Abstract
Background and Objectives: Proteomics encompasses the exploration of protein composition, regulation, function, and pathways. Its influence spans diverse clinical fields and holds promise in addressing various women’s health conditions, including cancers, osteoporosis, and cardiovascular disorders. However, no comprehensive summary of proteomics and menopausal health exists. Our objective was to summarize proteomic profiles associated with diseases and disorders in peri- and postmenopausal women. Materials and Methods: We conducted a comprehensive search of databases including PubMed, Google Scholar, the Cochrane database, Elsevier, and ScienceDirect until 2022. A total of 253 studies were identified, and 41 studies met the inclusion criteria to identify data of interest. These included the study design, disease, and proteomics/proteins of significance, as described by the authors. Results: The 41 studies covered diverse areas, including bone disorders (10 studies), cardiovascular diseases (5 studies), oncological malignancies (10 studies), and various conditions, such as obesity, nonalcoholic liver disease, the effects of hormone replacement therapy, and neurological diseases (16 studies). The results of our study indicate that proteomic profiles correlate with heart disease in peri- and postmenopausal women, with distinct sex differences. Furthermore, proteomic profiles significantly differ between women with and without osteoporosis. Additionally, patients with breast, ovarian, and endometrial cancer exhibit notable variations in proteomic profiles compared to those without these conditions. Conclusions: Proteomics has the potential to enhance risk assessment and disease monitoring in peri- and postmenopausal women. By analyzing unique protein profiles, clinicians can identify individuals with heightened susceptibility to specific diseases or those already affected by established conditions. This review suggests that there is sufficient preliminary data related to proteomics in peri- and postmenopausal women for early identification of cardiovascular disease, osteoporosis, and cancers, disease monitoring, and tailoring individualized therapies. Rigorous validation studies involving large populations are essential before drawing definitive conclusions regarding the clinical applicability of proteomic findings.
1. Introduction
Menopause, which occurs on average between the ages of 45 and 55, marks the end of a woman’s reproductive period. It is generally preceded by a perimenopausal phase characterized by ovarian hormonal fluctuations and irregular menstrual cycles [1]. Common symptoms during menopause include hot flashes, sleep disturbances, mood changes, and cognitive dysfunction [2,3]. Menopause can also be surgically induced in women who undergo bilateral salpingo-oophorectomy (BSO), which results in a sudden cessation of ovarian hormone production. Menopausal symptoms in these women tend to be more severe in comparison with natural menopause [2,4]. Medical conditions linked to menopause include osteoporosis, endometrial cancer, breast cancer, and cardiovascular diseases [5,6].
The decline in estrogen levels during the menopause transition is reported to increase the risk of cardiovascular disease and inflammation [7]. The menopause transition also affects various lipid parameters, including total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and apolipoprotein, independent of aging [7]. Additionally, the prevalence of metabolic syndrome increases during the menopausal transition, also independent of aging [8,9,10,11]. The menopause transition period also adversely impacts measures of body composition and vascular health [7]. These changes contribute to a higher risk of plaque buildup, atherosclerosis, and cardiovascular disease reported in postmenopausal women [8]. Women who undergo surgical menopause are reported to have a higher risk of coronary artery diseases and stroke due to the rapid decline in estrogen, further strengthening this association [2,4]. Estrogen loss during the menopause transition also accelerates changes in bone density, heightening the risk of osteopenia and osteoporosis, a condition with significant morbidity in postmenopausal women [12]. As estrogen levels drop, bone resorption surpasses bone formation, leading to reduced bone mineral density and increased fracture susceptibility [12]. Women with menopause induced surgically are reported to have a higher risk of osteoporosis compared to women with natural menopause as a result of the rapid loss of estrogen [2,4]. Although the hormonal changes associated with the menopause transition are not directly linked to an increased risk of cancers, the prevalence of multiple cancers increases in the postmenopausal years, which is likely a function of age. The most common cancers reported in postmenopausal women are breast, lung, colon, endometrial, and ovarian cancers. The menopausal transition, which is associated with an increased risk of cardiovascular disease, osteoporosis, and cancers, presents a crucial window for early disease detection and treatment using novel and innovative techniques. Recent proteomics studies suggest that examining disease mechanisms at the proteome level could be instrumental in identifying biomarkers for conditions before they manifest [13,14]. Therefore, proteomic research offers an opportunity to identify biomarkers related to menopausal conditions prior to disease onset.
Protein activity, localization, and turnover are the primary processes that regulate physiological changes in the human body. Because the proteome is dynamic and it changes over time depending on physiological conditions, i.e., a healthy physiological state versus a pathological stage, the study of proteins has the potential for identifying disease-specific biomarkers and elucidating molecular mechanisms underlying disease states [13,15]. Proteomic studies involve collecting serum or plasma samples followed by processing proteins ex vivo and digesting them into peptides. Following this, separation techniques, including liquid chromatography (LC), Matrix-Assisted Laser Desorption/Ionization (MALDI), surface-enhanced laser desorption/ionization (SELDI), single-cell proteomics, and spectrometry-based glycoproteomics and glycomics assays, aid in protein identification [16,17,18]. The protein data generated from these techniques undergo computational analysis whereby proteins are matched against established proteomic databases [17,18]. Bioinformatics tools are employed to analyze and interpret the data in the context of biological pathways. Interpretation of proteomic data often involves integrating findings with existing biological knowledge and experimental validation [17,18]. Researchers correlate changes in protein expression or modification with known physiological functions or disease mechanisms to deduce pathways that are dysregulated in specific conditions. Peptide platforms are therefore powerful tools for clinical and biological inquiries, and they can guide individualized therapies, thus enhancing personalized medicine [19,20].
