Efficacy and Safety of Anakinra in Colchicine-Resistant or -Intolerant Familial Mediterranean Fever: A Single-Center Real-Life Experience
Abstract
1. Introduction
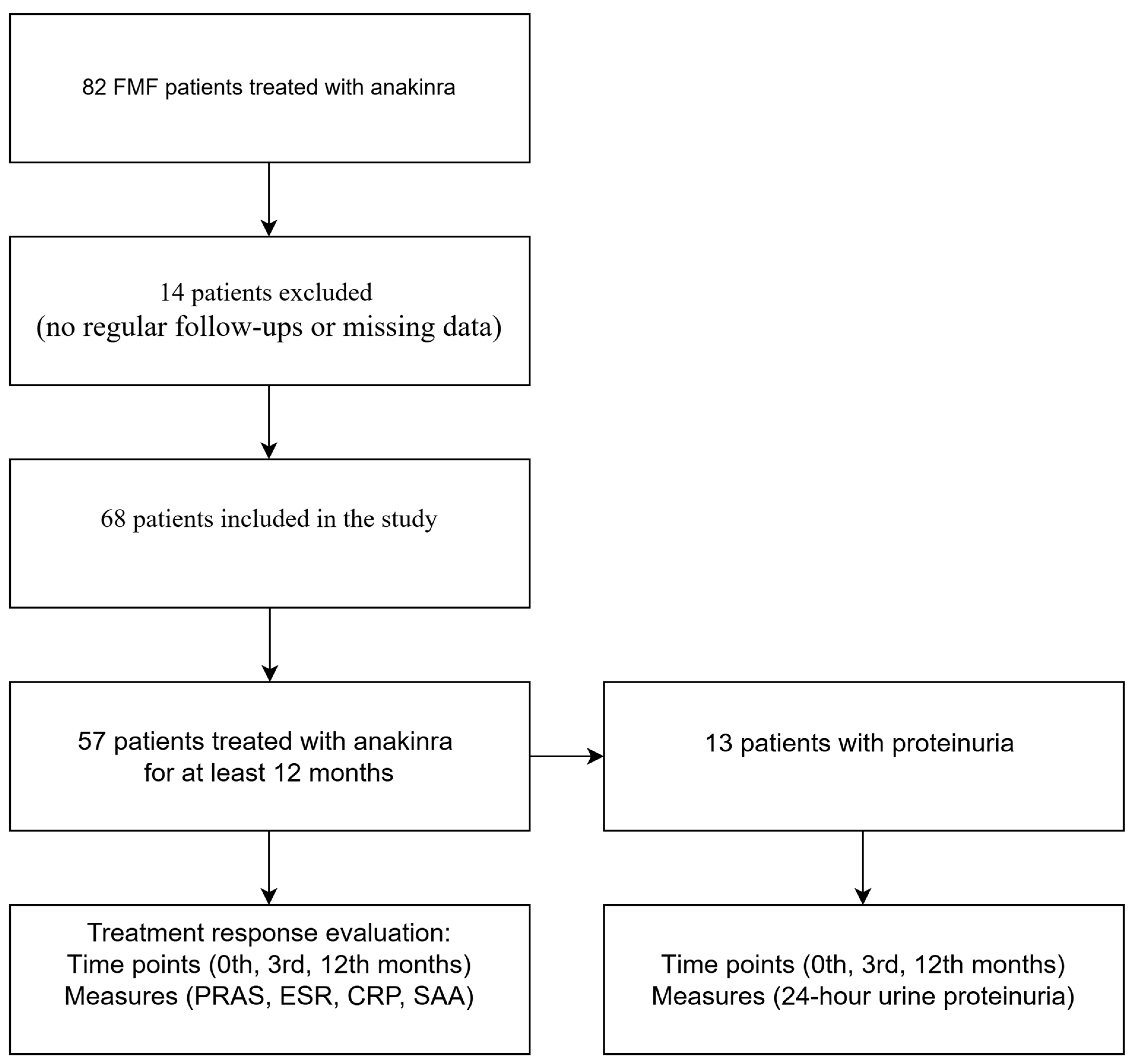
2. Materials and Methods
2.1. Study Population
2.2. Study Design and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Efficacy and Side Effects
3.2. Pregnancy and Other Conditions
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masters, S.L.; Simon, A.; Aksentijevich, I.; Kastner, D.L. Horror autoinflammaticus: The molecular pathophysiology of autoinflammatory disease. Annu. Rev. Immunol. 2009, 27, 621–668. [Google Scholar] [CrossRef] [PubMed]
- Livnehneh, A.; Langevitz, P.; Zemer, D.; Padeh, S.; Migdal, A.; Sohar, E.; Pras, M. The changing face of familial Mediterranean fever. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 1996; pp. 612–627. [Google Scholar]
- Van Gorp, H.; Saavedra, P.H.; de Vasconcelos, N.M.; Van Opdenbosch, N.; Vande Walle, L.; Matusiak, M.; Prencipe, G.; Insalaco, A.; Van Hauwermeiren, F.; Demon, D. Familial Mediterranean fever mutations lift the obligatory requirement for microtubules in Pyrin inflammasome activation. Proc. Natl. Acad. Sci. USA 2016, 113, 14384–14389. [Google Scholar] [CrossRef] [PubMed]
- Jamilloux, Y.; Lefeuvre, L.; Magnotti, F.; Martin, A.; Benezech, S.; Allatif, O.; Penel-Page, M.; Hentgen, V.; Sève, P.; Gerfaud-Valentin, M. Familial Mediterranean fever mutations are hypermorphic mutations that specifically decrease the activation threshold of the Pyrin inflammasome. Rheumatology 2018, 57, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Zemer, D.; Pras, M.; Sohar, E.; Modan, M.; Cabili, S.; Gafni, J. Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever. N. Engl. J. Med. 1986, 314, 1001–1005. [Google Scholar] [CrossRef]
- Babaoglu, H.; Varan, O.; Kucuk, H.; Atas, N.; Satis, H.; Salman, R.; Ozturk, M.A.; Goker, B.; Tufan, A.; Haznedaroglu, S. Effectiveness of canakinumab in colchicine-and anakinra-resistant or-intolerant adult familial Mediterranean fever patients: A single-center real-life study. JCR J. Clin. Rheumatol. 2020, 26, 7–13. [Google Scholar] [CrossRef]
- Santarlasci, V.; Cosmi, L.; Maggi, L.; Liotta, F.; Annunziato, F. IL-1 and T helper immune responses. Front. Immunol. 2013, 4, 182. [Google Scholar] [CrossRef]
- Ben-Zvi, I.; Kukuy, O.; Giat, E.; Pras, E.; Feld, O.; Kivity, S.; Perski, O.; Bornstein, G.; Grossman, C.; Harari, G. Anakinra for colchicine-resistant familial Mediterranean fever: A randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2017, 69, 854–862. [Google Scholar] [CrossRef]
