Abstract
Background and Objectives: Acute limb ischemia (ALI) is a life-threatening vascular emergency that requires immediate intervention to restore perfusion and prevent limb loss or mortality. Management strategies primarily include thrombolysis and surgical revascularization, each with distinct risks and benefits. This review evaluates and compares the outcomes of thrombolysis and surgical revascularization in ALI management, emphasizing their efficacy, safety, and patient selection criteria. Materials and Methods: A systematic review was conducted in adherence to PRISMA guidelines, analyzing data from 15 studies, including randomized controlled trials and large retrospective analyses, encompassing over 3500 patients with varying demographics and clinical presentations. Study quality was assessed using the Cochrane risk of bias tool and the Newcastle–Ottawa Scale. Results: Thrombolysis, utilizing agents such as urokinase or recombinant tissue plasminogen activator (rt-PA), demonstrated limb salvage rates up to 90% in acute cases, with 30-day mortality rates of 4–6%. It was particularly effective in patients with embolic occlusions or short symptom durations. However, bleeding complications associated with thrombolysis were reported in up to 47% of cases. Conversely, surgical revascularization remains crucial for those with advanced ischemia or contraindications to thrombolysis, offering reliable perfusion restoration but with higher perioperative morbidity, especially in older patients with significant comorbidities. Recent advancements, including hybrid approaches combining catheter-directed thrombolysis with percutaneous mechanical thrombectomy, have shown promise in improving outcomes by reducing procedure times and enhancing clot resolution. Conclusions: While thrombolysis and surgical revascularization are effective, optimizing patient selection remains a key challenge. Future research should focus on refining treatment algorithms, investigating novel thrombolytic agents, and expanding the role of minimally invasive techniques to improve long-term outcomes while mitigating complications such as bleeding and reperfusion injuries.
1. Introduction
Acute limb ischemia (ALI) is a critical vascular emergency defined by a rapid drop in arterial blood flow, which can lead to limb loss or death without immediate intervention [1]. Embolism, thrombosis, or trauma typically causes this condition, manifesting with the classic “6 Ps”: pain, pallor, pulselessness, paresthesia, paralysis, and poikilothermia [1,2]. It necessitates immediate intervention to restore blood flow and reduce ischemic damage [3]. ALI often results from peripheral artery disease (PAD), a chronic condition marked by progressive atherosclerotic narrowing of the peripheral arteries. This condition presents a considerable challenge for vascular surgeons as the management of patients becomes increasingly intricate [1,3].
Historically, surgical revascularization procedures, encompassing thrombectomy, embolectomy, and bypass grafting, have constituted the foundation of ALI management [4,5]. Although these surgical techniques demonstrate effectiveness, they frequently present considerable perioperative risks, including wound complications, infection, and a heightened mortality rate, particularly among older individuals or those with comorbidities [6].
Over the past few decades, thrombolysis or endovascular treatment has emerged as a less invasive alternative to surgery [7,8,9,10]. These techniques provide numerous advantages, including the potential for clot dissolution in distal arterial beds and the identification and treatment of underlying lesions, while contributing to a reduced prevalence of long-term mortality and morbidity [9]. Several studies, such as the crucial TOPAS (Thrombolysis or Peripheral Arterial Surgery) trials [11], have demonstrated that thrombolysis effectively enhances limb salvage and decreases mortality rates in specific patient groups.
While thrombolysis offers several benefits, it carries certain risks, notably bleeding complications such as intracranial hemorrhage [12]. Furthermore, Kwok et al. [13] show that using percutaneous aspiration thrombectomy as a primary treatment reduces the need for thrombolysis [11], thereby reducing the associated risk of bleeding. Nonetheless, although non-surgical treatments benefit patients with ALI, the meta-analysis by Enezate et al. [14], which examined five prospective randomized trials and one retrospective observational study, showed no differences in short-term and one-year mortality and amputation rates when comparing patients who underwent endovascular treatment to those who received surgical intervention.
Recent advancements in endovascular techniques have significantly enhanced the management of ALI [15,16,17,18]. Hybrid approaches, which combine thrombolysis with mechanical thrombectomy or surgical revascularization, demonstrate considerable potential for improving clinical outcomes by decreasing procedure duration and optimizing clot removal [16]. Incorporating these techniques into clinical practice has transitioned the paradigm of ALI management towards more personalized and minimally invasive strategies [16,17,18].
Given the ongoing debate and developing therapeutic options, there is an urgent need to consolidate new research on the comparative efficacy and safety of thrombolysis vs. surgical revascularization in ALI. This systematic review and pairwise meta-analysis primarily aims to evaluate the comparative effectiveness of thrombolysis versus surgical revascularization in treating ALI, focusing on outcomes such as limb salvage, mortality, and complications. By reviewing data from fifteen crucial studies, this research intends to gain a comprehensive understanding of the changing treatment landscape and to offer insights into enhancing care for ALI patients.
2. Materials and Methods
This systematic review evaluates the efficacy and safety of thrombolysis versus surgical revascularization in the management of ALI. The study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18]. We registered our study in the PROSPERO database with the CRD420251019298 number.