Proteomic studies related to menopause have shown promise in addressing menopausal-related conditions. By identifying and quantifying different proteins from biological samples using peptide platforms, researchers can compare the proteomes of premenopausal and postmenopausal women and determine the changes that occur and their physiological and pathophysiological implications [4]. For example, a study examined 71 biomarkers and identified seven proteins that were significantly associated with early menopause [21]. In another study, postmenopausal women with osteoporosis exhibited distinct proteomic profiles in comparison to postmenopausal women with osteopenia [22]. Proteomics has also been explored in breast, ovarian, and endometrial cancer, obesity, and cardiovascular disease in menopausal women. While these studies suggest a promising role for proteomics in menopause-related diseases, no comprehensive summary of proteomics and menopausal health exists to date. Our study aims to fill this gap by identifying relevant proteomic research and summarizing key findings and clinical implications.
2. Materials and Methods
2.1. Protocol and Search Strategy
A comprehensive search from 2010 to 28 October 2022 was conducted. The databases searched included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process and Other Non-Indexed Citations, and Daily, Ovid EMBASE, the Ovid Cochrane Central Register of Controlled Trials, the Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced research librarian with input from the study’s principal investigator. Controlled vocabulary supplemented with keywords was used to search for proteomic testing in menopausal women and animal models. MESH terms were added when applicable to retrieve relevant studies, including menopause, perimenopause, postmenopause, proteomics, glycoproteomic, etc.
2.2. Eligibility Criteria
We included original papers that discussed serum or plasma proteomics in peri- and postmenopausal women. The study types included case-control studies, cohorts, cross-sectional analysis, randomized clinical trials, non-randomized clinical trials, and diagnostic-test-accuracy assessments. Studies were excluded if (1) only tissue proteomics were studied, (2) the study was conducted on animals or cell culture, (3) the study population was restricted to include only men or premenopausal women, (4) the studies were not original papers (review articles, editorials, commentaries, etc.), or (5) the studies were published in languages other than English.
2.3. Screening and Data Extraction
All retrieved studies were imported into COVIDENCE, a standardized platform for systematic and narrative reviews [23]. Following this, duplicates were eliminated, and abstracts were screened for eligibility criteria. All studies that met the eligibility criteria were evaluated to identify data of interest. This included the study design, the disease studied, and proteomics/proteins of significance, as described by the authors.
3. Results
3.1. Study Identification, Screening, and Inclusion
Our searches identified 253 studies. After the removal of duplicates, 249 studies were eligible for abstract screening, and 69 underwent full-text screening. In total, 41 studies met the study criteria and were included in this review. Details of the screening process and the inclusion of studies are reported in Figure 1.
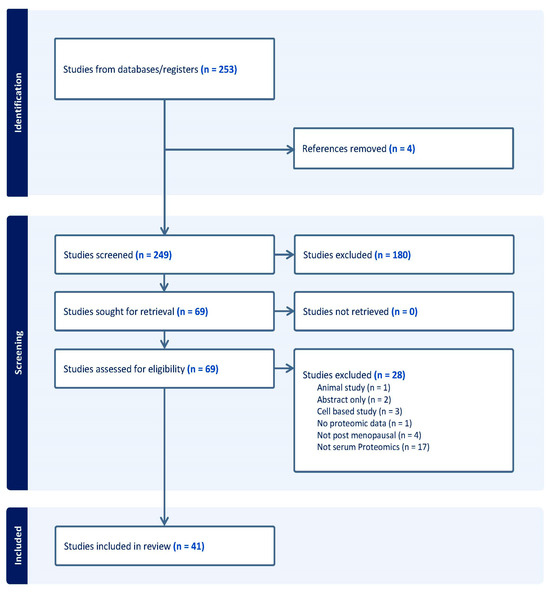
Figure 1.
Study identification screening and inclusion.
3.2. Characteristics of Included Studies
Of the 41 studies, most were case-control studies (23 studies), followed by cohort studies (12 studies), non-randomized clinical trials (3 studies), randomized clinical trials (2 studies), and one diagnostic test accuracy study [5,22,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. The majority of these studies were conducted in the United States (21 studies), followed by Asia (7 studies) and Europe (5 studies). We included studies published until 2022, and the studies had a median (IQR) of 102 (26.5–388) participants. These studies explored a wide range of diseases and pathologies affecting the population. Specifically, five studies focused on proteomics, biomarkers, and pathways involved in cardiovascular health, ten focused on various cancers, and ten focused on bone diseases. The remaining 16 studies addressed topics including aging, migraine, weight loss, premature ovarian failure, and nonalcoholic fatty liver disease. The details of the included studies are presented in Table 1.

Table 1.
Summary of proteomic studies.
3.3. Proteomics in Cardiovascular Health
We identified five studies identifying over 50 protein markers and pathways associated with CVD. The most significant proteomic findings identified by these authors are reported in Table 1. Prentice et al. (2010, 2013) identified proteins, such as B2M, ORM1, and IGFALS, as potential CVD diagnostic markers for stroke and heart disease and B2M, IGFBP1, THBS1, and CFD as predictors of hyperlipidemia and hypertension [50,51]. Protein markers influencing cholesterol efflux capacity predicting cardiovascular disease were studied by Jin et al. (2019), who identified apolipoproteins AI, CI, CII, CIII, and CIV as potential biomarkers of CVD [38]. Lau et al. (2019) reported sex-based differences in the proteins associated with CVD. They identified apolipoprotein B, CD14, and pro-basic platelet protein as proteomic markers associated with an increased incidence of cardiovascular disease in women compared to men [41]. Similarly, Appiah et al. (2022) identified several proteins associated with atherosclerosis in peri- and postmenopausal women [5]. In summary, proteomics studies related to cardiovascular health and disease suggest that proteomics testing may potentially detect and predict coronary artery disease and stroke. Additionally, there may be gender differences in men and women with coronary artery disease.
