Long-Term Clinical Outcomes of Ulcerative Colitis with Concurrent Endoscopic and Histologic Remission
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Endoscopic and Histologic Assessment
2.3. Follow-Up and Definition of Clinical Relapse
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
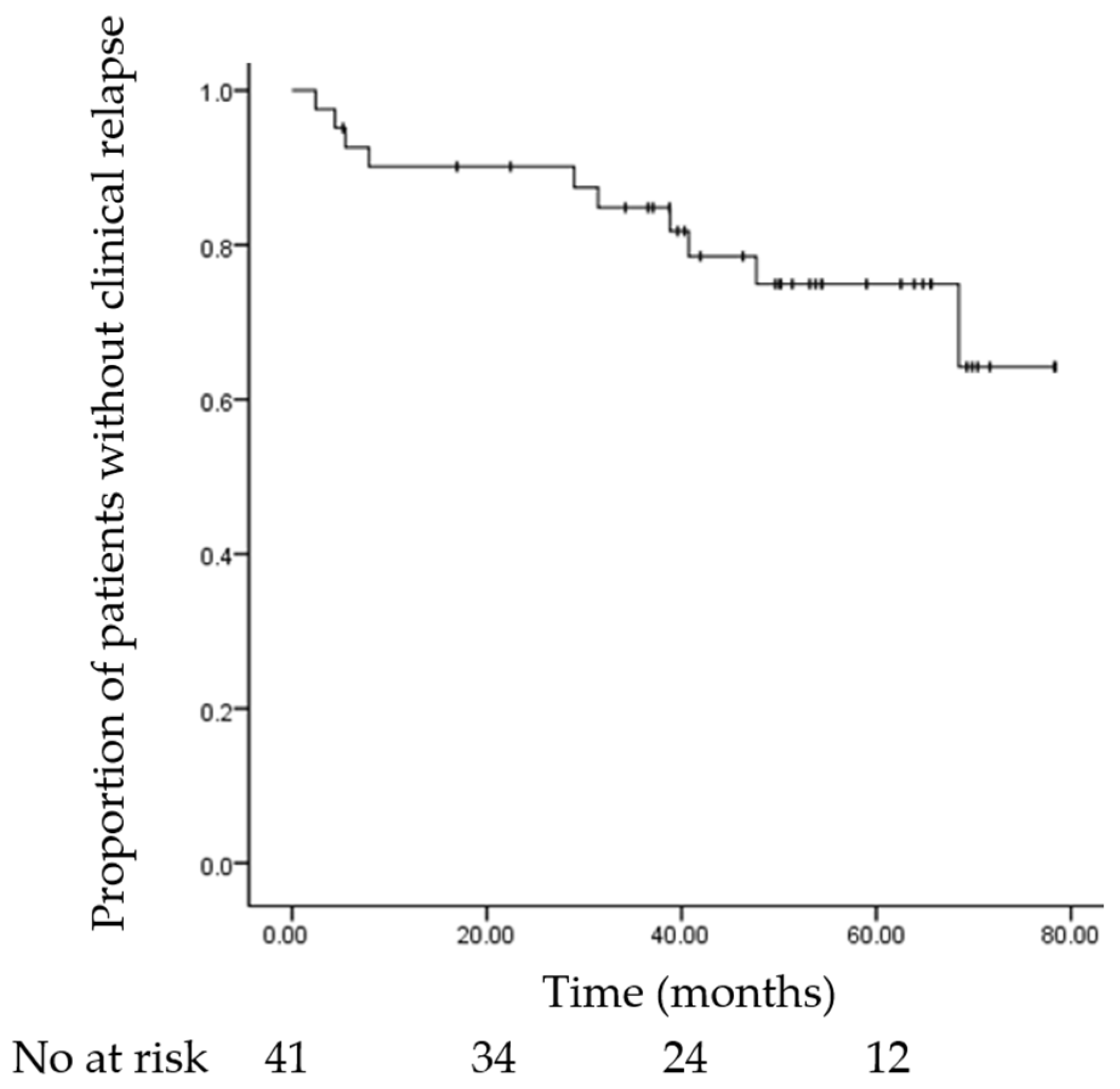
3.2. Relapse Outcomes
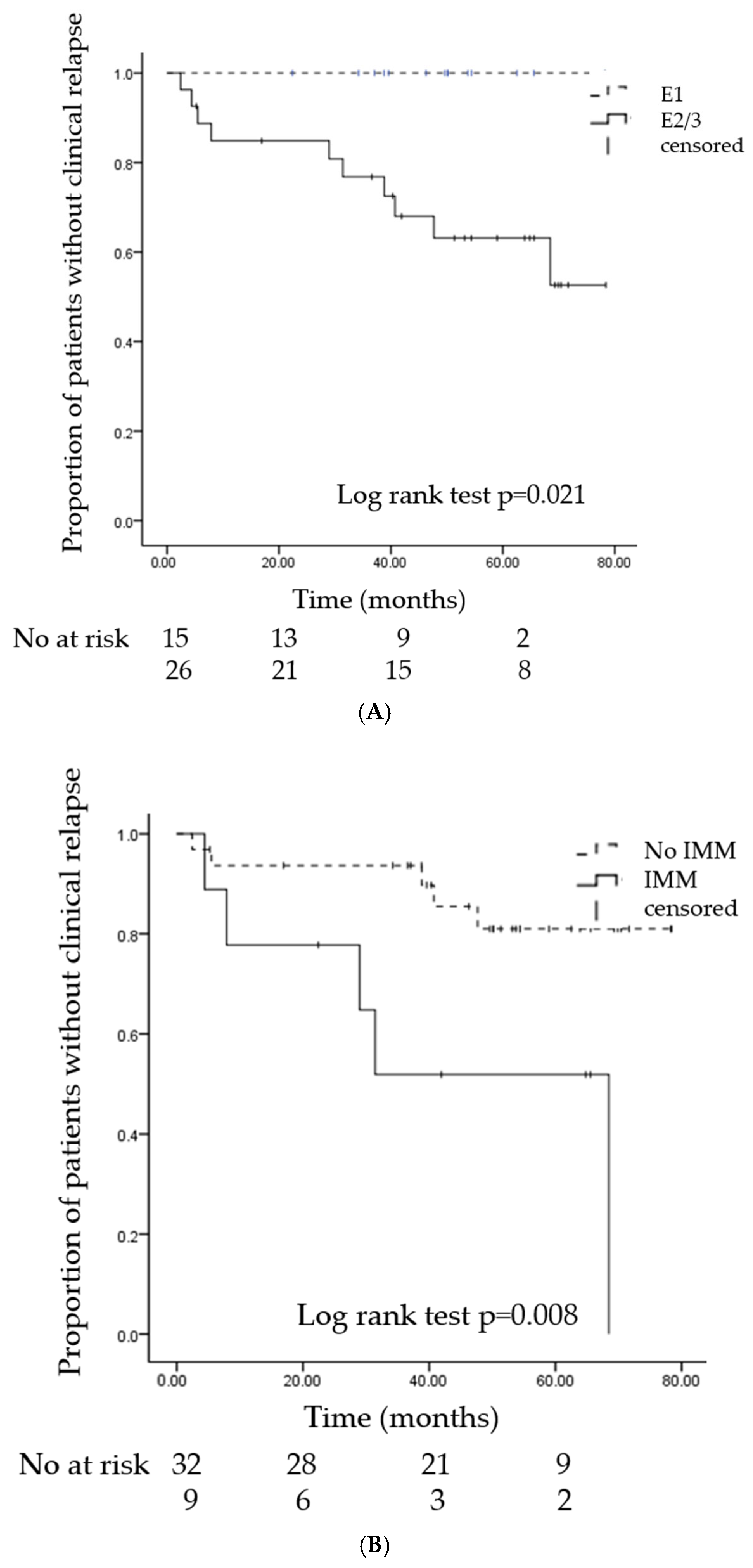
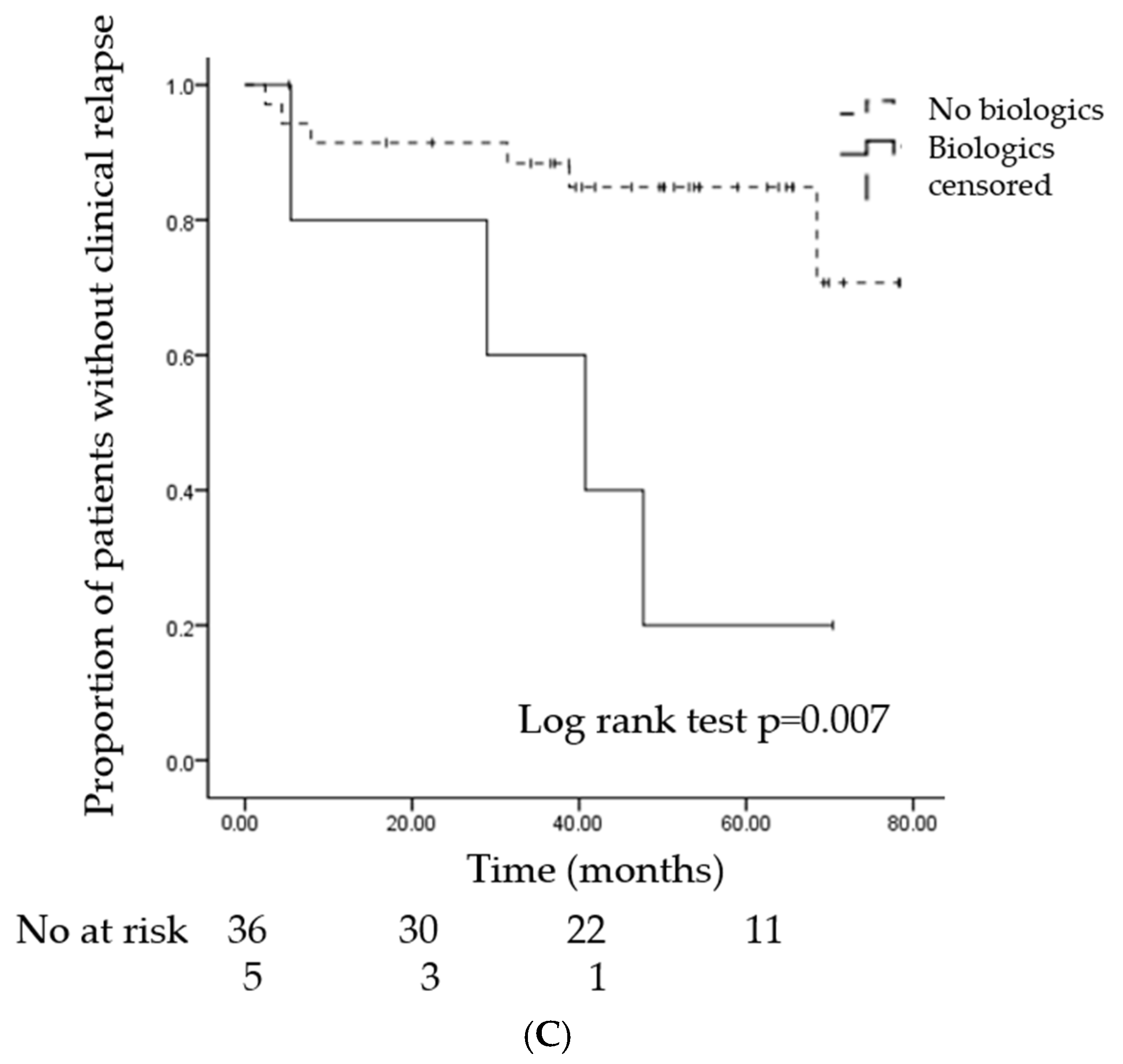
3.3. Risk Factors for Relapse
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UC | Ulcerative colitis |
| MES | Mayo endoscopic subscore |
| FC | fecal calprotectin |
References
- Neurath, M.F.; Travis, S.P. Mucosal healing in inflammatory bowel diseases: A systematic review. Gut 2012, 61, 1619–1635. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Rutgeerts, P.; Reinisch, W.; Esser, D.; Wang, Y.; Lang, Y.; Marano, C.W.; Strauss, R.; Oddens, B.J.; Feagan, B.G.; et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011, 141, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Hanauer, S.B.; Erlich, J.; Kassim, O.; Gibson, P.R.; Turner, J.R.; Hart, J.; Rubin, D.T. Histologic Normalization Occurs in Ulcerative Colitis and Is Associated with Improved Clinical Outcomes. Clin. Gastroenterol. Hepatol. 2017, 15, 1557–1564.e1551. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, B.; Arijs, I.; Van Assche, G.; Sagaert, X.; Geboes, K.; Ferrante, M.; Rutgeerts, P.; Vermeire, S.; De Hertogh, G. Correlation between the endoscopic and histologic score in assessing the activity of ulcerative colitis. Inflamm. Bowel Dis. 2013, 19, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.V.; Burger, D.C.; Delo, J.; Walsh, A.J.; Thomas, S.; von Herbay, A.; Buchel, O.C.; White, L.; Brain, O.; Keshav, S.; et al. Beyond endoscopic mucosal healing in UC: Histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016, 65, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Bressenot, A.; Kampman, W. Histologic remission: The ultimate therapeutic goal in ulcerative colitis? Clin. Gastroenterol. Hepatol. 2014, 12, 929–934.e922. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.K.; Jairath, V.; Vande Casteele, N.; Rieder, F.; Parker, C.E.; Lauwers, G.Y. The emerging role of histologic disease activity assessment in ulcerative colitis. Gastrointest. Endosc. 2018, 88, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, K.M.; Kang, H.S.; Koo, J.S.; Lee, H.S.; Jeong, S.H.; Kim, J.H.; Kim, D.B. Oral Sulfate Solution Is as Effective as Polyethylene Glycol with Ascorbic Acid in a Split Method for Bowel Preparation in Patients with Inactive Ulcerative Colitis: A Randomized, Multicenter, and Single-Blind Clinical Trial. Gut Liver 2023, 17, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Farrokhyar, F.; Marshall, J.K.; Easterbrook, B.; Irvine, E.J. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: Prevalence and impact on health. Inflamm. Bowel Dis. 2006, 12, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Sharara, A.I.; Malaeb, M.; Lenfant, M.; Ferrante, M. Assessment of Endoscopic Disease Activity in Ulcerative Colitis: Is Simplicity the Ultimate Sophistication? Inflamm. Intest. Dis. 2022, 7, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Marchal-Bressenot, A.; Salleron, J.; Boulagnon-Rombi, C.; Bastien, C.; Cahn, V.; Cadiot, G.; Diebold, M.D.; Danese, S.; Reinisch, W.; Schreiber, S.; et al. Development and validation of the Nancy histological index for UC. Gut 2017, 66, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Moon, W.; Kim, Y.S.; Kim, E.S.; Lee, B.I.; Jung, Y.; Yoon, Y.S.; Lee, H.; Park, D.I.; Han, D.S. Second Korean Guideline for the Management of Ulcerative Colitis. Korean J. Gastroenterol. Taehan Sohwagi Hakhoe Chi 2017, 69, 1–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Magro, F.; Langner, C.; Driessen, A.; Ensari, A.; Geboes, K.; Mantzaris, G.J.; Villanacci, V.; Becheanu, G.; Borralho Nunes, P.; Cathomas, G.; et al. European consensus on the histopathology of inflammatory bowel disease. J. Crohn’s Colitis 2013, 7, 827–851. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jangi, S.; Dulai, P.S.; Boland, B.S.; Prokop, L.J.; Jairath, V.; Feagan, B.G.; Sandborn, W.J.; Singh, S. Incremental Benefit of Achieving Endoscopic and Histologic Remission in Patients with Ulcerative Colitis: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 159, 1262–1275.e1267. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, J.; Arajol, C.; Armuzzi, A. Is histologic remission in ulcerative colitis ready for prime time? Dig. Liver Dis. 2017, 49, 1334–1335. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Magro, F.; Siegmund, B.; Kobayashi, T.; Kotze, P.G.; Solitano, V.; Caron, B.; Al Awadhi, S.; Hart, A.; Jairath, V.; et al. Disease Clearance as a New Outcome in Ulcerative Colitis: A Systematic