- van der Hilst, J.C.; Moutschen, M.; Messiaen, P.E.; Lauwerys, B.R.; Vanderschueren, S. Efficacy of anti-IL-1 treatment in familial Mediterranean fever: A systematic review of the literature. Biol. Targets Ther. 2016, 10, 75–80. [Google Scholar] [CrossRef]
- Ugurlu, S.; Ergezen, B.; Egeli, B.H.; Selvi, O.; Ozdogan, H. Anakinra treatment in patients with familial Mediterranean fever: A single-centre experience. Rheumatology 2021, 60, 2327–2332. [Google Scholar] [CrossRef]
- Marko, L.; Shemer, A.; Lidar, M.; Grossman, C.; Druyan, A.; Livneh, A.; Kivity, S. Anakinra for colchicine refractory familial Mediterranean fever: A cohort of 44 patients. Rheumatology 2021, 60, 2878–2883. [Google Scholar] [CrossRef]
- Atas, N.; Eroglu, G.A.; Sodan, H.N.; Ozturk, B.O.; Babaoglu, H.; Satis, H.; Karadeniz, H.; Guler, A.A.; Salman, R.B.; Goker, B. Long-term safety and efficacy of anakinra and canakinumab in patients with familial Mediterranean fever: A single-centre real-life study with 101 patients. Clin. Exp. Rheumatol. 2021, 39, 30–36. [Google Scholar] [CrossRef]
- Livneh, A.; Langevitz, P.; Zemer, D.; Kees, S.; Lidar, T.; Migdal, A.; Padeh, S.; Pras, M. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheumatol. 1997, 40, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Gattorno, M.; Hofer, M.; Federici, S.; Vanoni, F.; Bovis, F.; Aksentijevich, I.; Anton, J.; Arostegui, J.I.; Barron, K.; Ben-Cherit, E. Classification criteria for autoinflammatory recurrent fevers. Ann. Rheum. Dis. 2019, 78, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Özen, S.; Sag, E.; Ben-Chetrit, E.; Gattorno, M.; Gül, A.; Hashkes, P.J.; Kone-Paut, I.; Lachmann, H.J.; Tsitsami, E.; Twilt, M. Defining colchicine resistance/intolerance in patients with familial Mediterranean fever: A modified-Delphi consensus approach. Rheumatology 2021, 60, 3799–3808. [Google Scholar] [CrossRef]
- Pras, E.; Livneh, A.; Balow, J.E., Jr.; Pras, E.; Kastner, D.L.; Pras, M.; Langevitz, P. Clinical differences between North African and Iraqi Jews with familial Mediterranean fever. Am. J. Med. Genet. 1998, 75, 216–219. [Google Scholar] [CrossRef]
- Ozen, S.; Kone-Paut, I.; Gül, A. Colchicine resistance and intolerance in familial mediterranean fever: Definition, causes, and alternative treatments. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 2017; pp. 115–120. [Google Scholar]
- Alghamdi, M. Familial Mediterranean fever, review of the literature. Clin. Rheumatol. 2017, 36, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, N.; Lachmann, H.; Özen, S.; Woo, P.; Uziel, Y.; Modesto, C.; Koné-Paut, I.; Cantarini, L.; Insalaco, A.; Neven, B. Treatment of autoinflammatory diseases: Results from the Eurofever Registry and a literature review. Ann. Rheum. Dis. 2013, 72, 678–685. [Google Scholar] [CrossRef]