3.4. Proteomics in Oncology
We identified 10 studies that reported proteomic biomarkers associated with the detection, diagnosis, and prognosis of various oncological diseases, including ovarian cancer, endometrial cancer, adnexal mass, breast lesions, and breast cancer. The most significant proteomic findings identified by these authors are reported in Table 1. Bergen et al. (2003) highlighted the association between Fibrinopeptide-A concentrations and their correlation with ovarian cancer stages [25]. Tarney et al. (2019) and Celsi et al. (2022) investigated differences in biomarkers in postmenopausal women with and without endometrial cancer and reported a combined difference of 71 proteins between the two groups [28,57]. Among these six proteomic markers, namely, complement factor B, serotransferrin, catalase, proteasome subunit beta type-6, beta-2-microglobulin, and protocadherin-18, they were reported to have high diagnostic potential for endometrial cancer, and suprabasin was identified as a potential marker for poor-prognostic endometrial cancer [28,57]. Watrowski et al. (2022) identified CA125 and osteopontin as proteins with high discriminating power and diagnostic index for adnexal mass malignancy [60]. Delmonico et al. (2016) reported differences in nine proteins in pre- and postmenopausal women with either fibroadenoma or invasive ductal carcinoma in comparison to controls [32]. Among the identified proteins, five were significantly altered in both disease states and included α-2-macroglobulin, ceruloplasmin, haptoglobin, hemopexin, and vitamin D binding protein [32]. Five studies investigated proteomics associated with breast cancer [27,39,43,45,48]. Katayama et al. (2019) examined prognostic proteins in triple-negative breast cancer and identified 43 proteins and three genes with prognostic value [39]. Piterri et al. (2010), Henderson et al. (2019), Li et al. (2011), and Carlsson et al. (2008) focused on breast cancer detection and collectively identified 125 significant proteins that can be implicated in early breast cancer detection, diagnosis, or its metastasis [27,43,45,48]. Among the identified proteins, epidermal growth factor receptor was found to be a significant predictor by Li et al. (2011) and Piterri et al. (2010) [43,48]. In summary, proteomic research may be potentially beneficial for the early detection of breast, ovarian, and endometrial cancer. It may also help predict cancer risk among hormone-therapy users and guide personalized management of these conditions.
3.5. Proteomics in Bone Health
We identified a total of 10 studies providing insight into the role of proteomics in osteoporosis and osteopenia. The most significant proteomic findings identified by these authors are reported in Table 1. Soh et al. (2022), Li et al. (2013), and Zhang et al. (2016) investigated osteoporosis in postmenopausal women, identifying 138 protein peaks, seven gene sets, and three proteins predictive of osteoporosis [44,55,62]. Daswani et al. (2015) studied osteoporosis in pre- and postmenopausal women, identifying 35 proteins significantly altered in postmenopausal women, including tHSP25 and pHSP25, which play a role in monocyte migration and osteoporosis development and therefore can be beneficial in disease prediction [31]. Shi et al. (2015) and Shi et al. (2017) investigated primary osteoporosis in postmenopausal women and reported that 16 protein peaks and 87 proteins were differentially expressed in postmenopausal women with and without osteoporosis [52,53]. Among these, GAS6, SPP24, RAB7A, and TSP are known to play a role in fibroblast proliferation, angiogenesis, and immune response and may offer insight into the mechanism of osteoporosis [52]. He et al. (2016) identified 10 protein peaks predictive of osteopenia in postmenopausal women; among the proteins identified, secretin was reported to have the highest predictive power [37]. Martinez-Aguilar et al. (2019), Huang et al. (2020), and Pepe et al. (2022) focused on postmenopausal women with either osteoporosis or osteopenia, identifying several proteins and miRNAs that can be utilized in predicting, diagnosing, and differentiating osteoporosis and osteopenia in postmenopausal women [22,46,47]. Among the identified proteins, ceruloplasmin, fibrinogen, vitronectin, clusterin, and apolipoprotein were reported to be most significant [46,47]. In summary, proteomics may be a potentially beneficial diagnostic tool for osteoporosis and osteopenia in pre- and postmenopausal women.
3.6. Proteomics in Other Disease States
The remaining 11 studies provided insight into the role of proteomics in understanding various diseases and conditions, such as obesity, migraine, cognitive impairment, and nonalcoholic fatty liver disease. The most significant proteomic findings identified by these authors are reported in Table 1. Four studies examined the impact of hormone replacement therapy (HRT); one study examining HRT’s impact on CVD, one studied HRT’s impact on breast cancer risk, and another studied the reproducibility of biomarkers over time [36,49,58,59]. Collectively, these studies identified over 200 proteomic biomarkers that had the potential to distinguish between HRT users and non-users [36,49,58,59]. Gericke et al. (2005) identified several proteomic markers, including differences in endothelin I/II and endothelin-converting enzymes, in cardiovascular disease between HRT users and non-users and highlighted their potential use to monitor inflammatory responses [36]. Thomas et al. (2022) identified 54 proteins altered in postmenopausal women with a history of HRT and their possible use to detect breast cancer in patients with prior hormone therapy [58]. The study also stated that proteomic changes associated with HRT were visible even years after stopping HRT [59]. This was also concluded by Tworoger et al. (2008), indicating that biomarkers can serve as long-term indicators of women’s health status [59]. Pitteri et al. (2009) compared proteomic differences between postmenopausal women on estrogen-only or combined HRT therapy [49]. They identified approximately 192 proteins that differed between the groups and concluded that proteomic differences can provide insight into the varying health outcomes associated with different hormone therapies [49].
