Effects of Blood Flow Restriction Resistance Exercise Versus Traditional Resistance Exercise in Voluntary Exhaustion on Quadriceps Muscle Adaptations in Untrained Young Males: A Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Experimental Design
2.1.2. Participants
2.1.3. Procedures
2.2. Measurements
2.2.1. Height, Weight, and Body Mass Index Measurements
2.2.2. Blood Pressure and Heart Rate Measurements
2.2.3. Determination of Limb Occlusion Pressure
2.2.4. Determination of One-Repetition Maximum Strength
2.2.5. Leg Extension and Flexion Peak Power Test
2.2.6. Assessment of Thigh Circumference
2.2.7. Assessment of Muscle Thickness, Subcutaneous Fat Thickness, and Cross-Sectional Area
2.2.8. Assessments of Muscle Stiffness
2.2.9. Statistical Analyses
3. Results
3.1. Results of Baseline Tests, and Adherence to Intervention
3.2. Results of 1RM Muscle Strength and Muscle Power Tests
3.3. Results for Thigh Circumference and Subcutaneous Fat Thickness
3.4. Results for Muscle Thickness, Cross-Sectional Area, and Muscle Stiffness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BFR-RE | Resistance exercise with blood flow restriction |
| HL-RE | High-load resistance exercise |
| EMG | Electromyographic |
| CSA | Cross-sectional area |
| 1RM | One-repetition maximum |
| NSCA | National Strength and Conditioning Association |
| ECF | Extracellular fluid |
| MRI | Magnetic resonance imaging |
| ITT | Intent-to-treat |
| MCAR | Missing completely at random |
| ICC | Intraclass correlation coefficient |
| PPLEL | Peak power leg extension left |
| PPLER | Peak power leg extension right |
| PPLFR | Peak power leg flexion right |
| RFTL | Rectus femoris thickness on the left |
| RFTR | Rectus femoris thickness on the right |
| RFCSAL | Rectus femoris cross-sectional area on the left |
| RFCSAR | Rectus femoris cross-sectional area on the right |
| RFSL | Rectus femoris stiffness on the left |
| RFSR | Rectus femoris stiffness on the right |
References
- Scott, B.R.; Loenneke, J.P.; Slattery, K.M.; Dascombe, B.J. Exercise with Blood Flow Restriction: An Updated Evidence-Based Approach for Enhanced Muscular Development. Sports Med. 2015, 45, 313–325. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Wilson, J.M.; Marín, P.J.; Zourdos, M.C.; Bemben, M.G. Low Intensity Blood Flow Restriction Training: A Meta-Analysis. Eur. J. Appl. Physiol. 2012, 112, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Groennebaek, T.; Jespersen, N.R.; Jakobsgaard, J.E.; Sieljacks, P.; Wang, J.; Rindom, E.; Musci, R.V.; Bøtker, H.E.; Hamilton, K.L.; Miller, B.F.; et al. Skeletal Muscle Mitochondrial Protein Synthesis and Respiration Increase With Low-Load Blood Flow Restricted as Well as High-Load Resistance Training. Front. Physiol. 2018, 9, 1796. [Google Scholar] [CrossRef] [PubMed]
- Brandner, C.R.; Warmington, S.A.; Kidgell, D.J. Corticomotor Excitability Is Increased Following an Acute Bout of Blood Flow Restriction Resistance Exercise. Front. Hum. Neurosci. 2015, 9, 652. [Google Scholar] [CrossRef]
- Rolnick, N.; Kimbrell, K.; Cerqueira, M.S.; Weatherford, B.; Brandner, C. Perceived Barriers to Blood Flow Restriction Training. Front. Rehabil. Sci. 2021, 2, 697082. [Google Scholar] [CrossRef]
- Flocco, P.; Galeoto, G. Effect of Blood Flow Restriction Training on Physiological Outcomes in Healthy Athletes: A Systematic Review and Meta-Analysis. Muscle Ligaments Tendons J. 2021, 11, 101–117. [Google Scholar] [CrossRef]
- Herrington, L.; Ghulam, H.; Comfort, P. Quadriceps Strength and Functional Performance After Anterior Cruciate Ligament Reconstruction in Professional Soccer Players at Time of Return to Sport. J. Strength Cond. Res. 2021, 35, 769–775. [Google Scholar] [CrossRef]
- Culvenor, A.G.; Ruhdorfer, A.; Juhl, C.; Eckstein, F.; Elin Øiestad, B. Knee Extensor Strength and Risk of Structural, Symptomatic, and Functional Decline in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2017, 69, 649–658. [Google Scholar] [CrossRef]
- Miyamoto, N.; Wakahara, T.; Kawakami, Y. Task-Dependent Inhomogeneous Muscle Activities within the Bi-Articular Human Rectus Femoris Muscle. PLoS ONE 2012, 7, e34269. [Google Scholar] [CrossRef]
- Ema, R.; Sakaguchi, M.; Akagi, R.; Kawakami, Y. Unique Activation of the Quadriceps Femoris during Single- and Multi-Joint Exercises. Eur. J. Appl. Physiol. 2016, 116, 1031–1041. [Google Scholar] [CrossRef]