2.1. Search Strategy
A comprehensive literature search was conducted in PubMed, Scopus, and Web of Science for studies published from 1990 to 2024 (Figure 1). Keywords included “acute limb ischemia”, “thrombolysis”, “surgical revascularization”, “limb salvage”, and “mortality”. The reference lists of the included studies were also reviewed to identify additional relevant articles.
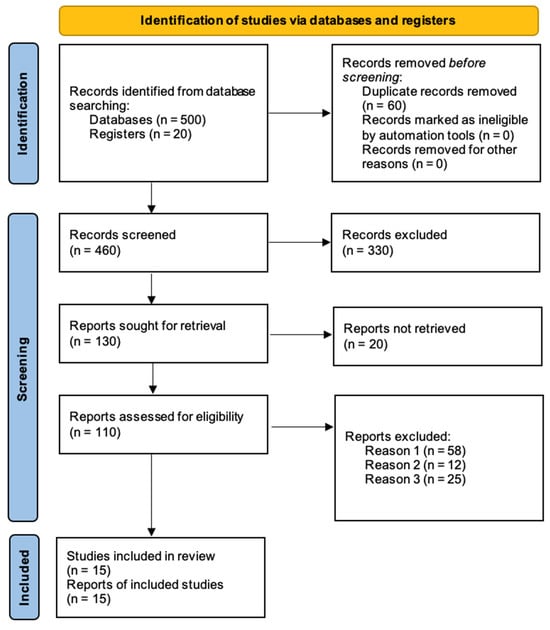
Figure 1.
PRISMA flow diagram for new systematic reviews, summarizing the study selection process (reason 1 = irrelevant outcomes; reason 2 = insufficient data; reason 3 = case reports).
2.2. Inclusion and Exclusion Criteria
Inclusion criteria:
- -
- Studies comparing thrombolysis and surgical revascularization in ALI.
- -
- Reported outcomes: limb salvage, mortality, complication rates.
- -
- Randomized controlled trials (RCTs), observational studies, and meta-analyses.
- -
- English language publications.
Exclusion criteria:
- -
- Case reports, editorials, and conference abstracts.
- -
- Studies with fewer than 20 patients.
- -
- Studies not reporting relevant outcomes.
2.3. Data Extraction and Quality Assessment
Two independent reviewers screened titles and abstracts, followed by full-text reviews. Discrepancies were resolved by consensus. Data were extracted on study design, sample size, patient demographics, intervention details, and outcomes [19]. The quality of the included studies was assessed using the Cochrane risk of bias tool [20] for RCTs and the Newcastle–Ottawa Scale [21] for observational studies.
2.4. Statistical Analysis
Descriptive statistics summarized study characteristics and outcomes. Results are presented as narrative syntheses with tabulated summaries. A quantitative meta-analysis was undertaken to evaluate the pooled effect of thrombolysis in contrast to surgical revascularization for managing ALI. For each study, odds ratios (ORs) and 95% confidence intervals (CIs) were extracted or computed for significant outcomes, such as limb salvage, mortality, and complication rates. Data were analyzed using a random-effects model, owing to the anticipated clinical and methodological heterogeneity among the included studies. Forest plots were generated to illustrate the distribution and precision of effect estimates. Supplementary Material Table S1 offers a comprehensive statistical summary, encompassing study-level effect sizes, standard errors, confidence intervals, and weight distribution.
3. Results
Table 1 below provides a comprehensive overview of 15 studies on managing ALI. Each study examines critical patient demographics, clinical presentations, and treatment strategies.

Table 1.
Comprehensive overview of 15 studies on managing ALI.
Table 1 summarizes data from 15 studies, involving a total of 3646 patients, with Table 2 presenting the risk factors from each study. Patient ages varied across studies, with median values ranging from 63.7 to 74 years, indicating a predominantly older population, reflective of the age group commonly affected by peripheral arterial disease and ALI. The proportion of male participants ranged from 50% to 72%, with most studies showing a slight male predominance. The largest cohort was reported by Baumgartner et al. [23] with 1738 patients, while Nilsson et al. [4] included only 20 patients.

Table 2.
Risk factors for the 15 studies on managing ALI.
The table highlights various treatment approaches, including thrombolysis, surgical revascularization, and hybrid procedures. Thrombolytic therapy utilizing agents such as rt-PA, urokinase, and alteplase was prevalent among the studies, with success rates in thrombus dissolution varying from 70% to 86%. Notably, Swischuk et al. [24] reported a 30-day amputation-free survival rate of 93%, whereas Conrad et al. [26] achieved successful lysis in 71% of treated patients. Mortality rates varied significantly, with some studies like Falkowski et al. [31] reporting a low mortality of 2.1%, while others such as Plate et al. [33] observed 21% mortality within one year.
