Effects of Intravenous Versus Intraosseous Adrenalin Administration on Morbidity and Mortality After Out-of-Hospital Cardiac Arrest: A Systematic Review
Abstract
1. Introduction
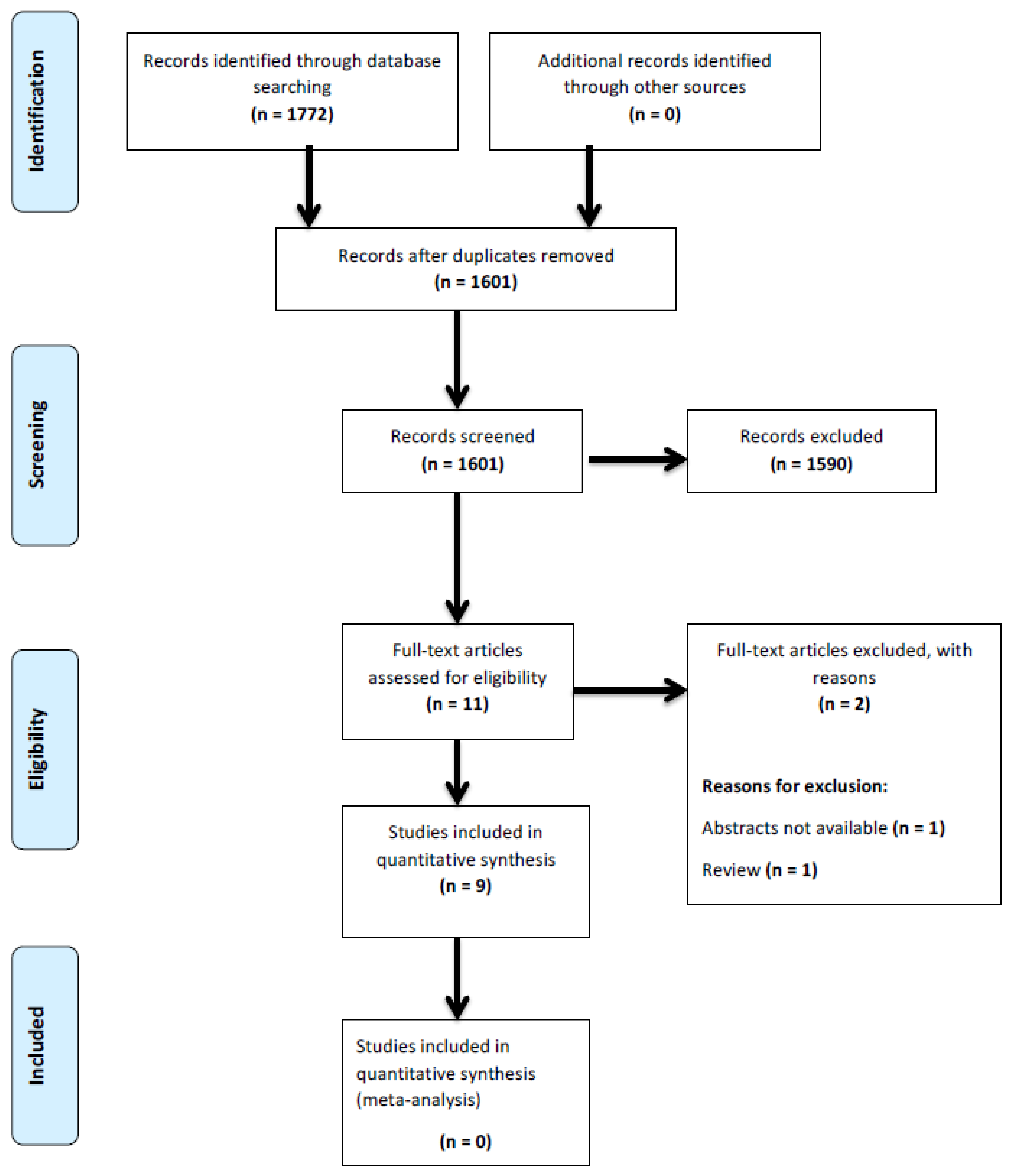
2. Materials and Methods
3. Results
3.1. Comparison Between IO and IV Access in OHCA
3.2. Return of Spontaneous Circulation (ROSC) as Primary Outcome Measurement
3.3. Neurological Outcome at Hospital Discharge
3.4. Comparing IO and IV Access as Sub-Analyses from Other Trials
4. Discussion
4.1. Pharmacokinetics
4.2. Infusion Site Location
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feinstein, B.A.; Stubbs, B.A.; Rea, T.; Kudenchuk, P.J. Intraosseous compared to intravenous drug resuscitation in out-of-hospital cardiac arrest. Resuscitation 2017, 117, 91–96. [Google Scholar] [PubMed]
- Mody, P.; Brown, S.P.; Kudenchuk, P.J.; Chan, P.S.; Khera, R.; Ayers, C.; Pandey, A.; Kern, K.B.; de Lemos, J.A.; Link, M.S.; et al. Intraosseous versus intravenous access in patients with out-of-hospital cardiac arrest: Insights from the resuscitation outcomes consortium continuous chest compression trial. Resuscitation 2019, 134, 69–75. [Google Scholar] [PubMed]
- Perkins, G.D.; Graesner, J.T.; Semeraro, F.; Olasveengen, T.; Soar, J.; Lott, C.; Van de Voorde, P.; Madar, J.; Zideman, D.; Mentzelopoulos, S.; et al. European Resuscitation Council Guidelines 2021: Executive summary. Resuscitation 2021, 161, 1–60. [Google Scholar]
- Merchant, R.M.; Topjian, A.A.; Panchal, A.R.; Cheng, A.; Aziz, K.; Berg, K.M.; Lavonas, E.J.; Magid, D.J.; MPH On behalf of the Adult Basic and Advanced Life Support, Pediatric Basic and Advanced Life Support, Neonatal Life Support, Resuscitation Education Science, and Systems of Care Writing Groups. Part 1: Executive Summary: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142 (Suppl. 2), S337–S357. [Google Scholar] [PubMed]
- Zhang, Y.; Zhu, J.; Liu, Z.; Gu, L.; Zhang, W.; Zhan, H.; Hu, C.; Liao, J.; Xiong, Y.; Idris, A.H. Intravenous versus intraosseous adrenaline administration in out-of-hospital cardiac arrest: A retrospective cohort study. Resuscitation 2020, 149, 209–216. [Google Scholar]
- Kawano, T.; Grunau, B.; Scheuermeyer, F.X.; Gibo, K.; Fordyce, C.B.; Lin, S.; Stenstrom, R.; Schlamp, R.; Jenneson, S.; Christenson, J. Intraosseous Vascular Access Is Associated With Lower Survival and Neurologic Recovery Among Patients With Out-of-Hospital Cardiac Arrest. Ann. Emerg. Med. 2018, 71, 588–596. [Google Scholar]
- Clemency, B.; Tanaka, K.; May, P.; Innes, J.; Zagroba, S.; Blaszak, J.; Hostler, D.; Cooney, D.; McGee, K.; Lindstrom, H.; et al. Intravenous vs. intraosseous access and return of spontaneous circulation during out of hospital cardiac arrest. Am. J. Emerg. Med. 2017, 35, 222–226. [Google Scholar]
- Nolan, J.P.; Deakin, C.D.; Ji, C.; Gates, S.; Rosser, A.; Lall, R.; Perkins, G.D. Intraosseous versus intravenous administration of adrenaline in patients with out-of-hospital cardiac arrest: A secondary analysis of the PARAMEDIC2 placebo-controlled trial. Intensive Care Med. 2020, 46, 954–962. [Google Scholar]