Fuchs et al. (2007) investigated the effects of dietary intervention, i.e., soy isoflavones, on cardiovascular diseases [34]. They reported that 29 proteins, including Heat Shock Protein 70, lymphocyte-specific protein phosphatase, α-enolase, and galectin-1, had potential as biomarkers of atherosclerosis-preventive activities induced by soy isoflavone consumption [34]. Cai et al. (2018) explored proteomic changes associated with nonalcoholic fatty liver disease, identifying 167 proteins and highlighting serum RBP4 and LGAL3BP as potential early diagnostic markers of alcoholic liver disease in postmenopausal women [26]. Lal et al. (2019) examined proteomic markers related to cognitive impairment in postmenopausal women with obstructive sleep apnea [40]. They identified 22 proteins, including ceruloplasmin and adiponectin, that had potential for early disease prediction [40]. Lee et al. (2019) studied proteomics profiles in women with premature ovarian failure, identifying 11 proteins, including Ceruloplasmin, Complement C3, Fibrinogen α, Fibrinogen β, and Sex-Hormone-Binding Globulin (SHBG), that were predictive of premature ovarian failure [42]. Shin et al. (2022) investigated aging-related proteomic changes, revealing three proteins associated with chronological aging, namely Growth-Differentiation Factor 15, insulin-like growth factor binding protein-2, and tumor necrosis factor-a, and two proteins associated with menopausal age, namely IL-8 and monocyte chemoattractant protein-1, in addition to ten proteins associated with both chronological and menopausal age [54]. Bellei et al. (2020) investigated proteins associated with migraines in peri- and postmenopausal women [24]. Among the 12 proteins significantly associated with migraines, apolipoprotein A-I was the most significant in perimenopausal women, and transthyretin was most significant in postmenopausal women [24]. Wong et al. (2008) explored proteomic profiles associated with weight loss in postmenopausal women with a recent history of HRT use [61]. Among the 57 identified proteins, sustained weight loss was associated with decreased IL-1, IL-6, and C-reactive protein [61]. Similarly, JAK-STAT and NF-κB pathways were identified as significant obesity-associated pathways in another study by Garrison et al. (2017) [35].
Sun et al. (2019) also reported that 173 proteins were differently expressed between lean and obese postmenopausal women with increased breast density who were on Lovaza [56]. Among the proteins identified, gelsolin, vitamin D binding protein isoform 1 precursor, and fibronectin isoform 10 precursor were noted to be associated with favorable breast densities [56]. These findings have similarities to the study by Fabian et al., who reported favorable proteomic profiles related to breast cancer risk in postmenopausal patients supplemented with an omega-3-rich diet [33]. Finally, Dalenc et al. (2010) and Chao et al. (2013) identified proteins that could discriminate between responses to immune therapies and hormone therapies, respectively, towards breast cancer [29,30]. In summary, numerous studies suggest that proteomic research may have diverse future applications for personalized health management in postmenopausal women. These varied in the detection of conditions, such as nonalcoholic fatty liver disease, obstructive sleep apnea, cognitive impairment, migraine, and premature ovarian failure. Additionally, proteomic profiles may be beneficial for predicting treatment response to therapies, such as omega-3 fatty acids, Lovaza, soy isoflavones, and hormone therapy.
4. Discussion
As we enter a new era of clinical science, biomarkers play a crucial role in identifying the underlying causes of diseases and guiding targeted therapies. Traditionally, clinicians have relied on routine blood tests, such as cell counts, biochemical profiles, hormonal levels, inflammatory markers, and lipid levels, to evaluate patients’ health. There has been an evolution of lab testing to the point that more than 7000 proteins can be tested quickly in a single sample, yet the interplay between these proteins and the occurrence of disease is an area we are just beginning to understand. This review elucidates the effectiveness of proteomic approaches in understanding, diagnosing, and predicting outcomes across diverse pathologies affecting the postmenopausal population, including osteoporosis, cancers (breast, ovarian, and endometrial), and cardiovascular diseases (stroke, coronary heart disease, and atherosclerosis), among others.
The results of our review demonstrate that proteomics could play a pivotal role in the future in advancing disease screening and the early detection of various conditions in asymptomatic patients. Multiple studies in this review support the role of proteomics in the early detection of coronary heart disease, breast, ovarian, and endometrial cancer, cognitive impairments, and premature ovarian failure [25,32,40,42,43,50,51,57]. For example, Bergen et al. (2003) demonstrated the role of Fibrinopeptide-A in the early detection of ovarian cancer, Li et al. (2011) demonstrated the role of EGFR in the early detection of early breast cancer, and Tarney et al. (2019) demonstrated the diagnostic potential of a 47-protein panel in the early diagnosis of endometrial cancer [25,43,57].
Cardiovascular disease, which is currently the leading cause of death in women, also contributes substantially to the health disparity between men and women in the United States [63]. Prentice et al. (2010, 2013) and Jin et al. (2019) demonstrated the diagnostic potential of proteomic profiles in the detection of cardiovascular disease in women [38,50,51]. Future strategies, such as validated predictive cardiovascular proteomics platforms, offer promising avenues for the early detection and prevention of cardiovascular disease in women, thus aiming to narrow the current health gap and improve long-term outcomes. Lau et al. (2019) suggested that the mechanisms of cardiovascular disease may be different in women and men. In their study, they noted significant differences in predictive proteins, such as apolipoprotein B, adipokines, and inflammatory markers, between men and women [41]. This highlights the need for sex-specific proteomic studies specific to cardiovascular health [41].
Breast cancer, the most common malignancy among women worldwide, remains a formidable health challenge [64]. It affects millions of women annually and ranks as one of the leading causes of cancer-related deaths. Beyond the physical toll, breast cancer imposes a substantial economic burden. Healthcare costs associated with diagnosis, treatment, and follow-up care contribute significantly to this impact. Additionally, lost productivity due to illness affects both patients and their families, further straining the economy. Ovarian and endometrial cancer, although less prevalent than breast cancer, also carry a significant mortality risk. Early detection remains a challenge, and treatment costs add to the economic burden. Currently, only breast cancer benefits from a clinically available screening tool—mammography. However, this method has limitations, including false positives and patient discomfort. For ovarian and endometrial cancers, no established screening methods exist. In this review, case-control studies by Piterii et al. (2010) and Li et al. (2011), and a cohort study by Henderson et al. (2019), demonstrated the potential utility of proteomics for breast cancer detection [43,45,48]. Similarly, case-control studies by Bergen et al. (2003) and Tarney et al. (2019) demonstrate the potential utility of proteomic biomarkers in the early detection of ovarian and endometrial cancer [25,57]. These approaches deserve further research and validation. Serum biomarkers specific to these cancers could revolutionize early detection by enabling timely intervention and improving outcomes.