- Eftestøl, E.; Egner, I.M.; Lunde, I.G.; Ellefsen, S.; Andersen, T.; Sjåland, C.; Gundersen, K.; Bruusgaard, J.C. Increased Hypertrophic Response with Increased Mechanical Load in Skeletal Muscles Receiving Identical Activity Patterns. Am. J. Physiol. Cell Physiol. 2016, 311, C616–C629. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Fisher, J.P.; Grgic, J.; Haun, C.T.; Helms, E.R.; Phillips, S.M.; Steele, J.; Vigotsky, A.D.; Schoenfeld, B.J. Resistance Training Recommendations to Maximize Muscle Hypertrophy in an Athletic Population: Position Stand of the IUSCA. Int. J. Strength Cond. 2021, 1. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Grgic, J.; Ogborn, D.; Krieger, J.W. Strength and Hypertrophy Adaptations Between Low- vs. High-Load Resistance Training: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2017, 31, 3508–3523. [Google Scholar] [CrossRef]
- Lacio, M.; Vieira, J.G.; Trybulski, R.; Campos, Y.; Santana, D.; Filho, J.E.; Novaes, J.; Vianna, J.; Wilk, M. Effects of Resistance Training Performed with Different Loads in Untrained and Trained Male Adult Individuals on Maximal Strength and Muscle Hypertrophy: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 11237. [Google Scholar] [CrossRef]
- Haun, C.T.; Mumford, P.W.; Roberson, P.A.; Romero, M.A.; Mobley, C.B.; Kephart, W.C.; Anderson, R.G.; Colquhoun, R.J.; Muddle, T.W.D.; Luera, M.J.; et al. Molecular, Neuromuscular, and Recovery Responses to Light versus Heavy Resistance Exercise in Young Men. Physiol. Rep. 2017, 5, e13457. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, E.; Dönmez, G.; Uzuner, K.; Babayeva, N.; Torgutalp, Ş.Ş.; Özçakar, L. Effects of Blood Flow Restriction Training on Muscle Strength and Architecture. J. Strength Cond. Res. 2022, 36, 1396–1403. [Google Scholar] [CrossRef]
- Kamiş, O.; Grses, V.V.; Şendur, H.N.; Altunsoy, M.; Pekel, H.A.; Yildirim, E.; Aydos, L. Low-Load Resistance Exercise with Blood Flow Restriction Versus High-Load Resistance Exercise on Hamstring Muscle Adaptations in Recreationally Trained Men. J. Strength. Cond. Res. 2024, 38, e541–e552. [Google Scholar] [CrossRef]
- Baz-Valle, E.; Fontes-Villalba, M.; Santos-Concejero, J. Total Number of Sets as a Training Volume Quantification Method for Muscle Hypertrophy: A Systematic Review. J. Strength Cond. Res. 2021, 35, 870–878. [Google Scholar] [CrossRef]
- Refalo, M.C.; Helms, E.R.; Trexler, E.T.; Hamilton, D.L.; Fyfe, J.J. Influence of Resistance Training Proximity-to-Failure on Skeletal Muscle Hypertrophy: A Systematic Review with Meta-Analysis. Sports Med. 2023, 53, 649–665. [Google Scholar] [CrossRef]
- Buckner, S.L.; Moreno, E.N.; Baxter, H.T. The Dose-Response Relationship between Resistance Training Volume and Muscle Hypertrophy: There Are Still Doubts. J. Trainology 2023, 12, 29–36. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Inami, T.; Ishida, H.; Morito, A.; Yamada, S.; Nagata, N.; Murayama, M. The Effect of Prior Creatine Intake for 28 Days on Accelerated Recovery from Exercise-Induced Muscle Damage: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients 2024, 16, 896. [Google Scholar] [CrossRef]
- Haun, C.T.; Vann, C.G.; Mobley, C.B.; Roberson, P.A.; Osburn, S.C.; Holmes, H.M.; Mumford, P.M.; Romero, M.A.; Young, K.C.; Moon, J.R.; et al. Effects of Graded Whey Supplementation During Extreme-Volume Resistance Training. Front. Nutr. 2018, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; for the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.; Snarr, R. NSCA’s Essentials of Personal Training; Human Kinetics: Champaign, IL, USA, 2021. [Google Scholar]
- Korakakis, P.A.; Wolf, M.; Coleman, M.; Burke, R.; Piñero, A.; Nippard, J.; Schoenfeld, B.J. Optimizing Resistance Training Technique to Maximize Muscle Hypertrophy: A Narrative Review. J. Funct. Morphol. Kinesiol. 2023, 9, 9. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Fahs, C.A.; Rossow, L.M.; Sherk, V.D.; Thiebaud, R.S.; Abe, T.; Bemben, D.A.; Bemben, M.G. Effects of Cuff Width on Arterial Occlusion: Implications for Blood Flow Restricted Exercise. Eur. J. Appl. Physiol. 2012, 112, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.M.; Triplett, N.T. Program Design for Resistance Training. In Essentials of Strength Training and Conditioning; Human Kinetics: Champaign, IL, USA, 2016; pp. 451–462. Available online: https://www.sciepub.com/reference/453011 (accessed on 25 March 2025).
