Abstract
Background and Objectives: Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a complex disease that involves changes in multiple organs and even the central nervous system (CNS). CP/CPPS may elevate seizure risk via neuroinflammatory mechanisms within the CNS. Neuroprotective effects of CO-releasing molecules (CORMs) were demonstrated in inflammation-driven conditions, while CORMs potential to ameliorate seizure susceptibility in inflammatory states, like CP/CPPS, remains unclear. Therefore, we investigated effects of CORM-A1 on susceptibility to lindane–induced seizures in rats with CP/CPPS through behavioral and electroencephalographic (EEG) study. Materials and Methods: Wistar rats were divided into four groups (n = 8/group): Sham-PBS, Sham-CORM, CP/CPPS-PBS and CP/CPPS-CORM. The CP/CPPS model was created by injection of 3% λ-carrageenan and its development assessed by mechanical pain threshold. CORM-A1 (2 mg/kg/day, i.p.) or vehicle (PBS) was given during seven postoperative days. Hereupon, subconvulsive dose of lindane (4 mg/kg, i.p.) was administered and behavioral features of seizures were observed alongside with EEG recordings. Results: Our data showed that the incidence and severity of lindane-induced seizures was significantly higher in the CP/CPPS-PBS group than in the Sham-PBS group. CORM-A1 treatment significantly decreased seizure incidence, prolonged seizure latency, and reduced seizure severity in CP/CPPS rats compared to vehicle treatment (CP/CPPS-CORM vs. CP/CPPS-PBS). Also, CORM-A1 treatment significantly reduced the number and duration of ictal periods induced by lindane in CP/CPPS animals compared to vehicle treatment. Conclusions: It could be concluded that CORM-A1 treatment reduced both behavioral and EEG signs of increased seizure susceptibility in rats with CP/CPPS, thus it could be a potential therapeutic target.
1. Introduction
Chronic prostatitis and chronic pelvic pain syndrome (CP/CPPS) represent significant clinical challenges due to their high prevalence, complex etiology, and notable impact on quality of life. CP/CPPS is characterized by persistent pain or discomfort in the pelvic region lasting at least three of the past six months, typically accompanied by lower urinary tract symptoms such as increased urinary frequency, nocturia, dysuria, urgency, weak stream, a sensation of incomplete bladder emptying, and pelvic discomfort [1,2].
Recent studies have shown that CPPS is not confined to peripheral pathology but also involves central nervous system (CNS) alterations [3,4]. Neuroinflammation plays a pivotal role in the pathogenesis of CP/CPPS [5]. Chronic pelvic inflammation induces biochemical changes in sensory nerve fibers, disrupting the functions of the immune, nervous, and endocrine systems, which contributes to peripheral sensitization [6]. This sensitization leads to further changes in the CNS, including altered synaptic plasticity, ultimately resulting in central sensitization and increased pain perception [7,8]. Additionally, neuroinflammation sustains chronic pelvic pain through activation of glial cells, the release of proinflammatory cytokines, and disturbances in neurotransmitter homeostasis [9].
Beyond lowering pain thresholds and perpetuating chronic pain, neuroinflammation has also been implicated in increasing susceptibility to epileptic seizures by promoting abnormal electrical activity in neurons [10,11]. Brain inflammation is initiated by the activation of microglia and astrocytes, which release proinflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6. These cytokines contribute to neuronal hyperexcitability and epileptiform discharges [12,13]. Furthermore, neuroinflammation can compromise the integrity of the blood-brain barrier (BBB), facilitating the entry of inflammatory molecules into the brain and sustaining epileptic activity [14]. In our previous study, we observed that rats with CP/CPPS had reduced seizure threshold and increased susceptibility to lindane-induced seizures by elevating the level of IL-1β and IL-6 in certain brain regions like hippocampus, cortex and thalamus. This suggests that CP/CPPS may elevate seizure risk via neuroinflammatory mechanisms within the CNS [15].
On the other hand, carbon monoxide-releasing molecules (CORMs) are compounds capable of releasing controlled amounts of carbon monoxide (CO) in biological systems and have gained interest due to their anti-inflammatory and neuroprotective properties [16,17]. CORMs have been reported to attenuate oxidative stress and exert anxiolytic effects [18], modulate inflammatory responses, reduce proinflammatory cytokine release [19]. It is also reported that CORM-A1 may act protectively in models of neonatal seizures by attenuating oxidative stress-induced neurovascular damage [20]. Additionally, CORM-A1 effectively prevented seizure-induced impairments in cerebrovascular responses when administered before or during seizures [21] suggesting that CORMs could modulate susceptibility to seizures in inflammatory states, like in CP/CPPS.
Having in mind these considerations, following mechanistic sequence could be hypothesized: CP/CPPS induces persistent peripheral nociceptive input, which promotes neuroinflammation and microglial activation. This inflammatory milieu may disrupt the excitatory—inhibitory balance, thereby reducing seizure threshold and increasing susceptibility to pronconvulsive stimuli. In this context, we hypothesized that CORM-A1 could interrupt this cascade by attenuating neuroinflammation and restoring neuronal homeostasis. However, this has not been investigated yet In this study we tested this hypothesis using both behavioral seizure outcomes and EEG measurements following lindane challenge.
Having this hypothesis in mind, the aim of our current study was to examine the therapeutic potential of CORM-A1 in modulating susceptibility to lindane—induced seizures in rats with CP/CPPS through behavioral and electroencephalographic (EEG) study.
2. Materials and Methods
2.1. Animals and Ethical Statement
Male Wistar Albino rats weighing between 250 and 300 g (total n = 32) were used in this study. The animals were maintained in a controlled environment with a temperature of 22 ± 2 °C, relative humidity of 50 ± 5%, and a 12 h: 12 h light: dark cycle, with lights on from 8:00 a.m. Throughout the study, the rats had unlimited access to standard food and water. Animals’ welfares were constantly monitored. Exclusion criteria were appearance of any signs of behavioral or physical impairments. There were no dropouts throughout the experiments. Experimental unit was a single animal. All animals were coded, and experimenters were blinded to its group allocation.
