Abstract
Natural antioxidants have renewed value for human health and the food industry. Green labeling is becoming an important attribute for consumers and is impacting food processing and formulations. Clean label is another attribute that ranked third after the “free-from” claims and “a good source” of nutrient claims. Clean label attributes also are ranked higher than local, seasonal, and organic. Techniques that are able to preserve the valuable characteristics of natural antioxidants, while eliminating even trace amounts of solvent residues from their extraction and processing, are important. Supercritical fluids (SCF) are an effective green technology that can be adopted for extraction of natural antioxidants. This review is focused on the application of supercritical carbon dioxide (SCCO2) for extracting hydrophobic antioxidant compounds with an emphasis on oilseed crops and carrots. The information provided about extraction parameters helps to guide optimization of the yield of tocopherols and carotenoids. Pressure is the most effective parameter for the extraction yield of tocopherol among the other parameters, such as temperature, time, and CO2 flow rate. For carotenoid extraction, both pressure and temperature have a large impact on extraction yield. Higher yields of antioxidants, greater purity of the extracts, and larger retention of bioactivity are the main advantages of supercritical fluid extraction (SFE) in comparison to other conventional techniques. The benefits of SCF technology may open new opportunities for extracting valuable, natural and effective antioxidant compounds from food processing co-streams for use as bioactive compounds.
1. Introduction
Antioxidants play a significant role in biological systems by slowing down or buffering the oxidation of other molecules and, in so doing, protect cells from damage caused by unstable molecules or free radicals [1,2]. In the popular press, antioxidants are frequently presented as supplements which prevent or cure human disease [1]. In the human body, natural antioxidants from plant sources have been shown to contribute to overall health and wellness and offer protection, at least in part, against health disorders such as cardiovascular, neurodegenerative, and gastrointestinal diseases, cancer, diabetes, arthritis, and retinal degeneration [3,4], as well as slowing the aging process [5].
Antioxidants have significant functions in many processed foods and beverages [6]. They prevent the foods we eat from spoiling and deteriorating due to oxidation and microbial activity, and, in so doing, preserve safety, flavor, texture, and nutritional integrity [6]. Antioxidants also help protect the sensory attributes of foods/beverages, such as odor, flavor, and color, as well as preserving nutritional integrity and quality and preventing spoilage by excluding oxygen [7].
Many antioxidants used by the food industry are synthetic antioxidants [6]. Their use as additives, however, elicited strong responses for their safety from increasingly concerned consumers [8]. Synthetic antioxidants may also impact health [9], and with names that are typically unfamiliar and difficult to pronounce are perceived as health risks [10]. As a result, there is notable increased interest in purchasing naturally based antioxidant products [11], with the food industry actively seeking new natural sources [7]. Consumer demand for “clean” labels for natural food ingredients and additives that are perceived as healthy is growing [7]. Such demands are reinforced by an increasing interest in sustainable sources of production using environmentally-friendly processing techniques [11]. Clean label attributes rank third behind “a good source” of nutrient claims and “free-from” claims as the top attributes that make foods and beverages healthy [7]. Clean label attributes are, therefore, ranked higher than local, seasonal, and organic [7].
Market Data Forecast has predicted global sale of the foods/beverages that have clean labels and include clean ingredients such as natural flavors and colors, fruit and vegetables, and others will increase from $38.8 billion in 2021 to $64.1 billion in 2026 at an annual growth rate of compound of 6.8% [12]. According to Innova Market Insights’ Innova Health & Nutrition Survey 2020, around 51% of consumers worldwide based their purchasing decision on whether or not a packaged food or beverage contains preservatives. At the same time, 46% of respondents reported trying to purchase “only natural ingredients”, 33% “organic” foods, and same rate 33% “sustainably sourced” ingredients [12].
Currently, there is no legal definition for “clean label”, but the term generally refers to foods that are minimally processed, processed without harsh solvents or harsh conditions, and do not contain artificial flavors, colors, sweeteners, artificial preservatives or additives, and unexpected allergens [13]. While no formal definition exists, several food retailers (Trader Joe’s, Whole Foods) have set internal standards by implementing in-store or brand bans for specific food ingredients or additives [14]. Although “clean labels” have a number of connotations, consumers are increasingly looking for natural preservatives or antioxidants extracted using “green” processing technologies [15].
SCCO2 is emerging as a “clean” or “green” option for extraction of oils and bioactive compounds for food, pharmaceuticals, and industrial applications as it is operable at moderate pressures and temperatures [16,17]. CO2 is inexpensive and readily available as a noncorrosive and nonflammable solvent with generally recognized as safe (GRAS) status [18,19,20]. SCCO2 is sustainably sourced and can be recycled and re-used without leaving chemical residues in the food [21,22]. Most importantly, SCCO2 protects antioxidants during extraction and/or reaction because it provides a non-oxidizing environment by preventing the formation of oxidation products [23,24], which is a very important aspect for preserving carotenoids [25]. SCCO2 is an excellent solvent for dissolving non-polar [26] or slightly polar compounds, although its solvent power for hydrophilic substrates is rather weak [27,28]. This can be overcome, however, by adding a polar co-solvent such as ethanol [27].
Carrots, a horticultural crop [29], and soybean, canola, sunflower, and rape oilseed, oleaginous field crops, are all rich in hydrophobic and lipophilic antioxidants [30]. These crops provide significant economic value in their growing regions. For example, canola has benefited the Canadian economy by creating $19.3 billion annually in economic activity and 249,000 jobs [31]. The press cake or meals from processing oilseeds, and processing by-products, are of particular interest as their increased utilization could provide an additional source of revenue for these under-utilized by-products [31]. These agricultural and industrial processing co-streams represent an inexpensive, renewable and highly accessible source of antioxidants [32].
The main focus of this review is the application of SCCO2 as a “green” process for extracting natural hydrophobic antioxidants from plant sources, specifically carotenoids from carrots and vitamin E from oleaginous field crops (soybean, canola, sunflower, and rapeseed oils). In addition, this paper introduces hydrophobic antioxidants, and reviews the advantages and disadvantages of various solvent extraction methods for vitamin E and carotenoids. The focus of vitamin E extraction is more on tocopherols than tocotrienols due to the high tocopherol content of canola, soybean, sunflower, and rapeseed oils. The effect of different variables, such as pressure, temperature, time and the flow rate of feed, modifier, and CO2, on yield of vitamin E during the extraction by SCCO2 are discussed in this review. Additionally, this review shows how the extraction yield of carotenoids from various sources was influenced by different pressures and temperatures during the SCCO2 extraction. Finally, the properties of SCCO2 extraction of vitamin E and carotenoid are compared to the extraction of these hydrophobic antioxidants by conventional techniques.
1.1. Hydrophobic Antioxidants
Vitamin E, ubiquinone, flavonoids, carotenoids, and retinoids are hydrophobic antioxidants with lipophilic properties [33,34]. Vitamin E, the collective name for eight constituents of this natural fat-soluble antioxidant, consists of three important functional groups [35]: (i) hydrophobic C16 side-chains that determine two different families, tocopherols and tocotrienols. Tocotrienols differ from the tocopherols by having an unsaturated C16 chain (Figure 1). (ii) Chroman-6-ol rings that distinguish four isomers (α, β, γ, and δ) that depend on the position and number of methyl groups attached to the chromane ring. (iii) A hydroxyl group on the ring that reduces free radicals when it donates a hydrogen atom, exists in all eight derivatives [33,36].
Sources of vitamin E include vegetable oils such, as canola, soy, peanut, sunflower, walnuts, and cereal grain oils (maize > wheat > barley > rye oils) [37,38]. Tocopherol concentrations in vegetable oils range from 20–1640 mg/kg for all four homologs [39]. The total amount of tocopherols and tocotrienols varies in different sources with walnut, soybean and peanut containing tocopherols at levels greater than 1000 mg/kg, while cottonseed, safflower, sunflower, and canola/oilseed rape sources contain over 680 mg/kg (canola typically 770 mg/kg) [39].
