX-2 & iNOS expression inhibition | 10 μM * | Undetermined | TWN | [122] |
| Anti-inflammatory | gyrosanolides B & C (125,126)/soft coral | Terpene f | Macrophage iNOS expression inhibition | 10 μM * | Undetermined | TWN | [123] |
| Anti-inflammatory | klysimplexin sulfoxide (127) soft coral | Terpene f | Macrophage COX-2 & iNOS expression inhibition | 10 μM * | Undetermined | TWN | [124] |
| Anti-inflammatory | L. crassum diterpenes (128,129)/soft coral | Terpene f | Macrophage NO release & iNOS expression inhibition | 3.8–4.0 μM | Undetermined | JPN | [125] |
| Anti-inflammatory | perthamides C & D (130,131)/sponge | Peptideg | Murine hind paw oedema inhibition | 0.3 mg/kg * | Undetermined | FRA, ITA | [126] |
| Anti-inflammatory | rossinones A & B(132,133)/ascidian | Terpene f | Neutrophil superoxide inhibition | 0.8–2.5 μM | Undetermined | MYS, NZL | [127] |
| Anti-inflammatory | nebrosteroid I (134)/soft coral | Steroid f | Macrophage iNOS expression inhibition | 10 μM * | Undetermined | TWN | [128] |
| Anti-inflammatory | sarcoehrenosides A & B (135,136)/soft coral | Glycolipid | Macrophage iNOS expression inhibition | 10 μM * | Undetermined | TWN | [129] |
| Anti-inflammatory | sarcocrassocolides A & B(137,138)/soft coral | Terpene f | Macrophage iNOS expression inhibition | 10 μM * | Undetermined | TWN | [130] |
| Anti-inflammatory | simplexin E (139)/soft coral | Terpene f | Macrophage COX-2 & iNOS expression inhibition | 10 μM * | Undetermined | TWN | [131] |
| Anti-inflammatory | terpioside B (140)/sponge | Glycolipid | Macrophage iNOS expression inhibition | <10 μM * | Undetermined | ITA | [132] |
| Immune system | grassystatins A–C (141–143)/bacterium | Peptide g | T cell antigen presentation inhibition | 10 μM * | Cathepsin E, IL-17 and IFN-γ inhibition | USA | [133] |
| Immune system | callyspongidiol (144) & 14,15-dihydrosiphonodiol (145)/sponge | Polyketide e | Dendritic cell activation | 10 μM * | IL-10 and Ag-presenting activity | DEU, JPN | [134] |
| Immune system | PFF-A (114)/alga | Polyketide e | Basophil IgE receptor inhibition | 25 μM * | Ca2+ influx and degranulation inhibition | S. KOR | [135] |
| Immune system | splenocin B (146)/bacterium | PKS/NRPS | Interleukin 5 and 13 Inhibition | 1.6–1.8 nM | Undetermined | USA | [136] |
| Immune system | HCLPS-1 (147)/clam | Polysaccharide h | In vivo & in vitro T and B cell activation | 20 mg/kg * | Undetermined | CHN | [137] |
| Immune system | yessotoxin (148)/alga | Polyketide(polyether) e | Macrophage phagocytosis inhibition | 1 nM * | TNF-α, MIP-1α & MIP-2 inhibition | ITA | [138] |
| Nervous system | calyculin A (149)/sponge | PKS/NRPS e | Hippocampal neuron neurite retraction | 100 mM * | Dependent on actomyosin activation | JPN | [139] |
| Nervous system | C. olemda purine (150)/sponge | Alkaloid g | Convulsion induction | 4 nm/mouse * | GABAergic transmission inhibition | JPN, USA | [140] |
| Nervous system | hoiamide B (151)/bacterium | Peptide g | Neocortical neuron Ca2+ oscillation inhibition | 79.8 nM | Stimulation of sodium influx | ITA, PNG, USA | [141] |
| Nervous system | palmyrolide A (152)/bacterium | Polyketide e | Neocortical neuron Ca2+ oscillation inhibition | 3.7 µM | Sodium influx inhibition | MEX, USA | [142] |
| Nervous system | xyloketal B (153)/fungus | Polyketide e | Ischemia-induced PC12 cell injury inhibition | 100 µM * | Free radical scavenging | CHN | [143] |
| Nervous system | alotamide (154)/bacterium | PKS/NRPS g | Neocortical neuron Ca2+ oscillation stimulation | 4.18 μM | Undetermined | MEX, USA | [144] |
| Nervous system | (−)-dibromophakellin (155)/sponge | Alkaloid g | Α2B adrenoreceptor agonist | 4.2 µM | Undetermined | AUS | [145] |
| Nervous system | dysideamine (156)/sponge | Terpene e | Hippocampal reactive oxygen species inhibition | 10 µM * | Undetermined | IDN, JPN | [146] |
| Nervous system | ircinialactams (157,158)/sponge | Terpene f | α1 & α3 glycine receptor potentiation | 0.5 µM * | Undetermined | AUS | [147] |
| Nervous system | eusynstyelamides B & C (159,160)/ascidian | Peptide g | Neuronal nitric oxide synthase inhibition | 4.3–5.8 µM | Undetermined | AUS | [148] |
| Nervous system | nanolobatolide (161)/soft coral | Terpene f | 6-hydroxy-dopamine neurotoxicity inhibition | 0.1 µM * | Undetermined | TWN | [149] |
| Nervous system | pulicatin A (162)/bacterium | Alkaloid g | Human serotonin 5-HT2B binding | 505 nM ** | Undetermined | PHL, USA | [150] |
a Organism: Kingdom Animalia: ascidian (Phylum Chordata), coral (Phylum Cnidaria), clam (Phylum Mollusca), fireworm (Phylum Polychaeta), sponge (Phylum Porifera); Kingdom Chromalveolata: dinoflagellates (Phylum Dinoflagellata); Kingdom Fungi: fungus; Kingdom Plantae: alga; Kingdom Monera: bacterium; b IC50: concentration of a compound required for 50% inhibition, *: apparent IC50; **: Ki; ND: not determined; c MMOA: molecular mechanism of action, NO: nitric oxide; d Country: AUS: Australia; BRA: Brazil; CHN: China; DEU: Germany; EGY: Egypt; ESP: Spain; FRA: France; IDN: Indonesia; ITA: Italy; JPN: Japan; MEX: Mexico; MYS: Malaysia; NLD: The Netherlands; NZL: New Zealand; PNG: Papua New Guinea; PHL: Phillipines; S.KOR: South Korea; TWN: Taiwan; e Chemistry: Polyketide; f Terpene; g Nitrogen-containing compound; h polysaccharide, modified as in the text.
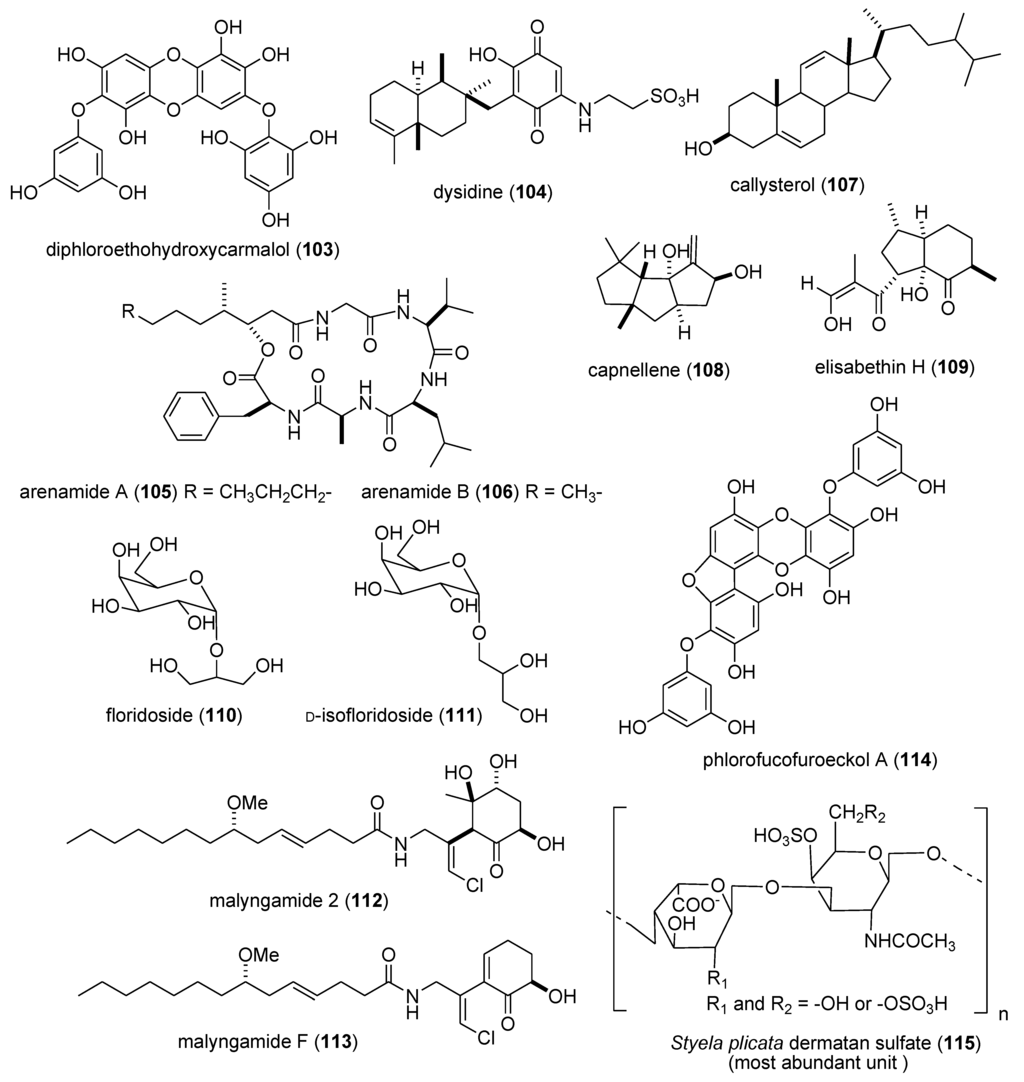
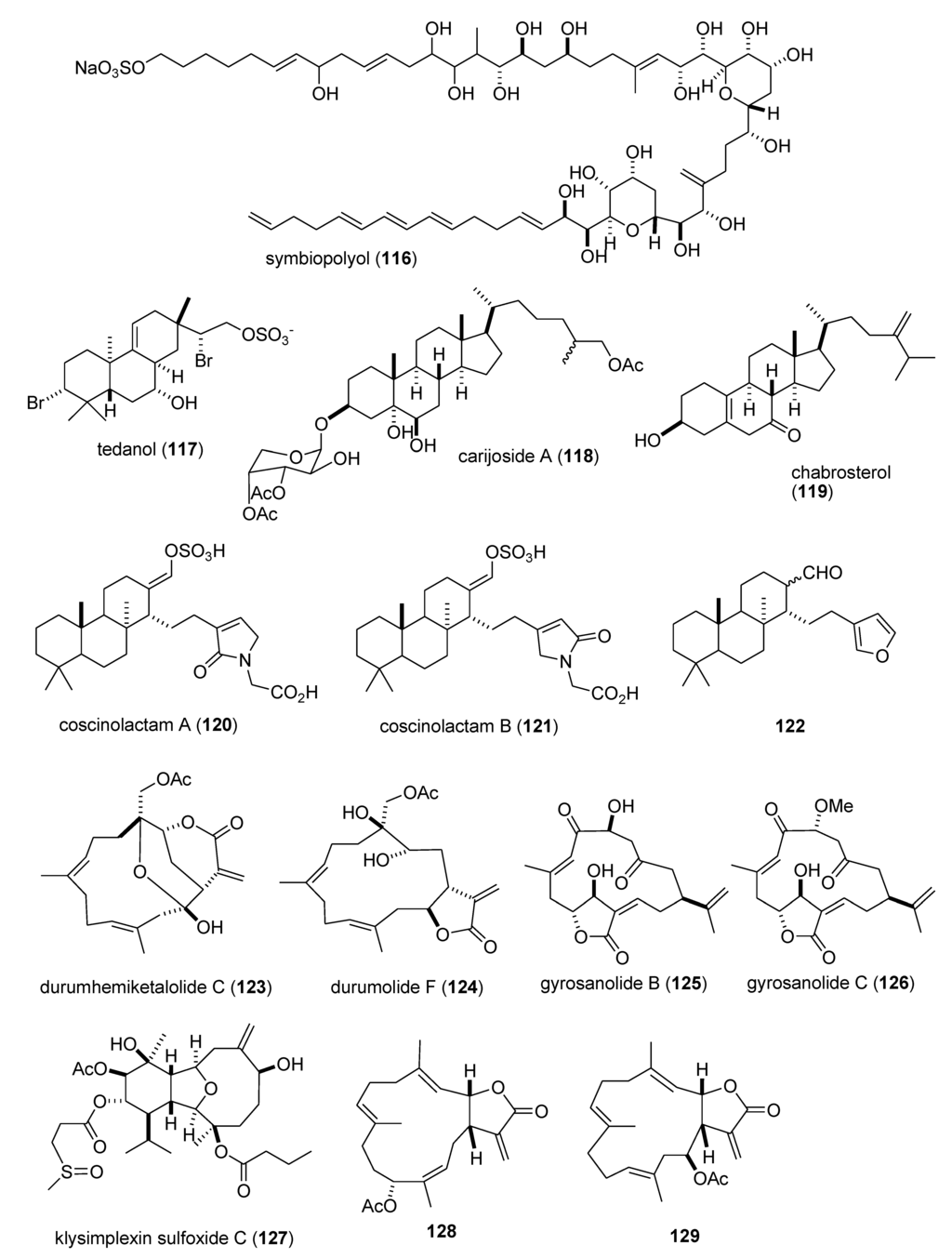
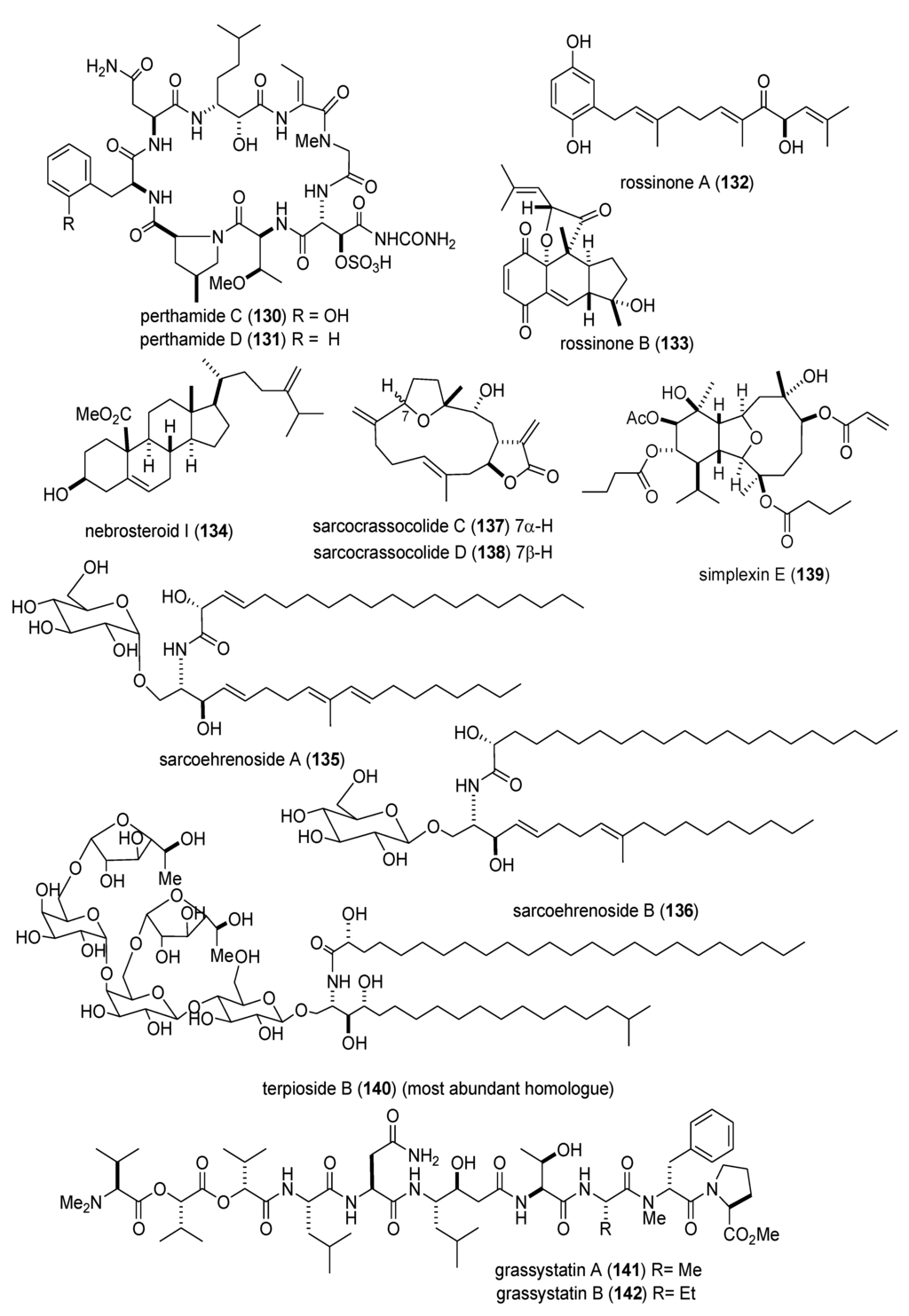
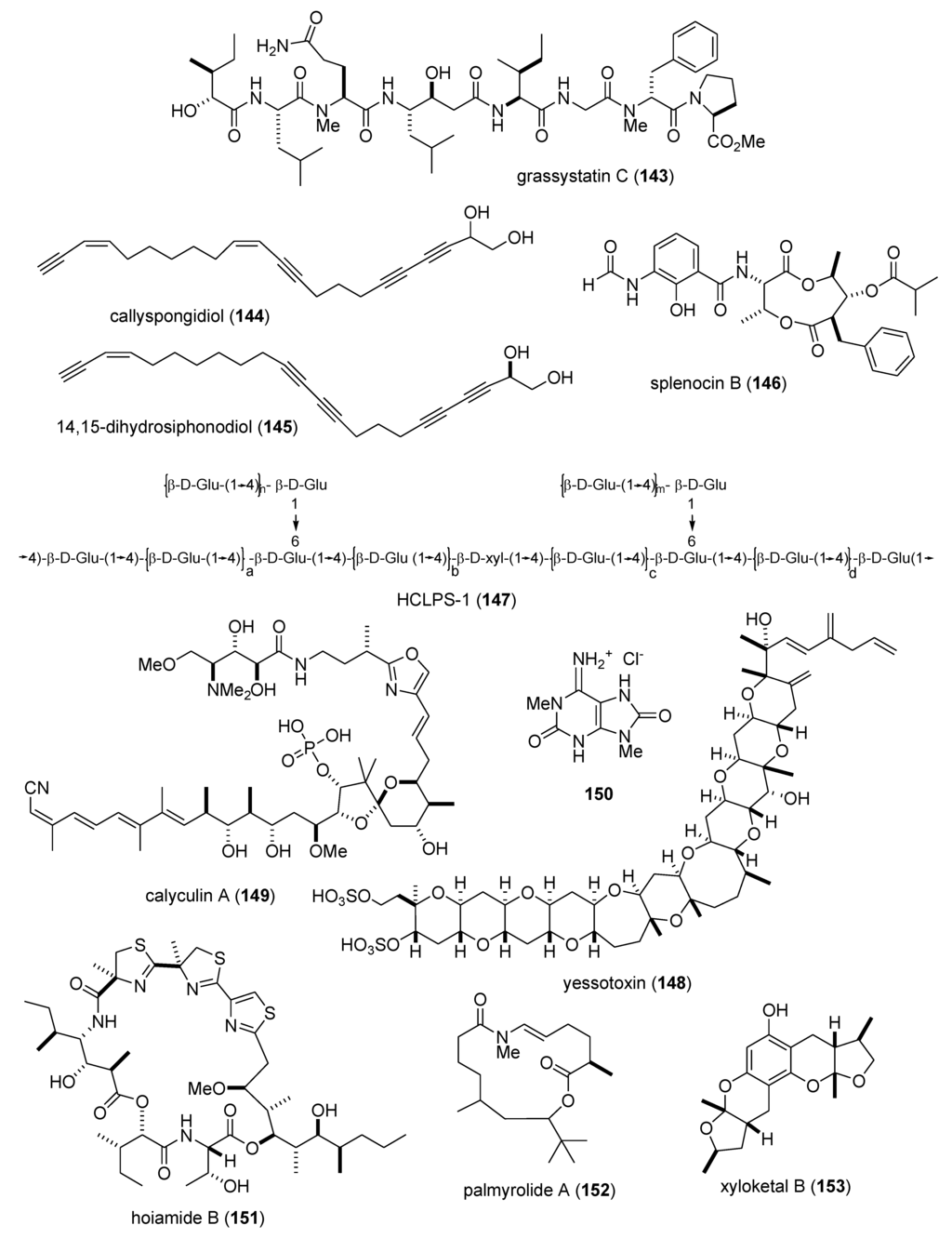
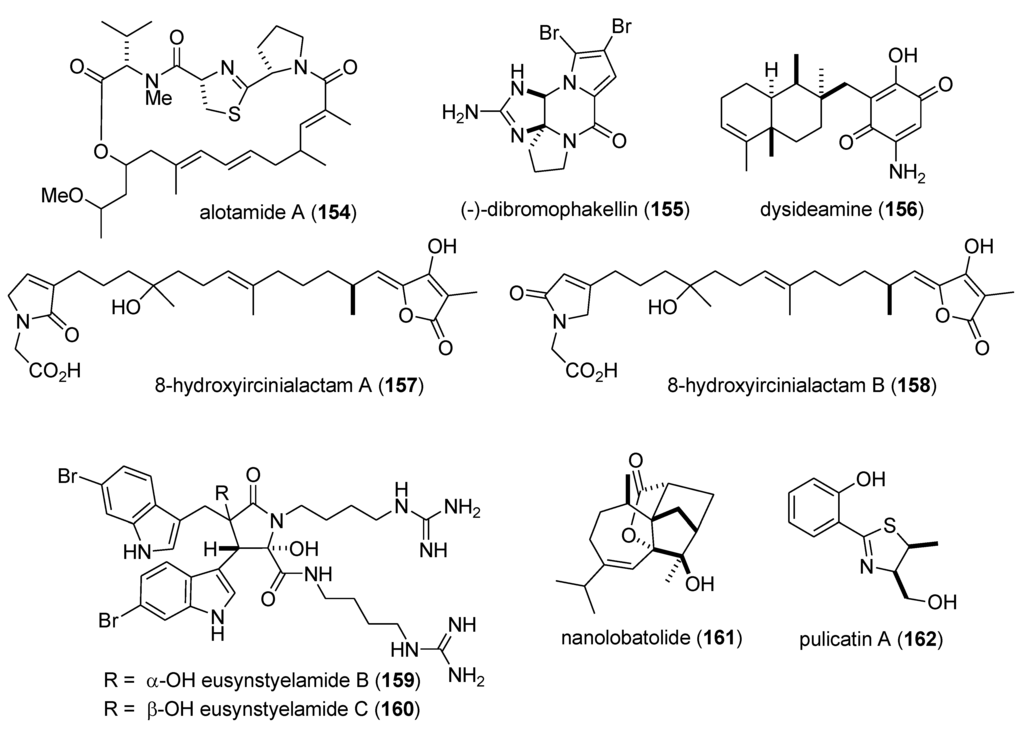
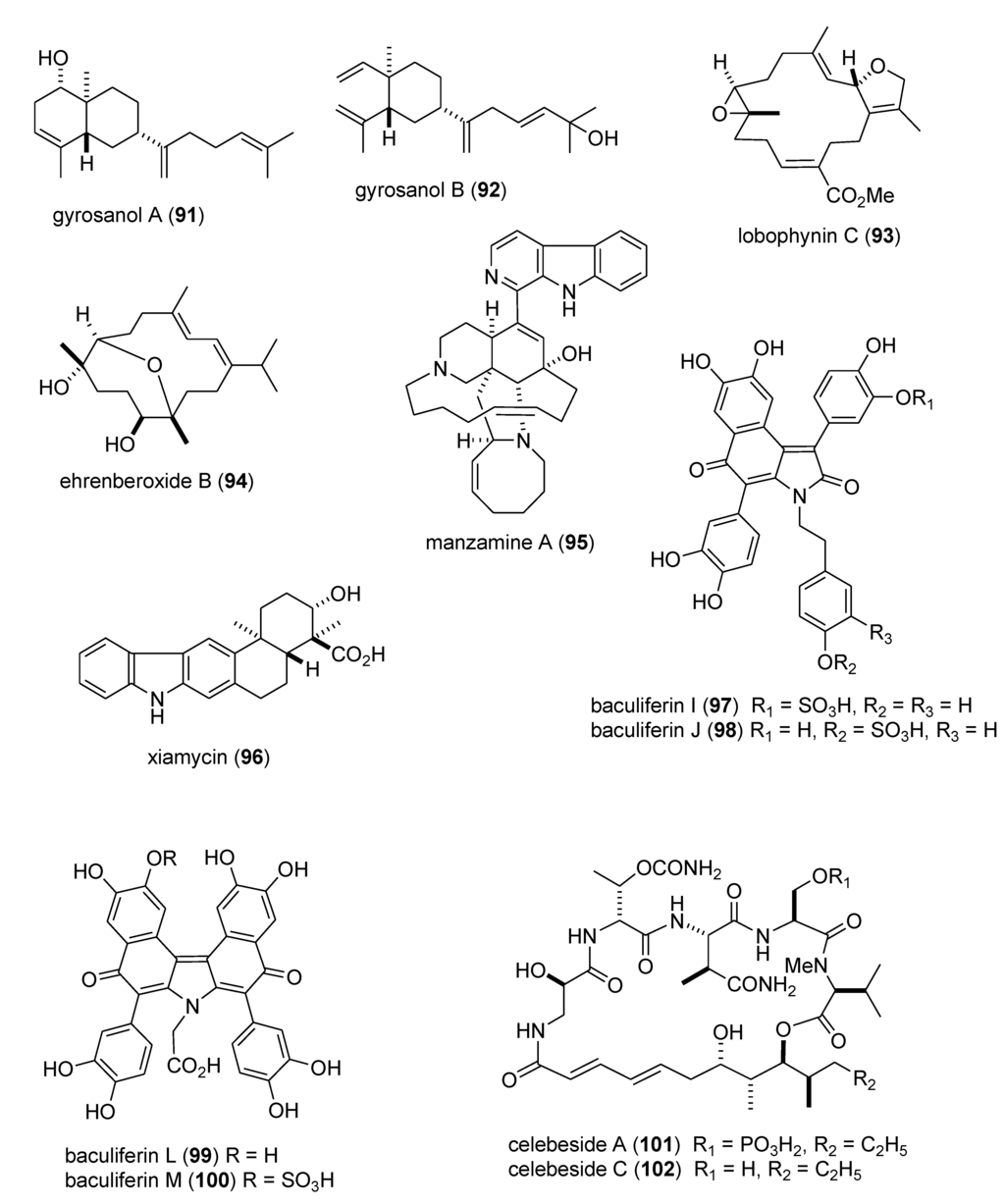
Figure 2.
