Lower Urinary Tract Symptoms in Uterine Myoma: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
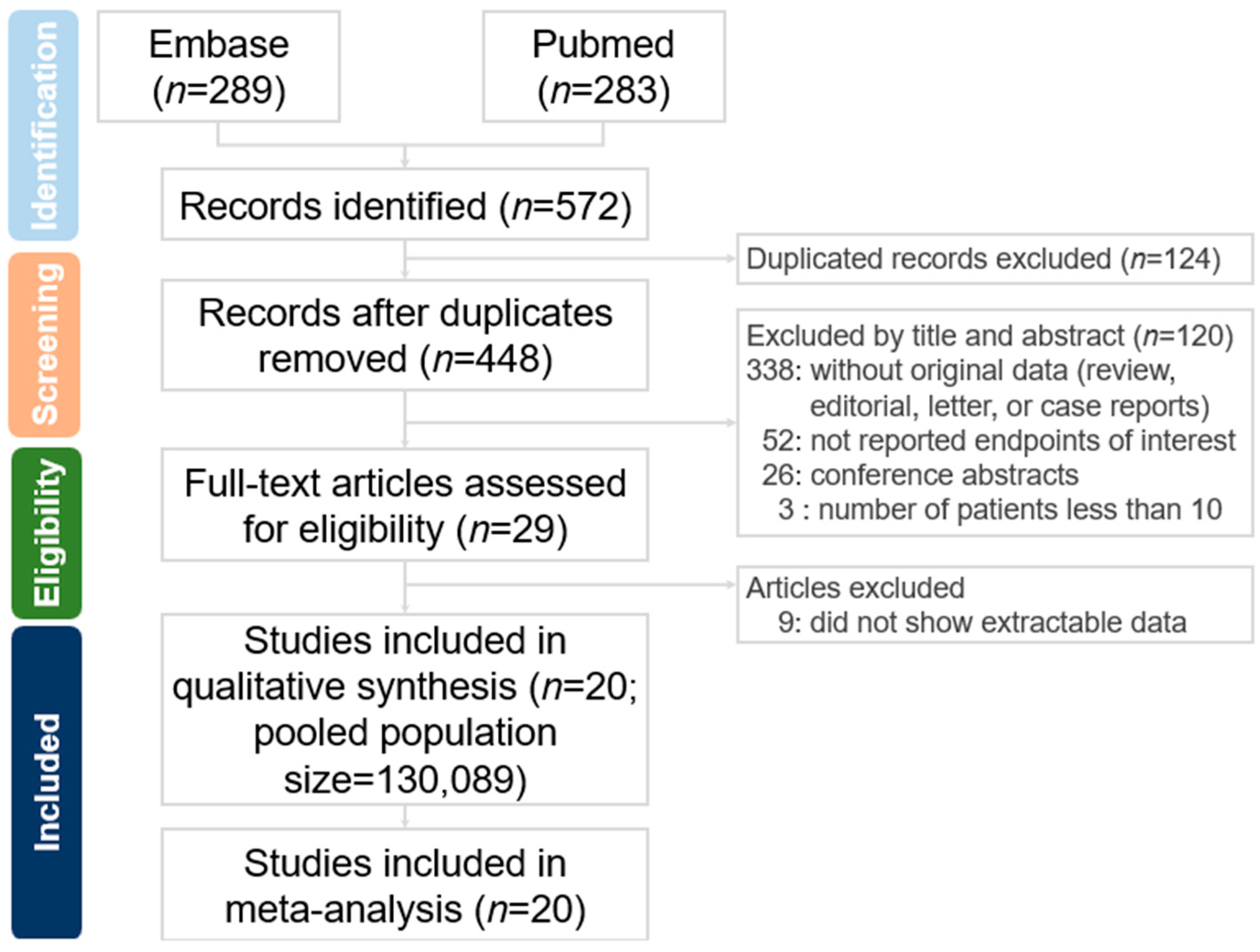
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Definition of Lower Urinary Tract Symptoms
2.3. Definition of Overactive Bladder
2.4. Data Abstraction
2.5. Data Analysis
3. Result
3.1. Overview of Enrolled Studies
3.2. Studies on LUTSs in UM Without Hysterectomy
3.3. Studies on LUTSs in UM After Hysterectomy
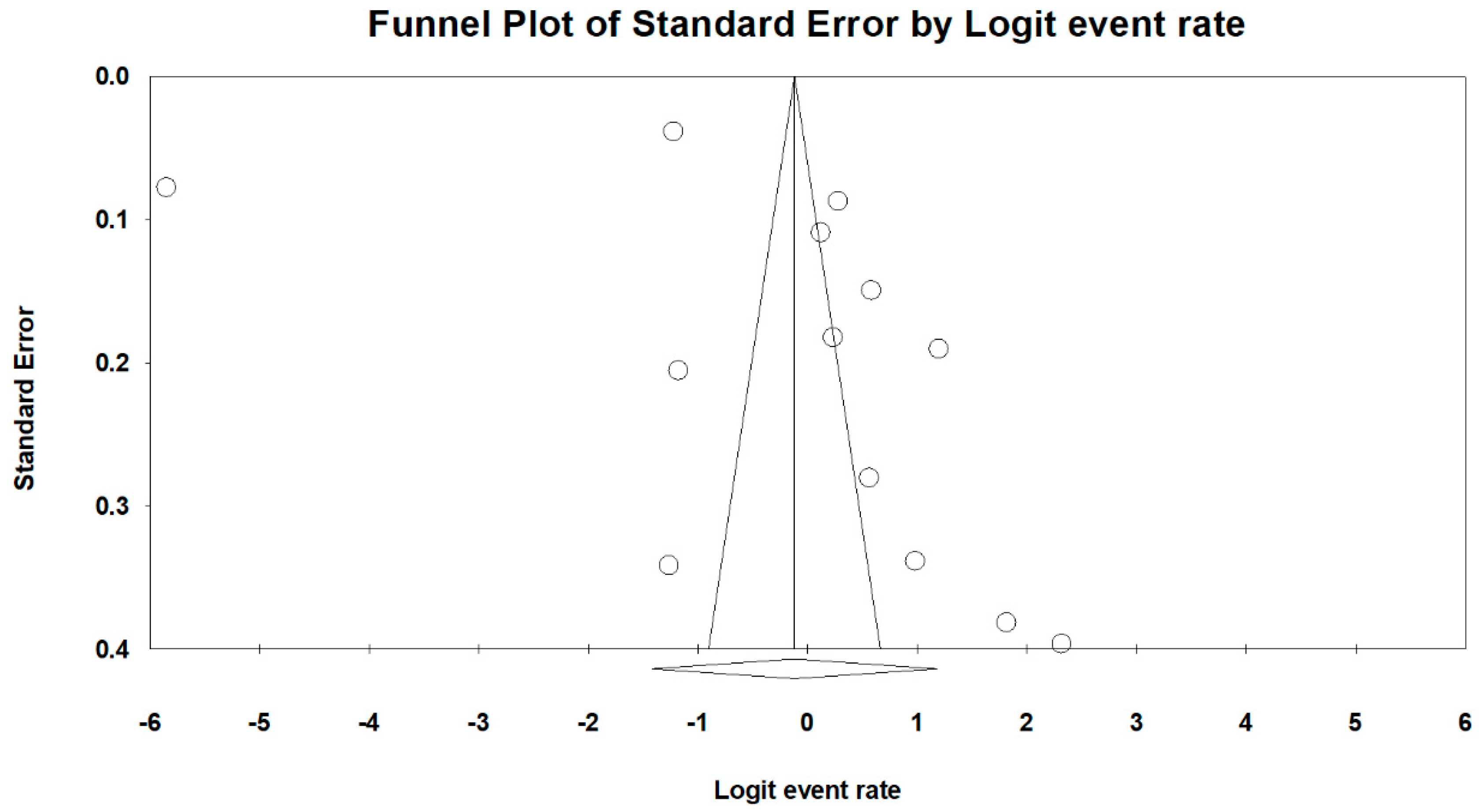
3.4. Risk Assessment of Bias
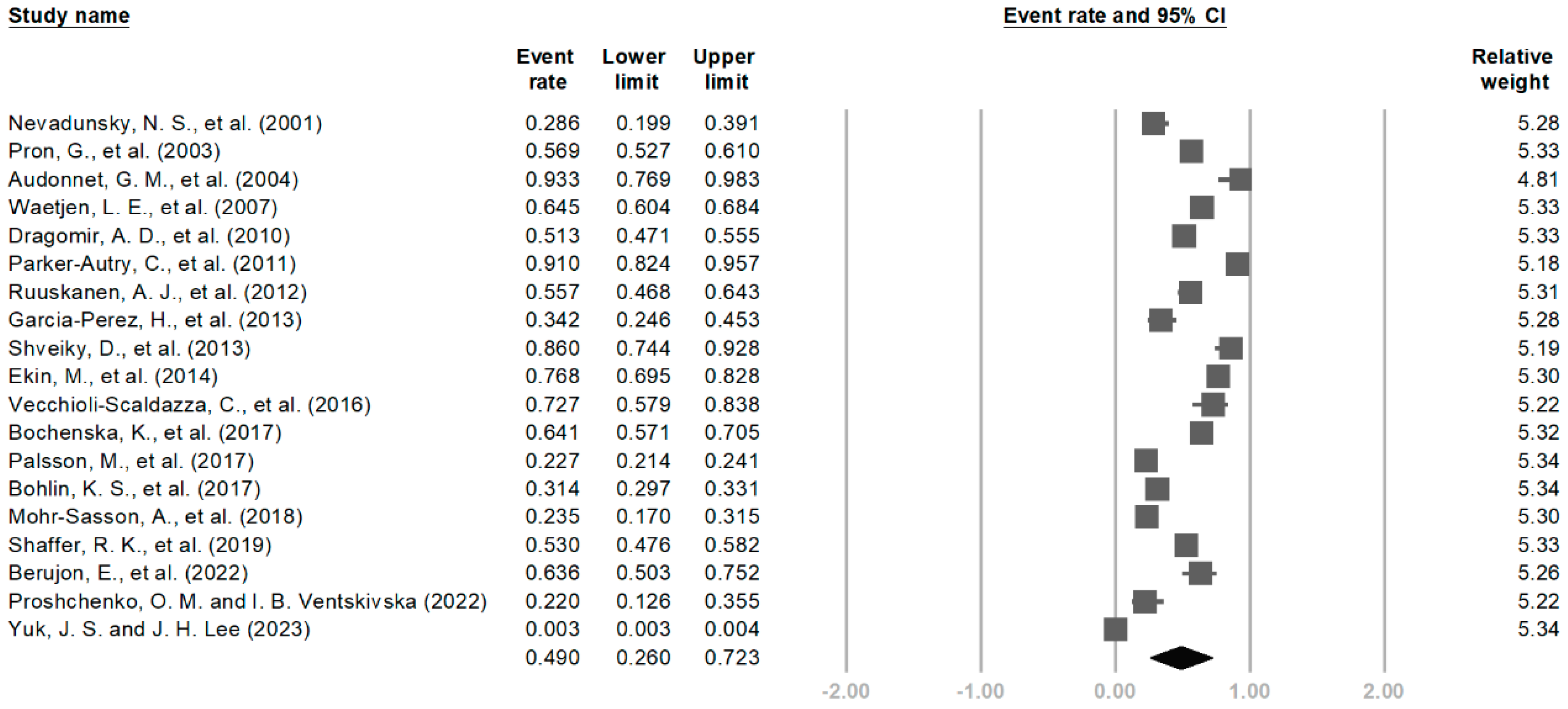
3.5. Meta-Analysis
4. Discussion
4.1. Prevalence of LUTSs in UM
4.2. Association with Hysterectomy and Other Interventions
4.3. Impact of UM Characteristics
4.4. Factors Influencing the Prevalence of LUTSs
4.5. LUTSs in Malignant vs. Benign Uterine Tumors
4.6. Emerging Evidence from Recently Published Studies
4.7. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giuliani, E.; As-Sanie, S.; Marsh, E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020, 149, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Huang, Y.; Li, S.; Luo, Z.; Li, C.; Chu, K.; Zhang, T.; Song, P.; Zhou, J. Global, regional, and national time trends in incidence, prevalence, years lived with disability for uterine fibroids, 1990–2019: An age-period-cohort analysis for the global burden of disease 2019 study. BMC Public Health 2023, 23, 916. [Google Scholar] [CrossRef] [PubMed]
- Sparic, R.; Mirkovic, L.; Malvasi, A.; Tinelli, A. Epidemiology of Uterine Myomas: A Review. Int. J. Fertil. Steril. 2016, 9, 424–435. [Google Scholar] [PubMed]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers 2016, 2, 16043. [Google Scholar] [CrossRef]
- Mourgues, J.; Villot, A.; Thubert, T.; Fauvet, R.; Pizzoferrato, A.C. Uterine myomas and lower urinary tract dysfunctions: A literature review. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 771–774. [Google Scholar] [CrossRef]
- Coyne, K.S.; Sexton, C.C.; Irwin, D.E.; Kopp, Z.S.; Kelleher, C.J.; Milsom, I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: Results from the EPIC study. BJU Int. 2008, 101, 1388–1395. [Google Scholar] [CrossRef]