All experimental procedures were approved by the local Ethics Committee authority (Approval No. 323-07-01339/2017-05/3) and fully in accordance with national and international standards in laboratory animal welfare.
2.2. Experimental Design
Upon laboratory acclimatizing of seven days, EEG electrodes were implanted to rats five days prior to the intraprostatic injection. Depending on the intraprostatic injection, total number of rats was firstly randomly divided into two cohorts using a randomization table. In the first cohort, CP/CPPS has been induced by intraprostatic 3% λ-carrageenan injection (CP/CPPS, n = 16 rats), while another cohort was Sham operated rats (intraprostatic 0.9% NaCl injection at time of surgery, Sham, n = 16 rats). Afterwards, one half of rats from each of these cohorts were administered CORM-A1 (2 mg/kg/day, i.p. for seven consecutive days upon intraprostatic injection). Phosphate-buffered saline (PBS, 0.1 mL/kg/day, i.p,) was given as the solvent to the other half of rats. Thus, the Sham-PBS, Sham-CORM, CP/CPPS-PBS, CP/CPPS-CORM groups were formed (n = 8 per each group). Animals were randomly assigned to treatment groups using a randomization table.
To evaluate convulsive behaviors and EEG responses, convulsion tests were performed by administering a sub-convulsive dose of 4 mg/kg lindane i.p. to all groups on the 7th postoperative day. Mechanical pain threshold was evaluated using the evF device on days −1 and 7 (in reference to intraprostatic injection, surgical operation) (Figure 1). All assessments, including seizure–related behavioral and EEG recordings, were conducted by investigators blinded to the group allocation.
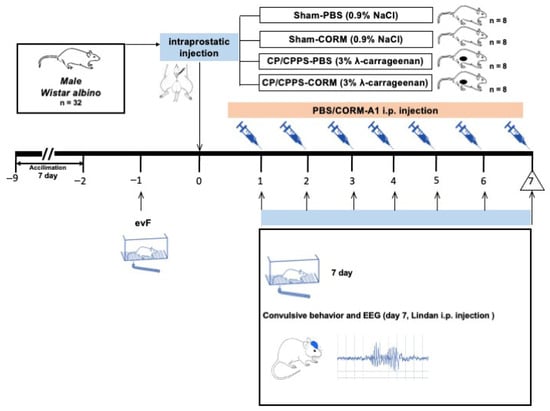
Figure 1.
Experimental design. To evaluate CORM treatment and convulsion responses together, the following four experimental groups were formed from each experimental arm: 1: Sham-PBS, 2: Sham-CORM, 3: CP/CPPS-PBS, 4: CP/CPPS-CORM. NaCl was applied intraprostatically to the Sham groups, and 3% λ-carrageenan solution was applied to the CP/CPPS groups. CORM-A1 (2 mg/kg/day) was administered intraperitoneally (i.p.) to the CORM groups every day from the 1st day to the 7th day after surgery. Convulsive tests were conducted to assess convulsive behavior and EEG responses, by administering subconvulsive dose of 4 mg/kg lindane i.p. to all groups on the 7th day. Pain threshold was measured on the—1 and 7th days.
2.3. Placement of Electrodes for EEG Recording
Electrodes for bipolar EEG registration were implanted five days prior to the intraprostatic injection to ensure proper recovery period for the animals. Before surgery, the surgical site was disinfected, and anesthesia was induced using sodium thiopental (40 mg/kg, i.p.). Using a stereotaxic apparatus, three gold-plated electrodes were positioned in specific cortical regions based on the Paxinos and Watson (2006) brain atlas: frontal cortex (2 mm anterior to bregma and 2 mm lateral to the midline), parietal cortex (2 mm anterior to lambda and 2 mm lateral), and occipital cortex (2 mm posterior to lambda) [22]. The electrodes were secured with dental acrylic and attached to the recording connector. Animals were further acclimated to the EEG recording setup 24 h prior to each data acquisition.
2.4. Experimental CP/CPPS Model Development and Surgical Procedure
In this study, the CP/CPPS model was developed as per previously reported protocols [20,23,24]. For general anesthesia, rats were administered sodium thiopental (40 mg/kg, i.p.). Hereupon, the scrotum and lower abdomen were sterilized, and the rats were placed in a supine position on a heating pad. After local anesthesia, a 1–1.5 cm vertical incision in the midline of the lower abdomen was performed and the ventral prostate lobes were exposed. 25 μL of 3% λ-carrageenan was injected into both prostate lobes of the animals in the CP/CPPS group, while 0.9% NaCl was given to the sham group.
2.5. Scrotal Pain Sensitivity Assessments: Mechanical Pain Threshold
To assess development of mechanical allodynia and progression of experimental CP/CPPS, the scrotal pain threshold was measured before (one day, −1) and after the intraprostatic injection (postoperative day 7). An electronic von Frey anesthesiometer (eVF) was used for these evaluations. Prior to testing, animals were acclimated to plexiglas chambers twice daily for 15–20 min and allowed 30 min of habituation during the measurement session. The eVF probe was applied perpendicularly to the scrotal skin, and pressure gradually increased until a withdrawal reflex was observed. The highest pressure recorded at the reflex response was noted as the pain threshold. Each animal’s threshold was calculated as the mean of three consecutive measurements. All evF measurements were performed by the same trained experimenter at a fixed time each day to minimize diurnal variability. Upon testing, the rats were returned to their home cages.
2.6. Behavioral and EEG Characteristics of Convulsive Behavior
To evaluate seizure susceptibility of rats subconvulsive dose of lindane (4 mg/kg, intraperitoneally) dissolved in dimethyl sulfoxide (DMSO) was administered to the animals on the seventh day after surgery. Lindane dosage has been chosen based on prior studies on dose-response findings demonstrated that lindane dose of 4 mg/kg produces low seizure incidence in control animals [25]. Pilot experiments in our current settings confirmed that this dose produces near—threshold seizure susceptibility under current conditions Convulsive behavior and EEG were recorded to evaluate brain hyperexcitability in these rats depending on the previous treatment.