Marine pharmacology in 2009–2011:Marine compounds with antidiabetic and anti-inflammatory activity; and affecting the immune and nervous system.
3.1. Antidiabetic Activity
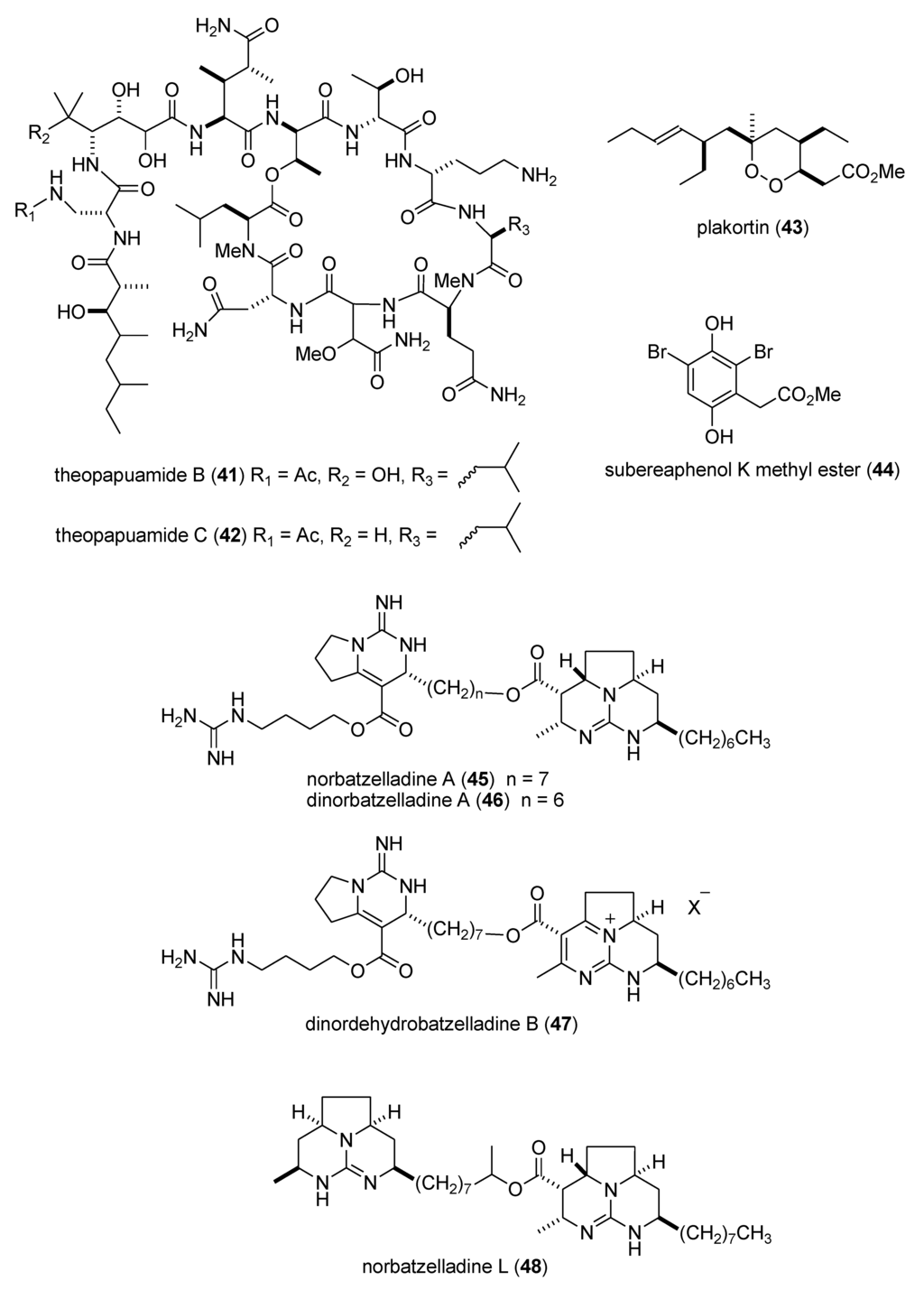
Heo and colleagues extended the pharmacology of diphlorethohydroxycarmalol [DPHC] (103), previously isolated from the marine brown alga Ishige okamurae, by showing that DPHC alleviated postprandial hyperglycemia in diabetic mice by potent inhibition of both α-glucosidase and α-amylase enzymes (IC50 = 0.16 and 0.53 nM, respectively) suggesting a possible use of DPHC “as a nutraceutical or functional food for diabetes” [104]. Li and colleagues evaluated the known sesquiterpene dysidine (104) from the Hainan marine sponge Dysidea villosa, and revealed that it activated the insulin pathway by inhibition of human protein phosphatase 1B (IC50 = 6.70 µM), a well characterized drug target for type-II diabetes and obesity treatment, as well as glucose uptake and glucose transporter 4 translocation in vitro [105,106].
3.2. Anti-Inflammatory Activity
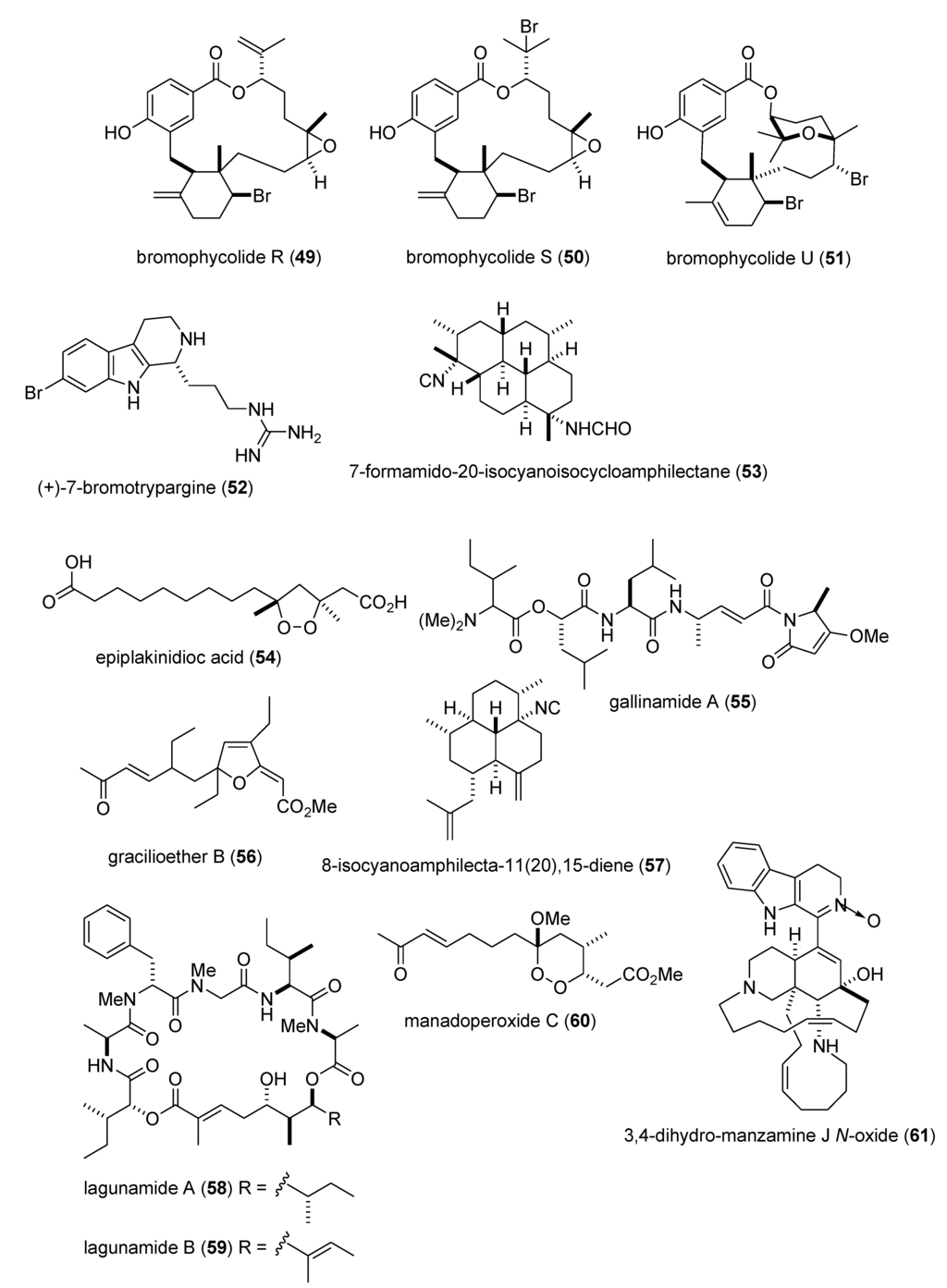
There was a remarkable increase in marine anti-inflammatory pharmacology research during 2009–2011. The molecular mechanism of action of several marine natural products, which were shown in preclinical pharmacological studies to target neutrophils and macrophages both in vitro and in vivo, was reported in several publications. Asolkar and colleagues described two new cyclohexadepsipeptides arenamides A and B (105,106), isolated from the Fijian bacterium Salinispora arenicola, that inhibited LPS-induced murine macrophage RAW 264.7 cells PGE2 and NO production in vitro, by affecting NFκB signaling activity (IC50 = 3.7 and 1.7 μM, respectively), thus highlighting their “anti-inflammatory characteristics” [107]. Three publications yielded potentially novel compounds targeting proinflammatory mediators released by activated brain microglia, a macrophage involved in neuroinflammation and neurodegeneration [151]: Youssef and colleagues described a new steroid callysterol (107) from the Red Sea sponge Callyspongia siphonella, which potently inhibited rat hind paw edema with an activity close to cortisone, and also reduced TXB2 release from LPS-activated rat brain microglia (apparent IC50 > 10 μM) [108]. Jean and colleagues observed that the sesquiterpene capnellene (108) isolated from the Indonesian soft coral Capnella imbricate, attenuated expression of inducible cyclooxygenase-2 both in activated microglia in vitro and in vivo, suggesting it might contribute to “the search for new therapeutic agents for treatment of neuroinflammatory diseases” [109]. Shi and colleagues isolated a new terpene, elisabethin H (109) from the Caribbean gorgonian octocoral Pseudopterogorgia elisabethae, which significantly inhibited superoxide anion (O2–) generation from E. coli LPS activated rat neonatal microglia in vitro (IC50 = 7 μM) [110]. Li and colleagues reported that the floridosides (110,111), isolated from the South Korean marine red alga Laurencia undulata, possessed significant antioxidant capacity and inhibited the proinflammatory matrix metalloproteinases MMP-2 and MMP-9, thus suggesting they might be candidates for further development as natural marine antioxidants [111]. Three publications investigated inhibition of pro-inflammatory mediators released by activated macrophage cell lines: Malloy and colleagues reported that the lipopeptide malyngamide 2 (112) from the Papua New Guinea marine cyanobacterium Lyngbya sordida inhibited nitric oxide production in LPS-primed RAW 264.7 macrophage cells (IC50 = 8.0 μM) [112]. In a detailed mechanistic study Villa and colleagues investigated the lipopeptide malyngamide F (113) from the marine cyanobacterium Lyngbya majuscula showing that it inhibited nitric oxide production in LPS-primed RAW 264.7 macrophage cells (IC50 = 7.1 μM) by selectively inhibiting the MyD88-dependent pathway of TLR4 and 9, thus potentially becoming a “useful tool” in cellular biology [113]. Kim and colleagues extended previous studies with the phlorotannin phlorofucofuroeckol A (PFF-A) (114), isolated from the Korean brown alga Ecklonia stolonifera, by demonstrating its antioxidant activity was of similar potency to vitamin C, and that it inhibited nitric oxide and PGE2 production (apparent IC50 = 5–10 μM) by downregulation of iNOS and COX-2 protein expression in LPS-primed RAW 264.7 macrophage cells [114]. Using an in vivo rat colitis model, Belmiro and colleagues provided a detailed molecular characterization of the anti-inflammatory properties of a dermatan sulfate (115), analog of mammalian heparin, purified from the Brazilian ascidian Styela plicata, which at 8 mg/kg per day significantly decreased lymphocyte and macrophage recruitment as well as TNF-α, TGF-β, and VEGF production in the inflamed rat colon [115]. Hanif and colleagues reported that the highly hydroxylated long-chain sulfate symbiopolyol (116), isolated from a symbiotic dinoflagellate of the jellyfish Mastigias papua significantly inhibited (K50 = 6.6 μM) the expression of the inducible adhesion vascular cell adhesion molecule-1 which binds to leukocytes present in early stages of inflammation, and thus might become a “potential anti-inflammatory agent” [116]. Costantino and colleagues reported that tedanol (117), a new brominated and sulfated pimarane diterpene isolated from the Caribbean sponge Tedania ignis, significantly reduced both the acute and subchronic phases of carrageenan-induced inflammation at 1 mg/kg with concomitant inhibition of both COX-2, iNOS expression and cellular infiltration [117].