Complication rates, including major bleeding and amputation, were consistently noted across studies. Swischuk et al. [24] reported major bleeding in 47% of patients, emphasizing the risks associated with thrombolytic therapy. The duration of hospital stays varied between 11 and 14 days in the studies that provided this metric, which underscores the intensive care necessary for these critical cases. Additionally, the research recorded comorbidities, indicating that hypertension (up to 88%) and diabetes (up to 49.2%) were common, emphasizing the intricate clinical profiles of these patients.
Table 3 summarizes the key findings from the 15 studies. Overall, thrombolytic therapy showed high success rates, with thrombus dissolution ranging from 70% to 86%, as reported in studies like Ouriel et al. [22] and Swischuk et al. [24]. Amputation-free survival rates were also notable, reaching up to 93.8% at 30 days in Falkowski et al. [31]. However, complications such as major bleeding were significant in some studies, with rates up to 47% in Swischuk et al. [24]. Surgical interventions were often more effective in chronic ischemic cases, as seen in STILE [8], where they outperformed thrombolysis for ischemia lasting more than 14 days. Mortality rates varied, with Weaver et al. [34] reporting 10.7% mortality in the thrombolysis group compared to 14.9% in the surgical group, highlighting comparable long-term outcomes between the two approaches.

Table 3.
Key findings from each study.
The forest plot presents the OR and CI for the studies included in this review (Figure 2). Notably, studies such as Ouriel et al. [22] (OR: 4.85; CI: 2.39–9.81, p = 0.001) and Swischuk et al. [24] (OR: 5.97; CI: 0.11–312.68, p = 0.38) show wide confidence intervals, suggesting a high variability. Meanwhile, Nilsson et al. [4] have a narrower CI (0.26–0.86) and a statistically significant result (p = 0.01), indicating more precise estimates. The overall effect has an OR of 1.33 (95% CI: 0.56–3.15), with a p-value of 0.52, indicating no statistically significant impact at the group level. Study weights range from 3.60% to 13.74%, highlighting the varying influence of each study on the overall analysis.
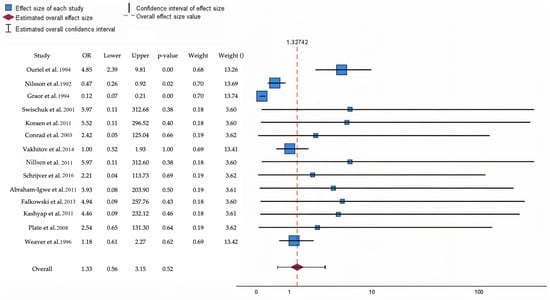
Figure 2.
The forest plot illustrates the odds ratio and predicts poor outcomes at follow-up for patients undergoing thrombolytic therapy compared to those receiving open surgical interventions [4,8,22,24,25,26,27,28,29,30,31,32,33,34]. Blue squares denote specific study estimates (size indicates study weight), while horizontal black lines illustrate 95% confidence intervals. The burgundy diamond denotes the pooled estimate, and the red dashed line indicates the summary risk ratio. The black horizontal line signifies the prediction interval, which reflects the anticipated range of effects in future studies.
In Supplementary Material Table S1 the study provides a detailed statistical summary of the effect sizes, standard errors, and confidence intervals for the aforementioned studies, along with their respective weights in the analysis. Notably, studies like Ouriel et al. [22] report a significant positive effect size (1.578, p < 0.001), indicating a strong intervention effect, with a relatively narrow CI (0.873 to 2.283). Conversely, STILE [8] demonstrates a significant negative effect size (−2.106, p < 0.001), having a poorer outcome in the context of the intervention. Studies such as Swischuk et al. [24] and Koraen et al. [25] have wide CI, indicating variability and less precision in their effect estimates, with non-significant p-values. The overall weights vary, with higher weights assigned to studies like Nilsson et al. [4] and Weaver et al. [34] (~13.4%), emphasizing their larger contribution to the meta-analysis.
4. Discussion
The comparative analysis of thrombolysis and surgical revascularization for acute limb ischemia highlights distinct outcomes critical in guiding clinical decision-making. This study evaluated over 3500 patients from 15 studies, revealing significant differences in limb salvage, mortality, and complication rates. The findings align with previous reports emphasizing the efficacy of thrombolysis in achieving superior limb salvage, particularly in patients presenting with embolic occlusions or symptoms of short duration [35,36].
Thrombolysis demonstrated remarkable success, with limb salvage rates exceeding 90% in several studies, as highlighted in Swischuk et al. [24], where the amputation-free survival at 30 days reached 93%. Similarly, Falkowski et al. [31] reported a thrombolytic success rate of 83.5% and a low mortality of 2.1%. This corroborates findings from previous meta-analyses showing that CDT significantly reduces the need for major amputations [37,38]. However, the risk of bleeding complications remains a concern, as evidenced by a 47% rate of major bleeding as reported by Swischuk et al. [24], consistent with literature citing bleeding as a major drawback of thrombolytic therapy [39,40].