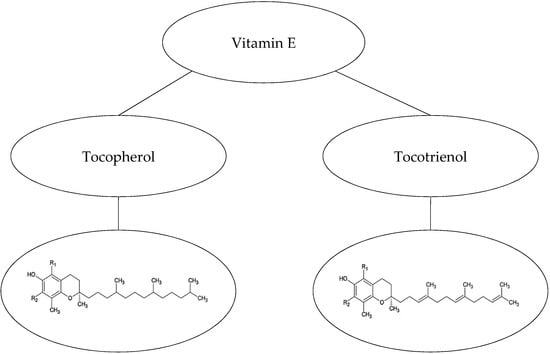
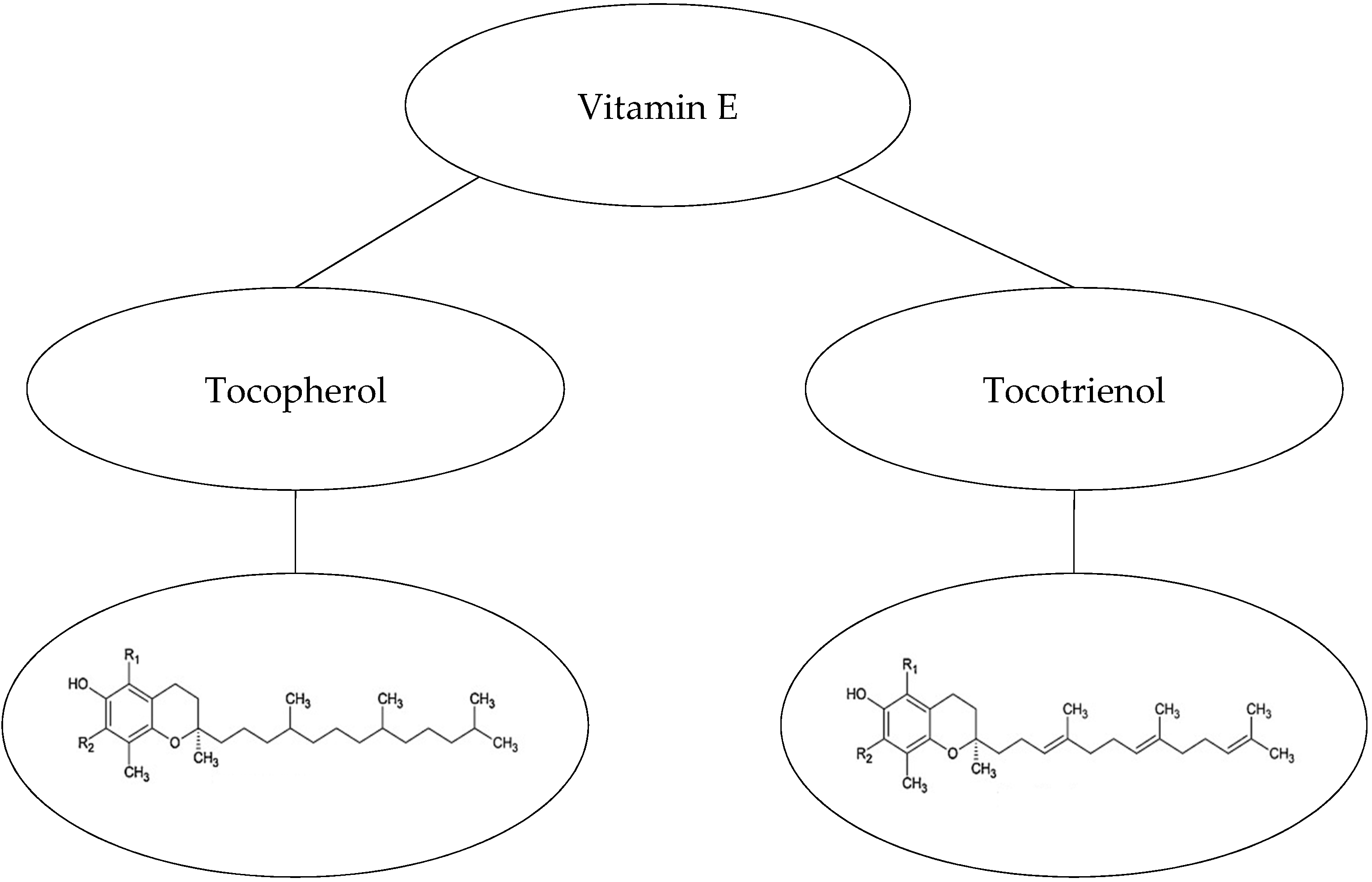

Figure 1.
Tocopherols and tocotrienols (vitamin E) [39,40].
Figure 1.
Tocopherols and tocotrienols (vitamin E) [39,40].

The most biologically active homolog among the isomers is α-tocopherol, which is preferred by the body for absorption and metabolization, while γ-tocopherol has more antioxidant activity [36,40]. Of the tocopherols, α and γ types represent the highest percentages in vegetable oils. For instance, canola oil has 210–272 mg/kg α-tocopherol and 423 kg/kg γ-tocopherol, while soybean oil contains 75 mg/kg α-tocopherol and 266 mg/kg γ-tocopherol [41,42]. Tocotrienols are not found in soybean and rapeseed oils [43,44].
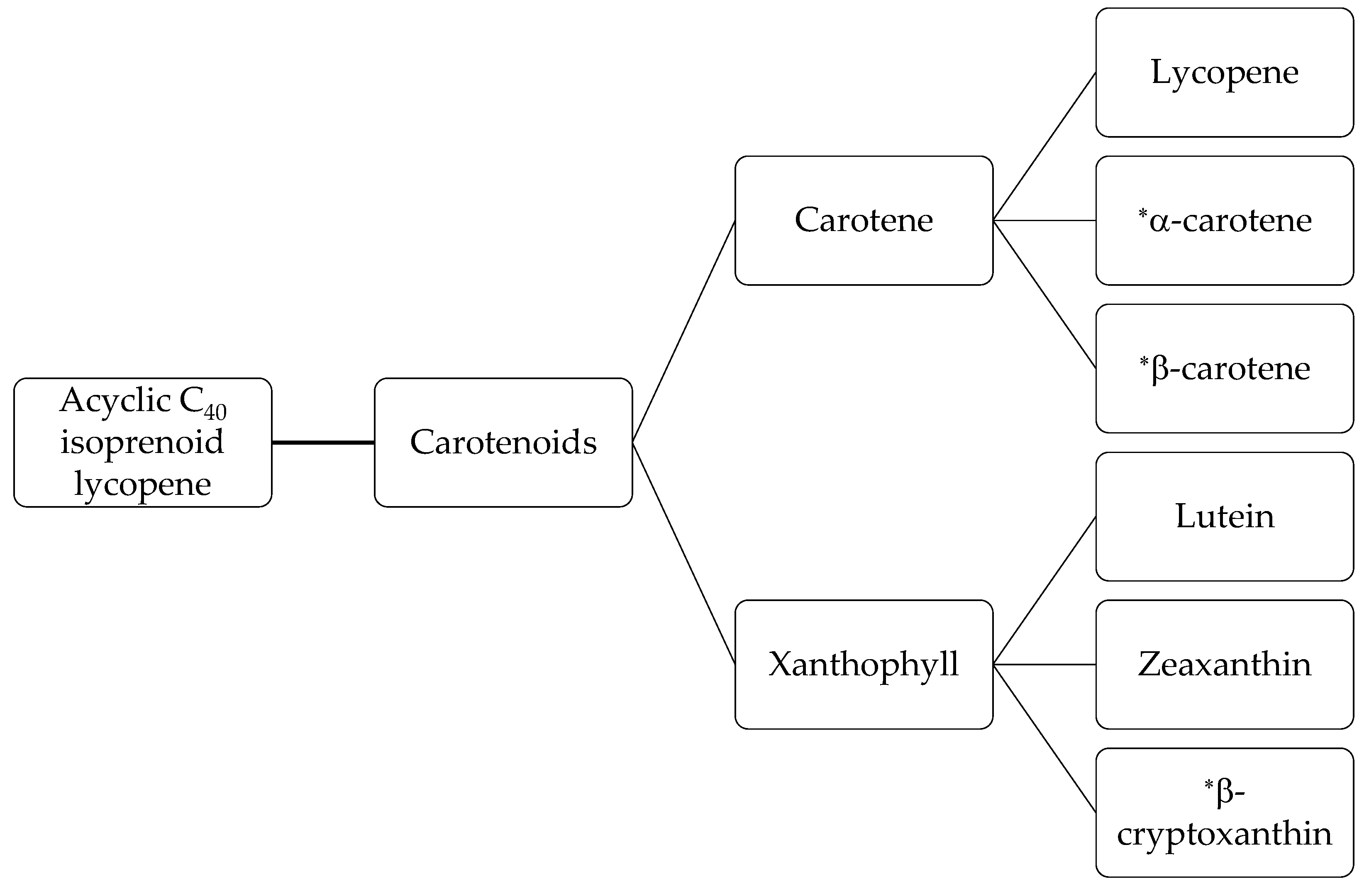
Carotenoids (tetraterpenes) are derived from the acyclic C40 isoprenoid lycopene [45]. They are known as functional and nutraceutical compounds due to their provitamin A activity and natural food colorant role [46]. Figure 2 shows the categories of carotenoids. Carotenoids are found in most yellow, orange and red fruits and vegetables. Carrots [47], peaches, nectarine [48], banana peels [49], tomatoes [50], and sweet potatoes [51] all contain this fat-soluble antioxidant. Carrots, in particular, are an excellent source of carotenoids, with values ranging from 600–1200 mg/kg carotene content [52]. The most significant carotenoids of carrots are lutein, lycopene, α-carotene, and β-carotene isomers [45]. Carotenoids exist naturally in the trans form in plants [45]. The vitamin A activity of trans-β-carotene and trans-α-carotene theoretically is 100% and 50%, respectively [42]. Carrots contain high percentages of the most active forms of carotenoids which includes 60–80% trans-β-carotene, 10–40% trans-α-carotene, and 1–5% lutein [42]. Carotenoid extraction is related to the recovery of lycopene and β-carotene as they are important economically because of their role as natural colorants [33]. In comparison with synthetic pigments, carotenoids are valued highly as food colorants and are recognized as a functional, medical, and nutraceutical food, as well as a natural additive [46].
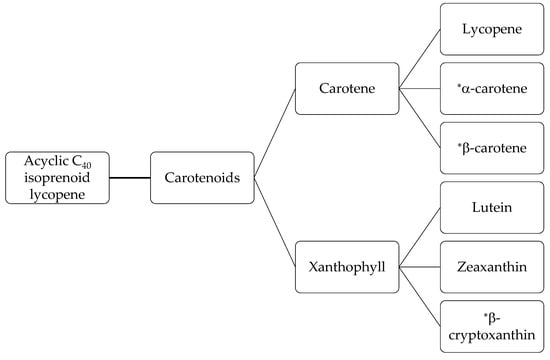
Figure 2.
Carotenoid fractions [45]. * promoters of vitamin A.