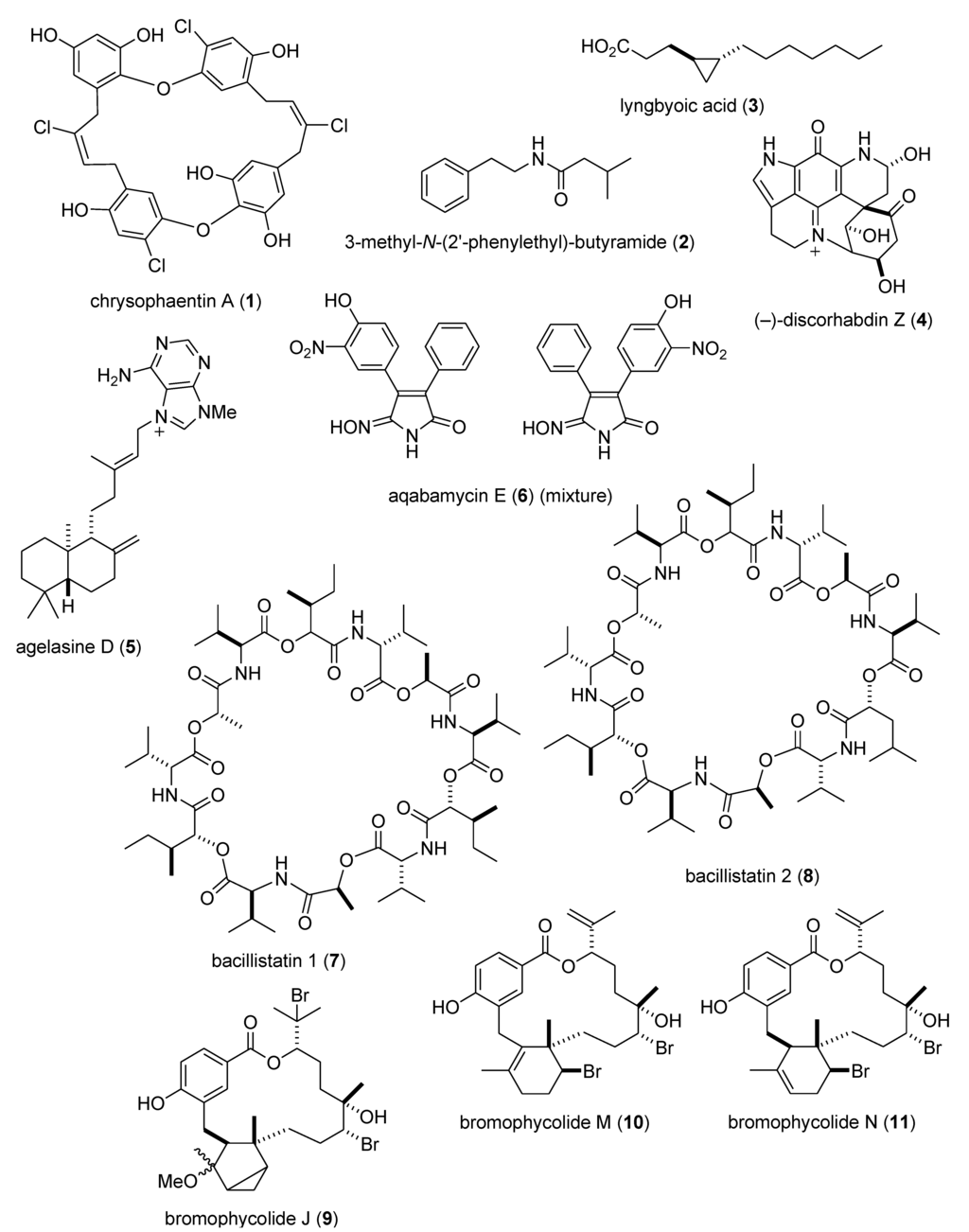
As shown in Table 2 and in contrast to the marine anti-inflammatory compounds previously discussed, while an anti-inflammatory activity and IC50 were reported, the molecular mechanism of action remained undetermined for the following marine compounds: carijoside A (118) [118]; chabrosterol (119) [119]; coscinolactams (120–122) [120]; durumhemiketalolide C (123) [121]; durumolide F (124) [122]; gyrosanolides B and C (125,126) [123]; klysimplexin sulfoxide C (127) [124]; L. crassum diterpenes (128,129) [125]; perthamides C and D (130,131) [126]; rossinones A and B (132,133) [127]; nebrosteroid I (134) [128]; sarcoehrenosides A and B (135,136) [129]; sarcocrassocolides A & B (137,138) [130]; simplexin E (139) [131]; and terpioside B (140) [132].
3.3. Marine Compounds with Activity on the Immune System
In 2009–2011, immune system pharmacology of marine compounds showed a considerable decrease from our previous review.
Kwan and colleagues isolated the linear peptides grassystatins A–C (141–143) from a marine cyanobacterium identified as Lyngbya cf. confervoides, demonstrating that the compounds selectively inhibited aspartic protease cathepsin E (IC50 = 0.3–0.8 nM) versus cathepsin D, as well as antigen-stimulated T cell proliferation, with a concomitant reduction of IL-17 and interferon γ [133]. Takei and colleagues reported that the known compounds callyspongidiol (144) and 14,15-dihydrosiphonodiol (145), isolated from a marine sponge Callyspongia sp., activated both functional and phenotypic maturation of human monocyte-derived dendritic cells, as well as higher interleukin-10 production by T cells, thus revealing potential use in autoimmune diseases and cancer [134]. Shim and colleagues observed that the phloroglucinol derivative phlorofucofuroeckol A (PFF-A) (114), purified from the Korean marine seaweed Ecklonia stolonifera, reduced the expression of the human basophil FcεR1 receptor (apparent IC50 = 25 µM), as well as intracellular [Ca2+]i and histamine release, findings which may be relevant for regulation of IgE-mediated allergic reactions [135]. Strangman and colleagues discovered novel splenocin B (146) isolated from the marine bacterium Streptomyces species strain CNQ431 that displayed potent inhibition of murine splenocyte-derived TH2 cytokines interleukin 5 and 13 (IC50 = 1.6–1.8 nM), thus contributing to the development of a “splenocin-derived drug” for allergic inflammation [136]. Dai and colleagues isolated a water soluble polysaccharide HCLPS-1 (147) from the Chinese pearl-producing mollusc Hyriopsis cumingii Lea, which stimulated murine spleen lymphocyte proliferation in vitro and in vivo in a concentration-dependent manner (apparent IC50 less than 20 mg/kg), suggesting HCLPS-1 might become a “potential natural immunomodulator” upon further pharmacological study [137]. Orsi and colleagues contributed to the immunopharmacology of the sulfated dinoflagellate polyether yessotoxin (148), by demonstrating that it decreased macrophage phagocytic activity against the fungus Candida albicans (apparent IC50 = 1 nM), affected the cytoskeleton by inducing F-actin re-organization, and enhanced release of the cytokine TNF-α and chemokines MIP-1α and MIP-2 [138].
3.4. Marine Compounds Affecting the Nervous System
As shown in Table 2, the nervous system pharmacology of marine natural products in 2009–2011 involved some areas of neuropharmacology, namely neuronal neurite retraction, neurotransmission inhibition, neuronal Ca2+ oscillations and free radical inhibition.
Marine natural products have previously been reported to affect neuritogenesis [7], a process required by neurons to respond to the extracellular environment to form synaptic connections. Inutsuka and colleagues contributed novel molecular studies on the effect of calyculin A (149) on neurons, demonstrating that rapid rat hippocampal neuron neurite retraction (apparent IC50 = 100 mM) induced by the toxin was dependent on actin filament polymerization or myosin II motor, yet independent of the microtubule polymerization status, and perhaps resulted from dephosphorylation of myosin light chain kinase [139]. Sakurada and colleagues reported that the novel Palauan sponge Cribrochalina olemda purine (150), which elicited convulsions upon intracerebroventricular injections in mice (4 nM/mouse), inhibited GABAergic transmission in hippocampal neurons [140]. Noteworthy was the author’s observation that this marine purine was “closely related in structure to endogenous neurosignaling molecules and commonly used CNS stimulants”.
As shown in Table 2, two marine compounds (151,152) identified as part of a drug discovery screening program, were shown to inhibit neuronal Ca2+ oscillations, a network phenomenon that appears to depend on voltage-gated sodium channel activation. Choi and colleagues reported that a cyclic depsipeptide hoiamide B (151), isolated from a Papua New Guinean cyanobacteria assemblage of Symploca sp. and Oscillatoria cf. sp., stimulated sodium influx (IC50 = 3.9 µM) in murine neuronal cells in vitro by putative activation of site 2 on the sodium channel, while suppressing spontaneous Ca2+ (IC50 = 79.8 nM) with greater potency, thus revealing that hoiamide B may have more than one molecular target [141]. Pereira and colleagues contributed a novel marine macrolide palmyrolide A (152) isolated from Northern Pacific Palmyra Atoll cyanobacteria assemblages of Leptolyngbya cf. and Oscillatoria sp. that inhibited both sodium influx (IC50 = 5.2 µM) in mouse neuroblastoma cells and spontaneous Ca2+ oscillations (IC50 = 3.7 µM) in primary cultures of murine cerebrocortical neurons, “making it an intriguing candidate for further pharmacological exploration” [142]. Zhao and colleagues reported that the known compound xyloketal B (153), isolated from the marine mangrove fungus Xylaria sp., inhibited ischemia-induced PC12 cell injury (IC50 = 100 µM) by a neuroprotective mechanism involving free radical scavenging and reduction of mitochondrial membrane potential and superoxide generation, suggesting further development for effective stroke therapy [143].
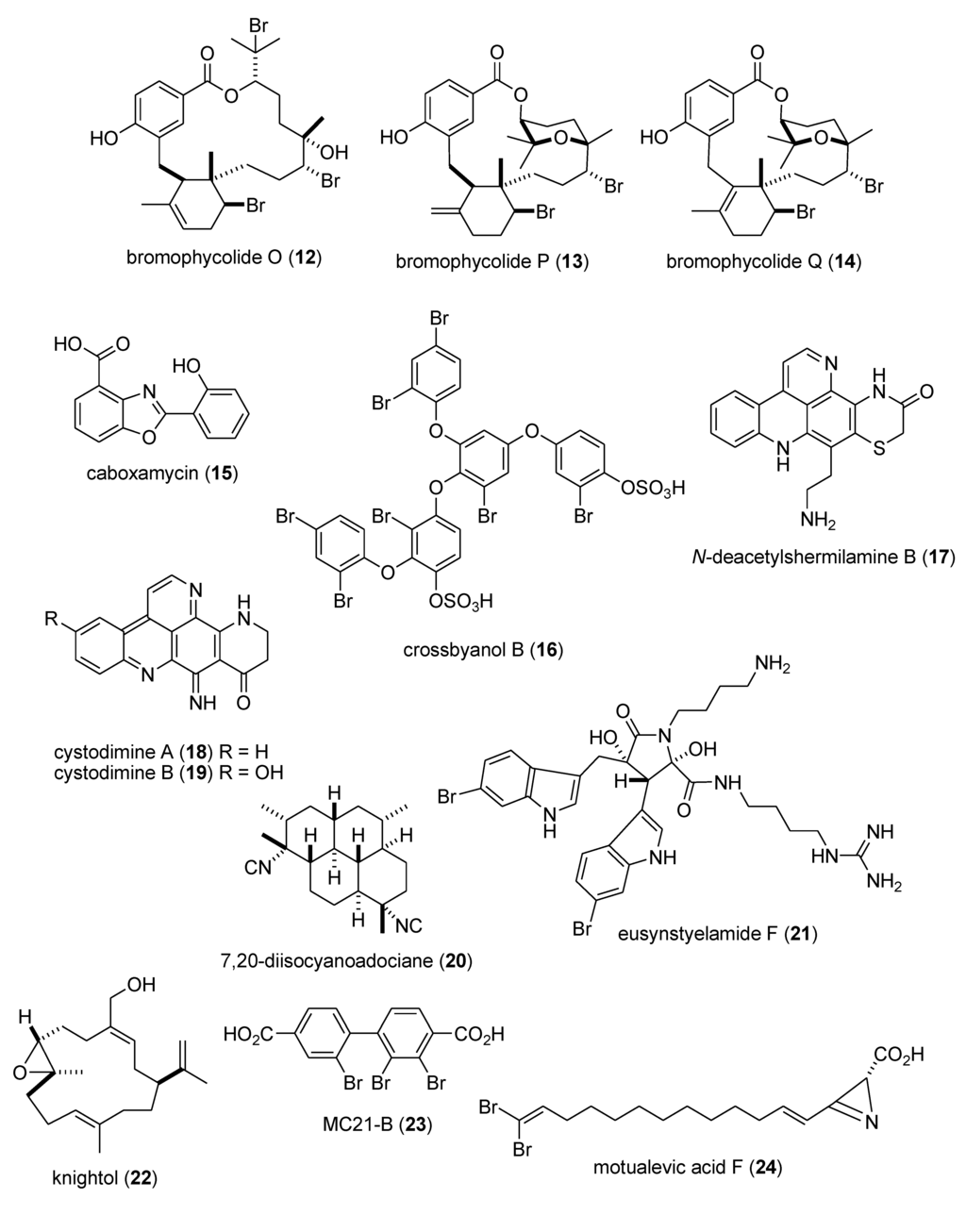
Finally, and as presented in Table 2, during 2009–2011, several other marine compounds were reported to affect the nervous system by demonstrating pharmacological activity on Ca2+ oscillations, several receptors, and neuronal nitric oxide synthase, yet the mechanism of action of these compounds remained undetermined: alotamide A (154) [144], (−)-dibromophakellin (155) [145], dysideamine (156) [146], ircinialactams (157,158) [147], eusynstyelamides B and C (159,160) [148], nanolobatolide (161) [149], and pulicatin A (162) [150].
4. Marine Compounds with Miscellaneous Mechanisms of Action
Table 3 presents the 2009–2011 preclinical pharmacology of 68 marine compounds (163–226) with miscellaneous mechanisms of action, with their respective structures shown in Figure 3. Because additional in vitro and in vivo pharmacological data for these compounds remained unpublished, assignment of these marine compounds to a particular drug class was not possible.
As shown in Table 3, the peer-reviewed literature reported a pharmacological activity, an IC50, and a molecular mechanism of action for 21 marine natural products: bisebromoamide (163) [152]; botryllamides I and J (164,165) [153]; dysidine (104) [106]; Ecklonia cava phlorotannins (166,167) [154]; fucophlorethols (168,169) [155]; gonad-stimulating substance (GSS) (170) [156]; halichlorine (171) [157]; hoiamide A (172) [158]; hypochromins A and B (173,174) [159]; mycothiazole (175) [160]; Mycale sp. pyrroles (176,177) [161]; neocomplanines A and B (178,179) [162]; pateamine A (180) [163]; polytheonamide B (181) [164]; and zampanolide (182) [165].
In contrast, although a pharmacological activity was described, and an IC50 for inhibition of an enzyme or receptor determined, detailed molecular mechanism of action studies were unavailable at the time of publication for the following 47 marine compounds included in Table 3: alotaketals A and B (183,184) [166]; aquastatin A (185) [167]; australin E (186) [168]; lyngbyastatins 9 & 10 (187,188) [169]; brunsvicamides A, B and C (189–191) [170]; carteriosulfonic acids A, B and C (192–194) [171]; Carteriospongia foliascens sesterterpenoids (195,196) [172]; clavatadines D and E (197,198) [173]; fibrosterol sulfates A and B (199,200) [174]; gracilin L (201) [175]; grassystatins A, B and C (141–143) [133]; 2-hydroxycircumdatin C (202) [176]; jaspaquinol (203) [177]; largamides A, B and C (204–206) [178]; largamide D oxazolidine (207) [179]; Laurencia similis brominated metabolites (208,209) [180]; molassamide (210) [181]; myrothenone A (211) [182]; 42-hydroxy-palytoxin (212) [183]; plectosphaeroic acids A, B and C (213–215) [184]; puupehenone (216) [177]; Sinularia numerosa oxylipin (217) [185]; sipholenone E (218) [186]; spartinoxide (219) [187]; 23-nor-spiculoic acid B (220) [188]; tanikolide dimer (221) [189]; tamulamides A and B (222,223) [190]; terretonins E and F (224,225) [191]; and tetrangulol methyl ether (226) [192].

Table 3.
Marine pharmacology in 2009–2011: Marine compounds with miscellaneous mechanisms of action.