Behavioral signs of seizures (i.e., convulsive behavior) were monitored for 30 min following Lindane administration. During this period, seizure incidence, latency to seizure onset, and seizure severity were assessed. Seizure incidence was calculated as the percentage of animals exhibiting seizures relative to the total number in each group. The latency period was defined as the time elapsed from lindane injection to the appearance of the first seizure sign; animals that did not develop seizures were assigned a latency of 30 min. Seizure severity was rated using a 0-to-4 scale [26]: 0—no seizures; 1—head shaking and/or jaw contractions; 2—myoclonic jerks or “kangaroo posture” (standing on hind limbs with forelimb clonus); 3—generalized clonic seizures involving tonic contractions of forelimbs, hind limbs, and tail; 4—severe clonic-tonic seizures lasting more than 20 s or recurrent seizures lasting over 5 min (status epilepticus).
Bipolar EEG recordings from freely moving rats were obtained using an 8-channel system (RIZ, Zagreb, Croatia). Signal acquisition was carried out via a 16-bit SCB-68 interface (National Instruments, Austin, TX, USA). Electrode impendence was checked prior to recording and impendence <10 kΩ were acceptable. Each animal was placed in a transparent plexiglas chamber (Elunit, Belgrade, Serbia) and connected to the EEG apparatus through a flexible tether, allowing for simultaneous behavioral monitoring during the recording period. Following Lindane administration, EEG activity was recorded for 30 min, after which the animals were returned to their cages. Data was sampled at 512 Hz. To minimize electrical interference, a 50 Hz notch filter, along with a 0.3 Hz high-pass and 100 Hz low-pass filter, was applied. Continuous EEG recordings were captured using NeuroSciLaBG software (version 1.2.1), a LabVIEW-based program developed in-house.
EEG data were initially examined visually to identify seizure-related activity. Screening for ictal periods and their identification were done by an evaluator blinded to group allocation. Subsequent quantitative analysis was conducted using NeuroSciLaBG software (Belgrade, Serbia). Ictal period was defined based on specific criteria indicative of lindane-induced epileptiform discharges [27], including spontaneous, widespread rhythmic spike-wave patterns; durations of at least one second; and wave amplitudes at least double the baseline EEG. For each subject, the total number and duration of ictal periods during the 30-min recording period were documented. EEG analysis in this study was focused on number and duration of ictal periods because these masseurs most directly reflect seizure susceptibility under acute lindane challenge. Quantitative spectral analysis was not included due to specific design of post-lindane recording window optimized for seizure detection rather than spectra characterization.
2.7. Substances
All chemicals of analytical grade used in this study, including PBS, DMSO, λ-carrageenan, CORM-A1, and Lindane, were obtained from Sigma Aldrich (St. Louis, MO, USA). Fresh solutions were prepared immediately prior to each administration. This is of particular importance for CORM-A1 solution.
2.8. Statistics
No missing data were spotted in this study as all rats completed the intended experimental protocol and their data were included in the final analysis. To test the normality of the data we used Kolmogorov-Smirnov test. Statistical significance of differences in seizure incidence was assessed by Fisher’s test. The statistical significance of differences in latency period, seizure intensity, ictal periods number and duration between groups were evaluated with the Kruskal-Wallis test and the Mann-Whitney post hoc test having in mind non-parametric distribution. Data were presented as medians with interquartile ranges. Significance of between groups differences in pain threshold were assessed using ANOVA with Tukey’s post hoc test for multiple comparisons. Significance of intra-group comparisons were assessed using the t test for repeated measurements. Data were expressed as mean ± SD. Statistical approach has been in line with Statistical strategies in other reports [28]. Statistical significance: p < 0.05 and p < 0.01.
3. Results
3.1. Effect of CORM-A 1 on Scrotal Pain Threshold
Scrotal pain threshold was measured before (day −1) and after surgery (day 7) in all groups. Baseline thresholds showed no significant differences among rats (p > 0.05). Pain thresholds remained stable in Sham groups throughout (p > 0.05). However, in CP/CPPS groups, the threshold dropped significantly on postoperative day 7 (p < 0.001), compared both to Sham groups and pre-surgery levels. Notably, CORM-A1 treatment in the CP/CPPS-CORM group significantly raised pain thresholds compared to CP/CPPS-PBS (p < 0.001). CORM-A1 had no significant effect in Sham animals (p > 0.05) (Table 1).

Table 1.
Scrotal pain threshold in experimental groups.
3.2. Effect of CO Modulation on Convulsive Behavior
The seizure incidence after a subconvulsive lindane dose was significantly higher in the CP/CPPS-PBS group (87.5%) compared to the Sham-PBS group (25%) (p < 0.05). In animals treated with CORM-A1, seizure incidence was 25% in the Sham-CORM group and 37.5% in the CP/CPPS-CORM group, with no significant difference between them (p > 0.05). Actually, CORM-A1 treatment significantly reduced seizure incidence in CP/CPPS animals compared to PBS-treated CP/CPPS controls (CP/CPPS-CORM vs CP/CPPS–PBS, 37.5% vs. 87.5%, p = 0.0455, Figure 2A).