- Kuo, H.-C. Videourodynamic Precision Diagnosis and Treatment of Lower Urinary Tract Symptoms in Women. Urol. Sci. 2021, 32, 94–101. [Google Scholar] [CrossRef]
- Andrada, A.O.; De Vicente, J.M.; Cidre, M.A. Pelvic plexus compression due to a uterine leiomyoma in a woman with acute urinary retention: A new hypothesis. Int. Urogynecol. J. 2014, 25, 429–431. [Google Scholar] [CrossRef]
- Parker, W.H. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil. Steril. 2007, 87, 725–736. [Google Scholar] [CrossRef]
- Parker-Autry, C.; Harvie, H.; Arya, L.A.; Northington, G.M. Lower urinary tract symptoms in patients with uterine fibroids: Association with fibroid location and uterine volume. Female Pelvic Med. Reconstr. Surg. 2011, 17, 91–96. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Leron, E.; Weintraub, A.Y.; Mastrolia, S.A.; Schwarzman, P. Overactive Bladder Syndrome: Evaluation and Management. Curr. Urol. 2018, 11, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Nevadunsky, N.S.; Bachmann, G.A.; Nosher, J.; Yu, T. Women’s decision-making determinants in choosing uterine artery embolization for symptomatic fibroids. J. Reprod. Med. 2001, 46, 870–874. [Google Scholar]
- Pron, G.; Bennett, J.; Common, A.; Wall, J.; Asch, M.; Sniderman, K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil. Steril. 2003, 79, 120–127. [Google Scholar] [CrossRef]
- Audonnet, G.M.; Valentini, F.A.; Nelson, P.P.; Breheret, J. Urodynamics and uterine fibroid. Is there a specific parameter? Ann. De Readapt. Et De Med. Phys. 2004, 47, 51–55. [Google Scholar] [CrossRef]
- Waetjen, L.E.; Liao, S.; Johnson, W.O.; Sampselle, C.M.; Sternfield, B.; Harlow, S.D.; Gold, E.B. Factors associated with prevalent and incident urinary incontinence in a cohort of midlife women: A longitudinal analysis of data: Study of women’s health across the nation. Am. J. Epidemiol. 2007, 165, 309–318. [Google Scholar] [CrossRef]