On the other hand, surgical revascularization displayed higher perioperative risks but remained the treatment of choice for patients with advanced ischemia or contraindications to thrombolysis. For instance, the STILE trial [8] demonstrated that surgical intervention was more effective for patients with ischemia duration exceeding 14 days, achieving superior long-term outcomes in this setting. Mortality rates in surgical cohorts were generally higher, ranging from 10.7% in Weaver et al. [34] to 21% in Plate et al. [33]. This reflects the risks of open vascular procedures, particularly in comorbid and elderly patients.
Recent hybrid approaches combining thrombolysis with surgical or mechanical thrombectomy have emerged as promising alternatives [41,42]. These methods aim to leverage the benefits of minimally invasive thrombolysis while addressing its limitations through adjunctive mechanical interventions [43,44]. Studies such as Vakhitov et al. [27] feature the utility of hybrid strategies, showing 77% thrombolysis success with additional endovascular or surgical procedures in a significant proportion of cases. This aligns with emerging evidence suggesting that hybrid interventions can improve clot resolution and reduce procedural time [45,46]. The present analysis also revealed a trend of improved outcomes in studies employing newer thrombolytic agents and advanced catheter systems. The TOPAS trial [10] previously demonstrated that rt-PA was superior to older agents like urokinase in terms of efficacy and safety.
Furthermore, beyond acute clinical results, it is critical to examine long-term quality of life and functional recovery after revascularization. Kahn et al. [47] found that patients with catheter-directed thrombolysis had superior walking distances and health-related quality of life scores than those undergoing surgical bypass. Furthermore, sex-based and age-related outcome discrepancies have been noted in recent investigations, with older females having lower limb salvage and survival rates, indicating the necessity for individualized therapy regimens [48,49].
In terms of cost-effectiveness, thrombolysis is often associated with lower initial hospitalization expenditures due to shorter ICU stays and fewer surgical procedures. Health economics research by Vaidya et al. [50] found that while thrombolysis incurs greater pharmacologic costs, it resulted in lower overall in-hospital expenditures than surgery in selected individuals.
Managing ALI presents a complex clinical challenge, influenced by the variety of patient presentations and comorbidities. Although the aforementioned studies thoroughly evaluated thrombolysis against open surgery, recent findings indicate that a personalized approach, tailored to each patient’s unique characteristics, might yield better results. For instance, improvements in pharmacomechanical devices and the use of lower-dose thrombolytic therapies administered directly to lesions have allowed for more focused clot extraction, potentially minimizing bleeding risks while maintaining limb viability [51,52]. Emerging technologies like ultrasound-accelerated thrombolysis and microcatheter-guided delivery systems are promising in shortening reperfusion times while minimizing systemic complications [53,54,55]. Clinical insights from Schanzer et al. suggest that combining swift imaging protocols with prompt catheter-directed therapy enhances outcomes for patients with embolic ALI, emphasizing the importance of timely treatment [56]. Surgical revascularization is still critical, especially when thrombotic involvement coincides with severe pre-existing PAD featuring advanced stenotic lesions. Notably, hybrid approaches enable vascular surgeons to switch between endovascular and open techniques within the same session, proving especially advantageous in anatomically challenging or limb-threatening situations [57,58,59,60].
Strenghts and Limitations and Perspectives for Practical Application
This study provides a comprehensive review of thrombolysis and surgical revascularization in the management of acute limb ischemia, drawing from 15 diverse studies. One of its key strengths lies in its systematic approach, adhering to PRISMA guidelines to ensure robust study selection and data synthesis. The inclusion of a wide range of studies, from small cohorts to large-scale analyses, enables a detailed comparison of outcomes across different treatment modalities, including thrombolysis, surgical interventions, and hybrid approaches.
The detailed analysis of limb salvage, mortality, and complication rates strengthens the validity of the findings. Furthermore, the exploration of hybrid strategies reflects the evolving clinical landscape, emphasizing the need for personalized treatment approaches.
However, several limitations must be acknowledged. The included studies show heterogeneity in design, sample size, and outcome measures, which may introduce bias and limit direct comparability. Additionally, the lack of standardized reporting across studies complicates data synthesis, particularly for long-term outcomes such as re-intervention rates and functional recovery.
Despite these limitations, this study provides valuable insights into ALI management, highlighting the strengths and weaknesses of current treatment strategies and laying the groundwork for future research. Perspectives for practical application include the development of clinical algorithms for patient stratification, the incorporation of hybrid strategies into standard practice, and the further exploration of minimally invasive techniques. Tailoring treatment protocols based on ischemia duration, etiology (thrombotic vs. embolic), and comorbid conditions may enhance clinical outcomes and resource allocation.
5. Conclusions
This study points out the critical role of both thrombolysis and surgical revascularization in managing ALI. Thrombolysis demonstrated superior outcomes in terms of limb salvage, with success rates up to 90% in acute settings, as observed in multiple studies. Moreover, thrombolysis was associated with lower short-term mortality rates, ranging from 1% to 6%, highlighting its potential for minimizing systemic risks, particularly in patients with embolic occlusions or short ischemia duration. Conversely, surgical revascularization remains essential for patients with complex or chronic ischemic conditions. Although it presented higher perioperative mortality, ranging from 11% to 21%, it offered durable outcomes in specific patient cohorts, especially when ischemia extended beyond 14 days. These findings align with the existing literature and reaffirm the need for personalized therapeutic strategies. Future research should focus on refining patient selection criteria and leveraging advanced techniques to improve outcomes further.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina61050828/s1, Table S1. Effect size estimates for individual studies [4,8,22,24,25,26,27,28,29,30,31,32,33,34].