- Granfeldt, A.; Avis, S.R.; Lind, P.C.; Holmberg, M.J.; Kleinman, M.; Maconochie, I.; Hsu, C.H.; de Almeida, M.F.; Wang, T.L.; Neumar, R.W.; et al. Intravenous vs. intraosseous administration of drugs during cardiac arrest: A systematic review. Resuscitation 2020, 149, 150–157. [Google Scholar]
- Hoskins, S.L.; do Nascimento, P., Jr.; Lima, R.M.; Espana-Tenorio, J.M.; Kramer, G.C. Pharmacokinetics of intraosseous and central venous drug delivery during cardiopulmonary resuscitation. Resuscitation 2012, 83, 107–112. [Google Scholar]
- Burgert, J.M.; Johnson, A.D.; O’Sullivan, J.C.; Blalock, W.J.; Duffield, B.C.; Albright, B.P.; Herzog, C.C.; Moore, M.S.; Dempster, K.S.; Rauch, J.W. Pharmacokinetic effects of endotracheal, intraosseous, and intravenous epinephrine in a swine model of traumatic cardiac arrest. Am. J. Emerg. Med. 2019, 37, 2043–2050. [Google Scholar] [CrossRef]
- Hampton, K.; Wang, E.; Argame, J.I.; Bateman, T.; Craig, W.; Johnson, D. The effects of tibial intraosseous versus intravenous amiodarone administration in a hypovolemic cardiac arrest procine model. Am. J. Disaster Med. 2016, 11, 253–260. [Google Scholar] [CrossRef]
- Wong, M.R.; Reggio, M.J.; Morocho, F.R.; Holloway, M.M.; Garcia-Blanco, J.C.; Jenkins, C.; Johnson, A.D. Effects of intraosseous epinephrine in a cardiac arrest swine model. J. Surg. Res. 2016, 201, 327–333. [Google Scholar] [CrossRef]
- Baert, V.; Vilhelm, C.; Escutnaire, J.; Nave, S.; Hugenschmitt, D.; Chouihed, T.; Tazarourte, K.; Javaudin, F.; Wiel, E.; El Khoury, C.; et al. Intraosseous Versus Peripheral Intravenous Access During Out-of-Hospital Cardiac Arrest: A Comparison of 30-Day Survival and Neurological Outcome in the French National Registry. Cardiovasc. Drugs Ther. 2020, 34, 189–197. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Conell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011. [Google Scholar]
- Altman, D. Practical Statistics for Medical Research; Chapman and Hal: London, UK, 1991. [Google Scholar]
- Nguyen, L.; Suarez, S.; Daniels, J.; Sanchez, C.; Landry, K.; Redfield, C. Effect of Intravenous Versus Intraosseous Access in Prehospital Cardiac Arrest. Air Med. J. 2019, 38, 147–149. [Google Scholar] [CrossRef]
- Tan, B.K.K.; Chin, Y.X.; Koh, Z.X.; Md Said, N.; Rahmat, M.; Fook-Chong, S.; Ng, Y.Y.; Ong, M.E. Clinical evaluation of intravenous alone versus intravenous or intraosseous access for treatment of out-of-hospital cardiac arrest. Resuscitation 2021, 159, 129–136. [Google Scholar] [CrossRef]
- Ross, E.M.; Mapp, J.; Kharod, C.; Wampler, D.A.; Velasquez, C.; Miramontes, D.A. Time to epinephrine in out-of-hospital cardiac arrest: A retrospective analysis of intraosseous versus intravenous access. Am. J. Disaster Med. 2016, 11, 119–123. [Google Scholar] [CrossRef]