- Chen, Y.T.; Hsieh, Y.Y.; Ho, J.Y.; Lin, J.C. Effects of Running Exercise Combined With Blood Flow Restriction on Strength and Sprint Performance. J. Strength Cond. Res. 2021, 35, 3090–3096. [Google Scholar] [CrossRef]
- Berko, N.S.; Fitzgerald, E.F.; Amaral, T.D.; Payares, M.; Levin, T.L. Ultrasound Elastography in Children: Establishing the Normal Range of Muscle Elasticity. Pediatr. Radiol. 2014, 44, 158–163. [Google Scholar] [CrossRef]
- Wenz, H.; Dieckmann, A.; Lehmann, T.; Brandl, U.; Mentzel, H.J. Strain Ultrasound Elastography of Muscles in Healthy Children and Healthy Adults. Fortschr Röntgenstr 2019, 191, 1091–1098. [Google Scholar] [CrossRef]
- Ahn, E.; Kang, H. Intention-to-Treat versus as-Treated versus per-Protocol Approaches to Analysis. Korean J. Anesthesiol. 2023, 76, 531–539. [Google Scholar] [CrossRef]
- Little, R.J.A. A Test of Missing Completely at Random for Multivariate Data with Missing Values. J. Am. Stat. Assoc. 1988, 83, 1198–1202. [Google Scholar] [CrossRef]
- Andridge, R.R.; Little, R.J.A. A Review of Hot Deck Imputation for Survey Non-Response. Int. Stat. Rev. 2010, 78, 40–64. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef]
- Kush, J.M.; Konold, T.R.; Bradshaw, C.P. The Sampling Ratio in Multilevel Structural Equation Models: Considerations to Inform Study Design. Educ. Psychol. Meas. 2022, 82, 409–443. [Google Scholar] [CrossRef] [PubMed]
- Kruschke, J.K.; Liddell, T.M. The Bayesian New Statistics: Hypothesis Testing, Estimation, Meta-Analysis, and Power Analysis from a Bayesian Perspective. Psychon. Bull. Rev. 2018, 25, 178–206. [Google Scholar] [CrossRef]
- Kelter, R. Bayesian Alternatives to Null Hypothesis Significance Testing in Biomedical Research: A Non-Technical Introduction to Bayesian Inference with JASP. BMC Med. Res. Methodol. 2020, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- Dienes, Z. Using Bayes to Get the Most out of Non-Significant Results. Front. Psychol. 2014, 5, 781. [Google Scholar] [CrossRef]
- Lixandrão, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceição, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of Muscle Strength and Mass Adaptations Between High-Load Resistance Training Versus Low-Load Resistance Training Associated with Blood-Flow Restriction: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 361–378. [Google Scholar] [CrossRef]