Author Contributions
Conceptualization M.C.C. and S.V.Ș.; Methodology M.C.C. and S.V.Ș.; Writing—original draft preparation, M.C.C. and S.V.Ș.; writing—review and editing, M.C.C. and N.A.L.; software, E.C.A.; validation, M.C.C., N.A.L., S.V.Ș., B.C.B., E.C.A., C.C., C.T., R.G., D.C.D. and A.V.M.; formal analysis, C.C., B.C.B., D.C.D. and E.C.A.; investigation, resources, and data curation, C.T. and R.G.; visualization, supervision, project administration, and funding acquisition, A.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the George Emil Palade University of Medicine, Pharmacy, Science and Technology of Târgu Mureș, Romania, research grant number 164/13/10.01.2023.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
This work was supported by the George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Târgu Mureș, Romania, with research grant number 164/13/10.01.2023. All authors have agreed with the acknowledgment.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALI | Acute limb ischemia |
| rt-PA | Recombinant tissue plasminogen activator |
| TOPAS | Thrombolysis or peripheral arterial surgery |
| RCT | Randomized controlled trial |
| AH | Arterial hypertension |
| DM | Diabetes mellitus |
| HLD | Hyperlipidemia |
| CAD | Coronary artery disease |
| OR | Odds ratio |
| CI | Confidence interval |
| CDT | Catheter-directed thrombolysis |
References
- Creager, M.A.; Kaufman, J.A.; Conte, M.S. Clinical Practice. Acute Limb Ischemia. N. Engl. J. Med. 2012, 366, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Stefanou, N.; Arnaoutoglou, C.; Papageorgiou, F.; Matsagkas, M.; Varitimidis, S.E.; Dailiana, Z.H. Update in Combined Musculoskeletal and Vascular Injuries of the Extremities. World J. Orthop. 2022, 13, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e726–e779. [Google Scholar] [CrossRef]
- Nilsson, L.; Albrechtsson, U.; Jonung, T.; Ribbe, E.; Thorvinger, B.; Thörne, J.; Astedt, B.; Norgren, L. Surgical Treatment versus Thrombolysis in Acute Arterial Occlusion: A Randomised Controlled Study. Eur. J. Vasc. Surg. 1992, 6, 189–193. [Google Scholar] [CrossRef]
- Björck, M.; Earnshaw, J.J.; Acosta, S.; Gonçalves, F.B.; Cochennec, F.; Debus, E.S.; Hinchliffe, R.; Jongkind, V.; Koelemay, M.J.W.; Menyhei, G.; et al. Editor’s Choice–European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Acute Limb Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 173–218. [Google Scholar] [CrossRef]
- El-Sayed, A.; Murali, N.; Lee, A.; Aziz, I.; Abdallah, A.; Stather, P. Outcomes of Surgical Revascularization for Acute Upper Limb Ischemia—A Single-Center Retrospective Analysis. Ann. Vasc. Surg. 2025, 110, 506–512. [Google Scholar] [CrossRef]
- Acosta, S.; Kulezic, A.; Zarrouk, M.; Gottsäter, A. Management of Acute Lower Limb Ischemia Without Surgical Revascularization–A Population-Based Study. Vasc. Endovascular Surg. 2024, 58, 316–325. [Google Scholar] [CrossRef]
- Graor, R.; Camerota, A.J.; Douville, Y.; Turpie, A.G.G. Results of a Prospective Randomized Trial Evaluating Surgery versus Thrombolysis for Ischemia of the Lower Extremity. The STILE Trial. Ann. Surg. 1994, 220, 251–266; discussion 266–268. [Google Scholar] [CrossRef]
- Shi, T.; Zhang, Y.; Shen, C.; Fang, J. A Single-Centre Protocol Using Low-Dose Urokinase for Catheter-Directed Thrombolysis in the Treatment of Acute Lower Limb Ischaemia. Vascular 2024, 32, 1143–1149. [Google Scholar] [CrossRef]
- Maheta, D.; Desai, D.; Agrawal, S.P.; Dani, A.; Frishman, W.H.; Aronow, W.S. Acute Limb Ischemia Management and Complications: From Catheter-Directed Thrombolysis to Long-Term Follow-Up. Cardiol. Rev. 2024, 10–1097. [Google Scholar] [CrossRef]