- Köhler, B.M.; Lorenz, H.M.; Blank, N. IL1-blocking therapy in colchicine-resistant familial Mediterranean fever. Eur. J. Rheumatol. 2018, 5, 230–234. [Google Scholar] [CrossRef]
- Şahin, A.; Derin, M.E.; Albayrak, F.; Karakaş, B.; Karagöz, Y. Assessment of effectiveness of anakinra and canakinumab in patients with colchicine-resistant/unresponsive familial Mediterranean fever. Adv. Rheumatol. 2020, 60, 12. [Google Scholar] [CrossRef]
- Çakan, M.; Aktay Ayaz, N.; Keskindemirci, G.; Karadağ, Ş.G.; Tanatar, A.; Sönmez, H.E. Serum amyloid A as a biomarker in differentiating attacks of familial Mediterranean fever from acute febrile infections. Clin. Rheumatol. 2020, 39, 249–253. [Google Scholar] [CrossRef]
- Ozdogan, H.; Ugurlu, S. Familial Mediterranean Fever. Presse Med. 2019, 48, e61–e76. [Google Scholar] [CrossRef] [PubMed]
- Papa, R.; Lachmann, H.J. Secondary, AA, Amyloidosis. Rheum. Dis. Clin. N. Am. 2018, 44, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Westermark, G.T.; Fändrich, M.; Westermark, P. AA amyloidosis: Pathogenesis and targeted therapy. Annu. Rev. Pathol. 2015, 10, 321–344. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, K.S.; Hentgen, V.; Fellahi, S.; Georgin-Lavialle, S.; Amselem, S.; Grateau, G.; Bastard, J.P.; Steichen, O. Concordance between CRP and SAA in familial Mediterranean fever during attack-free period: A study of 218 patients. Clin. Biochem. 2017, 50, 206–209. [Google Scholar] [CrossRef]
- Berkun, Y.; Padeh, S.; Reichman, B.; Zaks, N.; Rabinovich, E.; Lidar, M.; Shainberg, B.; Livneh, A. A single testing of serum amyloid a levels as a tool for diagnosis and treatment dilemmas in familial Mediterranean fever. Semin. Arthritis Rheum. 2007, 37, 182–188. [Google Scholar] [CrossRef]
- Sargin, G.; Kose, R.; Senturk, T. Anti-interleukin-1 treatment among patients with familial Mediterranean fever resistant to colchicine treatment. Retrospective analysis. Sao Paulo Med. J. 2019, 137, 39–44. [Google Scholar] [CrossRef]
- Stankovic Stojanovic, K.; Delmas, Y.; Ureña Torres, P.; Peltier, J.; Pelle, G.; Jéru, I.; Colombat, M.; Grateau, G. Dramatic beneficial effect of interleukin-1 inhibitor treatment in patients with familial Mediterranean fever complicated with amyloidosis and renal failure. Nephrol. Dial. Transplant. 2012, 27, 1898–1901. [Google Scholar] [CrossRef]
- Varan, Ö.; Kucuk, H.; Babaoglu, H.; Guven, S.C.; Ozturk, M.A.; Haznedaroglu, S.; Goker, B.; Tufan, A. Efficacy and safety of interleukin-1 inhibitors in familial Mediterranean fever patients complicated with amyloidosis. Mod. Rheumatol. 2019, 29, 363–366. [Google Scholar] [CrossRef]