- Dragomir, A.D.; Schroeder, J.C.; Connolly, A.; Kupper, L.L.; Cousins, D.S.; Olshan, A.F.; Baird, D.D. Uterine leiomyomata associated with self-reported stress urinary incontinence. J. Women’s Health 2010, 19, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, A.J.; Hippeläinen, M.I.; Sipola, P.; Manninen, H.I. Association between magnetic resonance imaging findings of uterine leiomyomas and symptoms demanding treatment. Eur. J. Radiol. 2012, 81, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, H.; Harlow, S.D.; Sampselle, C.M.; Denman, C. Measuring urinary incontinence in a population of women in northern Mexico: Prevalence and severity. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2013, 24, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Shveiky, D.; Iglesia, C.B.; Antosh, D.D.; Kudish, B.I.; Peterson, J.; Huang, C.-C.; Spies, J.B. The effect of uterine fibroid embolization on lower urinary tract symptoms. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2013, 24, 1341–1345. [Google Scholar] [CrossRef]
- Ekin, M.; Cengiz, H.; Öztürk, E.; Kaya, C.; Yasar, L.; Savan, K. Genitourinary symptoms and their effects on quality of life in women with uterine myomas. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2014, 25, 807–810. [Google Scholar] [CrossRef]
- Vecchioli-Scaldazza, C.; Morosetti, C.; Vichi, M.; Bini, E.; Giannubilo, W.; Ferrara, V. Effect of Surgical Therapy on Urinary Symptoms and Urodynamic Findings in Patients with Uterine Myoma. J. Reprod. Med. 2016, 61, 436–440. [Google Scholar]
- Bochenska, K.; LeWitt, T.; Marsh, E.; Pidaparti, M.; Mendoza, G.; Lewicky-Gaupp, C.; Mueller, M.; Kenton, K. Fibroids and urinary symptoms study (FUSS). Am. J. Obstet. Gynecol. 2017, 216, S617. [Google Scholar] [CrossRef]
- Pålsson, M.; Stjerndahl, J.H.; Granåsen, G.; Löfgren, M.; Sundfeldt, K. Patient-reported lower urinary tract symptoms after hysterectomy or hysteroscopy: A study from the Swedish Quality Register for Gynecological Surgery. Int. Urogynecol. J. 2017, 28, 1341–1349. [Google Scholar] [CrossRef]
- Bohlin, K.S.; Ankardal, M.; Lindkvist, H.; Milsom, I. Factors influencing the incidence and remission of urinary incontinence after hysterectomy. Am. J. Obstet. Gynecol. 2017, 216, 53.e1–53.e9. [Google Scholar] [CrossRef]