Proteomic biomarkers could facilitate the monitoring of therapeutic efficacy and personalized interventions, thus guiding treatment optimization and individualized care. For instance, Sun et al. (2019) reported differential effects on proteomic profiles in obese and non-obese women, suggesting that proteomic biomarkers can facilitate the monitoring of therapeutic efficacy [56]. Additionally, Dalenc et al. (2010) reported that fibrinogen alpha could discriminate between responders and non-responders in a cohort of women with resistant metastatic breast cancer [30]. Similarly, Gericke et al. (2005) reported the differential influence of hormone therapy on inflammatory response associated with cardiovascular disease [36]. These insights underscore the importance of proteomics in tailoring treatment and improving patient outcomes.
Proteomic studies could also offer beneficial insight into underlying disease mechanisms and pathways, shedding light on disease pathogenesis and progression. For instance, Huang et al. (2020) found that proteins, such as Lysozyme C, glucosidase, and protein disulfide isomerase A5, were differentially associated with bone density [22]. Additionally, Shi et al. (2017) reported differential expression of proteins involved in fibroblast proliferation, angiogenesis, and immune response in postmenopausal women with osteoporosis [52]. Further study of these proteins and associated pathways could help us better understand the disease mechanism underlying osteoporosis. Similarly, Garrison et al. (2017) explored the role of specific pathways in obesity, identifying JAK-STAT and NF-κB pathways as important regulators [35]. Further study of these pathways could help us better understand the pathophysiology of obesity and develop personalized interventions.
5. Conclusions
Proteomics has the potential to facilitate early identification of diseases and advance our understanding of disease mechanisms, thus ultimately improving patient outcomes. This review suggests that there are sufficient preliminary data to recommend future research into proteomics for peri- and postmenopausal women across a variety of disease conditions. Rigorous validation studies involving large populations are essential before drawing definitive conclusions about the clinical applicability of proteomic findings.
6. Future Directions
The future of proteomics in women’s health looks promising in many regards. These include the development of targeted biomarker panels for early disease detection, leveraging proteomics to tailor targeted individualized treatments, optimizing drug response, and developing a deeper understanding of biological dysregulations that underlie important health concerns in women’s health.
Author Contributions
Conceptualization, B.E.K., A.V., B.T., M.W. and S.N.; methodology, B.E.K., A.V., B.T., M.W. and S.N.; software, not applicable; validation, B.E.K., P.F., A.V., B.T., M.W., A.R.H., M.A., S.N., C.A. and A.S.; investigation, B.E.K., P.F., A.V., B.T., M.W., A.R.H., M.A., S.N., C.A. and A.S.; data curation, B.E.K., P.F., A.V., B.T., M.W., A.R.H., M.A., S.N., C.A. and A.S.; writing—original draft preparation, B.E.K., P.F., A.V., B.T., M.W., A.R.H., M.A., S.N., C.A. and A.S.; writing—review and editing, B.E.K., P.F., A.V., B.T., M.W., A.R.H., M.A., S.N., C.A. and A.S.; visualization, A.V.; supervision, A.V.; project administration, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Santoro, N.; Roeca, C.; Peters, B.A.; Neal-Perry, G. The Menopause Transition: Signs, Symptoms, and Management Options. J. Clin. Endocrinol. Metab. 2020, 106, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pillay, O.C.; Manyonda, I. The surgical menopause. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 81, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, P.; Harlow, S.D.; Nan, B.; Greendale, G.A.; Santoro, N.; Crawford, S.L.; Gold, E.B.; Tepper, P.G.; Randolph, J.F.J. Duration of the menopausal transition is longer in women with young age at onset: The multiethnic Study of Women’s Health Across the Nation. Menopause 2017, 24, 142–149. [Google Scholar] [CrossRef]
- Garg, A.; Robinson, L. Surgical menopause: A toolkit for healthcare professionals. Post Reprod. Health 2021, 27, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Appiah, D.; Schreiner, P.J.; Pankow, J.S.; Brock, G.; Tang, W.; Norby, F.L.; Michos, E.D.M.; Ballantyne, C.M.; Folsom, A.R. Long-term changes in plasma proteomic profiles in premenopausal and postmenopausal Black and White women: The Atherosclerosis Risk in Communities study. Menopause 2022, 29, 1150–1160. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, L.; Zhu, W.; Xu, C.; He, H.; Zhou, Y.; Liu, Y.-Z.; Tian, Q.; Zhang, J.-G.; Deng, F.-Y.; et al. Quantitative proteomics and integrative network analysis identified novel genes and pathways related to osteoporosis. J. Proteom. 2016, 142, 45–52. [Google Scholar] [CrossRef]