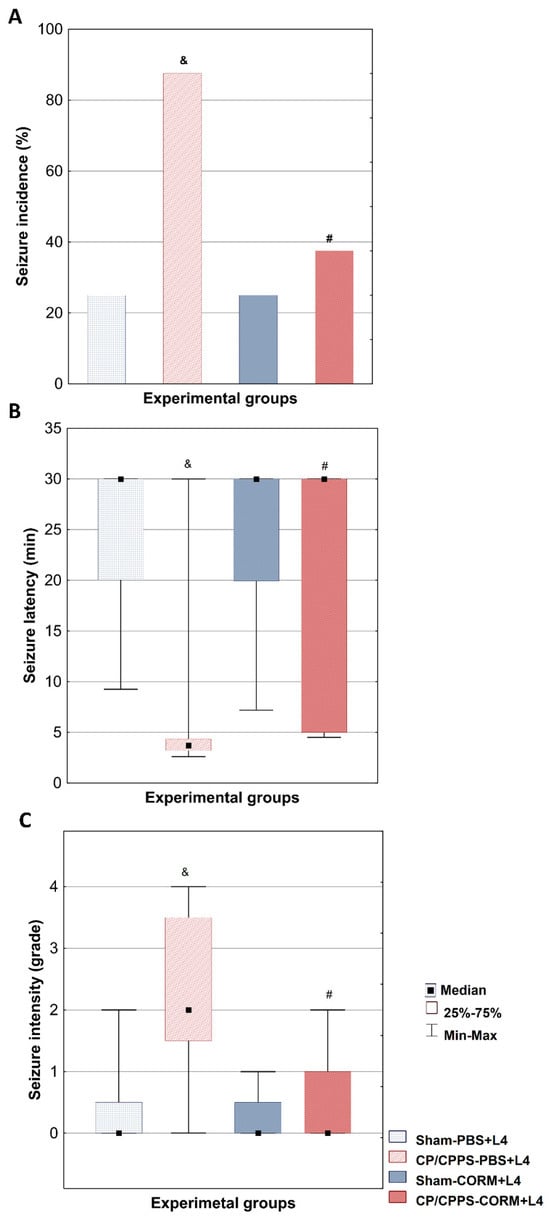
Figure 2.
Effect of CORM-A1 on convulsive behavior evoked by subconvulsive dose of lindane in rats with CP/CPPS. On the seventh day after surgery, subconvulsive dose of lindane (4 mg/kg) was administered intraperitoneally to rats to evaluate their susceptibility to seizures. Seizure incidence (A) was expressed as as a percentage (%) of the animals with convulsions. The latent period (B) was defined as the time from the injection of lindane to the appearance of the first observable convulsion sign. The intensity of seizures (C) was assessed on a descriptive scale with grades from 0 to 4. Statistical significance of differences in seizure incidence was assessed by Fisher’s test. The statistical significance of differences in latent period and seizure intensity between groups were evaluated with the Kruskal-Wallis test and the Mann-Whitney post hoc test (& p < 0.05 vs. Sham-PBS group; # p < 0.05 vs. CP/CPPS-PBS group). For details and abbreviations see captions in Figure 1.
Analysis of the latency period until the first convulsive seizure was significantly shorter in the CP/CPPS-PBS group compared to the Sham-PBS group (p < 0.05, Figure 2B). In addition, postoperative treatment with CO donor in prostatitis animals was shown to significantly prolong the median time to the first seizure compared to solvent-treated prostatitis animals (CP/CPPS-CORM vs. CP/CPPS-PBS, p = 0.0462, Figure 2B).
The seizure severity induced by lindane was significantly higher in the animals in the CP/CPPS-PBS group compared to the animals in the Sham-PBS group (p < 0.05, Figure 2C). In addition, the maximum seizure score observed in the CP/CPPS-PBS group was 4, while this value was 2 in the Sham-PBS group. Postoperative CORM-A1 treatment applied to the animals in the CP/CPPS-CORM group provided a statistically significant decrease in seizure severity compared to the animals in the CP/CPPS-PBS group (p = 0.0408, Figure 2C).
In the Sham-PBS group, 75% of animals showed no seizures (grade 0), while grades 1 and 2 occurred equally at 12.5%, and no grade 3 or 4 seizures were observed. In the CP/CPPS-PBS group, seizures were more severe: 25% had grade 4, 37.5% had grade 2, and grades 0, 1, and 3 each at 12.5%. Sham-CORM animals mainly had grades 0 (75%) and grade 1 (25%) seizures, with no higher grades. CP/CPPS-CORM animals mostly had grade 0 (62.5%), with grade 1 (25%) and 2 (12.5%) seizures; no grade 3 or 4 seizures occurred (Table 2). Postoperative CO donor treatment (CORM) in CP/CPPS animals significantly increased the frequency of grade 0 seizures compared to CP/CPPS-PBS (p < 0.05).

Table 2.
Effect of CORM-A1 on the distribution of seizure grades induced by a subconvulsive dose of lindane in rats with CP/CPPS.
3.3. Effect of CORM-A1 on EEG Ictal Activity Induced by Lindane in Animals with CP/CPPS
Representative ictal periods showed high-amplitude spike series in CP/CPPS-PBS animals and lower amplitude and frequency spikes in CP/CPPS-CORM animals during the 30-min EEG recording upon lindane administration in subconvousive dose (4 mg/kg, i.p.) (Figure 3).
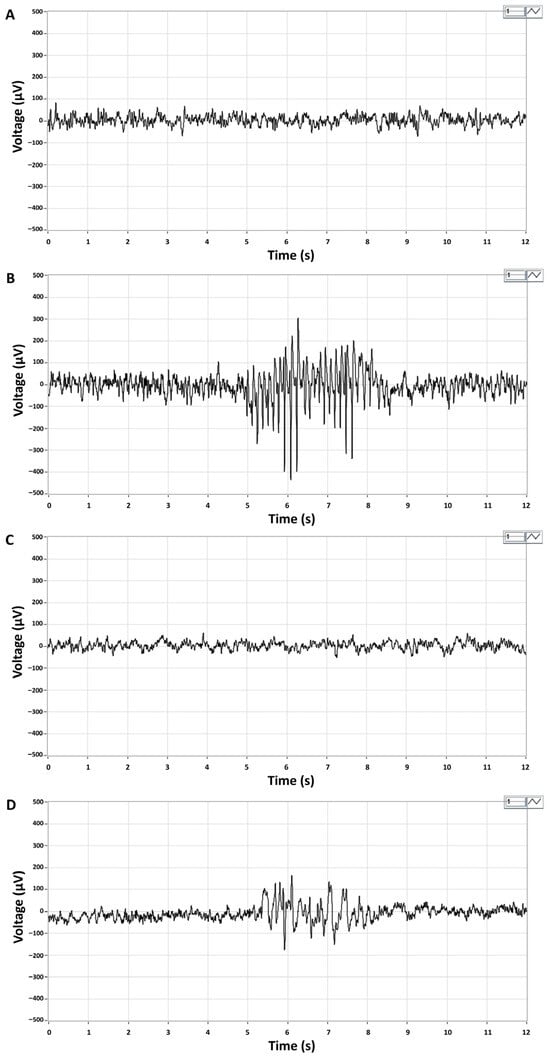
Figure 3.