| Compound/Organism a | Chemistry | Pharmacological Activity | IC50 b | MMOA c | Country d | References |
|---|---|---|---|---|---|---|
| bisebromoamide (163)/cyanobacterium | Peptide g | In vitro tumor growth inhibition | 0.040 μM | ERK inhibition | JPN | [152] |
| botryllamide I & J (164,165)/ascidian | Shikimate g | Multidrug resistance inhibition | 27–41 μM | ABCG2 transporter inhibition | USA | [153] |
| dysidine (104)/sponge | Terpene f | Insulin pathway activation | 10 μM | Protein tyrosine phosphatase 1B inhibition | CHN | [106] |
| E. cava phlorotannins (166,167)/alga | Polyketide e | In vitro antioxidants | DPPH, hydroxyl, peroxyl, & superoxide scavenging | CHN, S. KOR | [154] | |
| fucophlorethols (168,169)/alga | Polyketide e | DPPH radical scavenging | 10–14 μM | Cytochrome P450 CYP1A inhibition | DEU, ISR | [155] |
| GSS (170)/starfish | Peptide g | Oocyte maturation and ovulation | 2 nM | cAMP production | JPN | [156] |
| halichlorine (171)/sponge | Alkaloid (polyketide) g | Inhibition of vascular contractility | 3 μM * | L-type Ca2+ channel inhibition | JPN | [157] |
| hoiamide A (172)/bacterium | Peptide g | Voltage-gated sodium channel activator | 2.3 μM | Sodium channel site 2 activator | USA | [158] |
| hypochromin A & B (173,174)/fungus | Polyketide e | Angiogenesis inhibition | 13 & 50 μM | Tyrosine kinase inhibition | JPN | [159] |
| mycothiazole (175)/sponge | PKS/NRPS | Angiogenesis inhibition | 10 nM * | Mitochondrial complex 1 inhibition | USA | [160] |
| Mycale sp. metabolites (176,177)/sponge | Polyketide e | Hypoxia-inducible factor-1 inhibition | 7.8–8.6 μM | Mitochondrial electron transport chain inhibition | USA | [161] |
| neocomplanines A & B (178,179)/fireworm | Polyketide e | Murine footpad inflammation | ND | PKC activation | JPN | [162] |
| pateamine A (180)/sponge | PKS/NRPS | Nonsense-mediated mRNA inhibition | 100 nM * | Binding to eukaryotic initiation factor 4AIII | DEU, USA | [163] |
| polytheonamide B (181)/sponge | Peptide g | Cytotoxic mammalian channel formation | 14–29 nM | Selectivity towards Cs + cation | JPN | [164] |
| zampanolide (182)/sponge | Polyketide e | G2/M cell cycle arrest | 8 nM * | Microtubule bundle formation by tubulin polymerization | NZL | [165] |
| alotaketals A & B (183,184)/sponge | Terpene f | cAMP cell signaling activation | 18 & 240 nM | Undetermined | CAN, NLD, PAP | [166] |
| aquastatin (185)/fungus | Polyketide e | Protein phosphatase 1B inhibition | 0.19 μM | Undetermined | S. KOR | [167] |
| australin E (186)/soft coral | Terpene f | Inositol 5-phosphatase SHIP1 activation | >100 μM | Undetermined | CAN | [168] |
| lyngbyastatins 9 & 10 (187,188)/bacterium | Peptide g | Elastase and chymotrypsin inhibition | 0.2–9.3 μM | Undetermined | USA | [169] * |
| brunsvicamides A–C (189–191)/bacterium | Peptide g | Elastase inhibition | 2.0–4.4 μM | Undetermined | DEU | [170] |
| carteriosulfonic acids A, B & C (192–194)/sponge | Polyketide e | GSK-3β inhibition | 6.8–12.5 µM | Undetermined | SGP, USA | [171] |
| Carteriospongia foliascens sesterterpenoids (195,196)/sponge | Terpene f | Human Ras-converting enzyme inhibition | 4.2 μg/mL * | Undetermined | CAN, IDN,NLD, USA | [172] |
| clavatadines D & E (197,198)/sponge | Shikimate g | Factor XIa inhibition | 222 μM * | Undetermined | AUS | [173] |
| fibrosterol sulfates A & B (199,200)/sponge | Terpene f | Protein Kinase Cζ inhibition | 5.6 & 16.4 µM | Undetermined | PHL, USA | [174] |
| gracilin L (201)/sponge | Terpene f | EGF-R tyrosine kinase inhibition | <100 μM * | Undetermined | GBR, LUX | [175] |
| grassystatins A–C (141–143)/bacterium | Peptide g | cathepsin E inhibition | 0.3–43 nM | Undetermined | USA | [133] |
| 2-hydroxycircumdatin C (202)/fungus | Alkaloid g | DPPH radical scavenging activity | 9.9 µM | Undetermined | CHN | [176] |
| jaspaquinol (203)/sponge | Terpene f | 5-lipoxygenase inhibition | 0.45 µM | Undetermined | USA | [177] |
| largamides A–C (204–206)/bacterium | Peptide g | Elastase inhibition | 0.53–1.41 µM | Undetermined | USA | [178] |
| largamide D oxazolidine (207)/bacterium | Peptide g | Elastase and chymotrypsin inhibition | 0.9–1.5 μM | Undetermined | USA | [179] |
| Laurencia similis brominated metabolites (208,209)/alga | Polyketide e | Protein phosphatase 1B inhibition | 2.7–3 μM | Undetermined | CAN, CHN | [180] |
| molassamide (210)/bacterium | Peptide g | Elastase and chymotrypsin inhibition | 0.03 & 0.23 μM | Undetermined | USA | [181] |
| myrothenone A (211)/fungus | Polyketide e | Tyrosinase inhibition | 6.6 μM | Undetermined | S. KOR | [182] |
| 42-hydroxy-palytoxin (212)/soft coral | PKS/NRPS | Na+/K+ pump inhibition | 28 ± 7 nM | Undetermined | ITA, USA | [183] |
| plectosphaeroic acids A–C (213–215)/fungus | Alkaloid g | Indoleamine 2, 3 dioxygenase inhibtion | 2 μM * | Undetermined | CAN | [184] |
| puupehenone (216)/sponge | Terpene f | 5-lipoxygenase inhibition | 0.68 μM | Undetermined | USA | [177] |
| Sinularia numerosa oxylipin (217)/soft coral | Fatty acid e | Angiogenesis inhibition | 20–40 μM | Undetermined | JPN | [185] |
| sipholenone E (218)/sponge | Terpene f | P-glycoprotein multidrug resistance reversal | 5.7–62 nM | Undetermined | EGY, CHN, USA | [186] |
| spartinoxide (219)/fungus | Terpene f | Human elastase inhibition | 6.5 μM | Undetermined | DEU | [187] |
| 23-nor-spiculoic acid B (220)/sponge | Polyketide e | NFκB inhibition | 0.47 μM | Undetermined | VEN, USA | [188] |
| tanikolide dimer (221)/bacterium | Polyketide e | Human sirtuin type 2 inhibition | 0.176–2.4 μM | Undetermined | DEU, S. KOR, USA | [189] |
| tamulamide A & B (222,223)/dinoflagellate | Polyketide(polyether) e | Brevetoxin-3 binding inhibition | 0.2–2.5 μM | Undetermined | USA | [190] |
| terretonins E & F (224,225)/fungus | Terpene f | NADH oxidase inhibition | 2.9–3.9 μM | Undetermined | ESP, ITA | [191] |
| tetrangulol methyl ether (226)/bacterium | Polyketide e | Quinone reductase-2 inhibition | 0.16 μM | Undetermined | USA | [192] |
a Organism, Kingdom Animalia: ascidian (Phylum Chordata), fireworm (Phylum Annelida), soft corals (Phylum Cnidaria), starfish ( Phylum Echinodermata), sponge (Phylum Porifera); Kingdom Chromalveolata: dinoflagellates; Kingdom Fungi: fungus; Kingdom Plantae: alga; Kingdom Monera: bacterium; b IC50: concentration of a compound required for 50% inhibition in vitro; *: estimated IC50; c MMOA: molecular mechanism of action; d Country: AUS: Australia; CAN: Canada; CHN: China; DEU: Germany; EGY: Egypt; ESP: Spain; GBR: United Kingdom; IDN: Indonesia; ISR: Israel; ITA: Italy; JPN: Japan; LUX: Luxembourg; NZL: New Zealand; NLD: The Netherlands; PHL: Phillipines; PAP: Papua New Guinea; SGP: Singapore; S. KOR: South Korea; ESP: Spain; VEN: Venezuela; e Chemistry: Polyketide; f Terpene; g Nitrogen-containing compound; *: Bouillamides A and B are identical with lyngbyastatins 9 and 10. See [193].
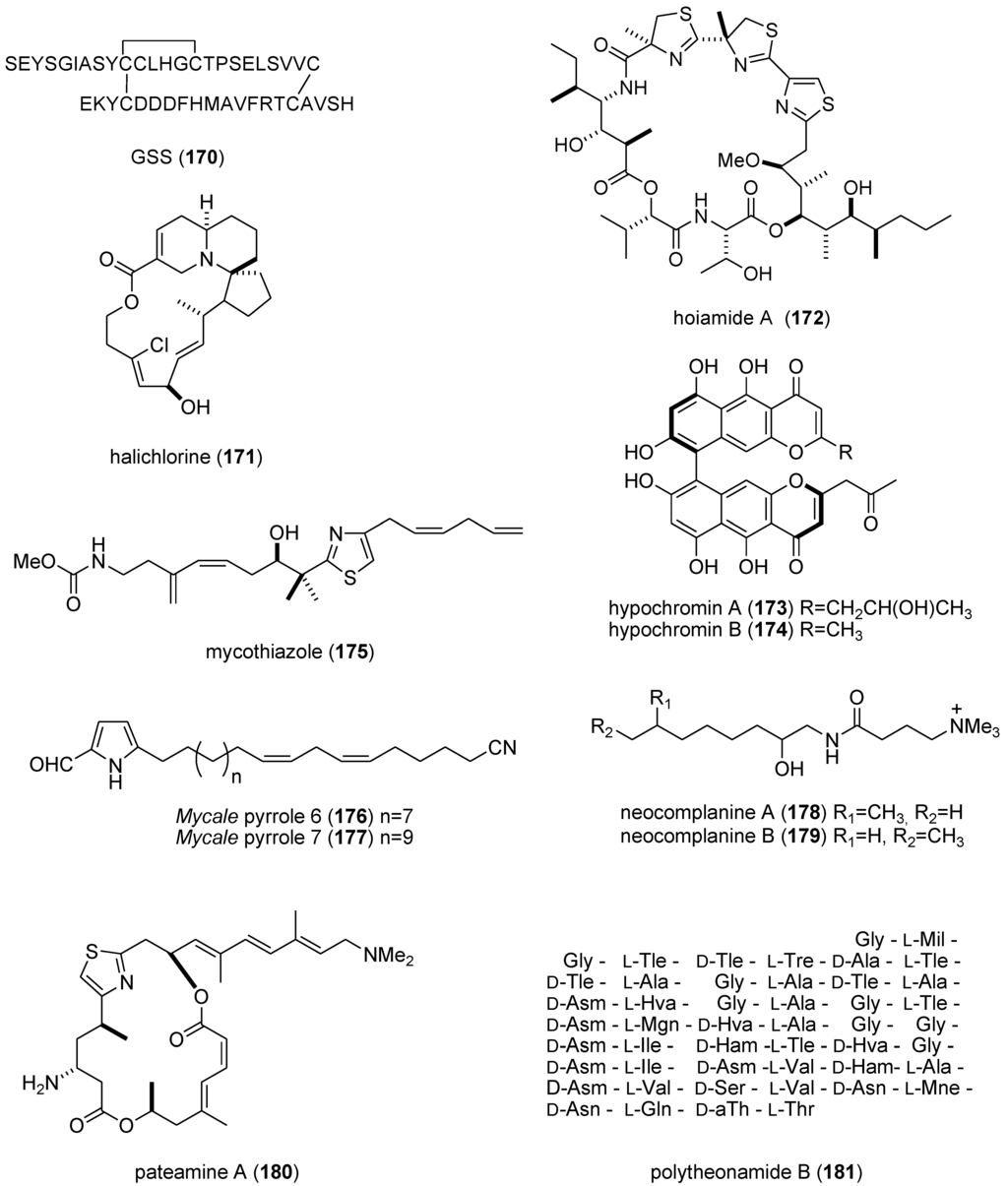
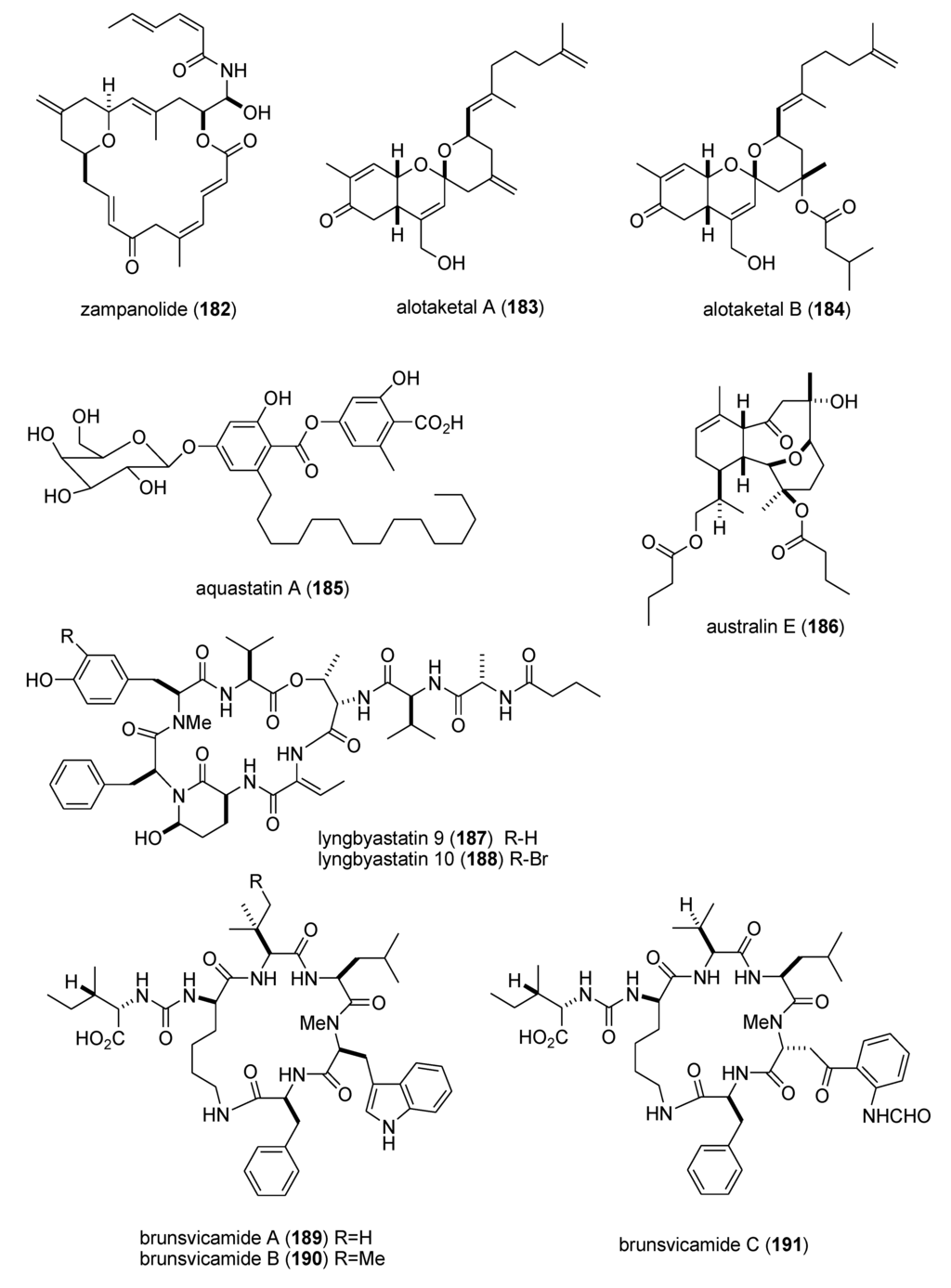
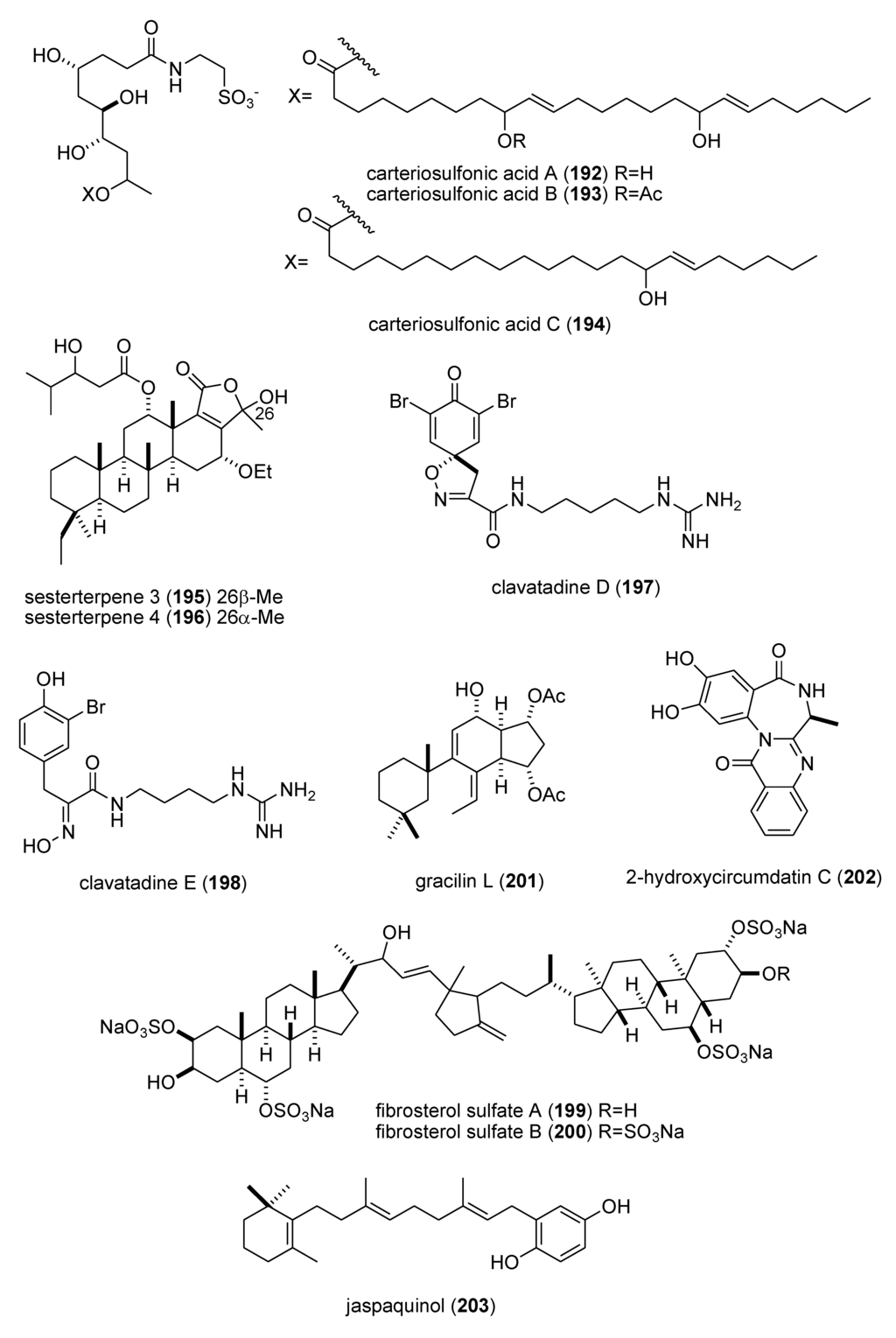
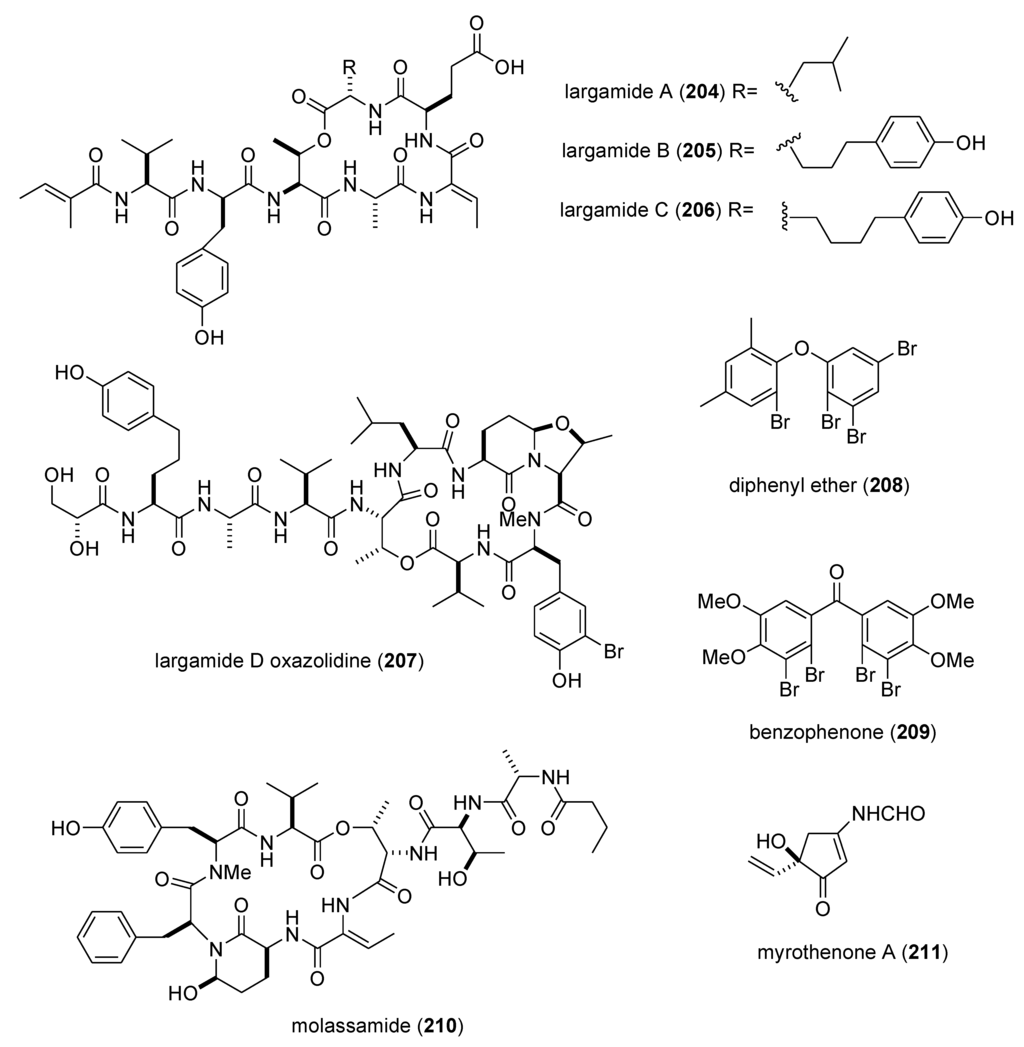
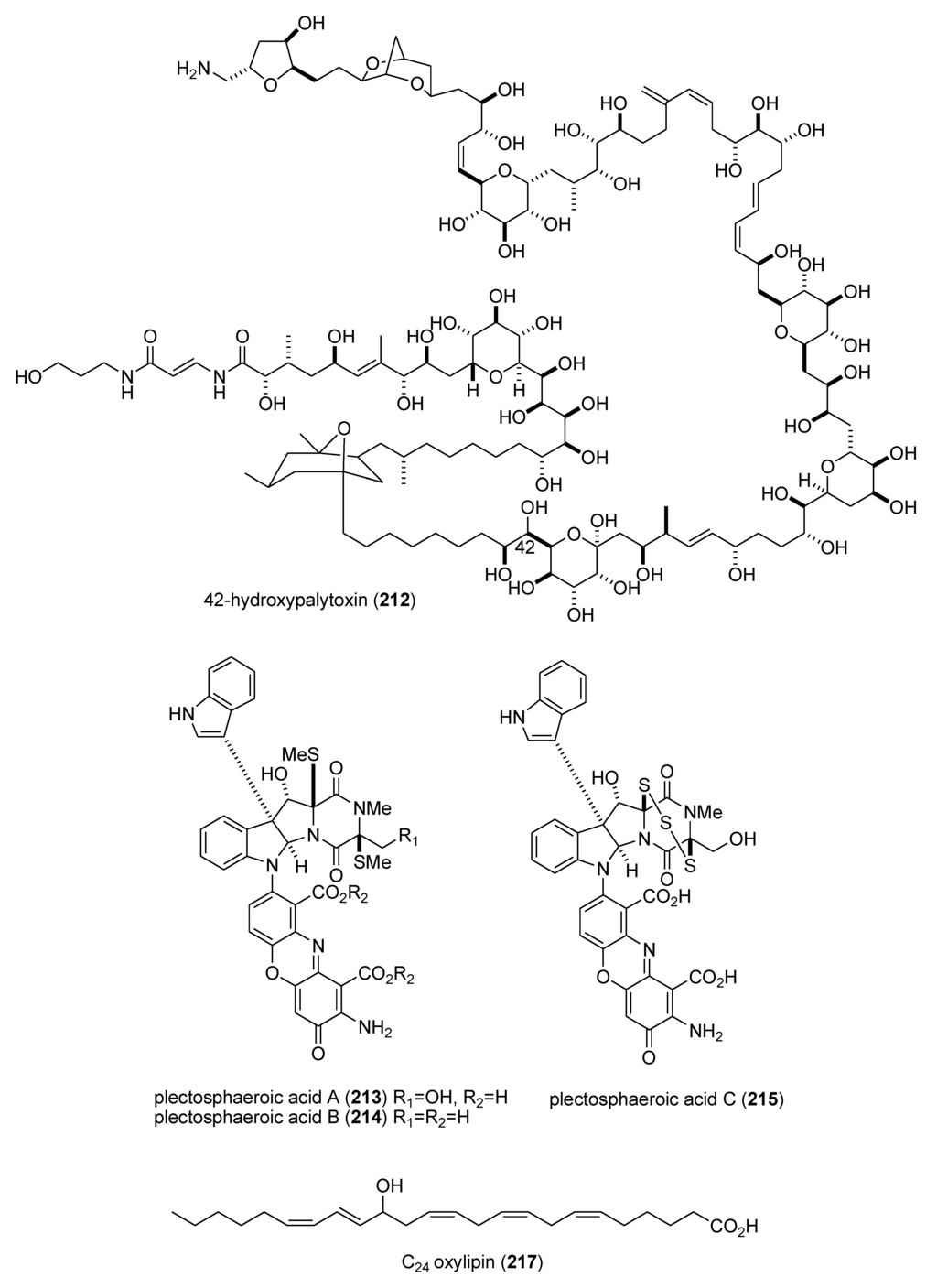
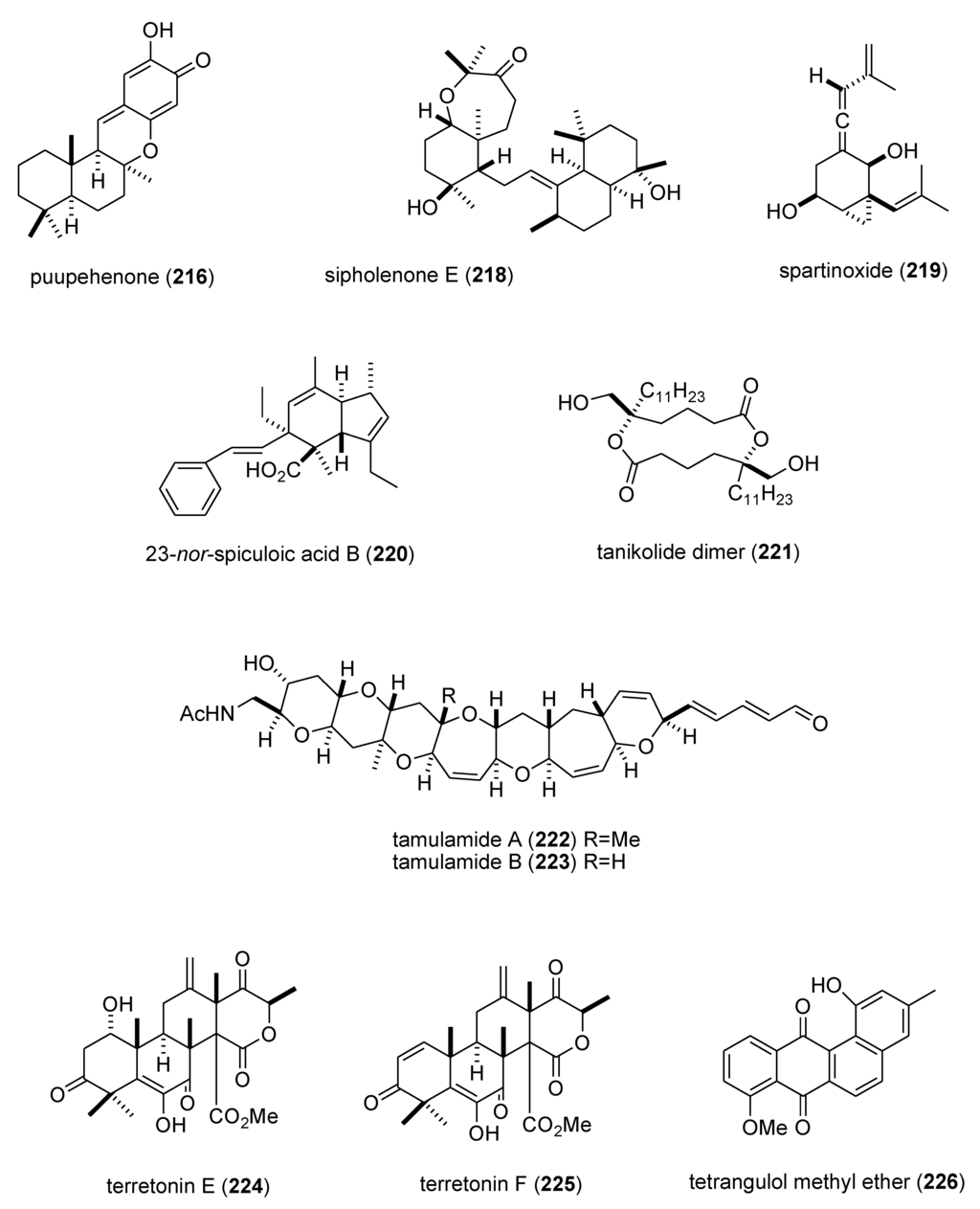
Figure 3.
Marine pharmacology in 2009–2011: Marine compounds with miscellaneous mechanisms of action.
5. Reviews on Marine Pharmacology
Several reviews covering both general and specific areas of marine preclinical pharmacology were published during 2009–2011: (a) marine pharmacology and marine pharmaceuticals: a renaissance in marine pharmacology: from preclinical curiosity to clinical reality [194]; biologically active marine natural products [195]; drug development from marine natural products [196]; biotechnological potential of marine natural products [197]; pharmaceuticals from marine natural products: surge or ebb? [198]; marine pharmacology in Australia: the Roche Research Institute [199]; the global marine pharmaceutical pipeline in 2010: U.S. Food and Drug Administration-approved compounds and those in Phase I, II and III of clinical development [200]; marine drugs from sponge-microbe associations [201]; cyanobacteria as an emerging source for drug discovery [202]; marine invertebrates as a future therapeutic treasure [203]; biodiversity conservation and marine natural products drug discovery [204]; marine invertebrates as a source of guanidines with chemical and pharmacological significance [205]; innovations in the field of marine natural products and a new wave of drugs [206]; (b) antimicrobial marine pharmacology: antibacterial marine natural products [207]; marine microbes and pharmaceutical development [208]; marine microbe-derived antibacterial agents [209]; antimicrobial peptides from marine invertebrates [210]; novel anti-infective compounds from marine bacteria [211]; conventional and unconventional antimicrobials from fish, marine invertebrates and microalgae [212]; (c) antiviral marine pharmacology: antiviral lead compounds from marine sponges [213]; potential anti-HIV agents from marine resources [214]; marine compounds and their antiviral activities [215]; marine organisms as a therapeutic source against herpes simplex virus infection [216]; (d) antiparasitic, antituberculosis, antimalarial and antifungal marine pharmacology: antiparasitic marine invertebrate-derived small molecules [217]; marine antileishmanial natural products [218]; antituberculosis leads from marine microbial metabolites [219]; antimalarial drug discovery from marine sources between January 2003 and December 2008 [220]; antimalarial marine natural products from 2006 to 2008 [221]; antimalarial marine compounds [222]; (e) immuno- and anti-inflammatory marine pharmacology: marine natural product leads for treatment of inflammation [223]; marine natural products targeting phospholipase A2 [224]; marine diterpene glycosides as anti-inflammatory agents [225]; anti-inflammatory compounds from marine algae [226]; (f) cardiovascular marine pharmacology: marine-derived angiotensin-I-converting enzyme inhibitors [227]; (g) nervous system marine pharmacology: conotoxins as natural products drug leads [228]; marine indole alkaloids as new drug leads for depression and anxiety [229]; marine natural products and ion channel pharmacology [230]; neuroprotective effects of marine algae [231]; conopeptides as novel options for pain management [232]; structure-activity studies with α-conotoxins as selective antagonists of nicotinic acetylcholine receptors [233]; (h) miscellaneous molecular targets: calyculins and related marine natural products as serine threonine protein phosphatase inhibitors [234]; NF-κB inhibition by marine natural products [235]; protein kinase inhibitors from marine sponges [236].
6. Conclusions
The global marine preclinical and clinical pharmaceutical pipelines remain remarkably active one year after U.S. Food and Drug Administration approval of brentuximab vedotin (Adcetris®), a conjugate between a monoclonal antibody that targets the cell-membrane protein CD30, an antigen which is highly expressed in lymphoid tumors, and several units of the potent antimitotic agent monomethyl auristatin E, a synthetic analog of the marine compound dolastatin 10 [237].
This review aims to continue contributing to the marine preclinical pipeline review series that was initiated in 1998 [1,2,3,4,5,6,7] and reveals the breadth of preclinical pharmacological research during 2009–2011, resulting from the global research effort of chemists and pharmacologists from Australia, Belgium, Brazil, Canada, China, Colombia, Cuba, Egypt, Fiji, France, Germany, Indonesia, Israel, Italy, Japan, Luxemburg, Malaysia, Mexico, the Netherlands, New Caledonia, New Zealand, Norway, Panama, Papua New Guinea, Philippines, South Africa, South Korea, Singapore, Spain, Switzerland, Taiwan, Thailand, United Kingdom, Venezuela, Vietnam, and the United States. Thus, we feel confident to predict that the marine preclinical pharmaceutical pipeline will most probably continue to provide novel pharmacological lead compounds that will enrich the marine clinical pharmaceutical pipeline [200], which currently consists of 6 U.S. Food and Drug Administration-approved pharmaceuticals and 11 compounds in Phase I, II and III of clinical development and which may be viewed at [238].
Acknowledgments
This review was made possible with financial support from Midwestern University to AMSM; and NIH-SC1 Award (Grant 1SC1GM086271-01A1) of the University of Puerto Rico to ADR, and EU project Bluegenics (Grant 311848) to OTS. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Article retrieval by library staff members, and students from the Chicago College of Pharmacy, Midwestern University, is gratefully acknowledged. The authors are especially thankful to Mary Hall for the careful review of the manuscript.
References
- Mayer, A.M.S.; Lehmann, V.K.B. Marine pharmacology in 1998: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities; with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Pharmacologist 2000, 42, 62–69. [Google Scholar]
- Mayer, A.M.S.; Hamann, M.T. Marine pharmacology in 1999: Compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities; Affecting the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C 2002, 132, 315–339. [Google Scholar]
- Mayer, A.M.S.; Hamann, M.T. Marine pharmacology in 2000: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; Affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar. Biotechnol. 2004, 6, 37–52. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2003–4: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; Affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C 2007, 145, 553–581. [Google Scholar]