- Centner, C.; Wiegel, P.; Gollhofer, A.; König, D. Effects of Blood Flow Restriction Training on Muscular Strength and Hypertrophy in Older Individuals: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 95–108. [Google Scholar] [CrossRef]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood Flow Restriction Training in Clinical Musculoskeletal Rehabilitation: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef]
- Grønfeldt, B.M.; Lindberg Nielsen, J.; Mieritz, R.M.; Lund, H.; Aagaard, P. Effect of Blood-Flow Restricted vs Heavy-Load Strength Training on Muscle Strength: Systematic Review and Meta-Analysis. Scand. J. Med. Sci. Sports 2020, 30, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Regalado, S.; Leon, J.; Padial, P.; Benavente, C.; Almeida, F.; Bonitch-Góngora, J.; de la Fuente, B.; Feriche, B. Exploring the Impact of Resistance Training at Moderate Altitude on Metabolic Cytokines in Humans: Implications for Adipose Tissue Dynamics. Int. J. Mol. Sci. 2024, 25, 11418. [Google Scholar] [CrossRef]
- Lorenz, D.S.; Bailey, L.; Wilk, K.E.; Mangine, R.E.; Head, P.; Grindstaff, T.L.; Morrison, S. Blood Flow Restriction Training. J. Athl. Train. 2021, 56, 937–944. [Google Scholar] [CrossRef]
- Viscor, G.; Torrella, J.R.; Corral, L.; Ricart, A.; Javierre, C.; Pages, T.; Ventura, J.L. Physiological and Biological Responses to Short-Term Intermittent Hypobaric Hypoxia Exposure: From Sports and Mountain Medicine to New Biomedical Applications. Front. Physiol. 2018, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Jessee, M.B.; Buckner, S.L.; Grant Mouser, J.; Mattocks, K.T.; Dankel, S.J.; Abe, T.; Bell, Z.W.; Bentley, J.P.; Loenneke, J.P. Muscle Adaptations to High-Load Training and Very Low-Load Training With and Without Blood Flow Restriction. Front. Physiol. 2018, 9, 1448. [Google Scholar] [CrossRef]
- Suga, T.; Okita, K.; Takada, S.; Omokawa, M.; Kadoguchi, T.; Yokota, T.; Hirabayashi, K.; Takahashi, M.; Morita, N.; Horiuchi, M.; et al. Effect of Multiple Set on Intramuscular Metabolic Stress during Low-Intensity Resistance Exercise with Blood Flow Restriction. Eur. J. Appl. Physiol. 2012, 112, 3915–3920. [Google Scholar] [CrossRef]
- Bielitzki, R.; Behrendt, T.; Weinreich, A.; Mittlmeier, T.; Schega, L.; Behrens, M. Acute Effects of Static Balance Exercise Combined with Different Levels of Blood Flow Restriction on Motor Performance Fatigue as Well as Physiological and Perceptual Responses in Young Healthy Males and Females. Eur. J. Appl. Physiol. 2024, 124, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Dankel, S.J.; Jessee, M.B.; Abe, T.; Loenneke, J.P. The Effects of Blood Flow Restriction on Upper-Body Musculature Located Distal and Proximal to Applied Pressure. Sports Med. 2016, 46, 23–33. