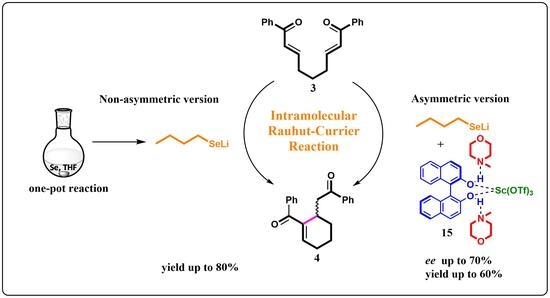
An Asymmetric Intramolecular Rauhut-Currier Reaction Initiated by Chiral Selenolate-BINOL Complexes
Abstract
:1. Introduction
2. Results and Discussion
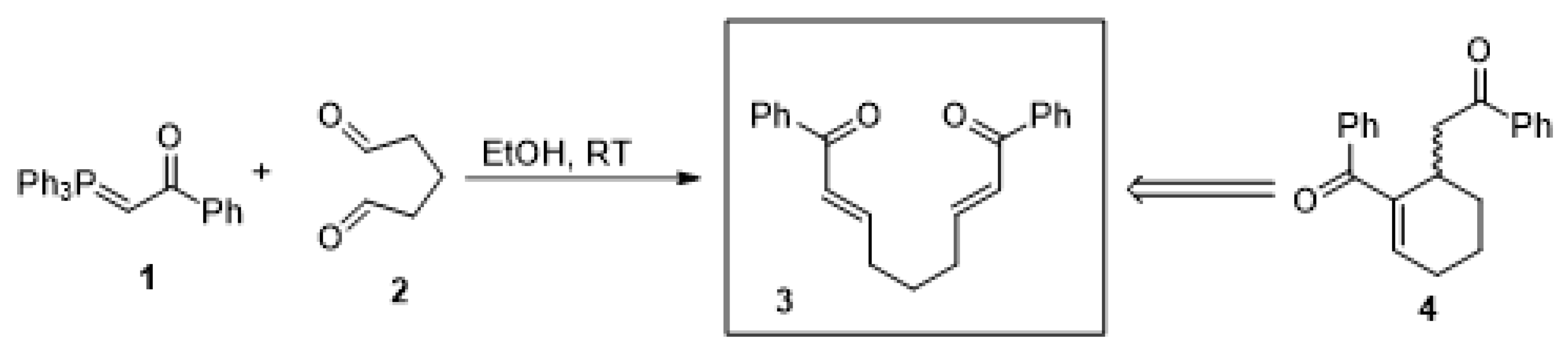
2.1. Preliminary Results Based on Reactions in the Literature
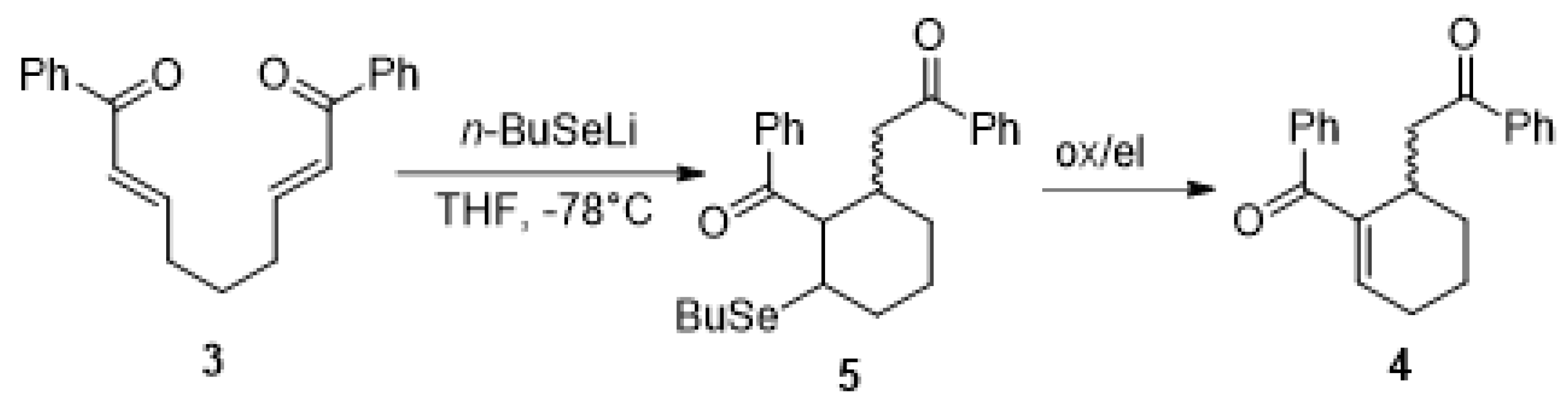
2.2. Investigations of the Reaction with n-BuSeLi
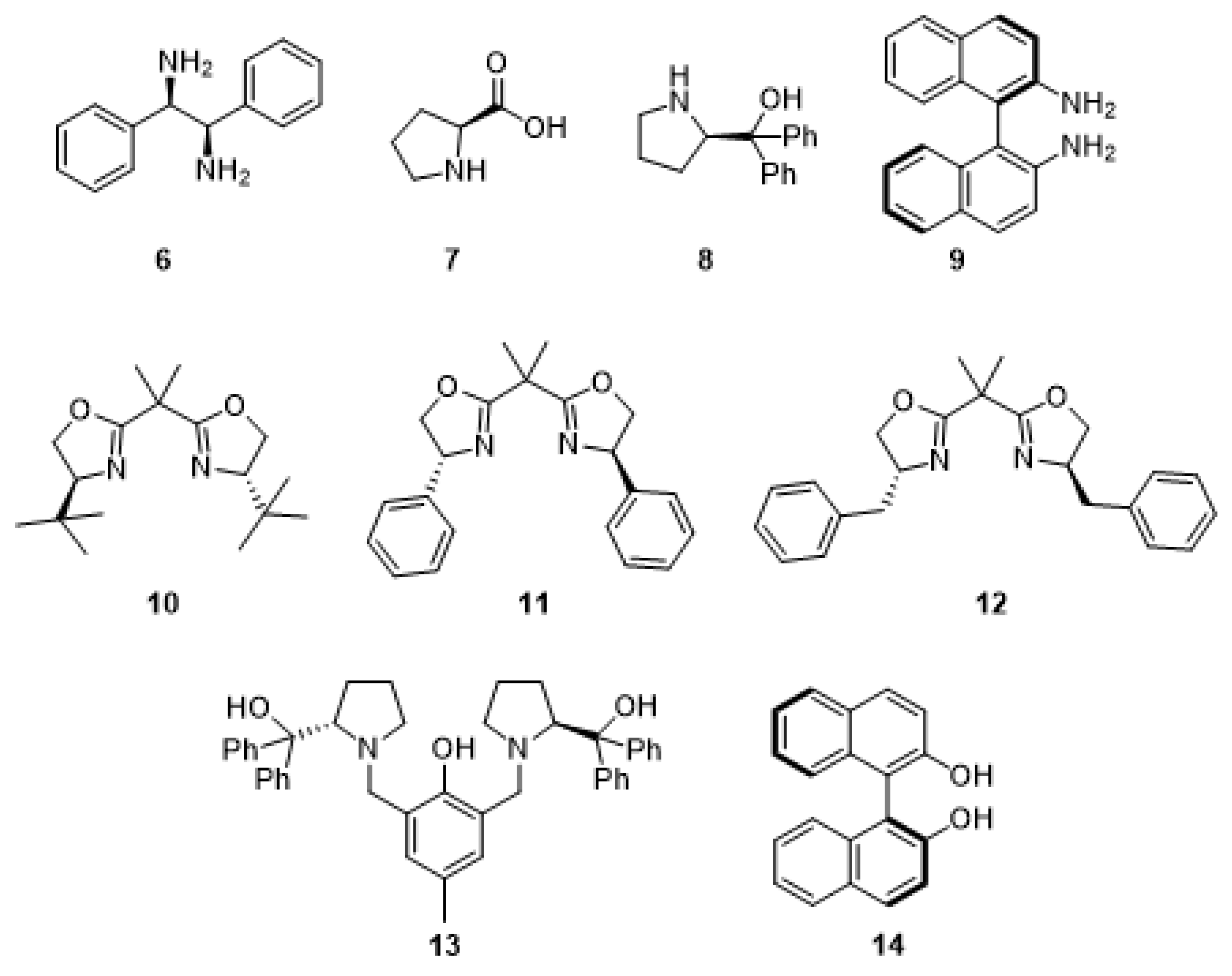
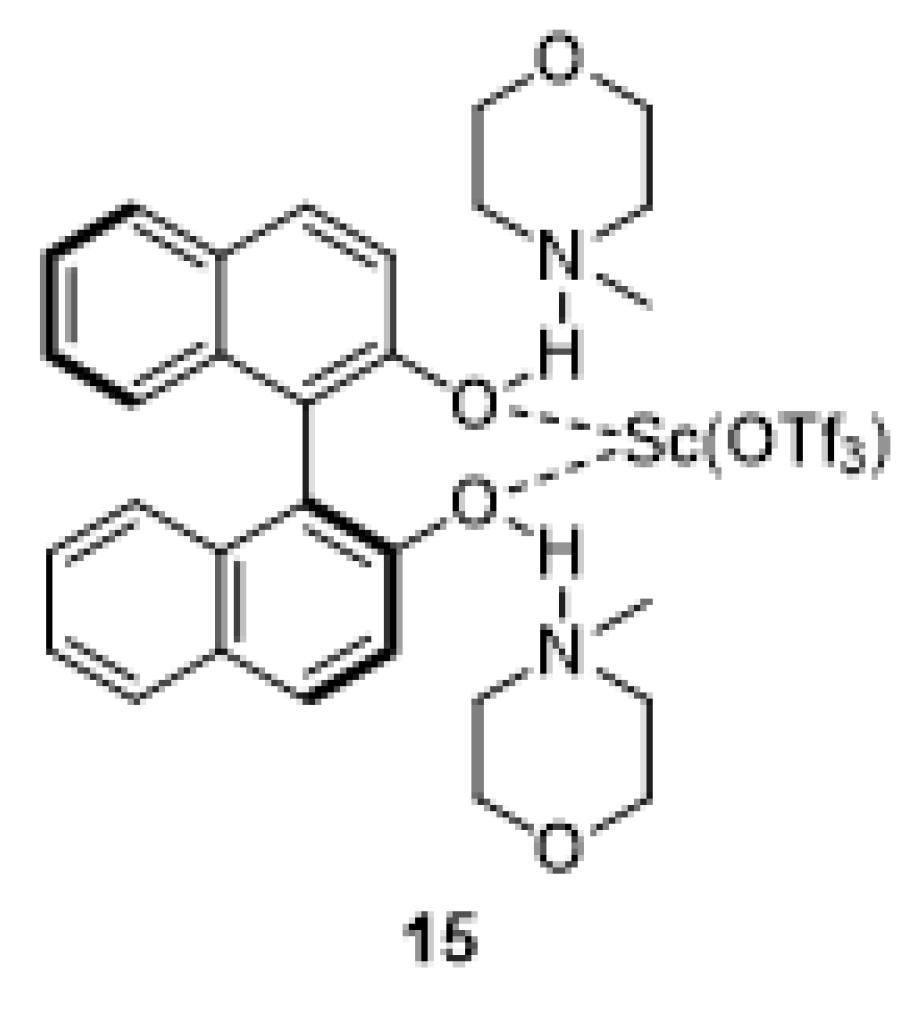
2.3. Searching of a Chiral Additive in Asymmetric Version
3. Conclusions
4. Experimental
4.1. General
4.2. General Procedure for Ylide Preparation
4.3. General Procedure for Synthesis of 1,9-Diphenylone-2,7-diene-1,9-dione (1)
4.4. General Procedure for the Non-Asymmetric IRC Reaction
4.5. General Procedure for an Asymmetric IRC Reaction
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbereviations
| IRC | intramolecular Rauhut-Currier |
| mCPBA | meta-chloroperoxybenzoic acid |
| DABCO | 1,4-diazabicyclo [2.2.2]octane |
| DBU | 1,8-diazabicyclo(5.4. 0)undec-7-ene |
| Hx | Hexane |
| AcOMeCys | methyl ester of acetylcysteine |
| Oxone | potassium peroxomonosulfate |
References
- Bharadwaj, K.C. Intramolecular Morita-Baylis-Hillman and Rauhut-Currier reactions. A catalytic and atom economic route for carbocycles and heterocycles. RSC Adv. 2015, 5, 75923–75946. [Google Scholar] [CrossRef]
- Rauhut, M.M.; Currier, H. U.S. Patent 3,074,999. 1963. Available online: https://patents.google.com/patent/US3074999A/en (accessed on 27 February 2022).