- Mayer, A.M.S.; Hamann, M.T. Marine pharmacology in 2001–2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; Affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C 2005, 140, 265–286. [Google Scholar]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; Affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.; Fusetani, N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; Affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C 2011, 153, 191–222. [Google Scholar]
- Schmitz, F.J.; Bowden, B.F.; Toth, S.I. Antitumor and Cytotoxic Compounds from Marine Organisms; Attaway, D.H., Zaborsky, O.R., Eds.; Plenum Press: New York, NY, USA, 1993. [Google Scholar]
- Schillaci, D.; Arizza, V.; Parrinello, N.; DiStefano, V.; Fanara, S.; Muccilli, V.; Cunsolo, V.; Haagensen, J.J.; Molin, S. Antimicrobial and antistaphylococcal biofilm activity from the sea urchin Paracentrotus lividus. J. Appl. Microbiol. 2010, 108, 17–24. [Google Scholar] [CrossRef]
- Desbois, A.P.; Mearns-Spragg, A.; Smith, V.J. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 2009, 11, 45–52. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Camara, R.B.; Nobre, L.T.; Costa, M.S.; Almeida-Lima, J.; Farias, E.H.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Mao, W.; Li, H.; Li, Y.; Zhang, H.; Qi, X.; Sun, H.; Chen, Y.; Guo, S. Chemical characteristic and anticoagulant activity of the sulfated polysaccharide isolated from Monostroma latissimum (Chlorophyta). Int. J. Biol. Macromol. 2009, 44, 70–74. [Google Scholar] [CrossRef]
- Ben Kahla-Nakbi, A.; Haouas, N.; El, O.A.; Guerbej, H.; Ben, M.K.; Babba, H. Screening of antileishmanial activity from marine sponge extracts collected off the tunisian coast. Parasitol. Res. 2010, 106, 1281–1286. [Google Scholar] [CrossRef]
- Lakshmi, V.; Srivastava, S.; Kumar, M.S.; Misra, S.; Verma, M.; Misra-Bhattacharya, S. In vitro and in vivo antifilarial potential of marine sponge, Haliclona exigua (Kirkpatrick), against human lymphatic filarial parasite brugia malayi: Antifilarial activity of H. exigua. Parasitol. Res. 2009, 105, 1295–1301. [Google Scholar] [CrossRef]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antiviral Res. 2009, 83, 282–289. [Google Scholar] [CrossRef]
- Rashid, Z.M.; Lahaye, E.; Defer, D.; Douzenel, P.; Perrin, B.; Bourgougnon, N.; Sire, O. Isolation of a sulphated polysaccharide from a recently discovered sponge species (Celtodoryx girardae) and determination of its anti-herpetic activity. Int. J. Biol. Macromol. 2009, 44, 286–293. [Google Scholar] [CrossRef]
- Dellai, A.; Laroche-Clary, A.; Mhadhebi, L.; Robert, J.; Bouraoui, A. Anti-inflammatory and antiproliferative activities of crude extract and its fractions of the defensive secretion from the mediterranean sponge. Spongia officinalis. Drug Dev. Res. 2010, 71, 412–418. [Google Scholar] [CrossRef]
- Jung, W.K.; Choi, I.; Oh, S.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, D.S.; Heo, S.J.; Jeon, Y.J.; Je, J.Y.; et al. Anti-asthmatic effect of marine red alga (Laurencia undulata) polyphenolic extracts in a murine model of asthma. Food Chem. Toxicol. 2009, 47, 293–297. [Google Scholar] [CrossRef]
- Kim, M.M.; Rajapakse, N.; Kim, S.K. Anti-inflammatory effect of Ishige okamurae ethanolic extract via Inhibition of NF-kappaB transcription factor in RAW 264. 7 cells. Phytother. Res. 2009, 23, 628–634. [Google Scholar] [CrossRef]
- Da Frota, M.L.J.; Braganhol, E.; Canedo, A.D.; Klamt, F.; Apel, M.A.; Mothes, B.; Lerner, C.; Battastini, A.M.; Henriques, A.T.; Moreira, J.C. Extracts of marine sponge Polymastia janeirensis induce oxidative cell death through a caspase-9 apoptotic pathway in human U138MG glioma cell line. Investig. New Drugs 2009, 27, 440–446. [Google Scholar] [CrossRef]
- Nappo, M.; Berkov, S.; Massucco, C.; Di Maria, V.; Bastida, J.; Codina, C.; Avila, C.; Messina, P.; Zupo, V.; Zupo, S. Apoptotic activity of the marine diatom Cocconeis scutellum and eicosapentaenoic acid in BT20 cells. Pharm. Biol. 2011, 50, 1–7. [Google Scholar]
- Grunewald, N.; Alban, S. Optimized and standardized isolation and structural characterization of anti-inflammatory sulfated polysaccharides from the red alga Delesseria sanguinea (Hudson) lamouroux (ceramiales, delesseriaceae). Biomacromolecules 2009, 10, 2998–3008. [Google Scholar] [CrossRef]
- Je, J.Y.; Ahn, C.B.; Oh, M.J.; Kang, S.Y. Antioxidant activity of a red seaweed Polysiphonia morrowii extract. Food Sci. Biotechnol. 2009, 18, 124–129. [Google Scholar]
- Mancini-Filho, J.; Novoa, A.V.; Gonzalez, A.E.; de Andrade-Wartha, E.R.; de O e Silva, A.M.; Pinto, J.R.; Mancini, D.A. Free phenolic acids from the seaweed Halimeda monile with antioxidant effect protecting against liver injury. Z. Naturforsch. C 2009, 64, 657–663. [Google Scholar]
- Sun, H.H.; Mao, W.J.; Chen, Y.; Guo, S.D.; Li, H.Y.; Qi, X.H.; Chen, Y.L.; Xu, J. Isolation, chemical characteristics and antioxidant properties of the polysaccharides from marine fungus Penicillium sp. F23-2. Carbohydr. Polym. 2009, 78, 117–124. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Shi, X.; Song, H.; Zhang, J. In vitro antioxidant activities of acetylated, phosphorylated derivatives of porphyran extracted from Porphyra haitanensis. Carbohydr. Polym. 2009, 78, 449–453. [Google Scholar] [CrossRef]
- Badiu, D.L.; Luque, R.; Dumitrescu, E.; Craciun, A.; Dinca, D. Amino acids from Mytilus galloprovincialis (L.) and Rapana venosa molluscs accelerate skin wounds healing via enhancement of dermal and epidermal neoformation. Protein J. 2010, 29, 81–92. [Google Scholar] [CrossRef]
- Suganthy, N.; Karutha, P.S.; Pandima, D.K. Neuroprotective effect of seaweeds inhabiting south indian coastal area (hare island, gulf of mannar marine biosphere reserve): Cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci. Lett. 2010, 468, 216–219. [Google Scholar] [CrossRef]
- Plaza, A.; Keffer, J.L.; Bifulco, G.; Lloyd, J.R.; Bewley, C.A. Chrysophaentins A–H, antibacterial bisdiarylbutene macrocycles that inhibit the bacterial cell division protein FtsZ. J. Am. Chem Soc. 2010, 132, 9069–9077. [Google Scholar] [CrossRef]
- Teasdale, M.E.; Liu, J.; Wallace, J.; Akhlaghi, F.; Rowley, D.C. Secondary metabolites produced by the marine bacterium Halobacillus salinus that inhibit quorum sensing-controlled phenotypes in gram-negative bacteria. Appl. Environ. Microbiol. 2009, 75, 567–572. [Google Scholar]
- Kwan, J.C.; Meickle, T.; Ladwa, D.; Teplitski, M.; Paul, V.; Luesch, H. Lyngbyoic acid, a “Tagged” fatty acid from a marine cyanobacterium, disrupts quorum sensing in Pseudomonas aeruginosa. Mol. Biosyst. 2011, 7, 1205–1216. [Google Scholar] [CrossRef]
- Jeon, J.E.; Na, Z.; Jung, M.; Lee, H.S.; Sim, C.J.; Nahm, K.; Oh, K.B.; Shin, J. Discorhabdins from the Korean marine sponge Sceptrella sp. J. Nat. Prod. 2010, 73, 258–262. [Google Scholar] [CrossRef]
- Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R.W.; de Voogd, N.J.; Wray, V.; Hentschel, U.; Kozytska, S.; Muller, W.E.; Proksch, P. From anti-fouling to biofilm inhibition: New cytotoxic secondary metabolites from two indonesian Agelas sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. [Google Scholar] [CrossRef]
- Al-Zereini, W.; Fotso Fondja Yao, C.B.; Laatsch, H.; Anke, H. Aqabamycins A–G: Novel nitro maleimides from a marine Vibrio species. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 2010, 63, 297–301. [Google Scholar] [CrossRef]
- Pettit, G.R.; Knight, J.C.; Herald, D.L.; Pettit, R.K.; Hogan, F.; Mukku, V.J.; Hamblin, J.S.; Dodson, M.J.; Chapuis, J.C. Antineoplastic agents. 570. Isolation and structure elucidation of bacillistatins 1 and 2 from a marine Bacillus silvestris. J. Nat. Prod. 2009, 72, 366–371. [Google Scholar] [CrossRef]
- Lane, A.L.; Stout, E.P.; Lin, A.S.; Prudhomme, J.; Le, R.K.; Fairchild, C.R.; Franzblau, S.G.; Hay, M.E.; Aalbersberg, W.; Kubanek, J. Antimalarial bromophycolides J–Q from the fijian red alga Callophycus serratus. J. Org. Chem. 2009, 74, 2736–2742. [Google Scholar] [CrossRef]
- Hohmann, C.; Schneider, K.; Bruntner, C.; Irran, E.; Nicholson, G.; Bull, A.T.; Jones, A.L.; Brown, R.; Stach, J.E.; Goodfellow, M.; et al. Caboxamycin, a new antibiotic of the benzoxazole family produced by the deep-sea strain Streptomyces sp. NTK 937. J. Antibiot. 2009, 62, 99–104. [Google Scholar] [CrossRef]
- Choi, H.; Engene, N.; Smith, J.E.; Preskitt, L.B.; Gerwick, W.H. Crossbyanols A–D, toxic brominated polyphenyl ethers from the hawai’ian bloom-forming cyanobacterium Leptolyngbya crossbyana. J. Nat. Prod. 2010, 73, 517–522. [Google Scholar] [CrossRef]
- Bontemps, N.; Bry, D.; Lopez-Legentil, S.; Simon-Levert, A.; Long, C.; Banaigs, B. Structures and antimicrobial activities of pyridoacridine alkaloids isolated from different chromotypes of the ascidian Cystodytes dellechiajei. J. Nat. Prod. 2010, 73, 1044–1048. [Google Scholar] [CrossRef]
- Wright, A.D.; McCluskey, A.; Robertson, M.J.; MacGregor, K.A.; Gordon, C.P.; Guenther, J. Anti-malarial, anti-algal, anti-tubercular, anti-bacterial, anti-photosynthetic, and anti-fouling activity of diterpene isonitriles from the tropical marine sponge Cymbastela hooperi. Org. Biomol. Chem. 2011, 9, 400–407. [Google Scholar] [CrossRef]
- Tadesse, M.; Tabudravu, J.N.; Jaspars, M.; Strom, M.B.; Hansen, E.; Andersen, J.H.; Kristiansen, P.E.; Haug, T. The antibacterial ent-eusynstyelamide B and eusynstyelamides D, E, and F from the arctic bryozoan Tegella cf. spitzbergensis. J. Nat. Prod. 2011, 74, 837–841. [Google Scholar] [CrossRef]
- Tello, E.; Castellanos, L.; Arevalo-Ferro, C.; Duque, C. Cembranoid diterpenes from the caribbean sea whip Eunicea knighti. J. Nat. Prod. 2009, 72, 1595–1602. [Google Scholar] [CrossRef]
- Isnansetyo, A.; Kamei, Y. Anti-methicillin-resistant Staphylococcus aureus (MRSA) activity of MC21-B, an antibacterial compound produced by the marine bacterium Pseudoalteromonas phenolica O-BC30T. Int. J. Antimicrob. Agents 2009, 34, 131–135. [Google Scholar] [CrossRef]
- Keffer, J.L.; Plaza, A.; Bewley, C.A. Motualevic acids A–F, antimicrobial Acids from the Sponge Siliquariaspongia sp. Org. Lett. 2009, 11, 1087–1090. [Google Scholar] [CrossRef]
- Engelhardt, K.; Degnes, K.F.; Kemmler, M.; Bredholt, H.; Fjaervik, E.; Klinkenberg, G.; Sletta, H.; Ellingsen, T.E.; Zotchev, S.B. Production of a new thiopeptide antibiotic, TP-1161, by a marine Nocardiopsis Species. Appl. Environ. Microbiol. 2010, 76, 4969–4976. [Google Scholar] [CrossRef]
- Stout, E.P.; Hasemeyer, A.P.; Lane, A.L.; Davenport, T.M.; Engel, S.; Hay, M.E.; Fairchild, C.R.; Prudhomme, J.; Le, R.K.; Aalbersberg, W.; et al. Antibacterial neurymenolides from the fijian red alga Neurymenia fraxinifolia. Org. Lett. 2009, 11, 225–228. [Google Scholar] [CrossRef]
- Feher, D.; Barlow, R.; McAtee, J.; Hemscheidt, T.K. Highly brominated antimicrobial metabolites from a marine Pseudoalteromonas sp. J. Nat. Prod. 2010, 73, 1963–1966. [Google Scholar] [CrossRef]
- Correa, H.; Aristizabal, F.; Duque, C.; Kerr, R. Cytotoxic and antimicrobial activity of pseudopterosins and seco-Pseudopterosins isolated from the octocoral Pseudopterogorgia elisabethae of san andres and providencia islands (Southwest Caribbean Sea). Mar. Drugs 2011, 9, 334–343. [Google Scholar] [CrossRef]
- Till, M.; Prinsep, M.R. 5-Bromo-8-methoxy-1-methyl-β-carboline, an alkaloid from the New Zealand marine bryozoan Pterocella vesiculosa. J. Nat. Prod. 2009, 72, 796–798. [Google Scholar] [CrossRef]
- Matsuda, S.; Adachi, K.; Matsuo, Y.; Nukina, M.; Shizuri, Y. Salinisporamycin, a novel metabolite from Salinispora arenicola. J. Antibiot. 2009, 62, 519–526. [Google Scholar] [CrossRef]
- Tadesse, M.; Strom, M.B.; Svenson, J.; Jaspars, M.; Milne, B.F.; Torfoss, V.; Andersen, J.H.; Hansen, E.; Stensvag, K.; Haug, T. Synoxazolidinones A and B: Novel bioactive alkaloids from the ascidian Synoicum pulmonaria. Org. Lett. 2010, 12, 4752–4755. [Google Scholar] [CrossRef]
- DiGirolamo, J.A.; Li, X.C.; Jacob, M.R.; Clark, A.M.; Ferreira, D. Reversal of fluconazole resistance by sulfated sterols from the marine sponge Topsentia sp. J. Nat. Prod. 2009, 72, 1524–1528. [Google Scholar] [CrossRef]
- Nishimura, S.; Arita, Y.; Honda, M.; Iwamoto, K.; Matsuyama, A.; Shirai, A.; Kawasaki, H.; Kakeya, H.; Kobayashi, T.; Matsunaga, S.; et al. Marine antifungal theonellamides target 3β-hydroxysterol to activate rho1 signaling. Nat. Chem. Biol. 2010, 6, 519–526. [Google Scholar] [CrossRef]
- Carroll, A.R.; Duffy, S.; Avery, V.M. Citronamides A and B, tetrapeptides from the australian sponge Citronia astra. J. Nat. Prod. 2009, 72, 764–768. [Google Scholar] [CrossRef]
- Yuan, W.H.; Yi, Y.H.; Tang, H.F.; Liu, B.S.; Wang, Z.L.; Sun, G.Q.; Zhang, W.; Li, L.; Sun, P. Antifungal triterpene glycosides from the sea cucumber Bohadschia marmorata. Planta Med. 2009, 75, 168–173. [Google Scholar] [CrossRef]
- El-Gendy, M.M.; El-Bondkly, A.M. Production and genetic improvement of a novel antimycotic agent, saadamycin, against dermatophytes and other clinical fungi from endophytic Streptomyces sp. Hedaya 48. J. Ind. Microbiol. Biotechnol. 2010, 37, 831–841. [Google Scholar] [CrossRef]
- Plaza, A.; Bifulco, G.; Keffer, J.L.; Lloyd, J.R.; Baker, H.L.; Bewley, C.A. Celebesides A-C and theopapuamides B-D, depsipeptides from an indonesian sponge that inhibit HIV-1 entry. J. Org. Chem. 2009, 74, 504–512. [Google Scholar] [CrossRef]
- Taglialatela-Scafati, O.; Fattorusso, E.; Romano, A.; Scala, F.; Barone, V.; Cimino, P.; Stendardo, E.; Catalanotti, B.; Persico, M.; Fattorusso, C. Insight into the mechanism of action of plakortins, Simple 1,2-Dioxane antimalarials. Org. Biomol. Chem. 2010, 8, 846–856. [Google Scholar] [CrossRef]