- Tepper, P.G.; Randolph, J.F.; McConnell, D.S.; Crawford, S.L.; El Khoudary, S.R.; Joffe, H.; Gold, E.B.; Zheng, H.; Bromberger, J.T.; Sutton-Tyrrell, K. Trajectory Clustering of Estradiol and Follicle-Stimulating Hormone during the Menopausal Transition among Women in the Study of Women’s Health across the Nation (SWAN). J. Clin. Endocrinol. Metab. 2012, 97, 2872–2880. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation 2020, 142, 506–532. [Google Scholar] [CrossRef]
- Freeman, E.W.; Sammel, M.D. Methods in a longitudinal cohort study of late reproductive age women: The Penn Ovarian Aging Study (POAS). Women’s Midlife Health 2016, 2, 1. [Google Scholar] [CrossRef]
- Gurka, M.J.; Vishnu, A.; Santen, R.J.; DeBoer, M.D. Progression of Metabolic Syndrome Severity During the Menopausal Transition. J. Am. Heart Assoc. 2016, 5, e003609. [Google Scholar] [CrossRef]
- Janssen, I.; Powell, L.H.; Crawford, S.; Lasley, B.; Sutton-Tyrrell, K. Menopause and the Metabolic SyndromeThe Study of Women’s Health Across the Nation. Arch. Intern. Med. 2008, 168, 1568–1575. [Google Scholar] [CrossRef]
- Blake, J.; Cosman, F.A.; Lewiecki, E.M.; McClung, M.R.; Pinkerton, J.; Shapiro, M. Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, J.-W.; Yun, J.W.; Chung, I.-S.; Cho, H.C.; Song, S.-E.; Im, S.-S.; Song, D.-K. Proteomics approach to identify serum biomarkers associated with the progression of diabetes in Korean patients with abdominal obesity. PLoS ONE 2019, 14, e0222032. [Google Scholar] [CrossRef]
- Pant, A.; Anjankar, A.P.; Shende, S.; Dhok, A.; Jha, R.K.; Manglaram, A.V. Early detection of breast cancer through the diagnosis of Nipple Aspirate Fluid (NAF). Clin. Proteom. 2024, 21, 45. [Google Scholar] [CrossRef]
- Williams, S.A.; Kivimaki, M.; Langenberg, C.; Hingorani, A.D.; Casas, J.P.; Bouchard, C.; Jonasson, C.; Sarzynski, M.A.; Shipley, M.J.; Alexander, L.; et al. Plasma protein patterns as comprehensive indicators of health. Nat. Med. 2019, 25, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Harsha, H.C.; Pinto, S.M.; Pandey, A. Proteomic Strategies to Characterize Signaling Pathways. Methods Mol. Biol. 2013, 1007, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.; Veenstra, T.D. Separation of Serum and Plasma Proteins for In-Depth Proteomic Analysis. Separations 2022, 9, 89. [Google Scholar] [CrossRef]
- Yates, J.R.; Ruse, C.I.; Nakorchevsky, A. Proteomics by Mass Spectrometry: Approaches, Advances, and Applications. Annu. Rev. Biomed. Eng. 2009, 11, 49–79. [Google Scholar] [CrossRef]
- Kanduc, D. The role of proteomics in defining autoimmunity. Expert Rev. Proteom. 2021, 18, 177–184. [Google Scholar] [CrossRef]
- McArdle, A.J.; Menikou, S. What is proteomics? Arch. Dis. Child.—Educ. Pract. Ed. 2020, 106, 178–181. [Google Scholar] [CrossRef]
- Ramirez, M.F.; Honigberg, M.; Wang, D.; Parekh, J.K.; Bielawski, K.; Courchesne, P.; Larson, M.D.; Levy, D.; Murabito, J.M.; Ho, J.E.; et al. Protein Biomarkers of Early Menopause and Incident Cardiovascular Disease. J. Am. Heart Assoc. 2023, 12, e028849. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Y.; Lv, J.; Yan, Y.; Hu, Y.; Liu, C.; Zhang, F.; Wang, J.; Hao, D. Proteomic profiling analysis of postmenopausal osteoporosis and osteopenia identifies potential proteins associated with low bone mineral density. PeerJ 2020, 8, e9009. [Google Scholar] [CrossRef] [PubMed]
- Covidence. Covidence Systematic Review Software. Available online: www.covidence.org (accessed on 22 July 2024).
- Bellei, E.; Rustichelli, C.; Bergamini, S.; Monari, E.; Baraldi, C.; Castro, F.L.; Tomasi, A.; Ferrari, A. Proteomic serum profile in menstrual-related and post menopause migraine. J. Pharm. Biomed. Anal. 2020, 184, 113165. [Google Scholar] [CrossRef] [PubMed]
- Bergen, H.R.; Vasmatzis, G.; Cliby, W.A.; Johnson, K.L.; Oberg, A.L.; Muddiman, D.C. Discovery of Ovarian Cancer Biomarkers in Serum Using NanoLC Electrospray Ionization TOF and FT-ICR Mass Spectrometry. Dis. Markers 2004, 19, 239–249. [Google Scholar] [CrossRef]
- Cai, H.; Lu, S.; Chen, Y.; Mrcog, S.D.M.; Niu, Z.; Zhuo, G.; Lai, L.; Zhang, Z. Serum retinol binding protein 4 and galectin-3 binding protein as novel markers for postmenopausal nonalcoholic fatty liver disease. Clin. Biochem. 2018, 56, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Wingren, C.; Ingvarsson, J.; Ellmark, P.; Baldertorp, B.; Fernö, M.; Olsson, H.; Borrebaeck, C.A. Serum proteome profiling of metastatic breast cancer using recombinant antibody microarrays. Eur. J. Cancer 2008, 44, 472–480. [Google Scholar] [CrossRef]
- Celsi, F.; Monasta, L.; Arrigoni, G.; Battisti, I.; Licastro, D.; Aloisio, M.; Di Lorenzo, G.; Romano, F.; Ricci, G.; Ura, B. Gel-Based Proteomic Identification of Suprabasin as a Potential New Candidate Biomarker in Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 2076. [Google Scholar] [CrossRef]