Representative EEG recordings of brain activity in Sham-PBS (A) and Sham-CORM (C) animals, and ictal EEG periods in CP/CPPS-PBS (B) and CP/CPPS-CORM (D) animals. On the 7th postoperative day, animals received an intraperitoneal injection of a subconvulsive dose of lindane (4 mg/kg, i.p.), and EEG activity was recorded for 30 min. Ictal periods are characterized by a series of high-amplitude spikes (200–300 μV) with a dominant frequency of 7–8 Hz in the alpha band. Recording leads: right frontal—left parietal. For details and abbreviations see caption in Figure 1.
The number of EEG ictal periods was significantly higher in the CP/CPPS-PBS group than in the Sham-PBS group (p < 0.05, Figure 4A). Postoperative CORM-A1 treatment significantly reduced the number of EEG ictal periods compared to PBS treatment in animals with prostatitis (p = 0.0239, Figure 4A).
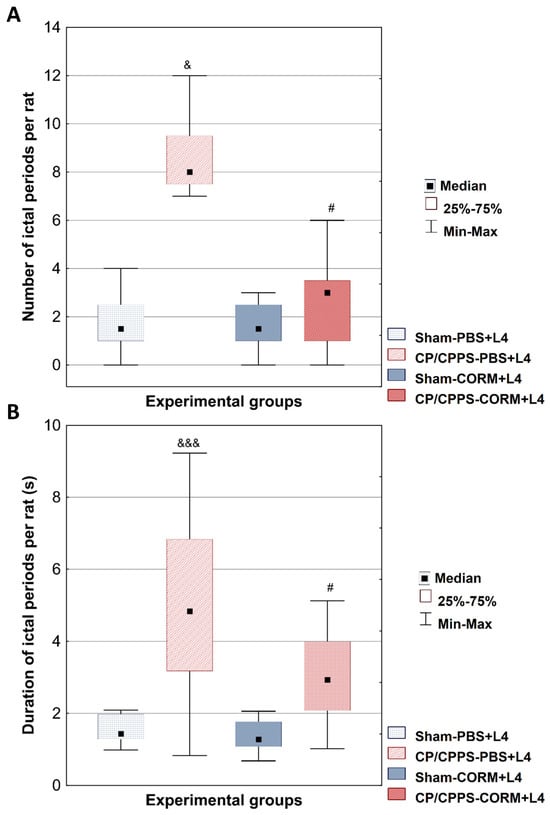
Figure 4.
Effect of CORM-A1 on the number (A) and duration (B) of EEG ictal periods per animal induced by a subconvulsive dose of lindane in rats with CP/CPPS. The number (A) and duration (B) of EEG ictal periods per rat was determined for all four groups. Statistical significance of differences between groups was assessed using the Kruskal-Wallis test with Mann-Whitney post hoc test (& p < 0.05; &&& p < 0.05 vs. Sham-PBS; # p < 0.05 vs. CP/CPPS-PBS). For details and abbreviations see caption in Figure 1.
Similar results were found in the analysis of EEG ictal period duration. The duration was significantly longer in the CP/CPPS-PBS group compared to Sham-PBS (p < 0.001, Figure 4B). Postoperative treatment with the CORM-A1 significantly reduced ictal period duration in CP/CPPS animals (CP/CPPS-CORM vs. CP/CPPS-PBS, p = 0.0203, Figure 4B).
4. Discussion
In our study, we aimed to evaluate therapeutic potential of CORM-A1, chemical compound that slowly releases carbon monoxide gas, in amelioration of seizure susceptibility in CP/CPPS rats. Our data showed that the incidence of lindane-induced seizures was significantly higher in the CP/CPPS-PBS group (87.5%) than in the Sham-PBS group (25%). This is in agreement with our previous study showing increased seizure susceptibility in CP/CPPS rats compared to corresponding Sham controls [15]. CORM-A1 treatment significantly decreased seizure incidence, prolonged seizure latency and reduced seizure severity in CP/CPPS rats comparing to vehicle treatment (CP/CPPS- CORM vs. CP/CPPS-PBS, Figure 2). Actually, seizures were generally mild (grade 0–1) in the Sham-PBS and Sham-CORM groups and no high-severity seizures were observed (Table 2), while seizure severity increased significantly in the CP/CPPS-PBS group and the maximum score reached 4. CORM-A1 treatment reduced seizure severity in the CP/CPPS group to levels close to the sham groups. Thus, CORM-A1 ameliorated seizure susceptibility induced by CP/CPPS in rats.
EEG analyses in our study also supported findings from convulsive behavior monitoring. Ictal activity was low in terms of number and duration of ictal periods in the sham groups upon lindane chalange. On the contrary, the number and duration of ictal periods induced by lindane increased significantly in the CP/CPPP rats on vehicle treatment (CP/CPPS + PBS). However, CORM-A1 treatment significantly reduced these parameters in CP/CPPS animals compared to vehicle treatment (Figure 4). Thus, these results speak in favor of CORM-A1 beneficial effects on seizure susceptibility induced by CP/CPPS in rats.
4.1. Possible Mechanisms
In our previous study [15], we have demonstrated that increased susceptibility to seizures in rats with CP/CPPS is associated with increased level of proinflammatory cytokines IL-1β and IL-6 in brain regions like the cerebral cortex and thalamus, thus indicating that CPPS-induced neuroinflammation is a possible mediator of increased seizure susceptibility in these rats.