- Frank, S.A.; Mergott, D.J.; Roush, W.R. The Vinylogous Intramolecular Morita−Baylis−Hillman Reaction: Synthesis of Functionalized Cyclopentenes and Cyclohexenes with Trialkylphosphines as Nucleophilic Catalysts. J. Am. Chem. Soc. 2002, 124, 2404–2405. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-C.; Luis, A.L.; Agapiou, K.; Jang, H.-Y.; Krische, M.J. Organocatalytic Michael cycloisomerization of bis(enones): The intramolecular Rauhut-Currier reaction. J. Am. Chem. Soc. 2002, 124, 2402–2403. [Google Scholar] [CrossRef] [PubMed]
- MacKay, J.A.; Landis, Z.C.; Motika, S.E.; Kench, M.H. The Intramolecular Allenolate Rauhut-Currier Reaction. J. Org. Chem. 2012, 77, 7768–7774. [Google Scholar] [CrossRef] [PubMed]
- Tello-Aburto, R.; Lucero, A.N.; Rogelj, S. A catalytic approach to the MH-031 lactone: Application to the synthesis of geralcin analogs. Tetrahedron Lett. 2014, 55, 6266–6268. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, G. DABCO catalyzed Cross-rauhut-currier/transesterification reactions of activated alkenes with phenyl acrylates: Scope and mechanistic insight. Org. Biomol. Chem. 2014, 12, 832–835. [Google Scholar] [CrossRef]
- Li, K.; Jin, Z.; Chan, W.-L.; Lu, Y. Enantioselective Construction of Bicyclic Pyran and Hydrindane Scaffolds via Intramolecular Rauhut-Currier Reactions Catalyzed by Thiourea-Phosphines. ACS Catal. 2018, 8, 8810–8815. [Google Scholar] [CrossRef]
- Bania, N.; Mondal, B.; Ghosh, S.; Pan, S.C. DMAP Catalyzed Domino Rauhut-Currier Cyclization Reaction between Alkylidene Pyrazolones and Nitro-olefins: Access to Tetrahydropyrano[2,3-c]pyrazoles. J. Org. Chem. 2021, 86, 4304–4312. [Google Scholar] [CrossRef]
- Pitchumani, V.; Breugst, M.; Lupton, D.W. Enantioselective Rauhut-Currier Reaction with β-Substituted Acrylamides Catalyzed by N-Heterocyclic Carbenes. Org. Lett. 2021, 23, 9413–9418. [Google Scholar] [CrossRef]
- Bharadwaj, K.C. Chemoselective and Highly Rate Accelerated Intramolecular Aza-Morita-Baylis-Hillman Reaction. J. Org. Chem. 2018, 83, 14498–14506. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, H.; Feng, L.; Yu, Q.; Hao, J.-C.; Zhu, R.; Wang, Y. Dual Chalcogen–Chalcogen Bonding Catalysis. J. Am. Chem. Soc. 2020, 142, 3117–3124. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, Y.; He, Q.; Du, W.; Chen, Y.-C. Asymmetric Intramolecular Rauhut-Currier Reaction and Its Desymmetric Version via Double Thiol/Phase-Transfer Catalysis. J. Org. Chem. 2020, 85, 10760–10771. [Google Scholar] [CrossRef] [PubMed]
- Banachowicz, P.; Mlynarski, J.; Buda, S. Intramolecular Tandem Seleno-Michael/Aldol Reaction: A Simple Route to Hydroxy Cyclo-1-ene-1-carboxylate Esters. J. Org. Chem. 2018, 83, 11269–11277. [Google Scholar] [CrossRef] [PubMed]