- Reades, R.; Studnek, J.R.; Vandeventer, S.; Garrett, J. Intraosseous versus intravenous vascular access during out-of-hospital cardiac arrest: A randomized controlled trial. Ann. Emerg. Med. 2011, 58, 509–516. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Kuhn, J.G.; Burris, H.A., 3rd; Miller, L.J. Does intraosseous equal intravenous? A pharmacokinetic study. Am. J. Emerg. Med. 2008, 26, 31–38. [Google Scholar] [CrossRef]
- Delguercio, L.R.; Coomaraswamy, R.P.; State, D. Cardiac output and other hemodynamic variables during external cardiac massage in man. N. Engl. J. Med. 1963, 269, 1398–1404. [Google Scholar] [PubMed]
- Michael, J.R.; Guerci, A.D.; Koehler, R.C.; Shi, A.Y.; Tsitlik, J.; Chandra, N.I.; Niedermeyer, E.; Rogers, M.C.; Traystman, R.J.; Weisfeldt, M.L. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation 1984, 69, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, L.D.; Baragchizadeh, A.; Johnson, C.; Johnson, D. Effects of tibial and humerus intraosseous administration of epinephrine in a cardiac arrest swine model. Am. J. Disaster Med. 2016, 11, 243–251. [Google Scholar] [PubMed]
- Pirracchio, R.; Payen, D.; Plaisance, P. The impedance threshold valve for adult cardiopulmonary resuscitation: A review of the literature. Curr. Opin. Crit. Care 2007, 13, 280–286. [Google Scholar]
- Zive, D.; Koprowicz, K.; Schmidt, T.; Stiell, I.; Sears, G.; Van Ottingham, L.; Idris, A.; Stephens, S.; Daya, M.; ROC Investigators. Variation in out-of-hospital cardiac arrest resuscitation and transport practices in the Resuscitation Outcomes Consortium: ROC Epistry-Cardiac Arrest. Resuscitation 2011, 82, 277–284. [Google Scholar] [PubMed]
| Author | Type of Study | N | Intervention | Primary Outcome | Secondary Outcome | Unadjusted Analysis First | Adjusted Analysis First | Unadjusted Analysis Second | Adjusted Analysis Second |
|---|---|---|---|---|---|---|---|---|---|
| Feinstein et al. [1] | Retrospective cohort study | N = 1800 IV 1525 IO 275 | Primary route of vascular access: first patent access for drug administration IV vs. IO | Survival to hospital discharge | Sustained ROSC Survival to hospital admission | IO less likely to survive hospital discharge 14.9% vs. 22.8%, p = 0.003 | No difference after adjusting for confounders in survival to discharge OR 95% CI 0.81, 0.55, 1.21, p = 0.31 | IO less likely to achieve ROSC 43.6% vs. 55.5% p < 0.001 Or be hospitalized 38.5% vs. 50.0%, p < 0.001 | IO access associated with lower likelihood of ROSC OR = 0.67 0.50, 0.88, p = 0.004 and survival to hospitalization OR 0.68 0.51, 0.91, p = 0.009 |
| Clemency et al. [7] | Retrospective chart review of EMS records | N = 1310 IV 788 IO 552 | First access type attempted First dose of parenteral epinephrine | ROSC at arrival ED | x | IO first approach non-inferior to IV first approach, ROSC 19.9% vs. 19.7%, p = 0.01 Epinephrine first IO vs. IV, ROSC 18.6% vs. 20.9% OR 0.86; 95% CI: 0.66–1.13 | IO first approach non-inferior to IV first approach, ROSC 19.9% vs. 19.7%, p = 0.01 Epinephrine first IO vs. IV, ROSC 18.6% vs. 20.9% OR 0.86; 95% CI: 0.66–1.13 | IO group superior 1st attempt success to IV group 81.6% vs. 94.8%, p < 0.01 | |