Review and Expert Consensus. Inflamm. Bowel Dis. 2024, 30, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Fiorino, G.; Solitano, V.; Massarini, E.; Guillo, L.; Allocca, M.; Furfaro, F.; Zilli, A.; Bonovas, S.; Magro, F.; et al. Ulcerative colitis: Impact of early disease clearance on long-term outcomes—A multicenter cohort study. United Eur. Gastroenterol. J. 2022, 10, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Theede, K.; Holck, S.; Ibsen, P.; Kallemose, T.; Nordgaard-Lassen, I.; Nielsen, A.M. Fecal Calprotectin Predicts Relapse and Histological Mucosal Healing in Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, S.; Nishijima, S.; Kojima, Y.; Kimura, M.; Ohsugi, M.; Ueki, K.; Mizokami, M.; Hattori, M.; Tsuchiya, K.; Uemura, N.; et al. Multi-biome analysis identifies distinct gut microbial signatures and their crosstalk in ulcerative colitis and Crohn’s disease. Nat. Commun. 2024, 15, 10291. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 41) | |
|---|---|
| Male Sex, n (%) | 23 (56.1) |
| Age, yr † | 54 ± 14 |
| Disease duration, months * | 55 [3, 262] |
| Follow-up duration, months * | 54 [17, 78] |
| Clinical activity, n (%) | |
| Mayo score 0 | 36 (87.8) |
| Mayo score 1 | 5 (12.2) |
| Extension, n(%) | |
| Proctitis (E1) | 15 (36.6) |
| Left-sided (E2) | 15 (36.6) |
| Extensive (E3) | 11 (26.8) |
| Current treatment, n (%) | |
| No medication | 1 (2.4) |
| 5-ASA | 40 (97.6) |
| Immunomodulators | 9 (22) |
| Biologics | 5 (12.2) |
| topical corticosteroids (budesonide enema) | 3 (7.3) |
| (A) | Relapse (n = 10) | No Relapse (n = 31) | p-Value |