1.2. Supercritical Fluid (SCF)
A fluid becomes supercritical when the temperature and pressure go above its critical point [19]. The three common states of matter are solid, liquid, and gas. Carbon dioxide exists in all three states, gas, liquid, and solid at its triple point which is at a temperature of −57 °C and 4.16 bar pressure [53]. Therefore, dry ice (solid CO2) sublimes directly to a vapor at room temperature and at atmospheric pressure. When temperature and pressure both increase, CO2 enters into a critical state at which no phase boundaries exist [26], so its liquid phase cannot be distinguished [52]. As CO2 approaches its critical temperature and pressure, the properties of its gas and liquid phase converge, resulting in a SCF [53]. The critical temperature of CO2 is 31°C (304 K) and the critical pressure is 74 bar. At this point, CO2 has the density of a liquid and dissolves materials like a liquid while it behaves like a gas and diffuses readily through solids [54]. Although the density of CO2 can be manipulated, above a temperature of 31 °C, it cannot be liquefied even with pressures as high as 3040 bar [53]. Table 1 illustrates the physical properties of CO2 at different states [19,55].

Table 1.
Physical properties of CO2 at different states [19,55].
Diffusion and dissolution are two essential stages in extraction by SCCO2 [56]. SCCO2 penetrates, diffuses, and is absorbed into a solid matrix, such as the vegetal material [57]. The solute, such as oil, is solubilized and is transported by the SCCO2 in two steps: (i) through the solid matrix to the outer layer, then (ii) from the outer surface of the solid to the bulk solution [58]. In extraction, an extractant should be separated from a solid or liquid matrix [58]. SCCO2 has two phases comprising a dense liquid and a low-density gas in equilibrium [59]. Close to the critical temperature (i.e., at 27 °C), the density of gas and liquid, at equilibrium state, becomes denser and lower, respectively. At the critical point (31 °C and 74 bar), there is only one phase and, therefore, no differences in the density [59]. Above the critical point, a small increase in pressure gives rise to a large increase in the density of the SCCO2 phase [58]. Many other physical properties, such as diffusivity, relative permittivity, solvent strength, and viscosity of a SCF, all of which affect its solubility, can be regulated by making relatively small changes in pressure or temperature [40,60].
SCFs are, therefore, proposed as an alternative to conventional methods of extraction, reaction, fractionation, and analysis due to their unique manipulable properties [55]. Supercritical fluid extraction (SFE) is a fast technique because it is a diffusion-based process and diffusivities are much faster in SCFs than in liquids [58]. SCF has very low surface tension, low viscosity, and moderately high diffusion coefficient, so it gives better small pore penetration [19,61,62]. In contrast, SCF has a density like a liquid that improves its solvent power to facilitate mass transfer [61]. All these characteristics provide excellent transport properties for the SCF compared to an organic solvent [23,61].
2. Extraction Methods for Hydrophobic Antioxidants Not Using SCF
2.1. Extraction of Vitamin E (Tocopherols)
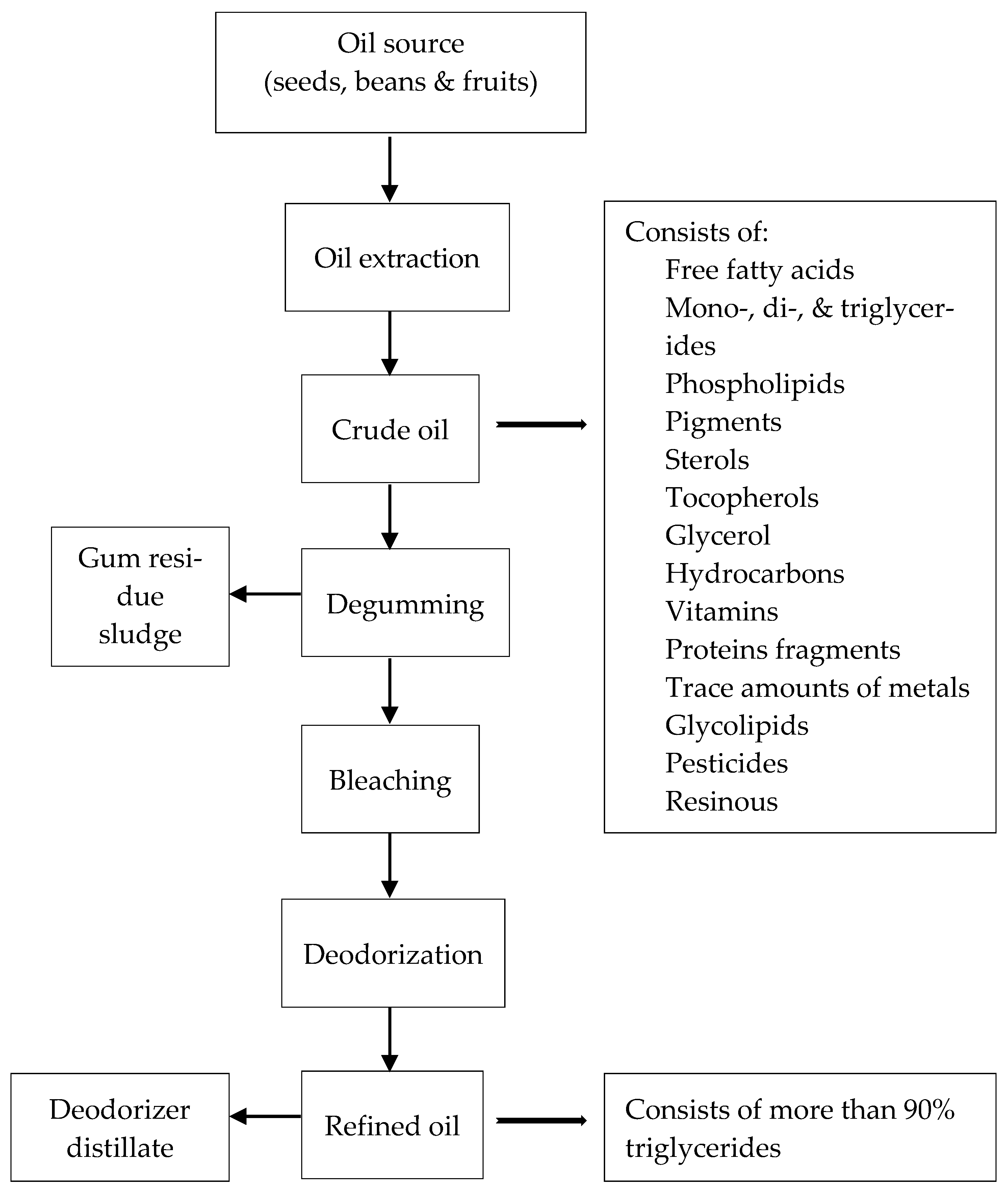
Production of edible oils in industry comprises two steps: the extraction of oils from the seeds and refining the crude oil. Converting the crude oil to refined oil occurs via physical and chemical refining stages (Figure 3) [63]. Tocopherols exist in refined oils, meal, and the deodorized sludge that is obtained from the deodorization step in conventional vegetable oil refining processes. Around 30–40% of the tocopherols and tocotrienols are removed from oil and end up in the bleached and deodorized sludge or in the gums during the process of manufacturing oils [64]. The presence of tocopherols in cooking oil has both functional and nutraceutical benefits as antioxidants by helping to increase the oil shelf life and maintain health benefits in the cooking oil. Furthermore, it is desirable for livestock feed consumption to keep the tocopherols in the meals after extracting oil from the beans or seeds. The deodorized distilled fraction, which is a by-product of steam distillation in the deodorization stage, may be the best source for extracting tocopherols in industry. Deodorized distillate is a rich source of vitamin E and sterols, especially campesterol, stigmasterol, and β-sitosterol [36]. Besides tocopherols and sterols, other components, such as aldehydes and ketones, can be isolated and used for nutritious products in the feed, cosmetic, and food industries [65].
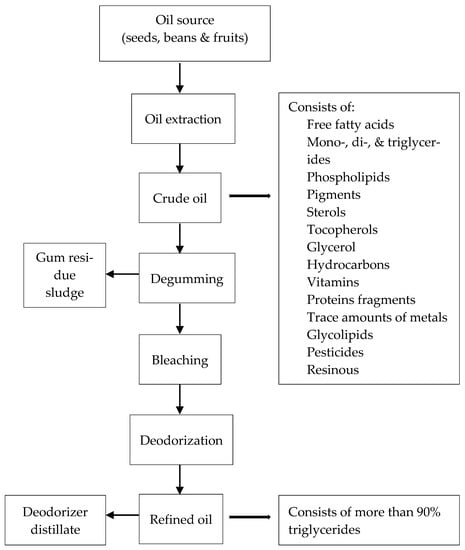
Figure 3.
Edible oil refining process [63,66].
Solvent Extraction Methods
The solvent extraction method is based on using a solvent in extraction and recovery of the sterols and tocopherols by crystallization [63]. Table 2 illustrates the results of some studies that used conventional solvent extraction techniques to extract tocopherols from the distillate fraction and most of them reported that recovery yield and purity did not exceed 89% and 76%, respectively. However, Zhou et al. [67] stated that there was no significant loss in tocopherol content during the extraction process. The solvent extraction method, however, requires some modifications so that the purity can be increased, as described below.

Table 2.
Different solvent extraction methods for tocopherols and sterol recovery from vegetable oil deodorizer distillate.
2.2. Modification to Increase the Purity
2.2.1. Extraction by Caustic Methanol
Tocopherol or tocotrienol-enriched organic materials are mixed with hexane. The resulting mixture is then mixed with methanol and sodium hydroxide. The mixture separates into two layers: (i) a tocopherol-containing caustic methanol phase and (ii) a phase containing impurities such as squalene, waxes, and sterols [40].