- Mohr-Sasson, A.; Machtinger, R.; Mashiach, R.; Nir, O.; Inbar, Y.; Maliyanker, N.; Goldenberg, M.; Rabinovici, J. Long-term outcome of MR-guided focused ultrasound treatment and laparoscopic myomectomy for symptomatic uterine fibroid tumors. Am. J. Obstet. Gynecol. 2018, 219, 375.e1–375.e7. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, R.K.; Dobberfuhl, A.D.; Vu, K.-N.; Fast, A.M.; Dababou, S.; Marrocchio, C.; Lum, D.A.; Hovsepian, D.M.; Ghanouni, P.; Chen, B. Are fibroid and bony pelvis characteristics associated with urinary and pelvic symptom severity? Am. J. Obstet. Gynecol. 2019, 220, 471.e1–471.e11. [Google Scholar] [CrossRef] [PubMed]
- Berujon, E.; Thubert, T.; Fauvet, R.; Villot, A.; Pizzoferrato, A.C. Impact of uterine fibroid surgery on lower urinary tract symptoms. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102355. [Google Scholar] [CrossRef]
- Shin, J.H.; Gwak, C.H.; Park, M.U.; Choo, M.S. Effects of different types of hysterectomies on postoperative urodynamics and lower urinary tract symptoms. Investig. Clin. Urol. 2022, 63, 207–213. [Google Scholar] [CrossRef]
- Proshchenko, O.M.; Ventskivska, I.B. Efficiency of treatment and diagnostic algorithms in the rehabilitation program of women after hysterectomy with opportunist salpingectomy due to uterine myoma. Reprod. Endocrinol. 2022, 66, 90–97. [Google Scholar] [CrossRef]
- Yuk, J.S.; Lee, J.H. Risk of overactive bladder after hysterectomy for uterine fibroids. Int. Urogynecol. J. 2023, 34, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.; Granath, F.; Cnattingius, S.; Falconer, C. Hysterectomy and risk of stress-urinary-incontinence surgery: Nationwide cohort study. Lancet 2007, 370, 1494–1499. [Google Scholar] [CrossRef]
- Christoffersen, N.M.; Klarskov, N.; Gradel, K.O.; Husby, K.R. Increased risk of stress urinary incontinence surgery after hysterectomy for benign indication-a population-based cohort study. Am. J. Obstet. Gynecol. 2023, 229, 149.e1–149.e9. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Law, K.-S.; Tai, H.-P.; Chen, H.L.; Tse, S.S.; Huang, Z.M.; Weng, W.C.; Huang, L.H.; Lee, I.Y.; Tung, M.C. Management of Urinary Tract Injuries Following Total Hysterectomy: A Single-Hospital Experience. Urol. Sci. 2018, 29, 12–19. [Google Scholar] [CrossRef]