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Fukuda, T.; Uchida, Y.; Iida, H.; Meguro, M.; Sato, Y.; Yamasoba, T.; Nakajima, T. Muscle Size and Arterial Stiffness after Blood Flow-Restricted Low-Intensity Resistance Training in Older Adults. Scand. J. Med. Sci. Sports. 2014, 24, 799–806. [Google Scholar] [CrossRef]
- Jones, E.J.; Bishop, P.A.; Woods, A.K.; Green, J.M. Cross-Sectional Area and Muscular Strength: A Brief Review. Sports Med. 2008, 38, 987–994. [Google Scholar] [CrossRef]
- Ruhdorfer, A.S.; Dannhauer, T.; Wirth, W.; Cotofana, S.; Roemer, F.; Nevitt, M.; Eckstein, F. Thigh Muscle Cross-Sectional Areas and Strength in Knees with Early vs Knees without Radiographic Knee Osteoarthritis: A between-Knee, within-Person Comparison. Osteoarthr. Cartil. 2014, 22, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Rockenfeller, R.; Günther, M.; Clemente, C.J.; Dick, T.J.M. Rethinking the Physiological Cross-Sectional Area of Skeletal Muscle Reveals the Mechanical Advantage of Pennation. R. Soc. Open Sci. 2024, 11, 240037. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Peterson, M.D.; Ogborn, D.; Contreras, B.; Sonmez, G.T. Effects of Low- vs. High-Load Resistance Training on Muscle Strength and Hypertrophy in Well-Trained Men. J. Strength Cond. Res. 2015, 29, 2954–2963. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front. Physiol. 2019, 10, 533. [Google Scholar]
- Namsawang, J.; Srijunto, W.; Werasirirat, P.; Snieckus, A.; Bradauskiene, K.; Kamandulis, S.; Muanjai, P. The Effects of 6-Week Home-Based Static Stretching, Dynamic Stretching, or Eccentric Exercise Interventions on Muscle-Tendon Properties and Functional Performance in Older Women. J. Exerc. Sci. Fit. 2024, 22, 117–126. [Google Scholar] [CrossRef]
- Suga, T.; Okita, K.; Morita, N.; Yokota, T.; Hirabayashi, K.; Horiuchi, M.; Takada, S.; Omokawa, M.; Kinugawa, S.; Tsutsui, H. Dose Effect on Intramuscular Metabolic Stress during Low-Intensity Resistance Exercise with Blood Flow Restriction. J. Appl. Physiol. 2010, 108, 1563–1567. [Google Scholar] [CrossRef]
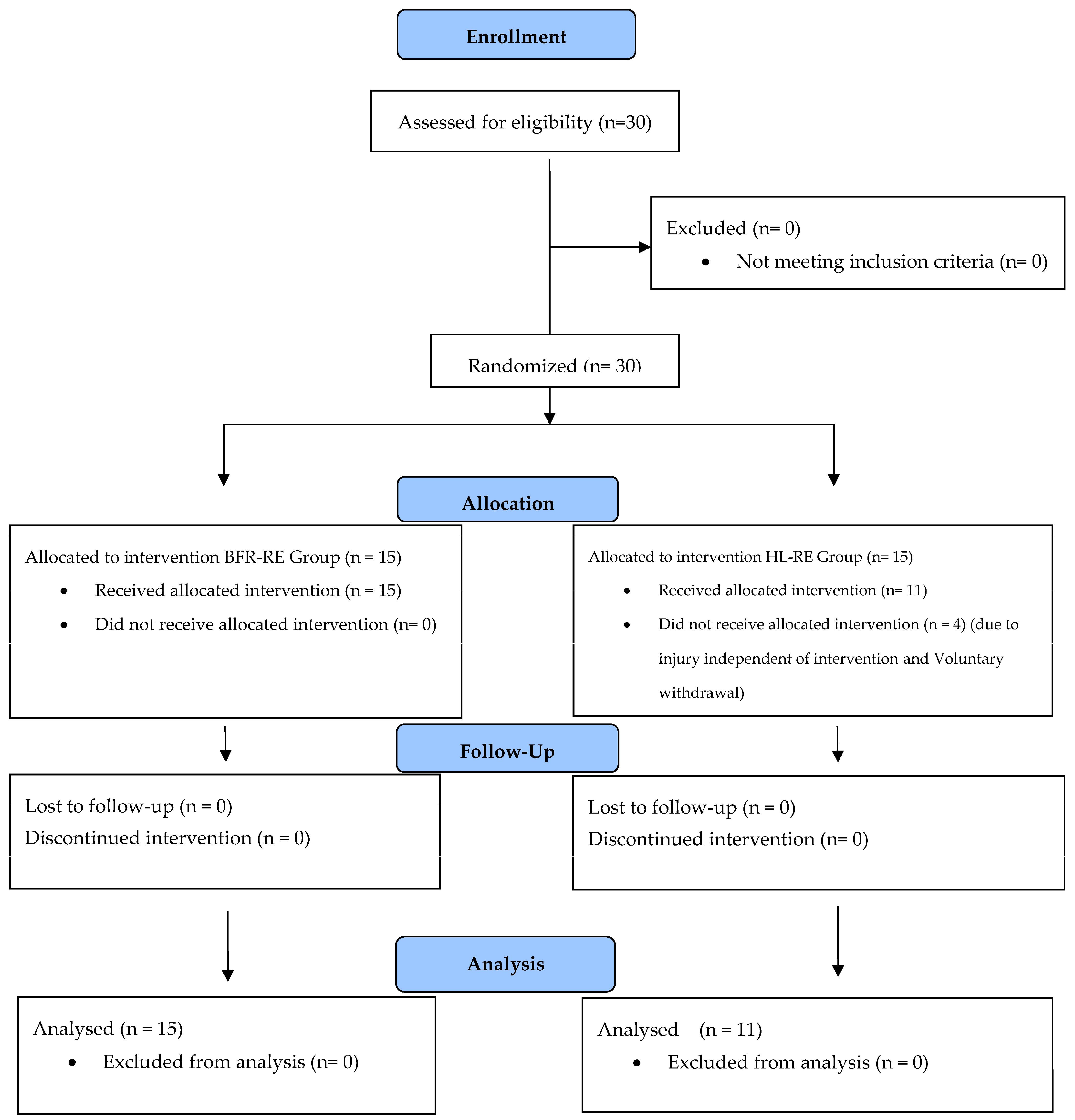

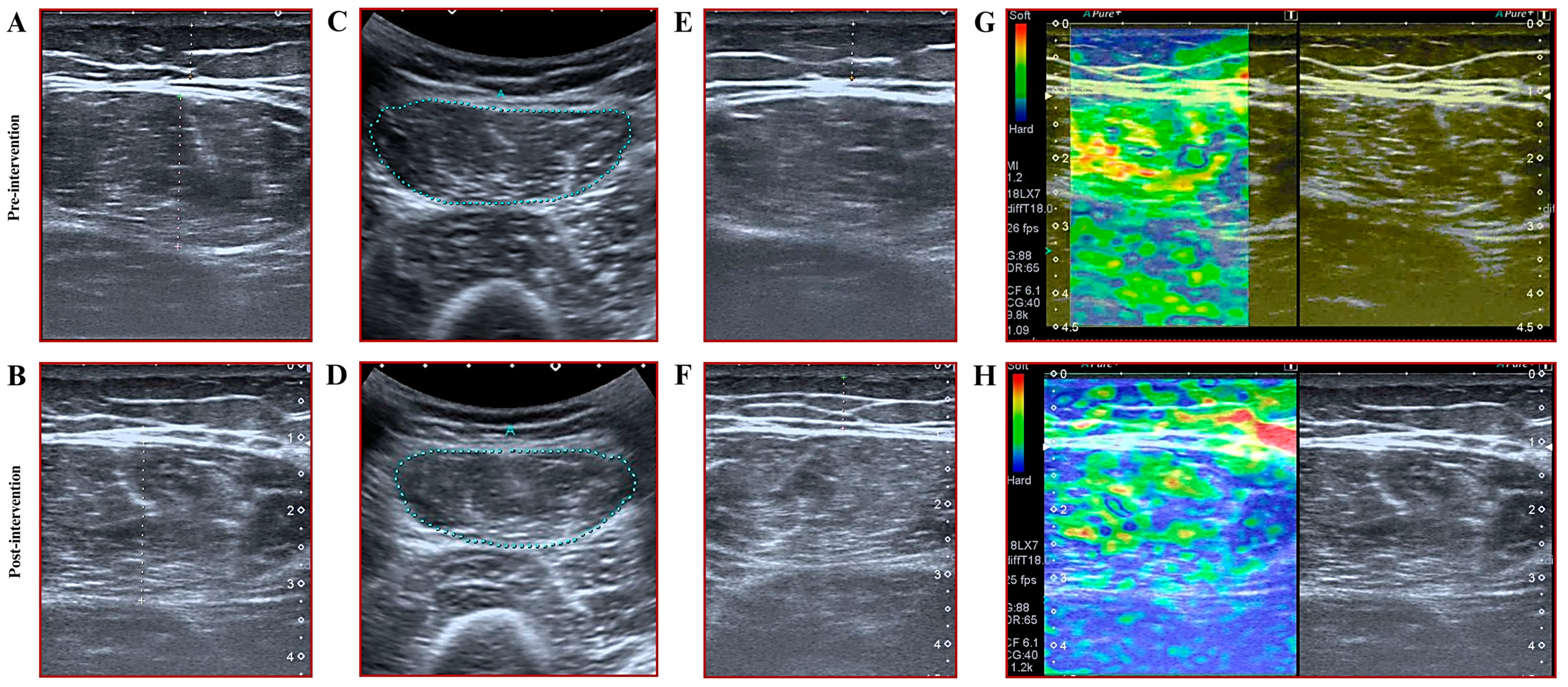
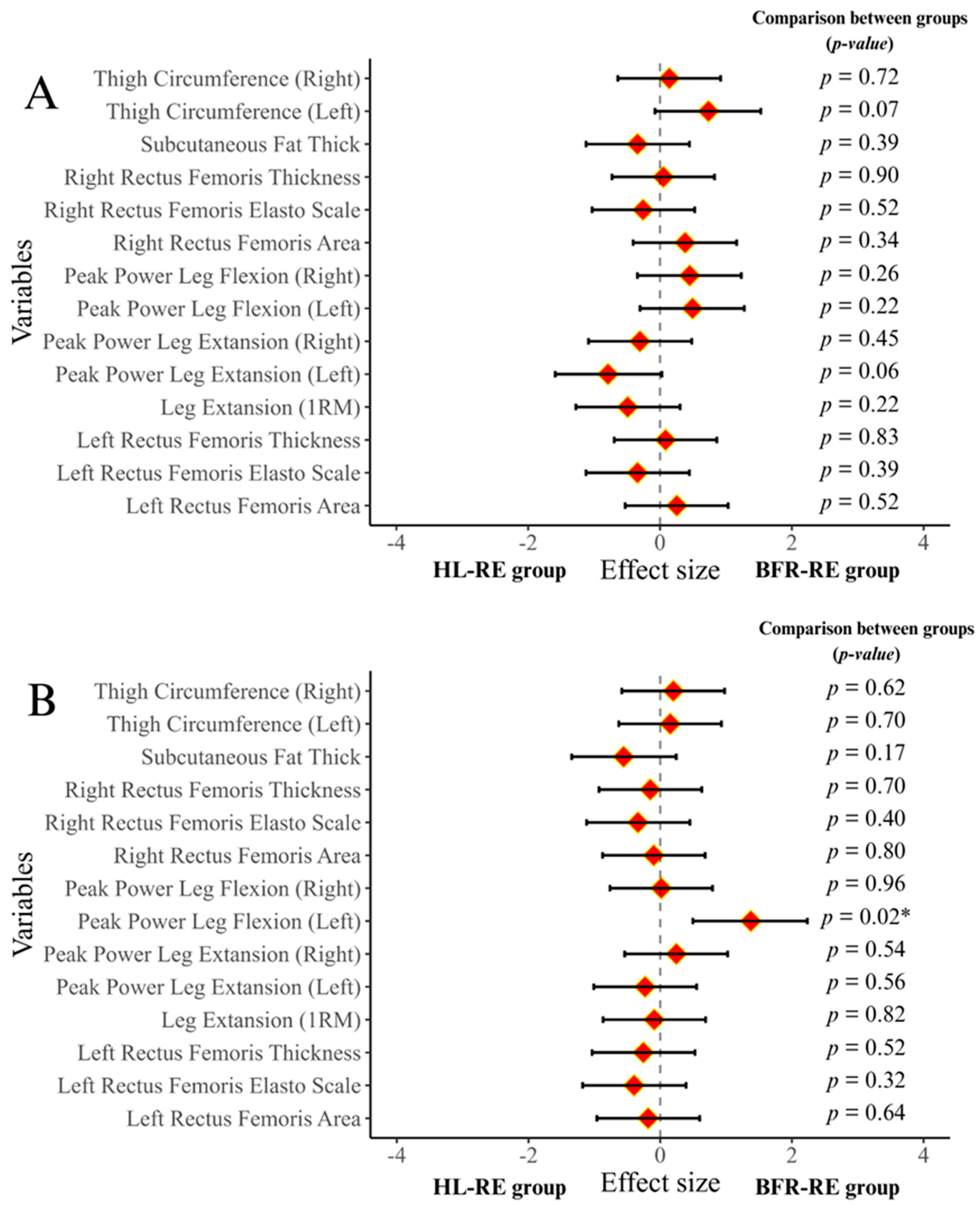
| Variables/Groups | BFR-RE Group (n = 15) | HL-RE Group (n = 11) | p-Value |
|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | ||
| Age (years) | 22.13 ± 3.29 | 20.72 ± 1.73 | 0.21 |