- Lebouvier, N.; Jullian, V.; Desvignes, I.; Maurel, S.; Parenty, A.; Dorin-Semblat, D.; Doerig, C.; Sauvain, M.; Laurent, D. Antiplasmodial activities of homogentisic acid derivative protein kinase inhibitors isolated from a vanuatu marine sponge Pseudoceratina sp. Mar. Drugs 2009, 7, 640–653. [Google Scholar] [CrossRef]
- Laville, R.; Thomas, O.P.; Berrue, F.; Marquez, D.; Vacelet, J.; Amade, P. Bioactive guanidine alkaloids from two caribbean marine sponges. J. Nat. Prod. 2009, 72, 1589–1594. [Google Scholar] [CrossRef]
- Lin, A.S.; Stout, E.P.; Prudhomme, J.; Le, R.K.; Fairchild, C.R.; Franzblau, S.G.; Aalbersberg, W.; Hay, M.E.; Kubanek, J. Bioactive bromophycolides R–U from the Fijian red alga Callophycus serratus. J. Nat. Prod. 2010, 73, 275–278. [Google Scholar] [CrossRef]
- Davis, R.A.; Duffy, S.; Avery, V.M.; Camp, D.; Hooper, J.N.A.; Quinn, R.J. (+)-7-bromotrypargine:An antimalarial β-Carboline from the australian marine sponge Ancorina sp. Tetrahedron Lett. 2010, 51, 583–585. [Google Scholar] [CrossRef]
- Wright, A.D.; Lang-Unnasch, N. Diterpene formamides from the tropical marine sponge Cymbastela hooperi and their antimalarial activity in vitro. J. Nat. Prod. 2009, 72, 492–495. [Google Scholar] [CrossRef]
- Jiménez-Romero, C.; Ortíz, I.; Vicente, J.; Vera, B.; Rodríguez, A.D.; Nam, S.; Jove, R. Bioactive cycloperoxides isolated from the puerto rican sponge Plakortis halichondrioides. J. Nat. Prod. 2010, 73, 1694–1700. [Google Scholar] [CrossRef]
- Linington, R.G.; Clark, B.R.; Trimble, E.E.; Almanza, A.; Urena, L.D.; Kyle, D.E.; Gerwick, W.H. Antimalarial peptides from marine cyanobacteria: Isolation and structural elucidation of gallinamide A. J. Nat. Prod. 2009, 72, 14–17. [Google Scholar] [CrossRef]
- Ueoka, R.; Nakao, Y.; Kawatsu, S.; Yaegashi, J.; Matsumoto, Y.; Matsunaga, S.; Furihata, K.; van Soest, R.W.; Fusetani, N. Gracilioethers A–C, antimalarial metabolites from the marine sponge Agelas gracilis. J. Org. Chem. 2009, 74, 4203–4207. [Google Scholar] [CrossRef]
- Wattanapiromsakul, C.; Plubrukarn, A.; Suwanborirux, K. 8-Isocyanoamphilecta-11(20),15-diene, a new antimalarial isonitrile diterpene from the sponge Ciocalapata sp. Can. J. Chem. 2009, 87, 612–618. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Tan, L.T. Lagunamides A and B: Cytotoxic and antimalarial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 1810–1814. [Google Scholar] [CrossRef]
- Fattorusso, C.; Persico, M.; Calcinai, B.; Cerrano, C.; Parapini, S.; Taramelli, D.; Novellino, E.; Romano, A.; Scala, F.; Fattorusso, E.; et al. Manadoperoxides A–D from the indonesian sponge Plakortis cfr. simplex. Further insights on the structure-activity relationships of simple 1,2-dioxane antimalarials. J. Nat. Prod. 2010, 73, 1138–1145. [Google Scholar] [CrossRef]
- Yamada, M.; Takahashi, Y.; Kubota, T.; Fromont, J.; Ishiyama, A.; Otoguro, K.; Yamada, H.; Omura, S.; Kobayashi, J. Zamamidine C, 3,4-dihydro-6-hydroxy-10,11-Epoxymanzamine A, and 3,4-Dihydromanzamine J N-oxide, new manzamine alkaloids from sponge Amphimedon sp. Tetrahedron 2009, 65, 2313–2317. [Google Scholar] [CrossRef]
- Wei, X.; Nieves, K.; Rodríguez, A.D. Neopetrosiamine A, biologically active bis-piperidine alkaloid from the caribbean sea sponge Neopetrosia proxima. Bioorg. Med. Chem. Lett. 2010, 20, 5905–5908. [Google Scholar] [CrossRef]
- Yang, X.; Davis, R.A.; Buchanan, M.S.; Duffy, S.; Avery, V.M.; Camp, D.; Quinn, R.J. Antimalarial bromotyrosine derivatives from the australian marine sponge Hyattella sp. J. Nat. Prod. 2010, 73, 985–987. [Google Scholar] [CrossRef]
- Dos Santos, A.O.; Britta, E.A.; Bianco, E.M.; Ueda-Nakamura, T.; Filho, B.P.; Pereira, R.C.; Nakamura, C.V. 4-Acetoxydolastane diterpene from the brazilian brown alga Canistrocarpus cervicornis as antileishmanial agent. Mar. Drugs 2011, 9, 2369–2383. [Google Scholar] [CrossRef]
- Vik, A.; Proszenyak, A.; Vermeersch, M.; Cos, P.; Maes, L.; Gundersen, L.L. Screening of agelasine D and analogs for inhibitory activity against pathogenic protozoa; Identification of hits for visceral leishmaniasis and chagas disease. Molecules 2009, 14, 279–288. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Lopez, D.; Vesely, B.A.; Della, T.G.; Gerwick, W.H.; Kyle, D.E.; Linington, R.G. Almiramides A–C: Discovery and development of a new class of leishmaniasis lead compounds. J. Med. Chem. 2010, 53, 4187–4197. [Google Scholar] [CrossRef]
- Davis, R.A.; Sykes, M.; Avery, V.M.; Camp, D.; Quinn, R.J. Convolutamines I and J, antitrypanosomal alkaloids from the bryozoan Amathia tortusa. Bioorg. Med. Chem. 2011, 19, 6615–6619. [Google Scholar] [CrossRef]
- Scala, F.; Fattorusso, E.; Menna, M.; Taglialatela-Scafati, O.; Tierney, M.; Kaiser, M.; Tasdemir, D. Bromopyrrole alkaloids as lead compounds against protozoan parasites. Mar. Drugs 2010, 8, 2162–2174. [Google Scholar] [CrossRef]
- Cantillo-Ciau, Z.; Moo-Puc, R.; Quijano, L.; Freile-Pelegrin, Y. The tropical brown alga Lobophora variegata: A source of antiprotozoal compounds. Mar. Drugs 2010, 8, 1292–1304. [Google Scholar] [CrossRef]
- Rubio, B.K.; Tenney, K.; Ang, K.H.; Abdulla, M.; Arkin, M.; McKerrow, J.H.; Crews, P. The marine sponge Diacarnus bismarckensis as a source of peroxiterpene inhibitors of Trypanosoma brucei, the causative agent of sleeping sickness. J. Nat. Prod. 2009, 72, 218–222. [Google Scholar] [CrossRef]
- Ma, W.S.; Mutka, T.; Vesley, B.; Amsler, M.O.; McClintock, J.B.; Amsler, C.D.; Perman, J.A.; Singh, M.P.; Maiese, W.M.; Zaworotko, M.J.; et al. Norselic acids A–E, highly oxidized anti-infective steroids that deter mesograzer predation, from the antarctic sponge Crella sp. J. Nat. Prod. 2009, 72, 1842–1846. [Google Scholar] [CrossRef]
- Regalado, E.L.; Tasdemir, D.; Kaiser, M.; Cachet, N.; Amade, P.; Thomas, O.P. Antiprotozoal steroidal saponins from the marine sponge Pandaros acanthifolium. J. Nat. Prod. 2010, 73, 1404–1410. [Google Scholar] [CrossRef]
- Feng, Y.; Davis, R.A.; Sykes, M.; Avery, V.M.; Camp, D.; Quinn, R.J. Antitrypanosomal cyclic polyketide peroxides from the australian marine sponge Plakortis sp. J. Nat. Prod. 2010, 73, 716–719. [Google Scholar] [CrossRef]
- Pimentel-Elardo, S.M.; Kozytska, S.; Bugni, T.S.; Ireland, C.M.; Moll, H.; Hentschel, U. Anti-parasitic compounds from Streptomyces sp. strains isolated from mediterranean sponges. Mar. Drugs 2010, 8, 373–380. [Google Scholar] [CrossRef]
- Vicente, J.; Vera, B.; Rodríguez, A.D.; Rodríguez-Escudero, I.; Raptis, R.G. Euryjanicin A: A new cycloheptapeptide from the caribbean marine sponge Prosuberites laughlini. Tetrahedron Lett. 2009, 50, 4571–4574. [Google Scholar] [CrossRef]
- Pruksakorn, P.; Arai, M.; Kotoku, N.; Vilcheze, C.; Baughn, A.D.; Moodley, P.; Jacobs, W.R., Jr.; Kobayashi, M. Trichoderins, novel aminolipopeptides from a marine sponge-derived Trichoderma sp., are active against dormant mycobacteria. Bioorg. Med. Chem. Lett. 2010, 20, 3658–3663. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chuang, C.T.; Wang, S.K.; Wen, Z.H.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. Antiviral and anti-inflammatory diterpenoids from the soft coral Sinularia gyrosa. J. Nat. Prod. 2010, 73, 1184–1187. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, S.K.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Chiang, M.Y.; Duh, C.Y. Cembranoids from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2010, 73, 197–203. [Google Scholar] [CrossRef]
- Palem, J.R.; Bedadala, G.R.; El Sayed, K.A.; Hsia, S.C. Manzamine a as a novel inhibitor of herpes simplex virus type-1 replication in cultured corneal cells. Planta Med. 2011, 77, 46–51. [Google Scholar] [CrossRef]
- Ding, L.; Munch, J.; Goerls, H.; Maier, A.; Fiebig, H.H.; Lin, W.H.; Hertweck, C. Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg. Med. Chem. Lett. 2010, 20, 6685–6687. [Google Scholar]
- Fan, G.; Li, Z.; Shen, S.; Zeng, Y.; Yang, Y.; Xu, M.; Bruhn, T.; Bruhn, H.; Morschhauser, J.; Bringmann, G.; et al. Baculiferins A–O, O-Sulfated pyrrole alkaloids with anti-HIV-1 activity, from the chinese marine sponge Iotrochota baculifera. Bioorg. Med. Chem. 2010, 18, 5466–5474. [Google Scholar] [CrossRef]
- Li, Y.; Carbone, M.; Vitale, R.M.; Amodeo, P.; Castelluccio, F.; Sicilia, G.; Mollo, E.; Nappo, M.; Cimino, G.; Guo, Y.W.; et al. Rare casbane diterpenoids from the Hainan soft coral Sinularia depressa. J. Nat. Prod. 2010, 73, 133–138. [Google Scholar] [CrossRef]
- Chakraborty, K.; Lipton, A.P.; Paulraj, R.; Chakraborty, R.D. Guaiane sesquiterpenes from seaweed Ulva fasciata delile and their antibacterial properties. Eur. J. Med. Chem. 2010, 45, 2237–2244. [Google Scholar] [CrossRef]
- Kemami Wangun, H.V.; Wood, A.; Fiorilla, C.; Reed, J.K.; McCarthy, P.J.; Wright, A.E. Gymnochromes E and F, cytotoxic phenanthroperylenequinones from a deep-water crinoid, Holopus rangii. J. Nat. Prod. 2010, 73, 712–715. [Google Scholar] [CrossRef]
- Mondol, M.A.; Kim, J.H.; Lee, M.A.; Tareq, F.S.; Lee, H.S.; Lee, Y.J.; Shin, H.J. Ieodomycins A–D, antimicrobial fatty acids from a marine Bacillus sp. J. Nat. Prod. 2011, 74, 1606–1612. [Google Scholar] [CrossRef]
- Yao, G.; Kondratyuk, T.P.; Tan, G.T.; Pezzuto, J.M.; Chang, L.C. Bioactive sulfated sesterterpene alkaloids and sesterterpene sulfates from the marine sponge Fasciospongia sp. J. Nat. Prod. 2009, 72, 319–323. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Kehraus, S.; Lindequist, U.; Sasse, F.; Shaaban, S.; Gutschow, M.; Josten, M.; Sahl, H.G.; König, G.M. Antimicrobial phenalenone derivatives from the marine-derived fungus Coniothyrium cereale. Org. Biomol. Chem. 2011, 9, 802–808. [Google Scholar] [CrossRef]
- Devi, P.; Wahidullah, S.; Rodrigues, C.; Souza, L.D. The sponge-associated bacterium Bacillus licheniformis SAB1: A source of antimicrobial compounds. Mar. Drugs 2010, 8, 1203–1212. [Google Scholar] [CrossRef]
- Kamei, Y.; Sueyoshi, M.; Hayashi, K.; Terada, R.; Nozaki, H. The novel anti-Propionibacterium acnes compound, sargafuran, found in the marine brown alga Sargassum macrocarpum. J. Antibiot. 2009, 62, 259–263. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Vagias, C.; Rahman, M.M.; Gibbons, S.; Roussis, V. Structure and antibacterial activity of brominated diterpenes from the red alga Sphaerococcus coronopifolius. Chem. Biodivers. 2010, 7, 186–195. [Google Scholar] [CrossRef]
- Mukai, H.; Kubota, T.; Aoyama, K.; Mikami, Y.; Fromont, J.; Kobayashi, J. Tyrokeradines A and B, new bromotyrosine alkaloids with an imidazolyl-quinolinone moiety from a verongid sponge. Bioorg. Med. Chem. Lett. 2009, 19, 1337–1339. [Google Scholar] [CrossRef]
- Li, C.; Haug, T.; Moe, M.K.; Styrvold, O.B.; Stensvag, K. Centrocins: Isolation and characterization of novel dimeric antimicrobial peptides from the green sea urchin, Strongylocentrotus droebachiensis. Dev. Comp. Immunol. 2010, 34, 959–968. [Google Scholar] [CrossRef]
- Galinier, R.; Roger, E.; Sautiere, P.E.; Aumelas, A.; Banaigs, B.; Mitta, G. Halocyntin and papillosin, two new antimicrobial peptides isolated from hemocytes of the solitary tunicate, Halocynthia papillosa. J. Pept. Sci. 2009, 15, 48–55. [Google Scholar] [CrossRef]
- Sperstad, S.V.; Haug, T.; Vasskog, T.; Stensvag, K. Hyastatin, a glycine-rich multi-domain antimicrobial peptide isolated from the spider crab (Hyas araneus) hemocytes. Mol. Immunol. 2009, 46, 2604–2612. [Google Scholar] [CrossRef]
- Heo, S.J.; Hwang, J.Y.; Choi, J.I.; Han, J.S.; Kim, H.J.; Jeon, Y.J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown alga, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Shen, X.; Guo, Y.W. A novel sesquiterpene quinone from hainan sponge Dysidea villosa. Bioorg. Med. Chem. Lett. 2009, 19, 390–392. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Guo, Y.W.; Jiang, H.L.; Shen, X. A sesquiterpene quinone, dysidine, from the sponge Dysidea villosa, activates the insulin pathway through inhibition of PTP ases. Acta Pharmacol. Sin. 2009, 30, 333–345. [Google Scholar] [CrossRef]
- Asolkar, R.N.; Freel, K.C.; Jensen, P.R.; Fenical, W.; Kondratyuk, T.P.; Park, E.J.; Pezzuto, J.M. Arenamides A–C, cytotoxic NFκB inhibitors from the marine actinomycete Salinispora arenicola. J. Nat. Prod. 2009, 72, 396–402. [Google Scholar]
- Youssef, D.T.; Ibrahim, A.K.; Khalifa, S.I.; Mesbah, M.K.; Mayer, A.M.; van Soest, R.W. New anti-inflammatory sterols from the red sea sponges Scalarispongia aqabaensis and Callyspongia siphonella. Nat. Prod. Commun. 2010, 5, 27–31. [Google Scholar]
- Jean, Y.H.; Chen, W.F.; Sung, C.S.; Duh, C.Y.; Huang, S.Y.; Lin, C.S.; Tai, M.H.; Tzeng, S.F.; Wen, Z.H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br. J. Pharmacol. 2009, 158, 713–725. [Google Scholar] [CrossRef]
- Shi, Y.P.; Wei, X.; Rodríguez, I.I.; Rodríguez, A.D.; Mayer, A.M.S. New terpenoid constituents of the Southwestern Caribbean sea whip Pseudopterogorgia elisabethae (Bayer) including a unique pentanorditerpene. Eur. J. Org. Chem. 2009, 4, 493–502. [Google Scholar]
- Li, Y.X.; Li, Y.; Lee, S.H.; Qian, Z.J.; Kim, S.K. Inhibitors of oxidation and matrix metalloproteinases, floridoside, and d-isofloridoside from marine red alga Laurencia undulata. J. Agric. Food Chem. 2010, 58, 578–586. [Google Scholar] [CrossRef]
- Malloy, K.L.; Villa, F.A.; Engene, N.; Matainaho, T.; Gerwick, L.; Gerwick, W.H. Malyngamide 2, an oxidized lipopeptide with nitric oxide inhibiting activity from a papua new guinea marine cyanobacterium. J. Nat. Prod. 2011, 74, 95–98. [Google Scholar] [CrossRef]