For recovery of the pure tocopherols, the first phase is mixed with hexane three times. These hexane layers are combined and then neutralized with glacial acetic acid (nonaqueous) and NaOH for three times to prevent loss of tocopherol. At this stage, all the caustic phases are combined, and the hexane layers collected and discarded. The resulting tocopherol-enriched caustic methanol is neutralized again with glacial acetic acid and separation occurs when water is added at this step. The results of this separation are (i) a tocopherol-enriched phase and (ii) a neutralized methanol phase. The second phase is washed with hexane. The two layers are combined and hexane removed under vacuum distillation; therefore, the product remains with 82% tocopherol and 7% sterol [40]. Molecular distillation helps separate tocopherols and sterols from saponified deodorized distilled oil at elevated temperature and high vacuum conditions [63].
2.2.2. Extraction by a Solution of Urea and Alcohol
This technique has three main steps including (i) melting the deodorizer distillates, (ii) adding to the mixture of urea and alcohol, and (iii) separating the free fatty acids. The outcome of this technique is increasing the tocopherol concentration [63].
2.2.3. Extraction by Anionic Exchange Resin
This method utilizes organic solvents and anionic resin. The mixture of polar organic solvent and the tocopherol-containing material is passed through a strongly anionic exchange resin. The adsorbed tocopherols are then eluted by acidic eluting solution [40,63].
2.2.4. Extraction Utilizing Liquid Fractionation Technique
Compared with the resin method, this technique isolates more purified tocopherol, by removing the free fatty acids from tocopherol-containing products; one or more organic solvents can be used. The mixture needs to stand for a while, followed by separation of the solvent layer. Tocopherols remain after evaporating the solvent from the recovered supernatant [40].
2.2.5. Extraction Utilizing Enzymatic Reaction Technique
Enzymatic reactions concentrate tocopherols by converting a class of components to another class, such as conversion of acylglycerols to free fatty acids, sterols to steryl esters, and free fatty acids to fatty acid methyl ester [63].
2.3. Extraction of Carotenoids
Approximately 70% of carrots cannot be marketed due to the fact that their shape and size do not meet market specifications [74]. These cull carrots are wasted unless other uses can be identified [75]. Carrots are a rich source of carotene (ranging from 600 to 1200 mg/kg, with some varieties as high as 3000 mg/kg); extracting of antioxidants from waste carrots will be valuable to industry and consumers [45]. Therefore, finding the best extraction method from an economic and environmentally-friendly point of view that produces the highest yield can add value to this waste product [76]. Enzymatic and non-enzymatic solvent extraction procedures are the two main industrial methods for carotenoid extractions. Canola, sunflower oil, and soybean oil can be used as solvents for extracting carotenoids from marine and vegetable wastes [38]. Sun and Temelli [77] discovered that using canola oil as a co-solvent in SFE increased the total carotenoid yield significantly higher (1904 µg/g) than that obtained by traditional solvent extraction (1136 µg/g). Chemical, biological, and physical pretreatments also improve the carotenoid extraction yield. Enzymatic pretreatments have been shown to enhance carotene extraction compared with chemical and chemical-physical extraction methods [38].
Solvent Extraction Methods
For analytical purposes, there are many methods for the extraction of antioxidants at a laboratory scale. Sun and Temelli [42] discussed one traditional carotenoid extraction method. The process started by washing and chopping the carrots to 5 mm followed by 5 days freeze-drying. After homogenization, an extraction medium of hexane/ acetone mixture containing 0.005% (w/v) of a synthetic antioxidant, butylated hydroxytoluene (BHT), was used. After filtration and washing the residue with acetone and then with hexane, Milli-Q water was used three times for washing the combined organic filtrate, then the organic phase was dried under nitrogen gas [42].
2.4. Modification to Increase the Purity
In order to improve the carotenoid yield, various techniques and methods have been used in solvent extraction methods.
2.4.1. Extraction by Soxhlet Technique
Extracting the carotenoids from a solid complex, such as grated carrots, can be performed using Soxhlet extraction [78]. Numerous organic solvents and different combined solvents have been used and tested to obtain the best recovery of carotenoids. It would be better to select a solvent according to the polarity of the pigments. Solvents with a lower boiling point are preferred [79]. Table 3 shows some solvents and their properties for extraction of different carotenoids. The process for extracting uncharacterized pigments involves solvent extraction (1:1 (v/v) hexane/acetone or hexane alone) and selective removal of compounds from the organic phase by filtration or by a strong basic resin [79].

Table 3.
Different solvents and their properties for carotenoid extraction [38].
For easier elimination of the water and more effective extraction, it is better to dehydrate plant samples using different methods such as: (i) low-temperature vacuum oven (for easily pulverizable vegetables); (ii) use of alcohol (for plant tissues containing lipids, waxes or sugars as they cannot be ground to powder); (iii) freeze-drying, which is an alternative for vacuum oven drying with slight pigment damage [79]; and (iv) pressure-cooking methods that can reduce the degradation [38].
One example of a solvent extraction procedure includes, (i) sample preparation (e.g., dehydration of plant samples), (ii) mixing with solvent, (iii) separation by vacuum filter [79] or centrifugation [80], (iv) continuing the extraction using smaller volumes of solvents, (v) concentration of extract by rotary evaporator, (vi) hot saponification at 56 °C with 40% (w/v) KOH in methanol, (vii) salting out in a separatory funnel with 10% (w/v) Na2SO4 solution, (viii) discarding the bottom layer and washing out the upper layer with water for three times, and (ix) removing moisture by anhydrous Na2SO4 and filtering using Whatman no.42 filter paper [79].
Carotenoids can be isolated by open-column chromatography (OCC) [81] or membrane separation and purified by crystallization or distillation [46].
2.4.2. Enzymatic Extraction
Biological methods have recently been evaluated for extracting carotenoids. Enzyme treatment can cause cell wall disintegration, releasing the carotenoids [82]. Raw enzymes generated from cultures of microorganisms are preferred versus commercial ones as they are cheaper and can reduce the processing time by 95% [38]. Cellulases, hemicellulases, glucoamylases, and pectinases are the most common enzymes that can be used in this extraction technique [38]. Water is necessary for enzymatic hydrolysis; however, excess water forms an aqueous phase and causes an inverse effect which reduces the speed of the extraction process and prevents the penetration of organic solvent into the vesicles of intracellular lipids leading to carotenoids [38]. Agitating is another important factor which improves carotene content and increases the extraction yield [38]. Enzyme cost, even in the case of using raw enzymes, low yields, and long processing times, is the main disadvantage of this technique [83]. The drying step can be replaced by an enzymatic pretreatment with conventional solvent extraction methods, and this aids in extraction of carotenoids at a lower temperature in comparison with drying techniques [79]. For example, Barzana et al. extracted carotenoids from Marigold flower (Tagetes erecta) by utilizing enzymes at room temperature (24–25 °C) [84].
2.4.3. Extraction by Pressurized Fluid Extraction (PFE) Technique
At elevated pressure and temperature, sometimes organic solvents can be used by utilizing the PFE technique [85]. This technique uses less solvent and the process takes place in a shorter time [79,85]. For example, Denery et al. [86] extracted carotenoids from Haematococcus pluvialis and Dunaliella salina and kavalactones from Piper methysticum by PFE using half the amount of solvent in 20 min instead of 90 min or longer for traditional extraction methods. A PFE system is composed of extraction cells and collection vials that are loaded onto an automated carousel. The stainless-steel extraction vessel fills up with three layers: first, cellulose filter (20 μm pore size) located on the bottom; second, a 2–3 cm3 layer of inert sand; and the upper layer, which is the mixture of the homogenized sample and sand. Extracts collect into glass vials by flushing the system with fresh solvent and at the end, nitrogen gas purges into the system. The range of pressure needed for this system is between 100 to 140 bar [83,86]. Denery et al. [86] showed that manipulating the pressure did not impact extraction yield.
2.4.4. Extraction by Microwave
The advantages and disadvantages of microwave-assisted extraction have been reported [87]. Extraction times using microwave methods are up to 1200 times shorter compared with the Soxhlet extraction technique. However, the contents of β-carotene and total carotenoid extractable from carrots, extracted by microwave-assisted extraction in 3 min, were less (23 and 52 mg/100 g dry basis) than those extracted by Soxhlet extraction method after 6 h (29 and 61 mg/100 g dry basis) [87]. Table 4 illustrates different solvent extraction methodologies and compares results based on the carrot or its by-product sources.

Table 4.
Solvent extraction methods for carotenoid recovery from carrots and carrots by-products.
2.5. Advantages and Disadvantages of Non-SFE Methods
The main advantages of solvent-based extraction methods are the low cost and high availability of solvent. Other benefits of such techniques are presented in Table 5. Amongst these methods, the pressurized fluid extraction technique is one of the new methods of extracting carotenoids that features lower operating temperature and reduction in the use of the solvent [85]. With reference to the literature illustrated in Table 5, there are considerable disadvantages for conventional extraction methods as they use a large amount of solvents that potentially damages the environment and can affect consumer health.

Table 5.
Different solvent extraction methods and their advantages and disadvantages for extracting vitamin E and carotenoids.
3. SFE of Hydrophobic Antioxidants
SFE is an ideal isolation technique for separating hydrophobic or non-polar compounds such as tocopherols compared to polar phenolics [93,94,95]. The molecular structure of the compounds determines which kind of technologies are applicable. Non-polar components like neutral lipids, essential oils, and carotenoids are readily extractable by SCCO2. Polar compounds such as polyphenols can be removed through the combination of SCCO2 and GRAS (Generally Recognized as Safe) solvents [94,96]. New technologies under the category of “Green Chemistry and Engineering” focus on using environmentally-friendly industrial processes [80,94]. Moreover, legal limits restrict the use of organic solvents in food and pharmaceuticals industries due to their potentially hazardous effects on human health [91]. The use of SCCO2 extraction is limited to separation of non-polar and lipophilic compounds; however, this drawback can be compensated by using a few polar co-solvents for enhancing the selectivity of extraction of polar compounds [91]. One of the potential applications of this technique is manufacturing novel products and extracting natural antioxidants [91].