- Smith, P.H.; Ballantyne, B. The neuroanatomical basis for denervation of the urinary bladder following major pelvic surgery. Br. J. Surg. 1968, 55, 929–933. [Google Scholar] [CrossRef]
- Siddique, S.A.; Gutman, R.E.; Schon Ybarra, M.A.; Rojas, F.; Handa, V.L. Relationship of the uterosacral ligament to the sacral plexus and to the pudendal nerve. Int. Urogynecol. J. Pelvic Floor. Dysfunct. 2006, 17, 642–645. [Google Scholar] [CrossRef] [PubMed]
- DeLancey, J.O. Anatomic aspects of vaginal eversion after hysterectomy. Am. J. Obstet. Gynecol. 1992, 166 Pt 1, 1717–1724; discussion 24–28. [Google Scholar] [CrossRef] [PubMed]
- Arleo, E.K.; Tal, M.G. Fibroid-induced acute urinary retention: Treatment by uterine artery embolization. Int. Urogynecol. J. Pelvic Floor. Dysfunct. 2008, 19, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Houlihan, S.; Koenig, N.; Friedman, B.; Lee, T.; Geoffrion, R. Fibroid surgery and improvement in bladder symptoms: The FAB study. Neurourol. Urodyn. 2018, 37, 1965–1970. [Google Scholar] [CrossRef]
- Koch, M.; Rauchenwald, T.; Kivaranovic, D.; Schwab, S.; Umek, W.; Koelbl, H.; Rauchenwald, M.; Helmy, S. Association of uterine leiomyoma and overactive bladder syndrome. Int. J. Gynaecol. Obstet. 2018, 142, 365–369. [Google Scholar] [CrossRef]
- Rivlin, M.E.; McGehee, R.P.; Patel, R.B.; Meeks, G.R. Acute urinary retention due to a free-standing broad ligament leiomyoma. J. Miss. State Med. Assoc. 1992, 33, 355–357. [Google Scholar]
- Huang, S.; He, Q.; Zhao, J.; Choi, S.; Gong, H. Association of weight-adjusted waist index (WWI) with overactive bladder (OAB): A cross-sectional study from NHANES 2005–2018. Sci. Rep. 2025, 15, 13207. [Google Scholar] [CrossRef]
- Azadzoi, K.M.; Tarcan, T.; Kozlowski, R.; Krane, R.J.; Siroky, M.B. Overactivity and structural changes in the chronically ischemic bladder. J. Urol. 1999, 162, 1768–1778. [Google Scholar] [CrossRef]
- Monica, F.Z.; Antunes, E. Stimulators and activators of soluble guanylate cyclase for urogenital disorders. Nat. Rev. Urol. 2018, 15, 42–54. [Google Scholar] [CrossRef]