- Villa, F.A.; Lieske, K.; Gerwick, L. Selective MyD88-dependent pathway inhibition by the cyanobacterial natural product malyngamide F acetate. Eur. J. Pharmacol. 2010, 629, 140–146. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Belmiro, C.L.; Castelo-Branco, M.T.; Melim, L.M.; Schanaider, A.; Elia, C.; Madi, K.; Pavao, M.S.; de Souza, H.S. Unfractionated heparin and new heparin analogues from ascidians (chordate-tunicate) ameliorate colitis in rats. J. Biol. Chem. 2009, 284, 11267–11278. [Google Scholar]
- Hanif, N.; Ohno, O.; Kitamura, M.; Yamada, K.; Uemura, D. Symbiopolyol, a VCAM-1 inhibitor from a symbiotic dinoflagellate of the jellyfish Mastigias papua. J. Nat. Prod. 2010, 73, 1318–1322. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Perinu, C.; Cirino, G.; de Gruttola, G.L.; Roviezzo, F. Tedanol: A potent anti-inflammatory ent-pimarane diterpene from the caribbean sponge Tedania ignis. Bioorg. Med. Chem. 2009, 17, 7542–7547. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hwang, T.L.; Lin, M.R.; Chen, Y.H.; Chang, Y.C.; Fang, L.S.; Wang, W.H.; Wu, Y.C.; Sung, P.J. Carijoside A, a bioactive sterol glycoside from an octocoral Carijoa sp. (Clavulariidae). Mar. Drugs 2010, 8, 2014–2020. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Huang, Y.C.; Wen, Z.H.; Chiou, S.F.; Wang, S.K.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. Novel sesquiterpenes and norergosterol from the soft corals Nephthea erecta and Nephthea chabroli. Tetrahedron Lett. 2009, 50, 802–806. [Google Scholar]
- De Marino, S.; Festa, C.; D’Auria, M.V.; Bourguet-Kondracki, M.L.; Petek, S.; Debitus, C.; Andres, R.M.; Terencio, M.C.; Paya, M.; Zampella, A. Coscinolactams A and B: New nitrogen-containing sesterterpenoid from the marine sponge Coscinoderma mathewsi exerting anti-inflammatory Properties. Tetrahedron 2009, 65, 2905–2909. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wen, Z.H.; Wang, S.K.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Chiang, M.Y.; Duh, C.Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral Lobophytum durum. J. Nat. Prod. 2009, 72, 152–155. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wen, Z.H.; Wang, S.K.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. Anti-inflammatory cembranolides from the soft coral Lobophytum durum. Bioorg. Med. Chem. 2009, 17, 3763–3769. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chuang, C.T.; Wen, Z.H.; Wang, S.K.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. Bioactive norditerpenoids from the soft coral Sinularia gyrosa. Bioorg. Med. Chem. 2010, 18, 3379–3386. [Google Scholar] [CrossRef]
- Chen, B.W.; Chao, C.H.; Su, J.H.; Wen, Z.H.; Sung, P.J.; Sheu, J.H. Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2010, 8, 2363–2366. [Google Scholar] [CrossRef]
- Wanzola, M.; Furuta, T.; Kohno, Y.; Fukumitsu, S.; Yasukochi, S.; Watari, K.; Tanaka, C.; Higuchi, R.; Miyamoto, T. Four new cembrane diterpenes isolated from an okinawan soft coral Lobophytum crassum with inhibitory effects on nitric oxide production. Chem. Pharm. Bull. 2010, 58, 1203–1209. [Google Scholar] [CrossRef]
- Festa, C.; de Marino, S.; Sepe, V.; Monti, M.C.; Luciano, P.; D’Auria, M.V. Perthamides C and D, two new potent anti-inflammatory cyclopeptides from a solomon lithistid sponge Theonella swinhoei. Tetrahedron 2009, 65, 10424–10429. [Google Scholar] [CrossRef]
- Appleton, D.R.; Chuen, C.S.; Berridge, M.V.; Webb, V.L.; Copp, B.R. Rossinones A and B, biologically active meroterpenoids from the antarctic ascidian, Aplidium species. J. Org. Chem. 2009, 74, 9195–9198. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Huang, Y.C.; Wen, Z.H.; Hsu, C.H.; Wang, S.K.; Dai, C.F.; Duh, C.Y. New 19-oxygenated and 4-methylated steroids from the formosan soft coral Nephthea chabroli. Steroids 2009, 74, 543–547. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wen, Z.H.; Chiou, S.F.; Tsai, C.W.; Wang, S.K.; Hsu, C.H.; Dai, C.F.; Chiang, M.Y.; Wang, W.H.; Duh, C.Y. Ceramide and cerebrosides from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2009, 72, 465–468. [Google Scholar] [CrossRef]
- Lin, W.Y.; Su, J.H.; Lu, Y.; Wen, Z.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Cytotoxic and anti-inflammatory cembranoids from the dongsha atoll soft coral Sarcophyton crassocaule. Bioorg. Med. Chem. 2010, 18, 1936–1941. [Google Scholar] [CrossRef]
- Wu, S.L.; Su, J.H.; Wen, Z.H.; Hsu, C.H.; Chen, B.W.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Simplexins A–I, eunicellin-based diterpenoids from the soft coral Klyxum simplex. J. Nat. Prod. 2009, 72, 994–1000. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Teta, R.; Panza, E.; Ianaro, A. Terpioside B, a difucosyl GSL from the marine sponge Terpios sp. is a potent inhibitor of NO release. Bioorg. Med. Chem. 2010, 18, 5310–5315. [Google Scholar] [CrossRef]
- Kwan, J.C.; Eksioglu, E.A.; Liu, C.; Paul, V.J.; Luesch, H. Grassystatins A–C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation. J. Med. Chem. 2009, 52, 5732–5747. [Google Scholar] [CrossRef]
- Takei, M.; Umeyama, A.; Shoji, N.; Hashimoto, T. Polyacetylenediols regulate the function of human monocyte-derived dendritic cells. Int. Immunopharmacol. 2010, 10, 913–921. [Google Scholar] [CrossRef]
- Shim, S.Y.; Choi, J.S.; Byun, D.S. Inhibitory effects of phloroglucinol derivatives isolated from Ecklonia stolonifera on FcεRI expression. Bioorg. Med. Chem. 2009, 17, 4734–4739. [Google Scholar] [CrossRef]
- Strangman, W.K.; Kwon, H.C.; Broide, D.; Jensen, P.R.; Fenical, W. Potent inhibitors of pro-inflammatory cytokine production produced by a marine-derived bacterium. J. Med. Chem. 2009, 52, 2317–2327. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, H.; Zhang, Y.; Wang, H. Chemical properties and immunostimulatory activity of a water-soluble polysaccharide from the clam of Hyriopsis cumingii lea. Carbohydr. Polym. 2009, 77, 365–369. [Google Scholar] [CrossRef]
- Orsi, C.F.; Colombari, B.; Callegari, F.; Todaro, A.M.; Ardizzoni, A.; Rossini, G.P.; Blasi, E.; Peppoloni, S. Yessotoxin inhibits phagocytic activity of macrophages. Toxicon 2010, 55, 265–273. [Google Scholar] [CrossRef]
- Inutsuka, A.; Goda, M.; Fujiyoshi, Y. Calyculin A-induced neurite retraction is critically dependent on actomyosin activation but not on polymerization state of microtubules. Biochem. Biophys. Res. Commun. 2009, 390, 1160–1166. [Google Scholar] [CrossRef]
- Sakurada, T.; Gill, M.B.; Frausto, S.; Copits, B.; Noguchi, K.; Shimamoto, K.; Swanson, G.T.; Sakai, R. Novel N-methylated 8-oxoisoguanines from pacific sponges with diverse neuroactivities. J. Med. Chem. 2010, 53, 6089–6099. [Google Scholar] [CrossRef]
- Choi, H.; Pereira, A.R.; Cao, Z.; Shuman, C.F.; Engene, N.; Byrum, T.; Matainaho, T.; Murray, T.F.; Mangoni, A.; Gerwick, W.H. The hoiamides, structurally intriguing neurotoxic lipopeptides from papua new guinea marine cyanobacteria. J. Nat. Prod. 2010, 73, 1411–1421. [Google Scholar] [CrossRef]
- Pereira, A.R.; Cao, Z.; Engene, N.; Soria-Mercado, I.E.; Murray, T.F.; Gerwick, W.H. Palmyrolide A, an unusually stabilized neuroactive macrolide from palmyra atoll cyanobacteria. Org. Lett. 2010, 12, 4490–4493. [Google Scholar] [CrossRef]
- Zhao, J.; Li, L.; Ling, C.; Li, J.; Pang, J.Y.; Lin, Y.C.; Liu, J.; Huang, R.; Wang, G.L.; Pei, Z.; et al. Marine compound xyloketal B protects PC12 cells against OGD-induced cell damage. Brain Res. 2009, 1302, 240–247. [Google Scholar]
- Soria-Mercado, I.E.; Pereira, A.; Cao, Z.; Murray, T.F.; Gerwick, W.H. Alotamide A, a novel neuropharmacological agent from the marine cyanobacterium Lyngbya bouillonii. Org. Lett. 2009, 11, 4704–4707. [Google Scholar] [CrossRef]
- Davis, R.A.; Fechner, G.A.; Sykes, M.; Garavelas, A.; Pass, D.M.; Carroll, A.R.; Addepalli, R.; Avery, V.M.; Hooper, J.N.; Quinn, R.J. (−)-Dibromophakellin: An α2B adrenoceptor agonist isolated from the australian marine sponge, Acanthella costata. Bioorg. Med. Chem. 2009, 17, 2497–2500. [Google Scholar] [CrossRef]
- Suna, H.; Arai, M.; Tsubotani, Y.; Hayashi, A.; Setiawan, A.; Kobayashi, M. Dysideamine, a new sesquiterpene aminoquinone, protects hippocampal neuronal cells against iodoacetic acid-induced cell death. Bioorg. Med. Chem. 2009, 17, 3968–3972. [Google Scholar] [CrossRef]
- Balansa, W.; Islam, R.; Fontaine, F.; Piggott, A.M.; Zhang, H.; Webb, T.I.; Gilbert, D.F.; Lynch, J.W.; Capon, R.J. Ircinialactams: Subunit-selective glycine receptor modulators from Australian sponges of the family irciniidae. Bioorg. Med. Chem. 2010, 18, 2912–2919. [Google Scholar] [CrossRef]
- Tapiolas, D.M.; Bowden, B.F.; Abou-Mansour, E.; Willis, R.H.; Doyle, J.R.; Muirhead, A.N.; Liptrot, C.; Llewellyn, L.E.; Wolff, C.W.; Wright, A.D.; et al. Eusynstyelamides A, B, and C, nNOS inhibitors, from the Ascidian Eusynstyela latericius. J. Nat. Prod. 2009, 72, 1115–1120. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Wen, Z.H.; Dai, C.F.; Chiang, M.Y.; Sheu, J.H. Nanolobatolide, a new C18 metabolite from the formosan soft coral Sinularia nanolobata. Org. Lett. 2009, 11, 5030–5032. [Google Scholar] [CrossRef]
- Lin, Z.; Antemano, R.R.; Hughen, R.W.; Tianero, M.D.; Peraud, O.; Haygood, M.G.; Concepcion, G.P.; Olivera, B.M.; Light, A.; Schmidt, E.W. Pulicatins A–E, neuroactive thiazoline metabolites from cone snail-associated bacteria. J. Nat. Prod. 2010, 73, 1922–1926. [Google Scholar] [CrossRef]
- Perry, V.H.; Nicoll, J.A.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef]
- Teruya, T.; Sasaki, H.; Fukazawa, H.; Suenaga, K. Bisebromoamide, a potent cytotoxic peptide from the marine cyanobacterium Lyngbya sp.: Isolation, stereostructure, and biological activity. Org. Lett. 2009, 11, 5062–5065. [Google Scholar] [CrossRef]
- Henrich, C.J.; Robey, R.W.; Takada, K.; Bokesch, H.R.; Bates, S.E.; Shukla, S.; Ambudkar, S.V.; McMahon, J.B.; Gustafson, K.R. Botryllamides: Natural product inhibitors of ABCG2. ACS Chem. Biol. 2009, 4, 637–647. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Ryu, B.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Parys, S.; Kehraus, S.; Krick, A.; Glombitza, K.W.; Carmeli, S.; Klimo, K.; Gerhauser, C.; Konig, G.M. In vitro chemopreventive potential of fucophlorethols from the brown alga Fucus vesiculosus L. by anti-oxidant activity and inhibition of selected cytochrome P450 enzymes. Phytochemistry 2010, 71, 221–229. [Google Scholar] [CrossRef]
- Mita, M.; Yoshikuni, M.; Ohno, K.; Shibata, Y.; Paul-Prasanth, B.; Pitchayawasin, S.; Isobe, M.; Nagahama, Y. A relaxin-like peptide purified from radial nerves induces oocyte maturation and ovulation in the starfish, Asterina pectinifera. Proc. Natl. Acad. Sci. USA 2009, 106, 9507–9512. [Google Scholar] [CrossRef]
- Tsubosaka, Y.; Murata, T.; Kinoshita, K.; Yamada, K.; Uemura, D.; Hori, M.; Ozaki, H. Halichlorine is a novel L-type Ca2+ channel inhibitor isolated from the marine sponge Halichondria okadai Kadota. Eur. J. Pharmacol. 2010, 628, 128–131. [Google Scholar] [CrossRef]
- Pereira, A.; Cao, Z.; Murray, T.F.; Gerwick, W.H. Hoiamide A, a sodium channel activator of unusual architecture from a consortium of two papua new guinea cyanobacteria. Chem. Biol. 2009, 16, 893–906. [Google Scholar] [CrossRef]
- Ohkawa, Y.; Miki, K.; Suzuki, T.; Nishio, K.; Sugita, T.; Kinoshita, K.; Takahashi, K.; Koyama, K. Antiangiogenic metabolites from a marine-derived fungus, Hypocrea vinosa. J. Nat. Prod. 2010, 73, 579–582. [Google Scholar] [CrossRef]
- Morgan, J.B.; Mahdi, F.; Liu, Y.; Coothankandaswamy, V.; Jekabsons, M.B.; Johnson, T.A.; Sashidhara, K.V.; Crews, P.; Nagle, D.G.; Zhou, Y.D. The marine sponge metabolite mycothiazole: A novel prototype mitochondrial complex I inhibitor. Bioorg. Med. Chem. 2010, 18, 5988–5994. [Google Scholar] [CrossRef]
- Mao, S.C.; Liu, Y.; Morgan, J.B.; Jekabsons, M.B.; Zhou, Y.D.; Nagle, D.G. Lipophilic 2,5-disubstituted pyrroles from the marine sponge Mycale sp. Inhibit mitochondrial respiration and HIF-1 Activation. J. Nat. Prod. 2009, 72, 1927–1936. [Google Scholar] [CrossRef]
- Nakamura, K.; Tachikawa, Y.; Ohno, O.; Kitamura, M.; Suganuma, M.; Uemura, D. Neocomplanines A and B, a complanine family isolated from the marine fireworm. J. Nat. Prod. 2010, 73, 303–305. [Google Scholar] [CrossRef]
- Dang, Y.; Low, W.K.; Xu, J.; Gehring, N.H.; Dietz, H.C.; Romo, D.; Liu, J.O. Inhibition of nonsense-mediated mRNA decay by the natural product pateamine a through eukaryotic initiation factor 4AIII. J. Biol. Chem. 2009, 284, 23613–23621. [Google Scholar] [CrossRef]
- Iwamoto, M.; Shimizu, H.; Muramatsu, I.; Oiki, S.A. Cytotoxic peptide from a marine sponge exhibits ion channel activity through vectorial-insertion into the membrane. FEBS Lett. 2010, 584, 3995–3999. [Google Scholar] [CrossRef]
- Field, J.J.; Singh, A.J.; Kanakkanthara, A.; Halafihi, T.; Northcote, P.T.; Miller, J.H. Microtubule-stabilizing activity of zampanolide, a potent macrolide isolated from the tongan marine sponge Cacospongia mycofijiensis. J. Med. Chem. 2009, 52, 7328–7332. [Google Scholar] [CrossRef]
- Forestier, R.; Merchant, C.E.; de Voogd, N.J.; Matainaho, T.; Kieffer, T.J.; Anderson, R.J. Aloketals A and B, sesterterpenoids from the marine sponge Hamigera species that activate the cAMP cell signalings pathway. Org. Lett. 2009, 11, 5166–5169. [Google Scholar] [CrossRef]
- Seo, C.; Sohn, J.H.; Oh, H.; Kim, B.Y.; Ahn, J.S. Isolation of the protein tyrosine phosphatase 1B inhibitory metabolite from the marine-derived fungus Cosmospora sp. SF-5060. Bioorg. Med. Chem. Lett. 2009, 19, 6095–6097. [Google Scholar] [CrossRef]
- Williams, D.E.; Amlani, A.; Dewi, A.S.; Patrick, B.O.; van Ofwegen, L.; Mui, A.L.F.; Andersen, R.J. Australin E isolated from the soft coral Cladiella sp. collected in pohnpei activates the inositol 5-phosphatase SHIP1. Aust. J. Chem. 2010, 63, 895–900. [Google Scholar] [CrossRef]