- Chao, T.; Ladd, J.J.; Qiu, J.; Johnson, M.M.; Israel, R.; Chin, A.; Wang, H.; Prentice, R.L.; Feng, Z.; Disis, M.L.; et al. Proteomic profiling of the autoimmune response to breast cancer antigens uncovers a suppressive effect of hormone therapy. Proteom.—Clin. Appl. 2013, 7, 327–336. [Google Scholar] [CrossRef]
- Dalenc, F.; Doisneau-Sixou, S.F.; Allal, B.C.; Marsili, S.; Lauwers-Cances, V.; Chaoui, K.; Schiltz, O.; Monsarrat, B.; Filleron, T.; Renée, N.; et al. Tipifarnib Plus Tamoxifen in Tamoxifen-Resistant Metastatic Breast Cancer: A Negative Phase II and Screening of Potential Therapeutic Markers by Proteomic Analysis. Clin. Cancer Res. 2010, 16, 1264–1271. [Google Scholar] [CrossRef]
- Daswani, B.; Gupta, M.K.; Gavali, S.; Desai, M.; Sathe, G.J.; Patil, A.; Parte, P.; Sirdeshmukh, R.; Khatkhatay, M.I. Monocyte Proteomics Reveals Involvement of Phosphorylated HSP27 in the Pathogenesis of Osteoporosis. Dis. Markers 2015, 2015, 196589. [Google Scholar] [CrossRef]
- Delmonico, L.; Bravo, M.; Silvestre, R.T.; Ornellas, M.H.F.; De Azevedo, C.M.; Alves, G. Proteomic profile of saliva and plasma from women with impalpable breast lesions. Oncol. Lett. 2016, 12, 2145–2152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fabian, C.J.; Kimler, B.F.; Phillips, T.A.; Nydegger, J.L.; Kreutzjans, A.L.; Carlson, S.E.; Hidaka, B.H.; Metheny, T.; Zalles, C.M.; Mills, G.B.; et al. Modulation of Breast Cancer Risk Biomarkers by High-Dose Omega-3 Fatty Acids: Phase II Pilot Study in Postmenopausal Women. Cancer Prev. Res. 2015, 8, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, D.; Vafeiadou, K.; Hall, W.L.; Daniel, H.; Williams, C.M.; Schroot, J.H.; Wenzel, U. Proteomic biomarkers of peripheral blood mononuclear cells obtained from postmenopausal women undergoing an intervention with soy isoflavones. Am. J. Clin. Nutr. 2007, 86, 1369–1375. [Google Scholar] [CrossRef]
- Garrison, C.B.; Lastwika, K.J.; Zhang, Y.; Li, C.I.; Lampe, P.D. Proteomic Analysis, Immune Dysregulation, and Pathway Interconnections with Obesity. J. Proteome Res. 2016, 16, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Gericke, B.; Koebnick, C.; Reimann, M.; Forterre, S.; Zunft, H.F.; Schweigert, F.J. Influence of hormone replacement therapy on proteomic pattern in serum of postmenopausal women. Maturitas 2005, 51, 334–342. [Google Scholar] [CrossRef]
- He, W.-T.; Liang, B.-C.; Shi, Z.-Y.; Li, X.-Y.; Li, C.-W.; Shi, X.-L. Weak cation exchange magnetic beads coupled with matrix-assisted laser desorption ionization-time of flight-mass spectrometry in screening serum protein markers in osteopenia. SpringerPlus 2016, 5, 679. [Google Scholar] [CrossRef]
- Jin, Z.; Collier, T.S.; Dai, D.L.; Chen, V.; Hollander, Z.; Ng, R.T.; McManus, B.M.; Balshaw, R.; Apostolidou, S.; Penn, M.S.; et al. Development and Validation of Apolipoprotein AI-Associated Lipoprotein Proteome Panel for the Prediction of Cholesterol Efflux Capacity and Coronary Artery Disease. Clin. Chem. 2019, 65, 282–290. [Google Scholar] [CrossRef]
- Katayama, H.; Tsou, P.; Kobayashi, M.; Capello, M.; Wang, H.; Esteva, F.; Disis, M.L.; Hanash, S. A plasma protein derived TGFβ signature is a prognostic indicator in triple negative breast cancer. NPJ Precis. Oncol. 2019, 3, 10. [Google Scholar] [CrossRef]
- Lal, C.; Hardiman, G.; Kumbhare, S.; Strange, C. Proteomic biomarkers of cognitive impairment in obstructive sleep apnea syndrome. Sleep Breath. 2018, 23, 251–257. [Google Scholar] [CrossRef]
- Lau, E.S.; Paniagua, S.M.; Guseh, J.S.; Bhambhani, V.; Zanni, M.V.; Courchesne, P.; Lyass, A.; Larson, M.G.; Levy, D.; Ho, J.E. Sex Differences in Circulating Biomarkers of Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 74, 1543–1553. [Google Scholar] [CrossRef]
- Lee, D.-H.; Pei, C.-Z.; Song, J.-Y.; Lee, K.-J.; Yun, B.-S.; Kwack, K.-B.; Lee, E.-I.; Baek, K.-H. Identification of serum biomarkers for premature ovarian failure. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2018, 1867, 219–226. [Google Scholar] [CrossRef]
- Li, C.I. Discovery and Validation of Breast Cancer Early Detection Biomarkers in Preclinical Samples. Horm. Cancer 2010, 2, 125–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, W.; Liu, C.; Wang, H. Screening for specific biomarkers in the serum of postmenopausal osteoporosis patients using proteomic fingerprint techniques. Biomed. Rep. 2012, 1, 129–133. [Google Scholar] [CrossRef]
- Henderson, M.C.; Silver, M.; Tran, Q.; Letsios, E.E.; Mulpuri, R.; Reese, D.E.; Lourenco, A.P.; LaBaer, J.; Anderson, K.S.; Alpers, J.; et al. A Noninvasive Blood-based Combinatorial Proteomic Biomarker Assay to Detect Breast Cancer in Women over age 50 with BI-RADS 3, 4, or 5 Assessment. Clin. Cancer Res. 2019, 25, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Aguilar, M.M.; Aparicio-Bautista, D.I.; Ramírez-Salazar, E.G.; Reyes-Grajeda, J.P.; De La Cruz-Montoya, A.H.; Antuna-Puente, B.; Hidalgo-Bravo, A.; Rivera-Paredez, B.; Ramírez-Palacios, P.; Quiterio, M.; et al. Serum Proteomic Analysis Reveals Vitamin D-Binding Protein (VDBP) as a Potential Biomarker for Low Bone Mineral Density in Mexican Postmenopausal Women. Nutrients 2019, 11, 2853. [Google Scholar] [CrossRef] [PubMed]