- Tala, M.R.Z.; Ficky, F.; Lubis, D.L.; Mirsya Warli, S. Efficacy of Body Weight Reduction in Improving Overactive Bladder Symptoms in Obese and Overweight Women: A Systematic Review. Nephro-Urol. Mon. 2024, 17, 154345. [Google Scholar] [CrossRef]
- Tung, M.-C.; Wu, C.-H.; Wu, R.C.; Kuo, W.W.T.; Mai, H.C.; Chen, S.H.; Chiang, C.Y.; Lin, V.C. Does Obesity Affect the Outcomes of Clinically Localized Prostate Cancer in the Era of Extraperitoneal Robot-Assisted Radical Prostatectomy? Urol. Sci. 2022, 33, 136–144. [Google Scholar] [CrossRef]
- Subak, L.L.; Wing, R.; West, D.S.; Franklin, F.; Vittinghoff, E.; Creasman, J.M.; Richter, H.E.; Myers, D.; Burgio, K.L.; Gorin, A.A.; et al. Weight loss to treat urinary incontinence in overweight and obese women. N. Engl. J. Med. 2009, 360, 481–490. [Google Scholar] [CrossRef]
- Burgio, K.L.; Richter, H.E.; Clements, R.H.; Redden, D.T.; Goode, P.S. Changes in urinary and fecal incontinence symptoms with weight loss surgery in morbidly obese women. Obstet. Gynecol. 2007, 110, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kang, M.Y. Psychosocial risk factors of lower urinary tract symptoms among working women. Int. J. Urol. 2025, 32, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Siu, K.C.; Lin, K.H. Impact of lower urinary tract symptoms on work productivity in female workers: A systematic review and meta-analysis. Neurourol. Urodyn. 2018, 37, 2323–2334. [Google Scholar] [CrossRef]
- Anderson, C.; Olshan, A.F.; Park, J.; Bae-Jump, V.L.; Brewster, W.R.; Lund, J.L.; Nichols, H.B. Adverse Urinary System Diagnoses among Older Women with Endometrial Cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1368–1375. [Google Scholar] [CrossRef]
- Erekson, E.A.; Sung, V.W.; DiSilvestro, P.A.; Myers, D.L. Urinary symptoms and impact on quality of life in women after treatment for endometrial cancer. Int. Urogynecol. J. Pelvic Floor. Dysfunct. 2009, 20, 159–163. [Google Scholar] [CrossRef]
- Agu, I.; Das, R.; Geller, E.J.; Carey, E.T.; Chu, C.M. Prevalence of Lower Urinary Tract Symptoms in Women Planning to Undergo Hysterectomy for Uterine Leiomyoma and Abnormal Uterine Bleeding. J. Womens Health 2024, 33, 798–804. [Google Scholar] [CrossRef]