- Ouriel, K.; Veith, F.J.; Sasahara, A.A. A Comparison of Recombinant Urokinase with Vascular Surgery as Initial Treatment for Acute Arterial Occlusion of the Legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N. Engl. J. Med. 1998, 338, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Giannini, D.; Balbarini, A. Thrombolytic Therapy in Peripheral Arterial Disease. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004, 4, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.H.R.; Fleming, S.; Chan, K.K.C.; Tibballs, J.; Samuelson, S.; Ferguson, J.; Nadkarni, S.; Hockley, J.A.; Jansen, S.J. Aspiration Thrombectomy versus Conventional Catheter-Directed Thrombolysis as First-Line Treatment for Noniatrogenic Acute Lower Limb Ischemia. J. Vasc. Interv. Radiol. 2018, 29, 607–613. [Google Scholar] [CrossRef]
- Enezate, T.H.; Omran, J.; Mahmud, E.; Patel, M.; Abu-Fadel, M.S.; White, C.J.; Al-Dadah, A.S. Endovascular versus Surgical Treatment for Acute Limb Ischemia: A Systematic Review and Meta-Analysis of Clinical Trials. Cardiovasc. Diagn. Ther. 2017, 7, 264–271. [Google Scholar] [CrossRef]
- Ouriel, K.; Veith, F.J.; Sasahara, A.A. Thrombolysis or Peripheral Arterial Surgery: Phase I Results. TOPAS Investigators. J. Vasc. Surg. 1996, 23, 64–73; discussion 74–75. [Google Scholar] [CrossRef]
- Heller, S.; Lubanda, J.-C.; Varejka, P.; Chochola, M.; Prochazka, P.; Rucka, D.; Kuchynkova, S.; Horakova, J.; Linhart, A. Percutaneous Mechanical Thrombectomy Using Rotarex® S Device in Acute Limb Ischemia in Infrainguinal Occlusions. BioMed Res. Int. 2017, 2017, 2362769. [Google Scholar] [CrossRef]
- Kronlage, M.; Printz, I.; Vogel, B.; Blessing, E.; Müller, O.J.; Katus, H.A.; Erbel, C. A Comparative Study on Endovascular Treatment of (Sub)Acute Critical Limb Ischemia: Mechanical Thrombectomy vs Thrombolysis. DDDT 2017, 11, 1233–1241. [Google Scholar] [CrossRef]
- Liang, S.; Zhou, L.; Ye, K.; Lu, X. Limb Salvage After Percutaneous Mechanical Thrombectomy in Patients with Acute Lower Limb Ischemia: A Retrospective Analysis from Two Institutions. Ann. Vasc. Surg. 2019, 58, 151–159. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Vahedi, M.; Rahimzadeh, M. Sample Size Calculation in Medical Studies. Gastroenterol. Hepatol. Bed Bench 2013, 6, 14–17. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Gierisch, J.M.; Beadles, C.; Shapiro, A.; McDuffie, J.R.; Cunningham, N.; Bradford, D.; Strauss, J.; Callahan, M.; Chen, M.; Hemminger, A.; et al. Newcastle-Ottawa Scale Coding Manual for Cohort Studies. In Health Disparities in Quality Indicators of Healthcare Among Adults with Mental Illness; Department of Veterans Affairs (US): Washington, DC, USA, 2014. [Google Scholar]
- Ouriel, K.; Shortell, C.K.; DeWeese, J.A.; Green, R.M.; Francis, C.W.; Azodo, M.V.U.; Gutierrez, O.H.; Manzione, J.V.; Cox, C.; Marder, V.J. A Comparison of Thrombolytic Therapy with Operative Revascularization in the Initial Treatment of Acute Peripheral Arterial Ischemia. J. Vasc. Surg. 1994, 19, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, I.; Norgren, L.; Fowkes, F.G.R.; Mulder, H.; Patel, M.R.; Berger, J.S.; Jones, W.S.; Rockhold, F.W.; Katona, B.G.; Mahaffey, K.; et al. Cardiovascular Outcomes After Lower Extremity Endovascular or Surgical Revascularization: The EUCLID Trial. J. Am. Coll. Cardiol. 2018, 72, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Swischuk, J.L.; Fox, P.F.; Young, K.; Hussain, S.; Smouse, B.; Castañeda, F.; Brady, T.M. Transcatheter Intraarterial Infusion of Rt-PA for Acute Lower Limb Ischemia: Results and Complications. J. Vasc. Interv. Radiol. 2001, 12, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Koraen, L.; Kuoppala, M.; Acosta, S.; Wahlgren, C.-M. Thrombolysis for Lower Extremity Bypass Graft Occlusion. J. Vasc. Surg. 2011, 54, 1339–1344. [Google Scholar] [CrossRef]
- Conrad, M.F.; Shepard, A.D.; Rubinfeld, I.S.; Burke, M.W.; Nypaver, T.J.; Reddy, D.J.; Cho, J.-S. Long-Term Results of Catheter-Directed Thrombolysis to Treat Infrainguinal Bypass Graft Occlusion: The Urokinase Era. J. Vasc. Surg. 2003, 37, 1009–1016. [Google Scholar] [CrossRef]