| Extension: E2 + E3, n (%) | 10 (100) | 16 (52) | 0.02 |
| Immunomodulators, n (%) | 5 (50) | 4 (13) | 0.01 |
| Biologics, n(%) | 4 (40) | 1 (3) | 0.02 |
| Disease duration, months * | 66 [3, 204] | 51 [3, 262] | NS |
| CRP, mg/dL † | 0.06 ± 0.03 | 0.13 ± 0.14 | NS |
| Fecal calprotectin, μg/g † | 181.8 ± 370.1 | 108.4 ± 164.4 | NS |
| (B) | Hazard Ratio | 95% Confidence Interval | p-Value |
| Extension: E2 + E3 | 41.1 | 0.08–9963.2 ** | NS |
| Immunomodulators | 4.7 | 1.3–16.4 | 0.02 |
| Biologics | 4.9 | 1.4–17.5 | 0.01 |
| Disease duration | 1.0 | 1.0–1.1 | NS |
| CRP | 0.001 | 0–152.0 * | NS |
| Fecal calprotectin | 1.0 | 1.0–1.1 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.M.; Lee, K.-M.; Kim, D.B.; Jung, J.-H. Long-Term Clinical Outcomes of Ulcerative Colitis with Concurrent Endoscopic and Histologic Remission. Medicina 2025, 61, 1968. https://doi.org/10.3390/medicina61111968
Lee JM, Lee K-M, Kim DB, Jung J-H. Long-Term Clinical Outcomes of Ulcerative Colitis with Concurrent Endoscopic and Histologic Remission. Medicina. 2025; 61(11):1968. https://doi.org/10.3390/medicina61111968
Chicago/Turabian StyleLee, Ji Min, Kang-Moon Lee, Dae Bum Kim, and Ji-Han Jung. 2025. "Long-Term Clinical Outcomes of Ulcerative Colitis with Concurrent Endoscopic and Histologic Remission" Medicina 61, no. 11: 1968. https://doi.org/10.3390/medicina61111968
APA StyleLee, J. M., Lee, K.-M., Kim, D. B., & Jung, J.-H. (2025). Long-Term Clinical Outcomes of Ulcerative Colitis with Concurrent Endoscopic and Histologic Remission. Medicina, 61(11), 1968. https://doi.org/10.3390/medicina61111968