| Height (cm) | 180.93 ± 7.88 | 177.54 ± 7367 | 0.27 |
| Weight (kg) | 75.50 ± 12.39 | 71.74 ± 8.48 | 0.39 |
| Lean Body Mass (kg) | 63.16 ± 8.83 | 59.70 ± 6.10 | 0.27 |
| Skeletal Muscle Mass (kg) | 35.84 ± 5.33 | 33.87 ± 3.54 | 0.29 |
| Body Mass Index (%) | 23.04 ± 3.35 | 22.57 ± 2.48 | 0.69 |
| Body Fat Ratio (%) | 15.92 ± 5.49 | 16.50 ± 5.46 | 0.79 |
| SBP (mm Hg) | 115.33 ± 7.43 | 120.00 ± 8.94 | 0.15 |
| DBP (mm Hg) | 76.00 ± 7.36 | 78.18 ± 6.03 | 0.43 |
| Right LOP (mm Hg) | 157.86 ± 19.97 | Not applied | N/A |
| Left LOP (mm Hg) | 160.36 ± 20.39 | Not applied | N/A |
| Variables | ICC | 95%CI Lower | 95%CI Upper | Reliability Interpretation |
|---|---|---|---|---|
| Peak Power Leg Extansion Pre-Test (Right) | 0.75 | 0.44 | 0.89 | Good |
| Peak Power Leg Extansion Post-Test (Right) | 0.82 | 0.60 | 0.92 | Good |
| Peak Power Leg Extansion Pre-Test (Left) | 0.83 | 0.64 | 0.92 | Good |
| Peak Power Leg Extansion Post-Test (Left) | 0.87 | 0.70 | 0.95 | Good |
| Peak Power Leg Flexion Pre-Test (Right) | 0.83 | 0.62 | 0.93 | Good |
| Peak Power Leg Flexion Post-Test (Right) | 0.87 | 0.70 | 0.95 | Good |
| Peak Power Leg Flexion Pre-Test (Left) | 0.83 | 0.61 | 0.93 | Good |
| Peak Power Leg Flexion Post-Test (Left) | 0.84 | 0.65 | 0.93 | Good |
| Variables | Groups | Pre-Test | Post-Test | Frequentist RM-ANOVA | Bayesian RM-ANOVA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Group Comparisons | Time (Main Effect) | Group × Time (İnteraction) | Intra-Group Comparisons | Time (Main Effect) | Group × Time (İnteraction) | |||||||||||||
| Mean ± SD | Mean ± SD | p-Value (Cohen’d) | p-Value (Omesquare) | p-Value (Omegasquare) | H | BF10 (Level) | H | BFincl (Level) | H | BFincl (Level) | ||||||||
| Leg Extansion 1RM (kg) | BFR-RE | 111.58 ± 16.42 | 161.03 ± 31.92 | 0.01 * (2.21) | 0.01 * (0.52) | 0.479 (0.000) | H1 | 170902.68 (extreme) | H1 | 7.886 × 10+10 (extreme) | H0 | 0.57 (anecdotal) | ||||||
| HL-RE | 119.42 ± 15.33 | 163.50 ± 18.58 | 0.01 * (1.97) | H1 | 1562.85 (extreme) | |||||||||||||
| Thigh Circumference (Right) (cm) | BFR-RE | 54.60 ± 4.77 | 55.93 ± 4.87 | 0.81 (0.31) | 0.08 (0.014) | 0.857 (0.000) | H0 | 0.51 (anecdotal) | H0 | 0.86 (anecdotal) | H0 | 0.27 (anecdotal) | ||||||
| HL-RE | 54.00 ± 3.37 | 55.09 ± 3.08 | 1.00 (0.25) | H0 | 0.35 (anecdotal) | |||||||||||||
| Thigh Circumference (Left) (cm) | BFR-RE | 56.20 ± 4.61 | 55.60 ± 6.23 | 1.00 (0.13) | 0.38 (0.000) | 0.06 (0.010) | H0 | 0.32 (anecdotal) | H0 | 0.33 (anecdotal) | H0 | 0.59 (anecdotal) | ||||||