- Ugurlu, S.; Ergezen, B.; Egeli, B.H.; Selvi, O.; Ozdogan, H. Safety and efficacy of anti-interleukin-1 treatment in 40 patients, followed in a single centre, with AA amyloidosis secondary to familial Mediterranean fever. Rheumatology 2020, 59, 3892–3899. [Google Scholar] [CrossRef]
- Venhoff, N.; Voll, R.; Glaser, C.; Thiel, J. IL-1-blockade with Anakinra during pregnancy: Retrospective analysis of efficacy and safety in female patients with familial Mediterranean fever. Z. Rheumatol. 2018, 77, 127–134. [Google Scholar] [CrossRef]
- İlgen, U.; Küçükşahin, O. Anakinra use during pregnancy: Report of a case with familial Mediterranean fever and infertility. Eur. J. Rheumatol. 2017, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Youngstein, T.; Hoffmann, P.; Gül, A.; Lane, T.; Williams, R.; Rowczenio, D.M.; Ozdogan, H.; Ugurlu, S.; Ryan, J.; Harty, L. International multi-centre study of pregnancy outcomes with interleukin-1 inhibitors. Rheumatology 2017, 56, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Eshed, I.; Rosman, Y.; Livneh, A.; Kedem, R.; Langevitz, P.; Ben-Zvi, I.; Lidar, M. Exertional leg pain in familial Mediterranean fever: A manifestation of an underlying enthesopathy and a marker of more severe disease. Arthritis Rheumatol. 2014, 66, 3221–3226. [Google Scholar] [CrossRef]
- Kaiser, C.; Knight, A.; Nordström, D.; Pettersson, T.; Fransson, J.; Florin-Robertsson, E.; Pilström, B. Injection-site reactions upon Kineret (anakinra) administration: Experiences and explanations. Rheumatol. Int. 2012, 32, 295–299. [Google Scholar] [CrossRef] [PubMed]
| Age (years) | 40.2 (21.8–74.7) |
| Gender (F/M) | 39/29 |
| Age at FMF diagnosis (years) | 28.1 ± 15 |
| Age at onset of anakinra therapy (years) | 36.3 (19.7–71.1) |
| Indications of anakinra therapy | |
| Colchicine resistance, n (%) | 63 (92.6) |
| Colchicine intolerance/side effects, n (%) | 5 (7.4) |
| Time between diagnosis and anakinra therapy (years) | 8.2 (1.1–23.9) |
| Family history of FMF, n (%) | 36 (52.9) |
| Fever, n (%) | 40 (58.8) |
| Abdominal pain, n (%) | 61 (89.7) |
| Chest pain, n (%) | 10 (14.7) |
| Arthralgia/Arthritis, n (%) | 44 (64.7) |
| Myalgia, n (%) | 9 (13.2) |
| Prolonged febrile myalgia, n (%) | 1 (1.5) |
| Erysipelas-like erythema, n (%) | 3 (4.4) |
| Hepatomegaly, n (%) | 16 (23.5) |
| Splenomegaly, n (%) | 16 (23.5) |
| Amyloidosis, n (%) | 15 (22.1) |
| Comorbidities, n (%) | 46 (67.6) |
| Diabetes mellitus, n (%) | 7 (10.3) |
| Hypertension, n (%) | 19 (27.9) |
| Hyperlipidemia, n (%) | 18 (26.5) |