Moreover, further studies investigating the relationship between CP/CPPS and neuroinflammation have shown that CP/CPPS creates a neuroinflammatory environment in the CNS [27,29] Significant increase in the expression of inflammatory genes and cytokines have been demonstrated in CP/CPPS patients [30]. Also, microglial activation at the spinal cord level has been reported to increase the expression of proinflammatory cytokines such as IL-1β and CCL3, as well as neuromodulators including the P2X4 receptor and BDNF in experimental autoimmune prostatitis models [31].
It is now well-known that neuroinflammation disrupts the balance between inhibition and excitation within neural circuits, facilitates seizure formation, and promotes the consolidation of epileptic networks [32]. Microglial activation is proposed to play a key role in the onset of seizures by promoting the release of proinflammatory cytokines such as IL-1β and TNF-α [33]. Namely, the activation of P2X4 receptors on microglia leads to the release of BDNF, which binds to TrkB receptors on neurons, downregulating KCC2 expression. This reduction impairs GABAergic and glycinergic inhibition, resulting in increased neural excitability [34]. Thus, microglial and astrocyte activation observed in CP/CPPS may similarly increase neural excitability via inflammatory cytokines and neuromodulators [35] what is congruent with our current and previous findings [14]. On the other hand, CO-releasing molecules has been demonstrated to reduce neural excitability by blocking P2X4 receptors [36]. Considering these data, the effect of CORM-A1 in reducing seizure severity of CP/CPPS rats might be due to suppression of microglial activation and related neuroinflammation by inhibiting the activation of P2X4 receptors.
Zheng et al. (2019) showed that CORM-3 administration after spinal cord injury inhibited inflammasome activation and reduced pyroptosis, which is inflammation-associated cell death [37]. Lin et al. (2017) reported that CORM-3 suppressed IL-1β-induced inflammatory responses by increasing HO-1 expression in rat brain astrocytes [38]. Bani-Hani et al. (2006) reported that CORM-3 significantly reduced inflammatory responses in microglia cells [39]. Wang et al. (2018) showed that CORM-3 reduced neuronal damage and microglia activation and showed neuroprotective effect by protecting the blood-brain barrier in a transient middle cerebral artery occlusion model [11]. These studies, consistent with our findings, support that CORMs provide neuroprotective effects.
These CORMs effects could be the consequence of their role in redox homeostasis. It is known that oxidative stress plays an important role in the pathophysiology of epilepsy, as well as that ROS and inflammation are mutually reinforcing processes in epileptogenesis [40]. On the other hand, it was shown that CORM-A1 molecules reduce oxidative stress and improve mitochondrial functions through Nrf2 activation [41] since Nrf2 transcription factor protects cells against oxidative stress by activating antioxidant genes and has a neuroprotective effect in both human and animal studies [42]. It was reported that CORM-2 increased the expression of heme oxygenase-1 (HO-1) by activating the Nrf2/ARE pathway and thus reduced TNF-α-induced inflammation. As a result, CORM-2 protected cells against oxidative stress by suppressing inflammation [43]. Also, CORM-3 was shown to reduce oxidative stress-induced cell damage and protect mitochondrial functions via the activation of the Nrf2/HO-1 pathway [44]. Actually, HO-1/CO system acts as an active intracellular defense mechanism in epileptic cells and provides a compensatory response to oxidative damage during epilepsy [10]. These studies suggest that activation of the Nrf2 pathway can provide both neuroprotective effects and slow down seizure progression by suppressing processes such as glial activation, inflammation, and mitochondrial dysfunction; in this context, the antioxidant and anti-inflammatory effects of CORMs via the Nrf2/HO-1 pathway support their potential as treatment candidates in epilepsy.
4.2. Limitations
Our study has several limitations. The use of a single, near-threshold dose of lindane (4 mg/kg) was based on prior dose-response studies demonstrating low seizure incidence in control animals. Pilot experiments in our laboratory confirmed that this dose produced minimal seizures in sham rats under our specific experimental conditions. Nonetheless, reliance on a single dose of lindane and time point limits our ability to generalize the findings to other seizure models, which should be explored in future work. In order to elucidate the exact mechanisms of herein observed outcomes in reducing seizure susceptibility in a model of CP/CPPS, further studies are needed with special emphasis on direct effects in the CNS, having in mind recent findings on direct effects on ion channels and CORMs properties (PMID 29500353). Further studies should also include spectral characterization of recorded EEG activity.
5. Conclusions
In our study, CORM-A1 significantly reduced severity of lindane-induced seizures in CP/CPPS rats, as well as number and duration of ictal periods in EEG, thus it reduces susceptibility to seizures in rats with CP/CPPS. CORM-A1 may be a potential therapeutic agent in reducing the brain hyberexcitability in model of CP/CPPS.
Author Contributions
Conceptualization, N.Š., O.S., A.R.-M. and D.H.; Methodology A.R.-M., DM., M.V. and D.H.; Validation N.Š. and D.H.; Formal Analysis N.Š., D.M., Z.N. and D.H.; Data Curation N.Š., A.R.-M., E.D., M.V. and D.H.; Writing—Original Draft Preparation, N.Š., N.E. and D.H.; Writing—Review & Editing O.S., D.M., M.V., E.D. and A.R.-M.; Visualization, N.Š., N.E., Z.N. and D.H.; Supervision, D.H. and O.S.; Funding Acquisition O.S. and D.H. All authors have read and agreed to the published version of the manuscript.
Funding
Ministry of Science, Innovation and Technological Development of the Republic of Serbia (NITRA, grant number 200110) supported this study. Additional funding was obtained from international grant No. FA4Lin by MNTR and TUBITAK. The corresponding author is MC member in EU COST Actions TEATIME (Action No. 20135) and ANDRONET (Action No. 20119). Erasmus mobility grant was awarded to N.E. Funders had no role in design of the study, interpretation of the results and its publication.
Institutional Review Board Statement
The animal study protocol was approved by the Local Ethics Committee (Approval No. 323-07-01339/2017-05/3) and conducted in full accordance with national and international standards for laboratory animal welfare.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Authors declare no conflict of interest.