| HL-RE | 53.27 ± 2.86 | 54.81 ± 3.02 | 0.43 (0.33) | H0 | 0.37 (anecdotal) | |||||||||||||
| Peak Power Leg Extansion (Right) | BFR-RE | 28.55 ± 4.61 | 35.96 ± 3.69 | 0.01 * (1.71) | 0.01 * (0.343) | 0.06 (0.014) | H1 | 61368.63 (extreme) | H1 | 4.968 × 10+7 (extreme) | H1 | 1.75 (anecdotal) | ||||||
| HL-RE | 29.95 ± 4.56 | 34.98 ± 4.43 | 0.01 * (1.16) | H1 | 132.59 (extreme) | |||||||||||||
| Peak Power Leg Extansion (Left) | BFR-RE | 24.86 ± 3.41 | 29.96 ± 3.52 | 0.01 * (1.15) | 0.01 * (0.151) | 0.01 * (0.020) | H1 | 8359.30 (extreme) | H1 | 68.00 (extreme) | H1 | 7.65 (moderate) | ||||||
| HL-RE | 28.54 ± 6.01 | 30.91 ± 4.93 | 0.03 * (0.53) | H1 | 5.34 (moderate) | |||||||||||||
| Peak Power Leg Flexion (Right) | BFR-RE | 23.84 ± 2.71 | 21.28 ± 2.04 | 0.01 * (1.08) | 0.01 * (0.148) | 0.07 (0.011) | H1 | 2317.33 (extreme) | H1 | 99.00 (extreme) | H1 | 2.00 (anecdotal) | ||||||
| HL-RE | 22.63 ± 2.65 | 21.25 ± 1.83 | 0.04 * (0.58) | H1 | 3.23 (moderate) | |||||||||||||
| Peak Power Leg Flexion (Left) | BFR-RE | 20.91 ± 1.26 | 21.21 ± 1.74 | 1.00 (0.21) | 0.15 (0.011) | 0.01 * (0.050) | H0 | 0.33 (anecdotal) | H0 | 0.99 (anecdotal) | H1 | 3.05 (moderate) | ||||||
| HL-RE | 20.25 ± 1.42 | 19.14 ± 1.07 | 0.08 (0.78) | H1 | 5.82 (moderate) | |||||||||||||
| Subcutaneous Fat Thick (cm) | BFR-RE | 5.65 ± 1.82 | 5.01 ± 1.87 | 0.01 * (0.32) | 0.01 * (0.010) | 0.08 (0.002) | H1 | 45.33 (extreme) | H1 | 20.42 (strong) | H1 | 2.48 (anecdotal) | ||||||
| HL-RE | 6.32 ± 2.15 | 6.13 ± 2.20 | 1.00 (0.09) | H0 | 0.43 (anecdotal) | |||||||||||||
| Rectus Femoris Thickness (Right) (mm) | BFR-RE | 20.04 ± 3.16 | 23.28 ± 3.41 | 0.01 * (1.19) | 0.01 * (0.298) | 0.44 (0.000) | H1 | 2330.98 (extreme) | H1 | 1.588 × 10+7 (extreme) | H0 | 0.63 (anecdotal) | ||||||
| HL-RE | 19.91 ± 1.52 | 23.70 ± 1.56 | 0.01 * (1.40) | H1 | 990.22 (extreme) | |||||||||||||
| Rectus Femoris CSA (Right) (cm2) | BFR-RE | 8.20 ± 1.86 | 9.65 ± 2.16 | 0.01 * (0.85) | 0.01 * (0.225) | 0.15 (0.007) | H1 | 159.74 (extreme) | H1 | 222.00 (extreme) | H0 | 0.94 (anecdotal) | ||||||
| HL-RE | 7.61 ± 1.02 | 9.82 ± 1.11 | 0.01 * (1.30) | H1 | 50.54 (extreme) | |||||||||||||
| Rectus Femoris Stiffness (Right) | BFR-RE | 2.80 ± 0.86 | 3.53 ± 0.64 | 0.04 * (1.07) | 0.01 * (0.213) | 0.98 (0.000) | H1 | 5.37 (moderate) | H1 | 209.15 (extreme) | H0 | 0.46 (anecdotal) | ||||||
| HL-RE | 3.00 ± 0.63 | 3.72 ± 0.46 | 0.12 (1.06) | H1 | 2.11 (anecdotal) | |||||||||||||