| Pulmonary disease, n (%) | 14 (20.6) |
| Chronic renal failure, n (%) | 11 (16.2) |
| Coronary artery disease, n (%) | 8 (11.8) |
| Spondyloarthropathies, n (%) | 5 (7.8) |
| Colchicine dose before anakinra (mg) | 2 (1–2) |
| Duration of anakinra, median (min–max range), (months) | 34.2 (1–118.9) |
| Age, years | 40.4 (21.8–74.7) |
| Gender (F/M) | 31/26 |
| Age at FMF diagnosis (years) | 28 ± 14.9 |
| Age at onset of anakinra therapy (years) | 37.9 (19.7–70.3) |
| Indications of anakinra therapy | |
| Colchicine resistance, n (%) | 54 (94.7) |
| Colchicine intolerance/side effects, n (%) | 3 (5.3) |
| Time between diagnosis and anakinra therapy (years) | 9.9 (1.1–23.9) |
| Family history of FMF, n (%) | 32 (56.1) |
| Fever, n (%) | 34 (59.6) |
| Abdominal pain, n (%) | 52 (91.2) |
| Chest pain, n (%) | 9 (15.8) |
| Arthralgia/Arthritis, n (%) | 36 (63.2) |
| Myalgia, n (%) | 9 (15.8) |
| Prolonged febrile myalgia, n (%) | 1 (1.8) |
| Erysipelas-like erythema, n (%) | 1 (1.8) |
| Hepatomegaly, n (%) | 15 (26.3) |
| Splenomegaly, n (%) | 16 (28.1) |
| Amyloidosis, n (%) | 13 (22.8) |
| Comorbidities, n (%) | 37 (64.9) |
| Diabetes mellitus, n (%) | 5 (8.8) |
| Hypertension, n (%) | 15 (26.3) |
| Hyperlipidemia, n (%) | 13 (22.8) |
| Pulmonary disease, n (%) | 10 (17.5) |
| Chronic renal failure, n (%) | 10 (17.5) |
| Coronary artery disease, n (%) | 6 (10.5) |
| Spondyloarthropathies, n (%) | 4 (7) |
| Colchicine dose before anakinra (mg) | 2 (1–2) |
| Duration of anakinra, median (min–max range), (months) | 39.5 (12.2–118.9) |
| Month 0 | Month 3 | Month 12 | p | 0th–3rd Month p | 3rd–12th Month p | 0th–12th Month p | |
|---|---|---|---|---|---|---|---|
| PRAS (n = 57) | 7 (2–11) | 4 (1–8) | 4 (1–7) | <0.001 | <0.001 | ≤0.001 | <0.001 |
| ESR (mm/h) (n = 57) | 25 (4–84) | 9 (2–68) | 9 (2–61) | <0.001 | <0.001 | <0.001 | <0.001 |
| CRP (mg/L) (n = 57) | 20 (2–124) | 6 (2–32) | 3 (2–21) | <0.001 | <0.001 | <0.001 | <0.001 |
| SAA (mg/L) (n = 57) | 81.7 (3–390) | 18 (3–150) | 6 (2.9–139) | <0.001 | <0.001 | <0.001 | <0.001 |
| 24 h urine proteinuria values (mg) (n = 13) | 5895 (710–15,059) | 5144 (180–10,868) | 900 (250–9200) | <0.001 | 0.011 | 0.006 | 0.007 |
| Remission | 8 |
| Insufficient response | 7 |
| Non-compliance | 1 |
| Pregnancy plan | 1 |
| Exitus | 1 |
| Side effects | 7 |
| Skin reaction | 5 |
| Diarrhea | 1 |
| Leukopenia | 1 |
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age at FMF diagnosis (years) | 8.2 | 23.8 | 17.4 | 33.3 |
| Age at onset of anakinra therapy (years) | 28.5 | 35 | 28 | 34.6 |
| Duration of anakinra (months) | 44.7 | 118.9 | 13.5 | 40.1 |
| 0th month PRAS | 8 | 8 | 9 | 5 |