- Biduś, N.; Banachowicz, P.; Buda, S. Application of a tandem seleno-michael/aldol reaction in the total syntheses of (+)-Pericosine B, (+)-Pericosine C, (+)-COTC and 7-chloro-analogue of (+)-Gabosine C. Tetrahedron 2020, 76, 131397. [Google Scholar] [CrossRef]
- Aroyan, C.E.; Miller, S.J. Enantioselective Rauhut−Currier Reactions Promoted by Protected Cysteine. J. Am. Chem. Soc. 2006, 129, 256–257. [Google Scholar] [CrossRef]
- Aroyan, C.E.; Dermenci, A.; Miller, S.J. Development of a Cysteine-Catalyzed Enantioselective Rauhut−Currier Reaction. J. Org. Chem. 2010, 75, 5784–5796. [Google Scholar] [CrossRef]
- Selig, P.S.; Miller, S.J. ortho-Acidic aromatic thiols as efficient catalysts of intramolecular Morita–Baylis–Hillman and Rauhut-Currier reactions. Tetrahedron Lett. 2011, 52, 2148–2151. [Google Scholar] [CrossRef]
- Thapa, B.; Schlegel, H.B. Theoretical calculation of pKa’s of selenols in aqueous solution using an implicit solvation model and explicit water molecules. J. Phys. Chem. A 2016, 120, 8916–8922. [Google Scholar] [CrossRef]
- Zhu, D.; Zheng, W.; Chang, H.; Xie, H. A theoretical study on the pKa values of selenium compounds in aqueous solution. New J. Chem. 2020, 44, 8325–8336. [Google Scholar] [CrossRef]
- Rabalakos, C.; Wulff, W.D. Enantioselective Organocatalytic Direct Michael Addition of Nitroalkanes to Nitroalkenes Promoted by a Unique Bifunctional DMAP-Thiourea. J. Am. Chem. Soc. 2008, 130, 13524–13525. [Google Scholar] [CrossRef]
- Reddy, C.R.; Reddy, M.D.; Haribabu, K. Organocatalyzed Intramolecular Michael Addition of Morita-Baylis-Hillman Adducts of β-Arylnitroethylenes: An Entry to 3-Aryl-4-nitrocyclohexanones. Eur. J. Org. Chem. 2012, 2012, 6414–6419. [Google Scholar] [CrossRef]
- Kobayashi, S.; Araki, M.; Hachiya, I. A Chiral Scandium Catalyst for Enantioselective Diels-Alder Reactions. J. Org. Chem. 1994, 59, 3758–3759. [Google Scholar] [CrossRef]
| Entry | Additives [1 Equiv] | Solvent | T [°C] | Time [h] | Yield [%] | Ref. |
|---|---|---|---|---|---|---|
| 1. a | PBu3 | Acetone | RT | 24 | 29 | [4] |
| 2. | PBu3 | t-BuOH | RT | 72 | - | [4] |
| 3. b,c | AcOMeCys | Acetonitrile | −40 | 5 | 33 | [19] |
| 4. | AcOMeCys | Acetonitrile | −40 | 24 | 30 | [19] |
| 5. d | AcOMeCys n-BuSeLi | Acetonitrile | −40 | 24 | 38 |
| Entry | n-BuSeLi [Equiv] | Solvent [1:6] | Tt [°C] | T2 [°C] | Yield of 4 [%] |
|---|---|---|---|---|---|
| 1. | 1 | THF:ACN | 0 | RT | 15 |
| 2. | 1 | THF:ACN | 0 | RT | 18 |
| 3. a | 1 | THF:ACN | −40 | RT | 52 |
| 4. b | 1 | THF:ACN | −40 | RT | 82 |
| 5. c | 1 | THF:ACN | −40 | RT | 35 |
| 6. | 1 | THF:ACN | −78 | RT | 18 |
| 7. | 0.2 | THF:ACN | −78 | RT | - |
| 8. | 0.2 | THF:ACN | −40 | RT | 15 |
| 9. | 0.2 | THF:ACN | 0 | RT | - |
| 10. | 1 | THF:ACN | −40 | RT | - |
| 11. | 1 | THF:DMF | 0 | RT | 15 |
| 12. | 1 | THF:DMF | −40 | RT | 24 |
| 13. | 1 | THF:DCM | −40 | RT | 75 |
| Entry | Oxidant | T1 [°C] | T2 [°C] | Yield of 4 [%] |