Abbreviations
| CORMs | CO-releasing molecules |
| CO | Carbon monoxide |
| EEG | Electroencephalography |
| CNS | Central nervous system |
References
- Magistro, G.; Stief, C.G.; Wagenlehner, F.M.E. Chronische Prostatitis/chronisches Beckenschmerzsyndrom [Chronic prostatitis/chronic pelvic pain syndrome]. Urologe. Ausg. A 2020, 59, 739–748. [Google Scholar] [CrossRef]
- Pendegast, H.J.; Leslie, S.W.; Rosario, D.J. Chronic Prostatitis and Chronic Pelvic Pain Syndrome in Men. In StatPearls Internet; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- He, H.; Luo, H.; Qian, B.; Xu, H.; Zhang, G.; Zou, X.; Zou, J. Autonomic nervous system dysfunction is related to chronic prostatitis/chronic pelvic pain syndrome. World J. Mens. Health 2024, 42, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Sadler, K.E.; Kolber, B.J. Urine trouble: Alterations in brain function associated with bladder pain. J. Urol. 2016, 196, 24–32. [Google Scholar] [CrossRef]
- Deng, G.C.; Lu, M.; Zhao, Y.Y.; Yuan, Y.; Chen, G. Activated spinal astrocytes contribute to the later phase of carrageenan-induced prostatitis pain. J. Neuroinflamm. 2019, 16, 189. [Google Scholar] [CrossRef] [PubMed]
- Pontari, M.A.; Ruggieri, M.R. Mechanisms in prostatitis/chronic pelvic pain syndrome. J. Urol. 2004, 172, 839–845. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Liang, Z.; Zheng, Z.; Guo, Q.; Tian, M.; Yang, J.; Liu, X.; Zhu, X.; Liu, S. The role of HIF-1α/HO-1 pathway in hippocampal neuronal ferroptosis in epilepsy. iScience 2023, 26, 108098. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, D.; Fu, X.; Yu, L.; Lu, Z.; Gao, Y.; Liu, X.; Man, J.; Li, S.; Li, N.; et al. Carbon monoxide-releasing molecule-3 protects against ischemic stroke by suppressing neuroinflammation and alleviating blood-brain barrier disruption. J. Neuroinflamm. 2018, 15, 188. [Google Scholar] [CrossRef]
- Dingledine, R.; Varvel, N.H.; Ravizza, T.; Vezzani, A. Neuroinflammation in epilepsy: Cellular and molecular mechanisms. In Jasper’s Basic Mechanisms of the Epilepsies, 5th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Vezzani, A., Delgado-Escueta, A.V., Eds.; Oxford University Press: New York, NY, USA, 2024; Chapter 30. [Google Scholar]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Huang, Y.; Deng, Z.; Qi, S.; Zhang, W.; Liu, Y.; Tan, G. Blood brain barrier dysfunction in epilepsy: Mechanisms, therapeutic strategies and future orientation. Int. J. Mol. Med. 2025, 56, 136. [Google Scholar] [CrossRef]
- Šutulović, N.; Grubač, Ž.; Šuvakov, S.; Jovanović, Đ.; Puškaš, N.; Macut, Đ.; Marković, A.R.; Simić, T.; Stanojlović, O.; Hrnčić, D. Chronic prostatitis/chronic pelvic pain syndrome increases susceptibility to seizures in rats and alters brain levels of IL-1β and IL-6. Epilepsy Res. 2019, 153, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R. Anti-inflammatory activities of carbon monoxide-releasing molecules (CO-RMs) in the brain. Springerplus 2015, 4, L41. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Bueno, S.M.; Kalergis, A.M. Carbon monoxide down-modulates Toll-like receptor 4/MD2 expression on innate immune cells and reduces endotoxic shock susceptibility. Immunology 2015, 144, 321–332. [Google Scholar] [CrossRef]
- Baskin, V.; Eroglu, E.; Harmanci, N.; Erol, K. Antinociceptive, anxiolytic, and depression-like effects of hydrogen sulfide, nitric oxide, and carbon monoxide in rats and the role of opioidergic and serotonergic systems in antinociceptive activity. Fundam. Clin. Pharmacol. 2022, 36, 674–686. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Vo, T.T.T.; Lee, C.W.; Chen, Y.L.; Lin, W.N.; Cheng, H.C.; Vo, Q.C.; Lee, I.T. Carbon monoxide releasing molecule-2 attenuates angiotensin II-induced IL-6/Jak2/Stat3-associated inflammation by inhibiting NADPH oxidase- and mitochondria-derived ROS in human aortic smooth muscle cells. Biochem. Pharmacol. 2022, 198, 114978. [Google Scholar] [CrossRef]
- Liu, J.; Fedinec, A.L.; Leffler, C.W.; Parfenova, H. Enteral supplements of a carbon monoxide donor CORM-A1 protect against cerebrovascular dysfunction caused by neonatal seizures. J. Cereb. Blood Flow Metab. 2015, 35, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, H.; Pourcyrous, M.; Fedinec, A.L.; Liu, J.; Basuroy, S.; Leffler, C.W. Astrocyte-produced carbon monoxide and the carbon monoxide donor CORM-A1 protect against cerebrovascular dysfunction caused by prolonged neonatal asphyxia. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H978–H988. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Cover, H., Ed.; Elsevier: New York, NY, USA, 2006. [Google Scholar]
- Šutulović, N.; Grubač, Ž.; Šuvakov, S.; Jerotić, D.; Puškaš, N.; Macut, D.; Rašić-Marković, A.; Simić, T.; Stanojlović, O.; Hrnčić, D. Experimental Chronic Prostatitis/Chronic Pelvic Pain Syndrome Increases Anxiety-Like Behavior: The Role of Brain Oxidative Stress, Serum Corticosterone, and Hippocampal Parvalbumin-Positive Interneurons. Oxid. Med. Cell. Longev. 2021, 2021, 6687493. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Nallu, R.S. Development and characterisation of a novel animal model of prostate inflammation-induced chronic pelvic pain. Inflammopharmacology 2009, 17, 23–28. [Google Scholar] [CrossRef]