| Kawano et al. [6] | Secondary analysis PRIMED study (Retrospective data analysis) | N = 13,155 IV 12,495 IO 660 | Initial route of vascular access IV vs. IO | Favorable neurologic outcome on hospital discharge | ROSC Survival to hospital discharge | IO associated with decreased probability of favorable neurological outcome OR 0.22, 95% CI 0.12–0.42 | Compared with IV, IO decreased probability of favorable neurological outcome OR 0.24; 95% CI 0.13–0.46 | IO associated with decreased probability of ROSC OR 0.53, 95% CI 0.44–0.66 And survival OR 0.42 95% CI 0.28–0.63 | Compared with IV, IO decreased probability of ROSC OR 0.60 95% CI 0.49–0.74 And survival 0.45 95% CI 0.29–0.69 |
| Nguyen et al. [18] | Retrospective cohort study | N = 795 IV 453 IO 342 | IV vs. IO access First access IV vs. IO (intention to treat) | ROSC | ROSC IV vs. IO 45.1% vs. 25.7%, p < 0.001 ROSC IV vs. IO first 42.4% vs. 26.6% | ROSC IV vs. IO 45.1% vs. 25.7%, p < 0.001 ROSC IV vs. IO first 42.4% vs. 26.6% | |||
| Mody et al. [2] | Retrospective cohort study | N = 19,731 IV 16,663 IO 3068 | Attempted IO vs. IV | Survival to hospital discharge | Rates of sustained ROSC Survival with favorable neurological outcome | IO vs. IV 4.6% vs. 5.7%, p = 0.01 | IO no longer associated with decreased survival vs. IV OR 0.88 95% CI 0.72–1.09, p = 0.24 | Favorable neurological status at discharge 2.8% vs. 4.2% Sustained ROSC IO vs. IV 17.9% vs. 23.5% | Favorable neurological status at discharge OR 0.87 95% CI 0.67–1.12, p = 0.29 Sustained ROSC IO vs. IV OR 0.80 95% CI 0.71–0.89, p < 0.001 |
| Zhang et al. [5] | Retrospective observational analysis | N = 35,733 IV 27,758 IO 7975 | First and only adrenaline route IV vs. IO | Survival to hospital discharge | ROSC Survival with good neurological outcome | IV vs. IO 5.8% vs. 3.1%, p < 0.05 | OR of IV vs. IO 1.468 95% CI, 1.264–1.705 | ROSC IV vs. IO 24.5% vs. 17.8%, p < 0.05 Survival with favorable neurological outcome IV vs. IO 4.3% vs. 1.8%, p < 0.05 | ROSC IV vs. IO OR 1.367 95% CI, 1.276–1.464 Survival with favorable neurological outcome IV vs. IO OR 1.849 95% CI 1.526–2.240 |
| Tan et al. [19] | Prospective parallel cluster-randomized study | N = 1016 IV only 478 IV + IO 529 | IV route at scene (max 2 attempts) IV or IO at scene (max 2 attempts IV then IO) | Any ROSC | Insertion success rate Proportion of patients who received first dose of adrenaline Time to first dose of adrenaline Survival outcome | IV + IO vs. IV OR 0.99 95% CI 0.75–1.29 | Post hoc per protocol analysis IV + IO 38.6% vs. 37.2%, p = 0.721 | Success rate IV + IO vs. IV 76.6% vs. 61.1%, p = 0.001 Prehospital adrenaline IV + IO vs. IV 71.3% vs. 55.4%, p = 0.001 IV + IO faster Adrenaline 23 vs. 25 min, p = 0.001 Survival outcome IV + IO vs. IV 4% vs. 3.4%, p = 0.630 | Post hoc per protocol analysis Success rate IV + IO vs. IV 100% vs. 61.1%, p < 0.001 Prehospital adrenaline IV + IO vs. IV 93.5% vs. 55.4%, p < 0.001 Survival outcome IV + IO vs. IV 4.9% vs. 8.4%, p = 0.054/3.3% vs. 4.0%, p = 0.713 |