| 0th month ESR (mm/h) | 51 | 29 | 28 | 23 |
| 0th month CRP (mg/L) | 124 | 37 | 15.6 | 2 |
| 0th month SAA (mg/L) | 272 | 178 | 81.7 | 87 |
| 3rd month PRAS | 6 | 5 | 5 | 3 |
| 3rd month ESR (mm/h) | 38 | 22 | 17 | 12 |
| 3rd month CRP (mg/L) | 22 | 12 | 4 | 2 |
| 3rd month SAA (mg/L) | 48 | 38 | 23 | 24 |
| 12th month PRAS | 6 | 5 | 4 | 3 |
| 12th month ESR (mm/h) | 24 | 28 | 9 | 2 |
| 12th month CRP (mg/L) | 5 | 7 | 2 | 2 |
| 12th month SAA (mg/L) | 41 | 14 | 3 | 6 |
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Gender | M | F | M | F | M | M |
| Age at FMF diagnosis (years) | 31.7 | 39.7 | 23.2 | 55.4 | 20.2 | 20.7 |
| Age at onset of anakinra therapy (years) | 53 | 41.3 | 39.6 | 57.8 | 26.4 | 42.9 |
| Duration of anakinra (months) | 68.8 | 82.2 | 54 | 96.3 | 45.1 | 12.4 |
| 0th month PRAS | 5 | 4 | 9 | 9 | 8 | 10 |
| 0th month ESR (mm/h) | 58 | 33 | 56 | 65 | 20 | 32 |
| 0th month CRP (mg/L) | 25 | 9.9 | 7.1 | 3 | 10.1 | 3.5 |
| 0th month SAA (mg/L) | 50 | 13 | 30 | 124 | 6.2 | 82 |
| 3rd month PRAS | 5 | 2 | 5 | 7 | 7 | 8 |
| 3rd month ESR (mm/h) | 24 | 14 | 20 | 23 | 12 | 24 |
| 3rd month CRP (mg/L) | 20 | 9 | 5 | 3 | 4 | 3 |
| 3rd month SAA (mg/L) | 40 | 8 | 23 | 8 | 4 | 12 |
| 12th month PRAS | 5 | 1 | 5 | 7 | 7 | 6 |
| 12th month ESR (mm/h) | 4 | 7 | 4 | 25 | 4 | 18 |
| 12th month CRP (mg/L) | 21 | 5.6 | 4 | 3.1 | 2 | 2 |
| 12th month SAA (mg/L) | 25 | 4 | 12 | 4 | 3 | 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocak, T.; Köse, H.N.; Yağız, B.; Coşkun, B.N.; Dalkılıç, E.; Pehlivan, Y. Efficacy and Safety of Anakinra in Colchicine-Resistant or -Intolerant Familial Mediterranean Fever: A Single-Center Real-Life Experience. Medicina 2025, 61, 792. https://doi.org/10.3390/medicina61050792
Ocak T, Köse HN, Yağız B, Coşkun BN, Dalkılıç E, Pehlivan Y. Efficacy and Safety of Anakinra in Colchicine-Resistant or -Intolerant Familial Mediterranean Fever: A Single-Center Real-Life Experience. Medicina. 2025; 61(5):792. https://doi.org/10.3390/medicina61050792
Chicago/Turabian StyleOcak, Tuğba, Havva Nur Köse, Burcu Yağız, Belkıs Nihan Coşkun, Ediz Dalkılıç, and Yavuz Pehlivan. 2025. "Efficacy and Safety of Anakinra in Colchicine-Resistant or -Intolerant Familial Mediterranean Fever: A Single-Center Real-Life Experience" Medicina 61, no. 5: 792. https://doi.org/10.3390/medicina61050792
APA StyleOcak, T., Köse, H. N., Yağız, B., Coşkun, B. N., Dalkılıç, E., & Pehlivan, Y. (2025). Efficacy and Safety of Anakinra in Colchicine-Resistant or -Intolerant Familial Mediterranean Fever: A Single-Center Real-Life Experience. Medicina, 61(5), 792. https://doi.org/10.3390/medicina61050792