3.1. Extraction of Vitamin E (Tocopherols) by SCCO2
Minor compounds of vegetable oils can be extracted by SFE systems. Optimizing the pressure and temperature can help improve the extraction yield of vitamin E [97] as the solubility of this compound changes with different operating parameters in a SCCO2 system [33]. Previous studies discussed some important information for reducing operating costs in implementation, scale-up of the SCF for extraction, purification and concentration [98]. A good example for implementation of SCF systems is the optimization for the separation of α-tocopherol and squalene from soybean deodorizer distillation on an industrial scale using response surface methodology by Wang et al. [98].
Table 6 illustrates the recovery yields of tocopherols reported in various studies. The highest yield (99%) belongs to tocopherol extraction from canola deodorizer distillate, which was achieved at a pressure of 250 bars and a temperature of 40 °C. Uquiche et al. [24] demonstrated that SCCO2 extraction of oil from canola press cake had higher oil yield and higher contents of tocopherol and stigmasterol relative to extraction by a solvent (hexane).

Table 6.
Extraction of vitamin E by SCCO2 technology.
3.2. Extraction of Carotenoids by SCCO2
SFE is a suitable technique for recovery of carotenoid compounds from carrot, carrot oil, and tomato skin, as they can have a high solubility in non-polar SCCO2 media [105]. Their large molecular weight, however, can cause solubility reductions. To remedy this problem, some modifiers, such as ethanol and canola oil, are utilized to improve the extraction yield [33]. Furthermore, heat, light, and oxygen accelerate isomerization and oxidation of conjugated bonds in carotenoids which can influence their pigmentation, antioxidant activity and instability [106]. Contrary to non-SFE methods, the cis-trans isomerization increases the solubility of carotenoids in SCCO2, while it degrades color concentration and the activity of pro-vitamin A [107]. Extraction aids, including unsaturated oils, organic solvents, peroxides, metals, and enzymes, can increase oxidation rates and decrease carotenoid stability and their antioxidant properties in various non-SFE methods [33,108]. SFE can prevent oxidation and degradation of the antioxidant activity of the carotenoids by optimizing the pressure and temperature and it can also improve the extraction of the more stable (trans) form of carotenoids [33,108].
According to Mattea et al. [46], extraction of carotenoids, specifically β-carotene, lutein, and lycopene, is achievable by SCF technology because CO2, a non-solvent fluid, can decrease the degradation of antioxidant activities. This attribute combined with increasing social interest for green extraction methods results in new commercial opportunities. Table 7 shows the effect of pressure and temperature on carotenoid extraction yield from different sources by SCCO2. From different sources of carrots, crude carrot oil provided much higher carotenoids compared to freeze dried and tray dried carrots (Table 7).

Table 7.
Carotenoid extraction by SCCO2 technique.
3.3. Comparison between SFE and Other Extraction Methods
According to Table 8, SCCO2 provides better mass transfer as it has a lower viscosity, surface tension, and density, and higher diffusivity than conventional solvents [63,91]. This technique allows selective extraction, fractionation, and purification by penetrating into micro and macro porous components [63]. Moreover, results from this review showed solvent extraction yield of the carrot was just 30% higher (52–61 µg/g) than SCCO2 extraction yield (25–43 µg/g). However, the vitamin E recovery yield from various plant-based oil sources shows higher yield (84–100%) [97,98,100,102,103,104] than solvent-extracted vitamin E (around 80%) (Table 8). Therefore, it can be concluded that SCCO2 extraction works effectively for extraction of tocopherols.
Many researchers have conducted lab-scale and modelling studies to define scale-up optimized parameters such as particle size [112,113]. Kinetic studies on the different parameters can suggest the most apt scale-up methodology [114,115]. Some studies have shown pilot scale level extraction provides a better yield than laboratory scale [116].


Table 8.
Advantages of SFE of vitamin E and carotenoid in comparison with non-SFE techniques.
Table 8.
Advantages of SFE of vitamin E and carotenoid in comparison with non-SFE techniques.
| Properties | SCCO2 | Non-SFE | Refs. |
|---|---|---|---|
| Energy consumption and its cost | Low temperature is needed because the critical temperature of CO2 is low (31.1 °C) High energy efficiency is obtained when SFE recovers butanol | For increasing solubility higher temperature is preferred Energy efficiency for butanol recovery, between 3.5 and 30-fold, is higher than SFE | [60,63,117] |
| Cost at high purity | Low | High | |
| Cost of manufacturing | Low and competitive with conventional technologies. | Solvent and its removing cost are larger than equipment cost | [33,36] |
| Toxicity | No | Yes | [60,91] |
| Flammability | No | Yes | [91] |
| Availability amount | Enormous | Less than CO2 | |
| Suitability for extracting heat labile, natural compounds with low volatility and polarity | Perfect as no thermal degradation and decomposition happens, no oxidation because of absence of light and oxygen | Lower stability because of higher temperature, longer time and oxidation, particularly for antioxidants, can be occur easily | [60] |
| Post-reaction separation | Rapid evaporation occurs as CO2 has high volatility | Solvent evaporation is more time and energy consuming and leaves solvent residue | [63,118,119] |
| Quality of extracted compounds | Keeps better natural flavor, fragrance in food supplements and nutraceutical products and biological properties in cosmetic and pharmaceutical products, has GRAS status | poorer reproduction of flavors and smell, the color quality of solvent extracted pigment is not as good as SCF extracted colorant | [46,91,120,121] |
| Compatibility with natural non-polar and low-polar compounds | Suitable for natural compounds that have low polarity and volatility | Organic solvents, such as hexane, are needed for low polar natural compounds | [38,91] |
| Antioxidant activity | Higher concentration of vitamin E is reported; for example, the SCCO2 extract of sesame seed contains up to 47 µg/mL | Hexane extractions have a significantly lower concentration of vitamin E; e.g., extracts of sesame seed has 25 µg/mL. | [33] |
| Purity | Total removal of free fatty acids from tocopherol mixtures is the main advantage | Free fatty acids may not separate completely | [63,119] |
| Control of physiochemical properties of fluid (density, dielectric constant and viscosity) | The physiochemical properties of CO2 can easily be regulated by altering pressure and temperature without passing phase boundaries | Solvents do not have this benefit | [60] |
| Solvent power | High-diffusion coefficient, low-viscosity, zero surface tension help solvent penetrate fast | Solvent molecules penetrate plant tissues with difficulty | [38,60] |
| Eco-friendliness | It is a green technology because of using CO2 gas | Due to high consumption of solvent | [43] |
4. Conclusions
This review has focused on the extraction of antioxidant compounds from a horticultural and a field crop (carrot and canola), and, specifically, processing by-products or co-streams that are underutilized in current process technologies. The data show that while solvent extraction is a simple and easy way to extract antioxidants from soybean deodorizer distillate and canola deodorizer distillate and carrot by-products, there are some important factors contributing to the suitability of SFE systems versus non-SFE methods. These factors include energy, time, chemical materials, product quality, and environmental safety. Two technological gaps in the extraction of bio-compounds by using SCF systems can be found. Many studies have carried out the optimization procedure for obtaining higher extraction yield based on five factors of time, temperature, pressure, modifier percentages, and CO2 flow rate. However, the particle size of an extractable substrate is one key factor which has been studied the least, and it may play a significant role in antioxidant extraction yield [122]. Another gap pertains specifically to canola. Khattab et al. [123] showed that canolol is a potentially lipid-soluble and antimutagenic compound which has potential antioxidant and anti-radical scavenging activities. Therefore, extraction of canolol, a non-polar compound, presents an opportunity for the industry and SCCO2 extraction of canolol from by-products of canola processing should be explored in the future. SCCO2 works better than other non-SFE methods for extracting tocopherols and carotenoids due to (i) equal or higher extraction yields and (ii) greater bioactivity of extracted antioxidants. Pressure, temperature, and time are three major and effective parameters in SFE. Optimization of these main variables, and, in some cases, other additional variables such as co-solvent percentage, can help in formulating statistical models for maximizing the extraction yield and reducing the cost. Pressure is more effective than other parameters for the extraction yield of tocopherol. For carotenoid extraction, both pressure and temperature have a large impact on extraction yield by SFE systems. SFE systems have a wide range of advantages, such as lower cost of extracted valuable compounds relative to non-SFE methods, eco-friendliness, and having good solubility characteristics for a range of compounds from simple to complicated. A critical future outlook on the use of SCF technologies offers an easy and selective separation technique to produce high-value, food-grade, and safe products with high-purity in an ecofriendly manner. The most economical method of production of high-quality antioxidants is integration and a combination of operation units. For example, for separating tocopherols, squalene, and phytosterols from olive pomace or extracted oil, SCF extraction and fractional separation can be conducted in two consecutive steps. Coupling processes reduces the cost of supercritical processing and increases the valuable minor components’ quality. A key issue for SCF systems is the effective re-using and recycling of CO2 in all the different process steps [124]. Other future studies are related to supercritical micronization and impregnation, which provide fine particles with customer design properties for application in the food, feed and pharmaceutical industries [125].