|---|---|---|---|---|
| 1. | X | −78 | RT | 11 |
| 2. | X | −40 | RT | 13 |
| 3. | H2O2 | −40 | RT | 84 |
| 4. a | O2 | −40 | RT | 27 |
| 5. b | O2 | −40 | RT | 30 |
| 6. c | Oxone® | −40 | RT | - |
| 7. | NaIO4 | −40 | RT | 58 |
| 8. d | mCPBA | −40 | RT | - |
| Entry | Solvent | Tt [°C] | T2 [°C] | Yield [%] |
|---|---|---|---|---|
| 1. | THF | −40 | RT | 62 |
| 2. | THF:DMF | −40 | RT | 35 |
| 3. | THF:DCM | −40 | RT | 68 |
| 4. | THF:ACN | −78 | RT | 57 |
| 5. | THF:ACN | −40 | RT | 76 |
| 6. a | THF:ACN | −40 | RT | 84 |
| 7. | THF:ACN | 0 | RT | - |
| 8. | THF:EtOH | −40 | RT | - |
| 9. | THF:Hx | −40 | RT | - |
| 10. | THF:CHCl3 | −40 | RT | - |
| Entry | Water [Equiv] | ee [%] |
|---|---|---|
| 1. | 0 | rac |
| 2. | 1 | rac |
| 3. | 5 | 10 |
| 4. a | 10 | 32 |
| 5. | 15 | 22 |
| 6. | 20 | 11 |
| 7. | 100 | rac |
| Entry | Solvent (1:3) | ee [%] |
|---|---|---|
| 1. | THF | 10 |
| 2. | THF:Hx | rac |
| 3. | THF:Tol | rac |
| 4. | THF:EtOH | 18 |
| 5. | THF:CHCl3 | 30 |
| 6. | THF:DMF | rac |
| 7. | THF:DCM | 28 |
| 8. a | THF:ACN | 32 |
| 9. b | THF:ACN | −29 |
| Entry | H2O [Equiv] | Amine | Solvent | T [°C] | ee [%] |
|---|---|---|---|---|---|
| 1. | 10 | DABCO | DCM | −40 | rac |
| 2. | 10 | DBU | DCM | −40 | rac |
| 3. | 10 | Et3N | DCM | −40 | rac |
| 4. | 10 | NMM | DCM | −40 | rac |
| 5. | 10 | NMM | DCM | −20 | rac |
| 6. | 10 | NMM | DCM | 0 | rac |
| 7. | 10 | NMM | DCM | RT | rac |
| 8. | 10 | NMM | ACN | −40 | rac |
| 9. | 10 | NMM | ACN | −20 | rac |
| 10. | 10 | NMM | ACN | 0 | rac |
| 11. | 1 | NMM | DCM | −40 | rac |
| 12. | 1 | NMM | ACN | −40 | rac |
| 13. a | 0 | NMM | DCM | −40 | 62 |
| Entry | Tt [°C] | T2 [°C] | ee [%] |
|---|---|---|---|
| 1. | −78 (6 h) | −15 (18 h) | 10 |
| 2. | −50 (6 h) | −15 (18 h) | 55 |
| 3. | −40 (6 h) | −15 (18 h) | 72 |
| 4. a | −40 (6 h) | −15 (66 h) | 70 |
| 5. b | −30 (6 h) | −15 (18 h) | 78 |
| 6. | −30 (6 h) | - | 82 |
| 7. | 0 (6 h) | −15 (18 h) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Całka-Kuc, G.; Buda, S. An Asymmetric Intramolecular Rauhut-Currier Reaction Initiated by Chiral Selenolate-BINOL Complexes. AppliedChem 2022, 2, 59-67. https://doi.org/10.3390/appliedchem2020004
Całka-Kuc G, Buda S. An Asymmetric Intramolecular Rauhut-Currier Reaction Initiated by Chiral Selenolate-BINOL Complexes. AppliedChem. 2022; 2(2):59-67. https://doi.org/10.3390/appliedchem2020004
Chicago/Turabian StyleCałka-Kuc, Gabriela, and Szymon Buda. 2022. "An Asymmetric Intramolecular Rauhut-Currier Reaction Initiated by Chiral Selenolate-BINOL Complexes" AppliedChem 2, no. 2: 59-67. https://doi.org/10.3390/appliedchem2020004
APA StyleCałka-Kuc, G., & Buda, S. (2022). An Asymmetric Intramolecular Rauhut-Currier Reaction Initiated by Chiral Selenolate-BINOL Complexes. AppliedChem, 2(2), 59-67. https://doi.org/10.3390/appliedchem2020004