| Baert et al. [14] | Retrospective comparative multi-center study | N = 28,856 IV 27,280 IO 1576 | IO vs. IV access | Survival at 30 days or hospital discharge | ROSC Survival at hospital admission Neurological outcome at day 30 or discharge | Survival day 30 1.9% vs. 3.8%, p < 0.001 | Survival discharge or day 30 IO vs. IV 1.8% vs. 2.4%, p = 0.266 | ROSC IO vs. IV 19.7% vs. 27.7%, p < 0.001 Survival at hospital admission IO vs. IV 14.8% vs. 23.4%, p < 0.001 Favorable neurological outcome 81.8% vs. 72.7%, p = 0.343 | ROSC IO vs. IV 19.8% vs. 25.3%, p < 0.001 Favorable neurological outcome IO vs. IV 85.2% vs. 65.7%, p = 0.082 |
| Nolan et al. [8] | Placebo controlled trial | N = 3631 IO 1116 IV 2515 | IO vs. IV adrenaline vs. placebo | Survival at 30 days | ROSC at handover hospital Survival at discharge Favorable neurological outcome | aHR IV vs. IO within 1 day survival 1.02 95% CI 0.94–1.10 aHR IV vs. IO over 1 day survival 1.30 95% CI 0.98, 1.72 | ROSC adrenaline vs. placebo IV aOR 4.07 95% CI 3.42–4.85 vs. IO aOR 3.98 95% CI 2.86–5.53, p = 0.90 |
| Criteria 1 | S1 | S2 | S3 | S4 | C1 | O1 | O2 | O3 | T |
|---|---|---|---|---|---|---|---|---|---|
| Feinstein et al. [1] | * | - | * | - | * | * | * | * | 6 |
| Clemency et al. [7] | * | * | * | - | ** | * | * | * | 8 |
| Kawano et al. [6] | * | * | * | - | ** | * | * | * | 8 |
| Nguyen et al. [18] | * | * | * | * | ** | * | * | * | 9 |
| Mody et al. [2] | * | * | * | * | ** | * | * | * | 9 |
| Zhang et al. [5] | * | - | * | - | ** | * | - | * | 6 |
| Tan et al. [19] | * | - | * | - | * | * | * | * | 6 |
| Baert et al. [14] | * | * | * | - | * | * | * | * | 7 |
| Nolan et al. [8] | * | * | * | - | * | * | * | * | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pouwels, S.; Johannes, E.; Scarano-Pereira, J.P. Effects of Intravenous Versus Intraosseous Adrenalin Administration on Morbidity and Mortality After Out-of-Hospital Cardiac Arrest: A Systematic Review. Medicina 2025, 61, 680. https://doi.org/10.3390/medicina61040680
Pouwels S, Johannes E, Scarano-Pereira JP. Effects of Intravenous Versus Intraosseous Adrenalin Administration on Morbidity and Mortality After Out-of-Hospital Cardiac Arrest: A Systematic Review. Medicina. 2025; 61(4):680. https://doi.org/10.3390/medicina61040680
Chicago/Turabian StylePouwels, Sjaak, Emschka Johannes, and Juan Pablo Scarano-Pereira. 2025. "Effects of Intravenous Versus Intraosseous Adrenalin Administration on Morbidity and Mortality After Out-of-Hospital Cardiac Arrest: A Systematic Review" Medicina 61, no. 4: 680. https://doi.org/10.3390/medicina61040680
APA StylePouwels, S., Johannes, E., & Scarano-Pereira, J. P. (2025). Effects of Intravenous Versus Intraosseous Adrenalin Administration on Morbidity and Mortality After Out-of-Hospital Cardiac Arrest: A Systematic Review. Medicina, 61(4), 680. https://doi.org/10.3390/medicina61040680