Author Contributions
Conceptualization, N.V. and C.B.R.; writing—original draft preparation, N.V. and C.B.R.; writing—review and editing, N.V., C.B.R., M.G.S., P.J.H.J. and M.N.A.E.; visualization, N.V.; supervision, M.G.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge support from the Canola AgriScience Cluster, a partnership between Agriculture and Agri-Food Canada and the canola industry under the Canadian Agricultural Partnership. Funding number: ASC-02.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Niciforovic, N.; Abramovic, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity: Sinapic Acid and Its Derivatives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R. The Role of Antioxidants in Human Health. In Oxidative Stress: Diagnostics, Prevention, and Therapy; Andreescu, S., Hepel, M., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1083, pp. 1–37. ISBN 978-0-8412-2683-8. [Google Scholar]
- Cui, X.; Lin, Q.; Liang, Y. Plant-Derived Antioxidants Protect the Nervous System From Aging by Inhibiting Oxidative Stress. Front. Aging Neurosci. 2020, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Kovač, V.; Milisav, I. Antioxidants, Food Processing and Health. Antioxidants 2021, 10, 433. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Varela, P.; Fiszman, S.M. Exploring Consumers’ Knowledge and Perceptions of Hydrocolloids Used as Food Additives and Ingredients. Food Hydrocoll. 2013, 30, 477–484. [Google Scholar] [CrossRef]
- Shah, N.A.; Khan, M.R.; Naz, K.; Khan, M.A. Antioxidant Potential, DNA Protection, and HPLC-DAD Analysis of Neglected Medicinal Jurinea Dolomiaea Roots. BioMed Res. Int. 2014, 2014, 726241. [Google Scholar] [CrossRef]
- Boye, J.I.; Arcand, Y. Current Trends in Green Technologies in Food Production and Processing. Food Eng. Rev. 2013, 5, 1–17. [Google Scholar] [CrossRef]
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural Antioxidants in Meat and Poultry Products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef]
- Brewster, E. Food Technology Magazine. 1 September 2021. Available online: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2021/september/columns/ingredients-clean-label (accessed on 20 April 2022).
- Greenwald, M. Consumer Attitudes Toward Food Safety, Nutrition & Health; International Food Information Council Foundation: Washington, DC, USA, 2012. [Google Scholar]
- Jacobson, M.F. Petition to Ban the Use of Yellow 5 and Other Food Dyes, in the Interim to Require a Warning on Foods Containing These Dyes, to Correct the Information the Food and Drug Administration Gives to Consumers on the Impact of These Dyes on the Behavior of Some Children, and to Require Neurotoxicity Testing of New Food Additives and Food Colors. 2008. Available online: https://www.cspinet.org/resource/cspi-petition-fda-re-food-dyes (accessed on 20 April 2022).
- Hensel, K. Clean Labels Showcased at IFT Food Expo; Institute of Food Technologists–IFT: New Orleans, LA, USA, 2014. [Google Scholar]
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of Supercritical Carbon Dioxide in Materials Processing and Synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Nautiyal, O.H. Food Processing by Supercritical Carbon Dioxide. EC Chem. 2016, 2, 111–135. [Google Scholar]
- Rizvi, S.S.H.; Yu, Z.R.; Bhaskar, A.R. Supercritical Fluid Processing of Food and Bio-Materials; Rizvi, S.S.H., Ed.; Chapman & Hall, Bishopbriggs: Glasgow, Scotland, 1994. [Google Scholar]
- Rozzi, N.L.; Singh, R.K. Supercritical Fluids and the Food Industry. Compr. Rev. Food Sci. Food Saf. 2002, 1, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, E.; Sun, Q.; Zhang, Z.; Zhang, C.; Gou, W. Mini-Review: Green Sustainable Processes Using Supercritical Fluid Carbon Dioxide. J. Environ. Sci. 2009, 21, 720–726. [Google Scholar] [CrossRef]
- Lopes, B.L.F.; Sánchez-Camargo, A.P.; Ferreira, A.L.K.; Grimaldi, R.; Paviani, L.C.; Cabral, F.A. Selectivity of Supercritical Carbon Dioxide in the Fractionation of Fish Oil with a Lower Content of EPA+DHA. J. Supercrit. Fluids 2012, 61, 78–85. [Google Scholar] [CrossRef]
- Otles, S. Handbook of Food Analysis Instruments; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-1-4200-4567-3. [Google Scholar]
- Rawson, A.; Tiwari, B.K.; Brunton, N.; Brennan, C.; Cullen, P.J.; O’Donnell, C.P. Application of Supercritical Carbon Dioxide to Fruit and Vegetables: Extraction, Processing, and Preservation. Food Rev. Int. 2012, 28, 253–276. [Google Scholar] [CrossRef]
- Uquiche, E.; Romero, V.; Ortíz, J.; del Valle, J.M. Extraction of Oil and Minor Lipids from Cold-Press Rapeseed Cake with Supercritical CO2. Braz. J. Chem. Eng. 2012, 29, 585–598. [Google Scholar] [CrossRef]
- Miękus, N.; Iqbal, A.; Marszałek, K.; Puchalski, C.; Świergiel, A. Green Chemistry Extractions of Carotenoids from Daucus Carota L.—Supercritical Carbon Dioxide and Enzyme-Assisted Methods. Molecules 2019, 24, 4339. [Google Scholar] [CrossRef]
- Friedrich, J.P.; List, G.R.; Heakin, A.J. Petroleum-Free Extraction of Oil from Soybeans with Supercritical CO2. J. Am. Oil Chem. Soc. 1982, 59, 288–292. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green Solvents for Green Technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Fattori, M.; Bulley, N.R.; Meisen, A. Carbon Dioxide Extraction of Canola Seed: Oil Solubility and Effect of Seed Treatment. J. Am. Oil Chem. Soc. 1988, 65, 968–974. [Google Scholar] [CrossRef]
- Que, F.; Hou, X.-L.; Wang, G.-L.; Xu, Z.-S.; Tan, G.-F.; Li, T.; Wang, Y.-H.; Khadr, A.; Xiong, A.-S. Advances in Research on the Carrot, an Important Root Vegetable in the Apiaceae Family. Hortic. Res. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Franke, S.; Fröhlich, K.; Werner, S.; Böhm, V.; Schöne, F. Analysis of Carotenoids and Vitamin E in Selected Oilseeds, Press Cakes and Oils. Eur. J. Lipid Sci. Technol. 2010, 112, 1122–1129. [Google Scholar] [CrossRef]
- LMC International Ltd. The Economic Impact of Canola on the Canadian Economy; LMC International, Canola Council of Canada: Winnipeg, MN, Canada, 2013. [Google Scholar]
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The Content of Phenolic Compounds and Radical Scavenging Activity Varies with Carrot Origin and Root Color. Plant Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.G.; Meireles, M.A.A. Supercritical Fluid Extraction of Bioactive Compounds: Fundamentals, Applications and Economic Perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- San Andres, M.P.; Otero, J.; Vera, S. High Performance Liquid Chromatography Method for the Simultaneous Determination of α-, γ- and δ-Tocopherol in Vegetable Oils in Presence of Hexadecyltrimethylammonium Bromide/n-Propanol in Mobile Phase. Food Chem. 2011, 126, 1470–1474. [Google Scholar] [CrossRef]
- Niki, E.; Abe, K. CHAPTER 1. Vitamin E: Structure, Properties and Functions. In Food Chemistry, Function and Analysis; Niki, E., Ed.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 1–11. ISBN 978-1-78801-240-9. [Google Scholar]
- Mendes, M.F.; Pessoa, F.L.P.; Uller, A.M.C. An Economic Evaluation Based on an Experimental Study of the Vitamin E Concentration Present in Deodorizer Distillate of Soybean Oil Using Supercritical CO2. J. Supercrit. Fluids 2002, 23, 257–265. [Google Scholar] [CrossRef]
- Bender, D.A. Dictionary. In Benders’ Dictionary of Nutrition and Food Technology (Eighth edition); Woodhead Publishing: Cambridge, UK, 2006; pp. 1–521. ISBN 978-1-84569-051-9. [Google Scholar]
- Arvayo-Enríquez, H.; Mondaca-Fernández, I.; Gortárez-Moroyoqui, P.; López-Cervantes, J.; Rodríguez-Ramírez, R. Carotenoids Extraction and Quantification: A Review. Anal. Methods 2013, 5, 2916. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2003; ISBN 0-203-50873-4. [Google Scholar]
- Willging, S.M.; Swanson, R.R. Purification of Tocopherols by Extraction. 1991. Available online: https://patents.google.com/patent/EP0171009B1/en (accessed on 20 April 2022).