- Vakhitov, D.; Suominen, V.; Korhonen, J.; Oksala, N.; Salenius, J.-P. Independent Factors Predicting Early Lower Limb Intra-Arterial Thrombolysis Failure. Ann. Vasc. Surg. 2014, 28, 164–169. [Google Scholar] [CrossRef]
- Løkse Nilssen, G.A.; Svendsen, D.; Singh, K.; Nordhus, K.; Sørlie, D. Results of Catheter-Directed Endovascular Thrombolytic Treatment of Acute Ischaemia of the Leg. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 91–96. [Google Scholar] [CrossRef]
- Schrijver, A.M.; de Vries, J.-P.P.M.; van den Heuvel, D.A.F.; Moll, F.L. Long-Term Outcomes of Catheter-Directed Thrombolysis for Acute Lower Extremity Occlusions of Native Arteries and Prosthetic Bypass Grafts. Ann. Vasc. Surg. 2016, 31, 134–142. [Google Scholar] [CrossRef]
- Abraham-Igwe, C.U.; Siddiqui, M.R.S.; Geddes, L.T.; Halls, J.; Irvine, A.; Browning, N. A Retrospective Study Examining Thrombolysis for Occluded Femoro-Popliteal Grafts-Is It Worthwhile? Int. J. Surg. 2011, 9, 632–635. [Google Scholar] [CrossRef]
- Falkowski, A.; Poncyljusz, W.; Samad, R.A.; Mokrzyński, S. Safety and Efficacy of Ultra-High-Dose, Short-Term Thrombolysis with Rt-PA for Acute Lower Limb Ischemia. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 118–123. [Google Scholar] [CrossRef]
- Kashyap, V.S.; Gilani, R.; Bena, J.F.; Bannazadeh, M.; Sarac, T.P. Endovascular Therapy for Acute Limb Ischemia. J. Vasc. Surg. 2011, 53, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Plate, G.; Oredsson, S.; Lanke, J. When Is Thrombolysis for Acute Lower Limb Ischemia Worthwhile? Eur. J. Vasc. Endovasc. Surg. 2009, 37, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Weaver, F.A.; Comerota, A.J.; Youngblood, M.; Froehlich, J.; Hosking, J.D.; Papanicolaou, G.; STILE Investigators. Surgical Revascularization versus Thrombolysis for Nonembolic Lower Extremity Native Artery Occlusions: Results of a Prospective Randomized Trial. J. Vasc. Surg. 1996, 24, 513–523. [Google Scholar] [CrossRef]
- Maldonado, T.S.; Powell, A.; Wendorff, H.; Rowse, J.; Nagarsheth, K.H.; Dexter, D.J.; Dietzek, A.M.; Muck, P.E.; Arko, F.R.; Chung, J.; et al. Safety and Efficacy of Mechanical Aspiration Thrombectomy for Patients with Acute Lower Extremity Ischemia. J. Vasc. Surg. 2024, 79, 584–592.e5. [Google Scholar] [CrossRef]
- Dammavalam, V.; Lin, S.; Nessa, S.; Daksla, N.; Stefanowski, K.; Costa, A.; Bergese, S. Neuroprotection during Thrombectomy for Acute Ischemic Stroke: A Review of Future Therapies. Int. J. Mol. Sci. 2024, 25, 891. [Google Scholar] [CrossRef]
- Kim, T.I.; Mena, C.; Sumpio, B.E. The Role of Lower Extremity Amputation in Chronic Limb-Threatening Ischemia. Int. J. Angiol. 2020, 29, 149–155. [Google Scholar] [CrossRef]
- Beschorner, U.; Boehme, T.; Noory, E.; Bollenbacher, R.; Salm, J.; Mashayekhi, K.; Westermann, D.; Zeller, T. Catheter-Directed Thrombolysis in the Management of Thrombotic Peripheral Artery Occlusions—Acute and Mid-Term Clinical Outcomes. J. Clin. Med. 2024, 13, 5732. [Google Scholar] [CrossRef]
- Baig, M.U.; Bodle, J. Thrombolytic Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Shafi, I.; Devarapally, S.R.; Gupta, N. Catheter-Directed Thrombolysis of Pulmonary Embolism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Segun-Omosehin, O.; Nasser, M.L.; Nasr, J.; Shi, A.; Bourdakos, N.E.; Seneviratne, S.; Than, C.A.; Tapson, V.F. Safety and Efficacy of Catheter-Directed Thrombectomy without Thrombolysis in Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2025, 420, 132707. [Google Scholar] [CrossRef]
- Zheng, M.; Li, L.; Chen, L.; Li, B.; Feng, C. Mechanical Thrombectomy Combined with Intravenous Thrombolysis for Acute Ischemic Stroke: A Systematic Review and Meta-Analyses. Sci. Rep. 2023, 13, 8597. [Google Scholar] [CrossRef]
- Saceleanu, V.M.; Toader, C.; Ples, H.; Covache-Busuioc, R.-A.; Costin, H.P.; Bratu, B.-G.; Dumitrascu, D.-I.; Bordeianu, A.; Corlatescu, A.D.; Ciurea, A.V. Integrative Approaches in Acute Ischemic Stroke: From Symptom Recognition to Future Innovations. Biomedicines 2023, 11, 2617. [Google Scholar] [CrossRef]