- Rubio, B.K.; Parrish, S.M.; Yoshida, W.; Schupp, P.J.; Schils, T.; Williams, P.G. Depsipeptides from a guamanian marine cyanobacterium, Lyngbya bouillonii, with selective inhibition of serine proteases. Tetrahedron Lett. 2010, 51, 6718–6721. [Google Scholar] [CrossRef]
- Sisay, M.T.; Hautmann, S.; Mehner, C.; Konig, G.M.; Bajorath, J.; Gutschow, M. Inhibition of human leukocyte elastase by brunsvicamides A–C: Cyanobacterial cyclic peptides. Chem. Med. Chem. 2009, 4, 1425–1429. [Google Scholar]
- McCulloch, M.W.; Bugni, T.S.; Concepcion, G.P.; Coombs, G.S.; Harper, M.K.; Kaur, S.; Mangalindan, G.C.; Mutizwa, M.M.; Veltri, C.A.; Virshup, D.M.; et al. Carteriosulfonic acids A–C, GSK-3β inhibitors from a Carteriospongia sp. J. Nat. Prod. 2009, 72, 1651–1656. [Google Scholar] [CrossRef]
- Williams, D.E.; Hollander, I.; Feldberg, L.; Frommer, E.; Mallon, R.; Tahir, A.; Van Soest, S.R.; Andersen, R.J. Scalarane-based sesterterpenoid RCE-protease inhibitors isolated from the indonesian marine sponge Carteriospongia foliascens. J. Nat. Prod. 2009, 72, 1106–1109. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Wessling, D.; Jobling, M.; Avery, V.M.; Davis, R.A.; Feng, Y.; Hooper, J.N.; Quinn, R.J. Clavatadines C–E, guanidine alkaloids from the australian sponge Suberea clavata. J. Nat. Prod. 2009, 72, 973–975. [Google Scholar] [CrossRef]
- Whitson, E.L.; Bugni, T.S.; Chockalingam, P.S.; Concepcion, G.P.; Feng, X.; Jin, G.; Harper, M.K.; Mangalindan, G.C.; McDonald, L.A.; Ireland, C.M. Fibrosterol sulfates from the philippine sponge Lissodendoryx (Acanthodoryx) fibrosa: Sterol dimers that inhibit PKCzeta. J. Org. Chem. 2009, 74, 5902–5908. [Google Scholar] [CrossRef]
- Rateb, M.E.; Houssen, W.E.; Schumacher, M.; Harrison, W.T.; Diederich, M.; Ebel, R.; Jaspars, M. Bioactive diterpene derivatives from the marine sponge Spongionella sp. J. Nat. Prod. 2009, 72, 1471–1476. [Google Scholar] [CrossRef]
- Cui, C.M.; Li, X.M.; Li, C.Y.; Sun, H.F.; Gan, S.S.; Wang, B.G. Benzodiazepine alkaloids from marine-derived endophytic fungus Aspergillus ochraceus. Helv. Chim. Acta 2009, 92, 1366–1370. [Google Scholar] [CrossRef]
- Robinson, S.J.; Hoobler, E.K.; Riener, M.; Loveridge, S.T.; Tenney, K.; Valeriote, F.A.; Holman, T.R.; Crews, P. Using enzyme assays to evaluate the structure and bioactivity of sponge-derived meroterpenes. J. Nat. Prod. 2009, 72, 1857–1863. [Google Scholar] [CrossRef]
- Matthew, S.; Paul, V.J.; Luesch, H. Largamides A–C, tiglic acid-containing cyclodepsipeptides with elastase-inhibitory activity from the marine cyanobacterium Lyngbya confervoides. Planta Med. 2009, 75, 528–533. [Google Scholar] [CrossRef]
- Matthew, S.; Ratnayake, R.; Becerro, M.A.; Ritson-Williams, R.; Paul, V.J.; Luesch, H. Intramolecular modulation of serine protease inhibitor activity in a marine cyanobacterium with antifeedant properties. Mar. Drugs 2010, 8, 1803–1816. [Google Scholar] [CrossRef]
- Qin, J.; Su, H.; Zhang, Y.; Gao, J.; Zhu, L.; Wu, X.; Pan, H.; Li, X. Highly brominated metabolites from marine red alga Laurencia similis inhibit protein tyrosine phosphatase 1B. Bioorg. Med. Chem. Lett. 2010, 20, 7152–7154. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Miller, M.W.; Kwan, J.C.; Luesch, H.; Paul, V.J. Molassamide, a depsipeptide serine protease inhibitor from the marine cyanobacterium Dichothrix utahensis. J. Nat. Prod. 2010, 73, 459–462. [Google Scholar] [CrossRef]
- Feng, Z.; Leutou, A.S.; Yang, G.; Nenkep, V.N.; Siwe, X.N.; Choi, H.D.; Kang, J.S.; Son, B.W. Bioactive cyclopentenone derivatives from marine isolates of fungi. Bull. Korean Chem. Soc. 2009, 30, 2345–2350. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Dello, I.E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Florio, C.; Lorenzon, P.; de Bortoli, M.; et al. Stereostructure and biological activity of 42-Hydroxy-palytoxin: A new palytoxin analogue from Hawaiian Palythoa subspecies. Chem. Res. Toxicol. 2009, 22, 1851–1859. [Google Scholar] [CrossRef]
- Carr, G.; Tay, W.; Bottriell, H.; Andersen, S.K.; Mauk, A.G.; Andersen, R.J. Plectosphaeroic Acids A, B, and C, indoleamine 2,3-Dioxygenase inhibitors produced in culture by a marine isolate of the fungus Plectosphaerella cucumerina. Org. Lett. 2009, 11, 2996–2999. [Google Scholar] [CrossRef]
- Yamashita, T.; Nakao, Y.; Matsunaga, S.; Oikawa, T.; Imahara, Y.; Fusetani, N. A new antiangiogenic C24 oxylipin from the soft coral Sinularia numerosa. Bioorg. Med. Chem. 2009, 17, 2181–2184. [Google Scholar] [CrossRef]
- Jain, S.; Abraham, I.; Carvalho, P.; Kuang, Y.H.; Shaala, L.A.; Youssef, D.T.; Avery, M.A.; Chen, Z.S.; El Sayed, K.A. Sipholane triterpenoids: Chemistry, reversal of ABCB1/P-Glycoprotein-Mediated multidrug resistance, and pharmacophore modeling. J. Nat. Prod. 2009, 72, 1291–1298. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Kehraus, S.; Gutschow, M.; Konig, G.M. Spartinoxide, a new enantiomer of A82775C with inhibitory activity toward HLE from the marine-derived fungus Phaeosphaeria spartinae. Nat. Prod. Commun. 2010, 5, 1071–1076. [Google Scholar]
- Ankisetty, S.; Gochfeld, D.J.; Diaz, M.C.; Khan, S.I.; Slattery, M. Chemical constituents of the deep reef caribbean sponges Plakortis angulospiculatus and Plakortis halichondrioides and their anti-inflammatory activities. J. Nat. Prod. 2010, 73, 1494–1498. [Google Scholar] [CrossRef]
- Gutierrez, M.; Andrianasolo, E.H.; Shin, W.K.; Goeger, D.E.; Yokochi, A.; Schemies, J.; Jung, M.; France, D.; Cornell-Kennon, S.; Lee, E.; et al. Structural and synthetic investigations of tanikolide dimer, a SIRT2 selective inhibitor, and tanikolide seco-acid from the madagascar marine cyanobacterium Lyngbya majuscula. J. Org. Chem. 2009, 74, 5267–5275. [Google Scholar] [CrossRef]
- Truxal, L.T.; Bourdelais, A.J.; Jacocks, H.; Abraham, W.M.; Baden, D.G. Characterization of tamulamides A and B, polyethers isolated from the marine dinoflagellate Karenia brevis. J. Nat. Prod. 2010, 73, 536–540. [Google Scholar] [CrossRef]
- Lopez-Gresa, M.P.; Cabedo, N.; Gonzalez-Mas, M.C.; Ciavatta, M.L.; Avila, C.; Primo, J. Terretonins E and F, inhibitors of the mitochondrial respiratory chain from the marine-derived fungus Aspergillus insuetus. J. Nat. Prod. 2009, 72, 1348–1351. [Google Scholar] [CrossRef]
- Choi, Y.; Jermihov, K.; Nam, S.J.; Sturdy, M.; Maloney, K.; Qiu, X.; Chadwick, L.R.; Main, M.; Chen, S.N.; Mesecar, A.D.; Farnsworth, N.R.; Pauli, G.F.; Fenical, W.; Pezzuto, J.M.; van Breemen, R.B. Screening natural products for inhibitors of quinone reductase-2 using ultrafiltration LC-MS. Anal. Chem. 2011, 83, 1048–1052. [Google Scholar] [CrossRef]
- Kwan, J.C.; Taori, K.; Paul, V.; Luesch, H. Lyngbyastatins 8–10, elastase inhibitors with cyclic depsipeptide scaffolds isolated from the marine cyanobacterium Lyngbya semiplena. Mar. Drugs 2009, 7, 528–538. [Google Scholar] [CrossRef]
- Glaser, K.B.; Mayer, A.M. A renaissance in marine pharmacology: From preclinical curiosity to clinical reality. Biochem. Pharmacol. 2009, 78, 440–448. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitamura, M.; Uemura, D. Biologically active marine natural products. Heterocycles 2009, 78, 1–17. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef]
- Fusetani, N. Biotechnological potential of marine natural products. Pure Appl. Chem. 2010, 82, 17–26. [Google Scholar] [CrossRef]
- Hill, R.T.; Fenical, W. Pharmaceuticals from marine natural products: Surge or Ebb? Curr. Opin. Biotechnol. 2010, 21, 777–779. [Google Scholar] [CrossRef]
- Rae, I.D. Marine pharmacology in Australia. The roche research institute at dee why, new South Wales, 1974–81. Aust. J. Chem. 2010, 63, 855–861. [Google Scholar] [CrossRef]
- Mayer, A.M.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Thomas, T.R.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, S.P.; Rai, A.K.; Mohapatra, T.M. Cyanobacteria: An emerging source for drug discovery. J. Antibiot. 2011, 64, 401–412. [Google Scholar] [CrossRef]
- Park, Y. Mining invertebrate natural products for future therapeutic treasure. Nat. Prod. Commun. 2011, 6, 1403–1408. [Google Scholar]
- Kingston, D.G. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2011, 74, 496–511. [Google Scholar]
- Ebada, S.S.; Proksch, P. Chemical and pharmacological significance of natural guanidines from marine invertebrates. Mini-Rev. Med. Chem. 2011, 11, 225–246. [Google Scholar] [CrossRef]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef]
- Hughes, C.C.; Fenical, W. Antibacterials from the Sea. Chem. Eur J. 2010, 16, 12512–12525. [Google Scholar] [CrossRef]
- Waters, A.L.; Hill, R.T.; Place, A.R.; Hamann, M.T. The expanding role of marine microbes in pharmaceutical development. Curr. Opin. Biotechnol. 2010, 21, 780–786. [Google Scholar] [CrossRef]
- Lu, X.; Cao, X.; Liu, X.; Jiao, B. Marine microbes-derived anti-bacterial agents. Mini-Rev. Med. Chem. 2010, 10, 1077–1090. [Google Scholar] [CrossRef]
- Otero-Gonzalez, A.J.; Magalhaes, B.S.; Garcia-Villarino, M.; Lopez-Abarrategui, C.; Sousa, D.A.; Dias, S.C.; Franco, O.L. Antimicrobial peptides from marine invertebrates as a new frontier for microbial infection control. FASEB J. 2010, 24, 1320–1334. [Google Scholar] [CrossRef]
- Rahman, H.; Austin, B.; Mitchell, W.J.; Morris, P.C.; Jamieson, D.J.; Adams, D.R.; Spragg, A.M.; Schweizer, M. Novel anti-infective compounds from marine bacteria. Mar. Drugs 2010, 8, 498–518. [Google Scholar] [CrossRef]
- Smith, V.J.; Desbois, A.P.; Dyrynda, E.A. Conventional and unconventional antimicrobials from fish, marine invertebrates and micro-algae. Mar. Drugs 2010, 8, 1213–1262. [Google Scholar]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.K. Potential anti-HIV agents from marine resources: An overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef]
- Yasuhara-Bell, J.; Lu, Y. Marine compounds and their antiviral activities. Antiviral Res. 2010, 86, 231–240. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Ta, Q.V.; Kim, S.K. Marine organisms as a therapeutic source against herpes simplex virus infection. Eur. J. Pharm. Sci. 2011, 44, 11–20. [Google Scholar] [CrossRef]
- Watts, K.R.; Tenney, K.; Crews, P. The structural diversity and promise of antiparasitic marine invertebrate-derived small molecules. Curr. Opin. Biotechnol. 2010, 21, 808–818. [Google Scholar] [CrossRef]
- Tempone, A.G.; de Oliveira, M.; Berlinck, R.G. Current approaches to discover marine antileishmanial natural products. Planta Med. 2011, 77, 572–585. [Google Scholar] [CrossRef]
- Ashforth, E.J.; Fu, C.; Liu, X.; Dai, H.; Song, F.; Guo, H.; Zhang, L. Bioprospecting for antituberculosis leads from microbial metabolites. Nat. Prod. Rep. 2010, 27, 1709–1719. [Google Scholar] [CrossRef]
- Peach, K.C.; Linington, R.G. New innovations for an old infection: Antimalarial lead discovery from marine natural products during the period 2003–2008. Future Med. Chem. 2009, 1, 593–617. [Google Scholar] [CrossRef]
- Gademann, K.; Kobylinska, J. Antimalarial natural products of marine and freshwater origin. Chem. Rec. 2009, 9, 187–198. [Google Scholar] [CrossRef]
- Fattorusso, E.; Taglialatela-Scafati, O. Marine antimalarials. Mar. Drugs 2009, 7, 130–152. [Google Scholar] [CrossRef]
- Villa, F.A.; Gerwick, L. Marine natural product drug discovery: Leads for treatment of inflammation, cancer, infections, and neurological disorders. Immunopharmacol. Immunotoxicol. 2010, 32, 228–237. [Google Scholar] [CrossRef]
- Folmer, F.; Jaspars, M.; Schumacher, M.; Dicato, M.; Diederich, M. Marine natural products targeting phospholipases A2. Biochem. Pharmacol. 2010, 80, 1793–1800. [Google Scholar] [CrossRef]
- Berrue, F.; McCulloch, M.W.; Kerr, R.G. Marine diterpene glycosides. Bioorg. Med. Chem. 2011, 19, 6702–6719. [Google Scholar] [CrossRef]
- Jaswir, I.; Monsur, H.A. Anti-inflammatory compounds of macro algae origin: A review. J. Med. Plants Res. 2011, 5, 7146–7154. [Google Scholar]
- Wijesekara, I.; Kim, S.K. Angiotensin-I-converting Enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef]
- Halai, R.; Craik, D.J. Conotoxins: Natural product drug leads. Nat. Prod. Rep. 2009, 26, 526–536. [Google Scholar] [CrossRef]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef]
- Teichert, R.W.; Olivera, B.M. Natural products and ion channel pharmacology. Future Med. Chem. 2010, 2, 731–744. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Daly, N.L.; Craik, D.J. Conopeptides as novel options for pain management. Drugs Future 2011, 36, 25–32. [Google Scholar]
- Muttenthaler, M.; Akondi, K.B.; Alewood, P.F. Structure-activity studies on alpha-conotoxins. Curr. Pharm. Des. 2011, 17, 4226–4241. [Google Scholar] [CrossRef]
- Fagerholm, A.E.; Habrant, D.; Koskinen, A.M. Calyculins and related marine natural products as serine-threonine protein phosphatase PP1 and PP2A inhibitors and total syntheses of calyculin A, B, and C. Mar. Drugs 2010, 8, 122–172. [Google Scholar] [CrossRef]
- Folmer, F.; Jaspars, M.; Solano, G.; Cristofanon, S.; Henry, E.; Tabudravu, J.; Black, K.; Green, D.H.; Küpper, F.C.; Aalbersberg, W.; et al. The inhibition of TNF-α-induced NF-κB activation by marine natural products. Biochem. Pharmacol. 2009, 78, 592–606. [Google Scholar] [CrossRef]
- Skropeta, D.; Pastro, N.; Zivanovic, A. Kinase inhibitors from marine sponges. Mar. Drugs 2011, 9, 2131–2154. [Google Scholar] [CrossRef]
- Senter, P.D.; Sievers, E.L. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 2012, 30, 631–637. [Google Scholar] [CrossRef]
- The Global Marine Pharmaceutical Pipeline. Available online: http://marinepharmacology.midwestern.edu/ (accessed on 3 June 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).