- Grilo, E.C.; Costa, P.N.; Gurgel, C.S.S.; Beserra, A.F.D.L.; Almeida, F.N.D.S.; Dimenstein, R. Alpha-Tocopherol and Gamma-Tocopherol Concentration in Vegetable Oils. Food Sci. Technol. Camp. 2014, 34, 379–385. [Google Scholar] [CrossRef]
- Sun, M.; Temelli, F. Supercritical CO2 Extraction of Carotenoids from Carrots and Evaluation of Products. J. Supercrit. Fluids 2002, 37, 397–408. [Google Scholar] [CrossRef]
- Malekbala, M.R.; Soltani, S.M.; Hosseini, S.; Eghbali Babadi, F.; Malekbala, R. Current Technologies in the Extraction, Enrichment and Analytical Detection of Tocopherols and Tocotrienols: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2935–2942. [Google Scholar] [CrossRef]
- Shahidi, F.; de Camargo, A. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Shivhare, U.S.; Basu, S.; Raghavan, G.S.V. Kinetics of Extraction of β-Carotene from Tray Dried Carrots by Using Supercritical Fluid Extraction Technique. Food Nutr. Sci. 2012, 3, 591–595. [Google Scholar] [CrossRef][Green Version]
- Mattea, F.; Martín, Á.; Cocero, M.J. Carotenoid Processing with Supercritical Fluids. J. Food Eng. 2009, 93, 255–265. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Stange, C. Biosynthesis of Carotenoids in Carrot: An Underground Story Comes to Light. Arch. Biochem. Biophys. 2013, 539, 110–116. [Google Scholar] [CrossRef]
- Di Vaio, C.; Graziani, G.; Marra, L.; Cascone, A.; Ritieni, A. Antioxidant Capacities, Carotenoids and Polyphenols Evaluation of Fresh and Refrigerated Peach and Nectarine Cultivars from Italy. Eur. Food Res. Technol. 2008, 227, 1225–1231. [Google Scholar] [CrossRef]
- Aquino, C.F.; Salomão, L.C.C.; Pinheiro-Sant’ana, H.M.; Ribeiro, S.M.R.; Siqueira, D.L.D.; Cecon, P.R. Carotenoids in the Pulp and Peel of Bananas from 15 Cultivars in Two Ripening Stages. Rev. Ceres 2018, 65, 217–226. [Google Scholar] [CrossRef]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.M.J.; Ortiz, G.M.D.; de Carvalho, J.L.V.; Smirdele, L.; Cardoso, F.d.S.N. Carotenoids in Yellow Sweet Potatoes, Pumpkins and Yellow Sweet Cassava. In Carotenoids; Cvetkovic, D.J., Nikolic, G.S., Eds.; IntechOpen: London, UK, 2017; ISBN 978-953-51-3211-0. [Google Scholar]
- Sone, M.; Uchiyama, H.; Chang, T.-F.M. Crystal Growth by Electrodeposition with Supercritical Carbon Dioxide Emulsion; IntechOpen: London, UK, 2013; ISBN 953-51-1010-1. [Google Scholar]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub-and Supercritical Fluid Extraction of Functional Ingredients from Different Natural Sources: Plants, Food-by-Products, Algae and Microalgae: A Review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- McHugh, M.; Krukonis, V.; Brenner, H. Supercritical Fluid Extraction: Principles and Practice; Butterworth-Heinemann Series in Chemical Engineering; Elsevier Science: Amsterdam, The Netherlands, 2013; ISBN 978-0-08-051817-6. [Google Scholar]
- Schütz, E. Supercritical Fluids and Applications–A Patent Review. Chem. Eng. Technol. 2007, 30, 685–688. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Wang, D. Solubility and Diffusion Coefficient of Supercritical CO2 in Polystyrene Dynamic Melt. e-Polymers 2020, 20, 659–672. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, M. Natural Extracts Using Supercritical Carbon Dioxide; CRC Press: Boca Raton, FL, USA, 2000; ISBN 1-4200-4169-X. [Google Scholar]
- Hrnčič, M.K.; Cör, D.; Verboten, M.T.; Knez, Ž. Application of Supercritical and Subcritical Fluids in Food Processing. Food Qual. Saf. 2018, 2, 59–67. [Google Scholar] [CrossRef]
- Shilpi, A.; Shivhare, U.; Basu, S. Supercritical CO2 Extraction of Compounds with Antioxidant Activity from Fruits and Vegetables Waste-A Review. Focus. Mod. Food Ind. 2013, 2, 43–62. [Google Scholar]
- Jenab, E. Lipase-Catalyzed Interesterification between Canola Oil and Fully-Hydrogenated Canola Oil in Contact with Supercritical Carbon Dioxide. Ph.D. Thesis, Bioresource and Food Engineering, Department of Agricultural: Food and Nutritional Science, University of Alberta, Edmonton, AB, Canada, 2013. [Google Scholar]
- Srivastava, S.; Madras, G.; Modak, J. Esterification of Myristic Acid in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2003, 27, 55–64. [Google Scholar] [CrossRef]
- Dumont, M.-J.; Narine, S.S. Soapstock and Deodorizer Distillates from North American Vegetable Oils: Review on Their Characterization, Extraction and Utilization. Food Res. Int. 2007, 40, 957–974. [Google Scholar] [CrossRef]
- Yuan, X. Evaluation of Antioxidants Activities of the Soybean Oils and Gums. Master’s Thesis, Food science Department, Louisiana State University, Baton Rouge, LA, USA, 2006. [Google Scholar]
- Rempel, C.B.; Scanlon, M.G. Canola and Rapeseed: Production, Processing, Food Quality, and Nutrition; Thiyam-Holländer, U., Eskin, N.M., Matthäus, B., Eds.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 1-4665-1386-1. [Google Scholar]
- Zeldenrust, R.S. Alkali Refining. Available online: http://lipidlibrary.aocs.org/OilsFats/content.cfm?ItemNumber=40319 (accessed on 22 August 2019).
- Zhou, W.; Qin, D.; Qian, J. Optimisation of Enzymatic Pretreatment of Soybean Oil Deodoriser Distillate for Concentration of Tocopherols. Int. J. Food Sci. Technol. 2009, 44, 1429–1437. [Google Scholar] [CrossRef]
- Kasim, N.S.; Gunawan, S.; Yuliana, M.; Ju, Y.-H. A Simple Two-Step Method for Simultaneous Isolation of Tocopherols and Free Phytosterols from Soybean Oil Deodorizer Distillate with High Purity and Recovery. Sep. Sci. Technol. 2010, 45, 2437–2446. [Google Scholar] [CrossRef]
- Nagao, T.; Kobayashi, T.; Hirota, Y.; Kitano, M.; Kishimoto, N.; Fujita, T.; Watanabe, Y.; Shimada, Y. Improvement of a Process for Purification of Tocopherols and Sterols from Soybean Oil Deodorizer Distillate. J. Mol. Catal. B Enzym. 2005, 37, 56–62. [Google Scholar] [CrossRef]
- Shimada, Y.; Nakai, S.; Suenaga, M.; Sugihara, A.; Kitano, M.; Tominaga, Y. Facile Purification of Tocopherols from Soybean Oil Deodorizer Distillate in High Yield Using Lipase. J. Am. Oil Chem. Soc. 2000, 77, 1009–1013. [Google Scholar] [CrossRef]
- Martins, P.F.; Batistella, C.B.; Maciel-Filho, R.; Wolf-Maciel, M.R. Comparison of Two Different Strategies for Tocopherols Enrichment Using a Molecular Distillation Process. Ind. Eng. Chem. Res. 2006, 45, 753–758. [Google Scholar] [CrossRef]
- Ramamurthi, S.; McCurdy, A.R. Enzymatic Pretreatment of Deodorizer Distillate for Concentration of Sterols and Tocopherols. J. Am. Oil Chem. Soc. 1993, 70, 287–295. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagao, T.; Hirota, Y.; Kitano, M.; Shimada, Y. Purification of Tocopherols and Phytosterols by a Two-Step in Situ Enzymatic Reaction. J. Am. Oil Chem. Soc. 2004, 81, 339–345. [Google Scholar] [CrossRef]
- Cima, R. The Invention of the Baby Carrot; Priceonomics: San Francisco, CA, USA, 2015. [Google Scholar]
- FAO. Food Losses and Waste in the Context of Sustainable Food Systems; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. Available online: https://www.fao.org/3/i3901e/i3901e.pdf (accessed on 20 April 2022).