- Pepe, J.; Rossi, M.; Battafarano, G.; Vernocchi, P.; Conte, F.; Marzano, V.; Mariani, E.; Mortera, S.L.; Cipriani, C.; Rana, I.; et al. Characterization of Extracellular Vesicles in Osteoporotic Patients Compared to Osteopenic and Healthy Controls. J. Bone Miner. Res. 2020, 37, 2186–2200. [Google Scholar] [CrossRef]
- Pitteri, S.J.; Amon, L.M.; Buson, T.B.; Zhang, Y.; Johnson, M.M.; Chin, A.; Kennedy, J.; Wong, C.-H.; Zhang, Q.; Wang, H.; et al. Detection of Elevated Plasma Levels of Epidermal Growth Factor Receptor Before Breast Cancer Diagnosis among Hormone Therapy Users. Cancer Res. 2010, 70, 8598–8606. [Google Scholar] [CrossRef]
- Pitteri, S.J.; Hanash, S.M.; Aragaki, A.; Amon, L.M.; Chen, L.; Buson, T.B.; Paczesny, S.; Katayama, H.; Wang, H.; Johnson, M.M.; et al. Postmenopausal estrogen and progestin effects on the serum proteome. Genome Med. 2009, 1, 121. [Google Scholar] [CrossRef]
- Prentice, R.L.; Paczesny, S.; Aragaki, A.; Amon, L.M.; Chen, L.; Pitteri, S.J.; McIntosh, M.; Wang, P.; Busald, T.B.; Hsia, J.; et al. Novel proteins associated with risk for coronary heart disease or stroke among postmenopausal women identified by in-depth plasma proteome profiling. Genome Med. 2010, 2, 48. [Google Scholar] [CrossRef]
- Prentice, R.L.; Zhao, S.; Johnson, M.; Aragaki, A.; Hsia, J.; Jackson, R.D.; Rossouw, J.E.; E Manson, J.; Hanash, S.M. Proteomic risk markers for coronary heart disease and stroke: Validation and mediation of randomized trial hormone therapy effects on these diseases. Genome Med. 2013, 5, 112. [Google Scholar] [CrossRef]
- Shi, X.-L.; Liang, B.-c.; Shi, Z.-Y.; Wang, B.; Wu, P.; Kong, L.-C.; Yao, J.-l.; Li, C.-w. Discovery and identification of serum biomarkers for postmenopausal osteoporosis based on TMT labeling and HPLC-MS/MS technology. Int. J. Clin. Exp. Med. 2017, 10, 334–346. [Google Scholar]
- Shi, X.; Li, C.; Liang, B.; He, K.; Li, X. Weak cation magnetic separation technology and MALDI-TOF-MS in screening serum protein markers in primary type I osteoporosis. Evolution 2015, 14, 15285–15294. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Lee, E.; Han, S.; Choe, S.-A.; Jeon, O.H. Plasma Proteomic Signature of Cellular Senescence and Markers of Biological Aging Among Postmenopausal Women. Rejuvenation Res. 2022, 25, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Soh, G.T.; Mohammad, A.H.; Isa, S.N.L.S.; Chin, K.-Y.; Mohamed, N. Comparison of Cytokine Profile between Postmenopausal Women with and Without Osteoporosis—A Case-Control Study. Endocrine Metab. Immune Disord.—Drug Targets 2023, 23, 811–817. [Google Scholar] [CrossRef]
- Sun, Y.-W.; Xu, H.; Benitez, G.; Chen, K.-M.; Stanley, A.; Stanley, B.; Zhu, J.; Thompson, H.; Manni, A.; El-Bayoumy, K. Omega-3 Fatty Acids Responsive Proteins and Reduction in Breast Density in Obese Postmenopausal Women. J. Proteome Res. 2019, 18, 3461–3469. [Google Scholar] [CrossRef]
- Tarney, C.M.; Wang, G.; Bateman, N.W.; Conrads, K.A.; Zhou, M.; Hood, B.L.; Loffredo, J.; Tian, C.; Darcy, K.M.; Hamilton, C.A.; et al. Biomarker panel for early detection of endometrial cancer in the Prostate, Lung, Colorectal, and Ovarian cancer screening trial. Am. J. Obstet. Gynecol. 2019, 221, 472.e1–472.e10. [Google Scholar] [CrossRef]
- Thomas, C.E.; Dahl, L.; Byström, S.; Chen, Y.; Uhlén, M.; Mälarstig, A.; Czene, K.; Hall, P.; Schwenk, J.M.; Gabrielson, M. Circulating proteins reveal prior use of menopausal hormonal therapy and increased risk of breast cancer. Transl. Oncol. 2022, 17, 101339. [Google Scholar] [CrossRef] [PubMed]
- Tworoger, S.S.; Spentzos, D.; Grall, F.T.; Liebermann, T.A.; Hankinson, S.E. Reproducibility of Proteomic Profiles Over 3 Years in Postmenopausal Women Not Taking Postmenopausal Hormones. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1480–1485. [Google Scholar] [CrossRef]
- Watrowski, R.; Obermayr, E.; Wallisch, C.; Aust, S.; Concin, N.; Braicu, E.I.; Van Gorp, T.; Hasenburg, A.; Sehouli, J.; Vergote, I.; et al. Biomarker-Based Models for Preoperative Assessment of Adnexal Mass: A Multicenter Validation Study. Cancers 2022, 14, 1780. [Google Scholar] [CrossRef]
- Wong, E.; Freiberg, M.; Tracy, R.; Kuller, L. Epidemiology of Cytokines. Am. J. Epidemiol. 2008, 168, 443–453. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zeng, Y.; Zhu, W.; Zhao, Y.; Zhang, J.; Zhu, J.; He, H.; Shen, H.; Tian, Q.; et al. Network-based proteomic analysis for postmenopausal osteoporosis in Caucasian females. Proteomics 2015, 16, 12–28. [Google Scholar] [CrossRef] [PubMed]
- CDC. Heart Disease Facts. Available online: https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html#cdc_facts_stats_curr_actions-what-cdc-is-doing (accessed on 22 July 2024).
- Anderson, B.; World Health Organisation. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 22 July 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).