- Chen, S.; Fang, S.; Zhou, Y.; Huang, Z.; Yu, S.; Chen, D.; Wang, Z.; Xu, Y.; Liu, P.; Li, Y.; et al. A Low Bleeding Risk Thrombolytic Agent: citPA5. Cardiovasc. Res. 2024, 120, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Visualizing Thrombosis to Improve Thrombus Resolution. Res. Pract. Thromb. Haemost. 2021, 5, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.J.; Roy, T.L. Catheter-Directed Interventions for the Treatment of Lower Extremity Deep Vein Thrombosis. Life 2022, 12, 1984. [Google Scholar] [CrossRef]
- Kahn, S.R.; Julian, J.A.; Kearon, C.; Gu, C.S.; Cohen, D.J.; Magnuson, E.A.; Comerota, A.J.; Goldhaber, S.Z.; Jaff, M.R.; Razavi, M.K.; et al. Quality of Life after Pharmacomechanical Catheter-Directed Thrombolysis for Proximal Deep Vein Thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 8–23.e18. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Shu, C.; Zhao, J.; Dubois, L. The Effect of Gender on Outcomes after Lower Extremity Revascularization. J. Vasc. Surg. 2017, 65, 889–906.e4. [Google Scholar] [CrossRef]
- Chihade, D.B.; Lieb, K.R.; Wainwright, B.S.; Shaw, P.M. Sex-Related Disparities in Acute Limb Ischemia Treatment Outcomes. Ann. Vasc. Surg. 2023, 95, 133–141. [Google Scholar] [CrossRef]
- Vaidya, V.; Gangan, N.; Comerota, A.; Lurie, F. Cost-Effectiveness Analysis of Initial Treatment Strategies for Nonembolic Acute Limb Ischemia Using Real-Word Data. Ann. Vasc. Surg. 2017, 39, 276–283. [Google Scholar] [CrossRef]
- Erdoes, G.; Achermann, A.; Spahn, D.R. Update on management of acute limb ischemia. Vasa 2020, 49, 12–20. [Google Scholar]
- Auda, M.E.; Ratner, M.; Pezold, M.; Rockman, C.; Sadek, M.; Jacobowitz, G.; Berland, T.; Siracuse, J.J.; Teter, K.; Johnson, W.; et al. Short-term outcomes of endovascular management of acute limb ischemia using aspiration mechanical thrombectomy. Vascular 2024, 33, 34–41. [Google Scholar] [CrossRef]
- Chait, J.; Aurshina, A.; Marks, N.; Hingorani, A.; Ascher, E. Comparison of Ultrasound-Accelerated Versus Multi-Hole Infusion Catheter-Directed Thrombolysis for the Treatment of Acute Limb Ischemia. Vasc. Endovascular Surg. 2019, 53, 558–562. [Google Scholar] [CrossRef]
- Wang, Q.; Du, X.; Jin, D.; Zhang, L. Coupling magnetic torque and force for colloidal microbot assembly and transport. ACS Nano. 2022, 16, 262–271. [Google Scholar] [CrossRef]
- Doelare, S.A.N.; Jean Pierre, D.M.; Nederhoed, J.H.; Smorenburg, S.P.M.; Lely, R.J.; Jongkind, V.; Hoksbergen, A.W.J.; Ebben, H.P.; Yeung, K.K.; MUST Collaborators. Microbubbles and ultrasound accelerated thrombolysis for peripheral arterial occlusions: The outcomes of a single arm phase II trial. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Schanzer, A.; Messina, L.M. Evidence-based management of acute limb ischemia. Semin. Vasc. Surg. 2017, 29, 212–226. [Google Scholar] [CrossRef]
- Kwong, M.; Curtis, E.E.; Mell, M.W. The impact of hybrid revascularization strategies in the treatment of acute limb ischemia. Ann. Vasc. Surg. 2021, 72, 83–91. [Google Scholar] [CrossRef]
- Casian, D.; Predenciuc, A.; Culiuc, V. Clinical value of foot thermometry in patients with acute limb ischemia. Vascular 2025, 33, 58–65. [Google Scholar] [CrossRef]
- Konstantinou, N.; Argyriou, A.; Dammer, F.; Bisdas, T.; Chlouverakis, G.; Torsello, G.; Tsilimparis, N.; Stavroulakis, K. Outcomes After Open Surgical, Hybrid, and Endovascular Revascularization for Acute Limb Ischemia. J. Endovasc. Ther. 2023, 15266028231210232. [Google Scholar] [CrossRef]
- Arbănași, E.M.; Mureșan, A.V.; Coșarcă, C.M.; Kaller, R.; Bud, T.I.; Hosu, I.; Voidăzan, S.T.; Arbănași, E.M.; Russu, E. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio Impact on Predicting Outcomes in Patients with Acute Limb Ischemia. Life 2022, 12, 822. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).