- Mustafa, A.; Turner, C. Pressurized Liquid Extraction as a Green Approach in Food and Herbal Plants Extraction: A Review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Sun, M.; Temelli, F. Supercritical Carbon Dioxide Extraction of Carotenoids from Carrot Using Canola Oil as a Continuous Co-Solvent. J. Supercrit. Fluids 2006, 37, 397–408. [Google Scholar] [CrossRef]
- Yahaya, M.Z.B. Study on Factors Affecting Extraction of Carotene from Carrot by Using Soxhlet Extraction Method; Universiti Malaysia Pahang: Kuantan, Malyasia, 2013. [Google Scholar]
- Machmudah, S.; Goto, M. Methods for Extraction and Analysis of Carotenoids. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3367–3411. ISBN 978-3-642-22143-9. [Google Scholar]
- Pokorný, J. Preparation of Natural Antioxidants. In Antioxidants in Food; Elsevier: Amsterdam, The Netherlands, 2001; pp. 311–330. ISBN 978-1-85573-463-0. [Google Scholar]
- Nagy, V.; Agócs, A.; Turcsi, E.; Deli, J. Isolation and Purification of Acid-Labile Carotenoid 5,6-Epoxides on Modified Silica Gels. Phytochem. Anal. 2009, 20, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef]
- Mai, H.C.; Trunonng, V.; Debaste, F. Optimisation of Enzyme-Assisted Extraction of Oil Rich in Carotenoids from Gac Fruit (Momordica Cochinchinensis Spreng.). Food Technol. Biotechnol. 2013, 51, 488. [Google Scholar]
- Barzana, E.; Rubio, D.; Santamaria, R.I.; Garcia-Correa, O.; Garcia, F.; Ridaura Sanz, V.E.; López-Munguía, A. Enzyme-Mediated Solvent Extraction of Carotenoids from Marigold Flower (Tagetes erecta). J. Agric. Food Chem. 2002, 50, 4491–4496. [Google Scholar] [CrossRef]
- Li, J.; Pettinato, M.; Campardelli, R.; De Marco, I.; Perego, P. High-Pressure Technologies for the Recovery of Bioactive Molecules from Agro-Industrial Waste. Appl. Sci. 2022, 12, 3642. [Google Scholar] [CrossRef]
- Denery, J.R.; Dragull, K.; Tang, C.S.; Li, Q.X. Pressurized Fluid Extraction of Carotenoids from Haematococcus Pluvialis and Dunaliella Salina and Kavalactones from Piper Methysticum. Anal. Chim. Acta 2004, 501, 175–181. [Google Scholar] [CrossRef]
- Hiranvarachat, B.; Devahastin, S.; Chiewchan, N.; Vijaya Raghavan, G.S. Structural Modification by Different Pretreatment Methods to Enhance Microwave-Assisted Extraction of β-Carotene from Carrots. J. Food Eng. 2013, 115, 190–197. [Google Scholar] [CrossRef]
- Fikselova, M.; Silhar, S.; Marecek, J.; Francakova, H. Extraction of Carrot (Daucus Carota L.) Carotenes under Different Conditions. Czech J. Food Sci. 2008, 26, 268–274. [Google Scholar] [CrossRef]
- Moreira, E.A.; Baltanás, M.A. Recovery of Phytosterols from Sunflower Oil Deodorizer Distillates. J. Am. Oil Chem. Soc. 2004, 81, 161–167. [Google Scholar] [CrossRef]
- Torres, C.F.; Reglero, G.; Torrelo, G. Extraction and Enzymatic Modification of Functional Lipids from Soybean Oil Deodorizer Distillate; INTECH Open Access Publisher: London, UK, 2011; ISBN 953-307-533-3. [Google Scholar]
- Diaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 Extraction and Purification of Compounds with Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef] [PubMed]
- King, J. Supercritical Fluid Processing of Nutritionally Functional Lipids. In Healthful Lipids; AOCS Publishing: Urbana, IL, USA, 2005; ISBN 978-1-893997-51-6. [Google Scholar]
- Lee, H.; Chung, B.; Park, Y. Concentration of Tocopherols from Soybean Sludge by Supercritical Carbon Dioxide. J. Am. Oil Chem. Soc. 1991, 68, 571–573. [Google Scholar] [CrossRef]
- Vuorela, S. Analysis, Isolation, And Bioactivities of Rapeseed Phenolics; Department of Applied Chemistry and Microbiology, University of Helsinki: Helsinki, Finland, 2005. [Google Scholar]
- Quispe-Fuentes, I.; Uribe, E.; López, J.; Contreras, D.; Poblete, J. A Study of Dried Mandarin (Clementina orogrande) Peel Applying Supercritical Carbon Dioxide Using Co-solvent: Influence on Oil Extraction, Phenolic Compounds, and Antioxidant Activity. J. Food Process. Preserv. 2022, 46, e16116. [Google Scholar] [CrossRef]
- Pokorný, J. Introduction. In Antioxidants in Food; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1–3. ISBN 978-1-85573-463-0. [Google Scholar]
- Sun, Q.; Shi, J.; Scanlon, M.; Xue, S.J.; Lu, J. Optimization of Supercritical-CO2 Process for Extraction of Tocopherol-Rich Oil from Canola Seeds. LWT 2021, 145, 111435. [Google Scholar] [CrossRef]
- Wang, H.; Goto, M.; Sasaki, M.; Hirose, T. Separation of α-Tocopherol and Squalene by Pressure Swing Adsorption in Supercritical Carbon Dioxide. Ind. Eng. Chem. Res. 2004, 43, 2753–2758. [Google Scholar] [CrossRef]
- Brunner, G.; Malchow, T.; Stürken, K.; Gottschau, T. Separation of Tocopherols from Deodorizer Condensates by Countercurrent Extraction with Carbon Dioxide. J. Supercrit. Fluids 1991, 4, 72–80. [Google Scholar] [CrossRef]
- Chang, C.J.; Chang, Y.-F.; Lee, H.; Lin, J.; Yang, P.-W. Supercritical Carbon Dioxide Extraction of High-Value Substances from Soybean Oil Deodorizer Distillate. Ind. Eng. Chem. Res. 2000, 39, 4521–4525. [Google Scholar] [CrossRef]
- Nagesha, G.K.; Manohar, B.; Udaya Sankar, K. Enrichment of Tocopherols in Modified Soy Deodorizer Distillate Using Supercritical Carbon Dioxide Extraction. Eur. Food Res. Technol. 2003, 217, 427–433. [Google Scholar] [CrossRef]
- Mendes, M.; Pessoa, F.; Uller, A. Optimization of the Process of Concentration of Vitamin E from DDSO Using Supercritical CO2. Braz. J. Chem. Eng. 2005, 22, 83–91. [Google Scholar] [CrossRef]
- Vázquez, L.; Torres, C.F.; Fornari, T.; Grigelmo, N.; Señoráns, F.J.; Reglero, G. Supercritical Fluid Extraction of Minor Lipids from Pretreated Sunflower Oil Deodorizer Distillates. Eur. J. Lipid Sci. Technol. 2006, 108, 659–665. [Google Scholar] [CrossRef]
- Fang, T.; Goto, M.; Wang, X.; Ding, X.; Geng, J.; Sasaki, M.; Hirose, T. Separation of Natural Tocopherols from Soybean Oil Byproduct with Supercritical Carbon Dioxide. J. Supercrit. Fluids 2007, 40, 50–58. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef]
- Atencio, S.; Verkempinck, S.H.E.; Reineke, K.; Hendrickx, M.; Van Loey, A. Heat and Light Stability of Pumpkin-Based Carotenoids in a Photosensitive Food: A Carotenoid-Coloured Beverage. Foods 2022, 11, 485. [Google Scholar] [CrossRef]
- Shi, J.; Mittal, G.; Kim, E.; Xue, S.J. Solubility of Carotenoids in Supercritical CO2. Food Rev. Int. 2007, 23, 341–371. [Google Scholar] [CrossRef]
- Oman, M.; Škerget, M.; Knez, Z. Application of Supercritical Fluid Extraction for Separation of Nutraceuticals and Other Phytochemicals from Plant Material. Maced. J. Chem. Chem. Eng. 2013, 32, 183–226. [Google Scholar] [CrossRef][Green Version]
- Saldaña, M.D.A.; Sun, L.; Guigard, S.E.; Temelli, F. Comparison of the Solubility of β-Carotene in Supercritical CO2 Based on a Binary and a Multicomponent Complex System. J. Supercrit. Fluids 2006, 37, 342–349. [Google Scholar] [CrossRef]
- Topal, U.; Sasaki, M.; Goto, M.; Hayakawa, K. Extraction of Lycopene from Tomato Skin with Supercritical Carbon Dioxide: Effect of Operating Conditions and Solubility Analysis. J. Agric. Food Chem. 2006, 54, 5604–5610. [Google Scholar] [CrossRef]
- Zaghdoudi, K.; Framboisier, X.; Frochot, C.; Vanderesse, R.; Barth, D.; Kalthoum-Cherif, J.; Blanchard, F.; Guiavarc’h, Y. Response Surface Methodology Applied to Supercritical Fluid Extraction (SFE) of Carotenoids from Persimmon (Diospyros Kaki L.). Food Chem. 2016, 208, 209–219. [Google Scholar] [CrossRef]
- Reverchon, E.; Marrone, C. Modeling and Simulation of the Supercritical CO2 Extraction of Vegetable Oils. J. Supercrit. Fluids 2001, 19, 161–175. [Google Scholar] [CrossRef]
- Mezzomo, N.; Martínez, J.; Ferreira, S.R.S. Supercritical Fluid Extraction of Peach (Prunus persica) Almond Oil: Kinetics, Mathematical Modeling and Scale-Up. J. Supercrit. Fluids 2009, 51, 10–16. [Google Scholar] [CrossRef]
- Ghafoor, K.; AL-Juhaimi, F.Y.; Choi, Y.H. Supercritical Fluid Extraction of Phenolic Compounds and Antioxidants from Grape (Vitis labrusca B.) Seeds. Plant Foods Hum. Nutr. 2012, 67, 407–414. [Google Scholar] [CrossRef]
- Sookwong, P.; Suttiarporn, P.; Boontakham, P.; Seekhow, P.; Wangtueai, S.; Mahatheeranont, S. Simultaneous Quantification of Vitamin E, γ-Oryzanols and Xanthophylls from Rice Bran Essences Extracted by Supercritical CO2. Food Chem. 2016, 211, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.M.; Prado, G.H.C.; Meireles, M.A.A. Scale-up Study of Supercritical Fluid Extraction Process for Clove and Sugarcane Residue. J. Supercrit. Fluids 2011, 56, 231–237. [Google Scholar] [CrossRef]
- Manjare, S.D.; Dhingra, K. Supercritical Fluids in Separation and Purification: A Review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Albuquerque, C.L.C.; Meireles, M.A.A. Supercritical CO2 Extraction of Cupuassu Butter from Defatted Seed Residue: Experimental Data, Mathematical Modeling and Cost of Manufacturing. Food Bioprod. Process. 2016, 97, 48–62. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. Top. Curr. Chem. 2018, 376, 2–54. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; van Buren, L.; Wagner, E.; Wiseman, S.; van de Put, F.; Dacombe, C.; Rice-Evans, C.A. The Antioxidant Activity of Regularly Consumed Fruit and Vegetables Reflects Their Phenolic and Vitamin C Composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef]
- Grassmann, J. Terpenoids as Plant Antioxidants. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2005; Volume 72, pp. 505–535. ISBN 978-0-12-709872-2. [Google Scholar]
- Khattab, R.; Rempel, C.; Suh, M.; Thiyam, U. Quality of Canola Oil Obtained by Conventional and Supercritical Fluid Extraction. Am. J. Anal. Chem. 2012, 3, 966. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Eskin, M.N.A.; Thiyam-Hollander, U. Production of Canolol from Canola Meal Phenolics via Hydrolysis and Microwave-Induced Decarboxylation. J. Am. Oil Chem. Soc. 2014, 91, 89–97. [Google Scholar] [CrossRef]
- Lubary, M. Added-Value Milk Fat Derivatives from Integrated Processes Using Supercritical Technology; Universitat de Barcelona: Barcelona, Spain, 2011. [Google Scholar]
- Knez, Ž.; Pantić, M.; Cör, D.; Novak, Z.; Knez Hrnčič, M. Are Supercritical Fluids Solvents for the Future? Chem. Eng. Process.-Process Intensif. 2019, 141, 107532. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).