- Yuk, J.S.; Cho, I.C.; Lee, J.H. The Risk of Stress Urinary Incontinence After Hysterectomy for Uterine Fibroids. Int. Neurourol. J. 2023, 27, 252–259. [Google Scholar] [CrossRef]
- Zhu, L.; Qiang, Z.; Liu, Y. Pelvic Floor Functional Exercise Improves Lower Urinary Tract Symptoms after Uterine Fibroid Surgery. Arch. Esp. Urol. 2024, 77, 345–352. [Google Scholar] [CrossRef]


| Variables | Number of Studies | Coefficient (95% CI) | Prevalence (95% CI) | p-Value | I2 (%) | |
|---|---|---|---|---|---|---|
| By age | 19 | −0.09 (−0.52, 0.33) | 0.49 (0.26, 0.72) | 0.67 | 99.82 | |
| By parity | 6 | 0.58 (−0.69, 1.84) | 0.50 (0.35, 0.65) | 0.37 | 99.17 | |
| By BMI | 9 | 0.51 (0.29, 0.73) | 0.63 (0.49, 0.75) | <0.001 | 96.51 | |
| WHO regions | European | 10 | Ref | 0.53 (0.42, 0.64) | Ref | 96.57 |
| Americas | 8 | 0.15 (−0.45, 0.74) | 0.56 (0.48, 0.64) | 0.62 | ||
| Western Pacific | 1 | −5.85 (−7.01, −4.61) | 0.003 (0.003, 0.004) | <0.001 | ||
| Eastern Mediterranean | - | - | - | - | ||
| South-East Asia | - | - | - | - | ||
| African | - | - | - | - | ||
| Risk of bias | Low | 17 | Ref | 0.48 (0.24, 0.74) | Ref | 99.81 |
| Some concerns | 3 | 0.20 (−2.56, 2.95) | 0.53 (0.23, 0.80) | 0.89 | ||
| High | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.-H.; Tsai, L.-H. Lower Urinary Tract Symptoms in Uterine Myoma: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 890. https://doi.org/10.3390/medicina61050890
Tan L-H, Tsai L-H. Lower Urinary Tract Symptoms in Uterine Myoma: A Systematic Review and Meta-Analysis. Medicina. 2025; 61(5):890. https://doi.org/10.3390/medicina61050890
Chicago/Turabian StyleTan, Lek-Hong, and Li-Hsien Tsai. 2025. "Lower Urinary Tract Symptoms in Uterine Myoma: A Systematic Review and Meta-Analysis" Medicina 61, no. 5: 890. https://doi.org/10.3390/medicina61050890
APA StyleTan, L.-H., & Tsai, L.-H. (2025). Lower Urinary Tract Symptoms in Uterine Myoma: A Systematic Review and Meta-Analysis. Medicina, 61(5), 890. https://doi.org/10.3390/medicina61050890