| Rectus Femoris Thickness (Left) (mm) | BFR-RE | 19.03 ± 2.61 | 21.90 ± 2.63 | 0.01 * (1.32) | 0.01 * (0.360) | 0.13 (0.004) | H1 | 60172.78 (extreme) | H1 | 3.928 × 10+9 (extreme) | H0 | 0.97 (anecdotal) | ||||||
| HL-RE | 18.85 ± 1.04 | 22.47 ± 1.49 | 0.01 * (1.66) | H1 | 8766.54 (extreme) | |||||||||||||
| Rectus Femoris CSA (Left) (cm2) | BFR-RE | 7.65 ± 1.63 | 8.86 ± 1.80 | 0.01 * (0.82) | 0.01 * (0.211) | 0.18 (0.005) | H1 | 230.87 (extreme) | H1 | 8849.34 (extreme) | H0 | 0.79 (anecdotal) | ||||||
| HL-RE | 7.31 ± 0.79 | 9.15 ± 1.21 | 0.01 * (1.25) | H1 | 22.41 (strong) | |||||||||||||
| Rectus Femoris Stiffness (Left) | BFR-RE | 2.46 ± 0.74 | 3.60 ± 0.63 | 0.01 * (1.70) | 0.01 * (0.411) | 0.89 (0.000) | H1 | 668.61 (extreme) | H1 | 1.232 × 10+6 (extreme) | H0 | 0.54 (anecdotal) | ||||||
| HL-RE | 2.72 ± 0.78 | 3.81 ± 0.40 | 0.01 * (1.64) | H1 | 14.96 (strong) | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akgül, M.Ş.; Uysal, H.Ş.; Keskin, N.K.; Çetin, T.; Başdemirci, M.; Akgül, M.N.; Yıldız, Z.; Çiftçi, E.; Soslu, R. Effects of Blood Flow Restriction Resistance Exercise Versus Traditional Resistance Exercise in Voluntary Exhaustion on Quadriceps Muscle Adaptations in Untrained Young Males: A Randomized Trial. Medicina 2025, 61, 804. https://doi.org/10.3390/medicina61050804
Akgül MŞ, Uysal HŞ, Keskin NK, Çetin T, Başdemirci M, Akgül MN, Yıldız Z, Çiftçi E, Soslu R. Effects of Blood Flow Restriction Resistance Exercise Versus Traditional Resistance Exercise in Voluntary Exhaustion on Quadriceps Muscle Adaptations in Untrained Young Males: A Randomized Trial. Medicina. 2025; 61(5):804. https://doi.org/10.3390/medicina61050804
Chicago/Turabian StyleAkgül, Mustafa Şakir, Hüseyin Şahin Uysal, Nevin Köremezli Keskin, Tuğba Çetin, Merve Başdemirci, Melike Nur Akgül, Zehra Yıldız, Ebubekir Çiftçi, and Recep Soslu. 2025. "Effects of Blood Flow Restriction Resistance Exercise Versus Traditional Resistance Exercise in Voluntary Exhaustion on Quadriceps Muscle Adaptations in Untrained Young Males: A Randomized Trial" Medicina 61, no. 5: 804. https://doi.org/10.3390/medicina61050804
APA StyleAkgül, M. Ş., Uysal, H. Ş., Keskin, N. K., Çetin, T., Başdemirci, M., Akgül, M. N., Yıldız, Z., Çiftçi, E., & Soslu, R. (2025). Effects of Blood Flow Restriction Resistance Exercise Versus Traditional Resistance Exercise in Voluntary Exhaustion on Quadriceps Muscle Adaptations in Untrained Young Males: A Randomized Trial. Medicina, 61(5), 804. https://doi.org/10.3390/medicina61050804