- Hrnčić, D.; Rašić-Marković, A.; Djuric, D.; Sušić, V.; Stanojlović, O. The role of nitric oxide in convulsions induced by lindane in rats. Food Chem. Toxicol. 2011, 49, 947–954. [Google Scholar] [CrossRef]
- Stanojlović, O.; Rasić-Marković, A.; Hrncić, D.; Susić, V.; Macut, D.; Radosavljević, T.; Djuric, D. Two types of seizures in homocysteine thiolactone-treated adult rats, behavioral and electroencephalographic study. Cell. Mol. Neurobiol. 2009, 29, 329–339. [Google Scholar] [CrossRef]
- Rana, A.; Musto, A.E. The role of inflammation in the development of epilepsy. J. Neuroinflamm. 2018, 15, 144. [Google Scholar] [CrossRef]
- Yu, G.; Wu, X.; Liu, Z.; Shi, M.; Fan, H.; Liu, Y.; Kuang, N.; Peng, S.; Lian, Z.; Huang, C.; et al. Genetic influence and neural pathways underlying the dose-response relationships between wearable-measured physical activity and mental health in adolescence. Psychiatry Res. 2025, 349, 116503. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, L.; Moran, M.P.; Norato, G.; Thomas, B.; Dick, A.D.; Wells, E.; Suslovic, W.; Bumbut, A.; Chamberlain, J.M.; Theodore, W.H.; et al. Differential activation of neuroinflammatory pathways in children with seizures: A cross-sectional study. Seizure 2021, 91, 150–158. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Keslar, K.S.; Gotwald, P.; Berglund, R.; Vij, S. Neuroinflammatory gene expression in chronic prostatitis/chronic pelvic pain syndrome patients: Insights into etiology and phenotype biology. Transl. Androl. Urol. 2021, 10, 3340–3347. [Google Scholar] [CrossRef]
- Wong, L.; Done, J.D.; Schaeffer, A.J.; Thumbikat, P. Experimental autoimmune prostatitis induces microglial activation in the spinal cord. Prostate 2015, 75, 50–59. [Google Scholar] [CrossRef]
- Villasana-Salazar, B.; Vezzani, A. Neuroinflammation microenvironment sharpens seizure circuit. Neurobiol. Dis. 2023, 178, 106027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, C.; Huang, D. Microglial activation: An important process in the onset of epilepsy. Am. J. Transl. Res. 2018, 10, 2877–2889. [Google Scholar] [PubMed]
- Kohno, K.; Tsuda, M. Role of microglia and P2X4 receptors in chronic pain. Pain Rep. 2021, 6, e864. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, R.; Luo, J.; Zhong, C.; Pan, J. Involvement of NOTCH1-mediated microglia activation in neuromodulation of chronic prostatitis-related pain. In Vivo 2024, 38, 691–698. [Google Scholar] [CrossRef]
- Wilkinson, W.J.; Kemp, P.J. The carbon monoxide donor, CORM-2, is an antagonist of ATP-gated, human P2X4 receptors. Purinergic Signal. 2011, 7, 57–64. [Google Scholar] [CrossRef]
- Zheng, G.; Zhan, Y.; Wang, H.; Luo, Z.; Zheng, F.; Zhou, Y.; Wu, Y.; Wang, S.; Wu, Y.; Xiang, G.; et al. Carbon monoxide releasing molecule-3 alleviates neuron death after spinal cord injury via inflammasome regulation. eBioMedicine 2019, 40, 643–654. [Google Scholar] [CrossRef]
- Lin, C.C.; Yang, C.C.; Hsiao, L.D.; Chen, S.Y.; Yang, C.M. Heme oxygenase-1 induction by carbon monoxide releasing molecule-3 suppresses interleukin-1β-mediated neuroinflammation. Front. Mol. Neurosci. 2017, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Bani-Hani, M.G.; Greenstein, D.; Mann, B.E.; Green, C.J.; Motterlini, R. A carbon monoxide-releasing molecule (CORM-3) attenuates lipopolysaccharide- and interferon-gamma-induced inflammation in microglia. Pharmacol. Rep. 2006, 58, 132–144. [Google Scholar] [PubMed]
- Fabisiak, T.; Patel, M. Crosstalk between neuroinflammation and oxidative stress in epilepsy. Front. Cell Dev. Biol. 2022, 10, 976953. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Jadeja, R.N.; Vyas, H.S.; Pandya, B.; Joshi, A.; Vohra, A.; Thounaojam, M.C.; Martin, P.M.; Bartoli, M.; Devkar, R.V. Carbon monoxide releasing molecule-A1 improves nonalcoholic steatohepatitis via Nrf2 activation mediated improvement in oxidative stress and mitochondrial function. Redox Biol. 2020, 28, 101314. [Google Scholar] [CrossRef]
- Carmona-Aparicio, L.; Pérez-Cruz, C.; Zavala-Tecuapetla, C.; Granados-Rojas, L.; Rivera-Espinosa, L.; Montesinos-Correa, H.; Hernández-Damián, J.; Pedraza-Chaverri, J.; Sampieri, A.; Coballase-Urrutia, E.; et al. Overview of Nrf2 as therapeutic target in epilepsy. Int. J. Mol. Sci. 2015, 16, 18348–18367. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Hsiao, L.D.; Cho, R.L.; Yang, C.M. CO-Releasing molecule-2 induces Nrf2/ARE-dependent heme oxygenase-1 expression suppressing TNF-α-induced pulmonary inflammation. J. Clin. Med. 2019, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Lin, B.H.; Zheng, G.; Tan, K.; Liu, G.Y.; Yao, Z.; Xie, J.; Chen, W.K.; Chen, L.; Xu, T.H.; et al. CORM-3 attenuates oxidative stress-induced bone loss via the Nrf2/HO-1 pathway. Oxid. Med. Cell. Longev. 2022, 2022, 5098358. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.