Abstract
Background and Objectives: In the undertaken study, proteomics alterations of blood-borne XDR S. Typhi isolated from Pakistan were investigated using mass spectrometry. Materials and Methods: MDR and XDR S. Typhi total protein lysates were fractionated, digested, and processed for nanoflow LC-LTQ-Orbitrap MS analysis. Results: Among the 1267 identified proteins, 37 were differentially regulated, of which 28 were up-regulated, and 9 were down-regulated in XDR S. Typhi as compared to MDR S. Typhi. Based on the functional annotation, proteins found up-regulated are involved mainly in metabolic pathways (ManA, FadB, DacC, GpmA, AphA, PfkB, TalA, FbaB, OtsA, 16504242), the biosynthesis of secondary metabolites (ManA, FadB, GlpB, GpmA, PfkB, TalA, FbaB, OtsA), microbial metabolism in diverse environments (FadB, GpmA, PfkB, NfnB, TalA, FbaB), and ABC transporters (PstS, YbeJ, MglB, RbsB, ArtJ). Proteins found down-regulated are involved mainly in carbon metabolism (FadB, GpmA, PfkB, FalA, FbaB) and the biosynthesis of amino acids (GpmA, PfkB, TalA, FbaB). Most of the identified differential proteins were predicted to be antigenic, and matched with resistome data. Conclusions: A total of 28 proteins were up-regulated, and 9 were down-regulated in XDR S. Typhi. Further characterization of the identified proteins will help in understanding the molecular signaling involved in the emergence of XDR S. Typhi.
1. Introduction
Salmonella enterica serovar Typhi (S. Typhi) is a Gram-negative human pathogen that causes typhoid. The global data reflected almost 21 million people infected with S. Typhi. Among the South Asian countries, Pakistan has an estimated rate of 493.5 per 100,000 cases in 2018 [1].
The emergence of antimicrobial resistance (AMR), and even the augmented rate of extensively drug-resistant (XDR) S. Typhi, is a global threat to public health and leads to worse clinical outcomes, prolonged hospital stays, cost, and mortality rates [2,3]. Understanding the microbial resistance mechanism against the available antibiotics is the ultimate strategy for disease surveillance [4].
Proteomic techniques are currently in use for the elucidation of biomarker proteins, the identification of antigenic molecules, and the virulence markers of S. Typhi [5,6]. Proteomics is a rapidly emerging tool for identifying drug resistance pathways in infectious diseases caused by S. Typhi [7,8].
Previously, the molecular strain typing and drug resistance of S. Typhi from Pakistan were reported [9]; however, from literature mining, there is a lack of data on the differential proteome profile of XDR S. Typhi. This study aimed to investigate the proteome of blood-borne XDR S. Typhi hospital isolates in Pakistan. Findings from the work will be helpful in understanding the molecular mechanism leading to XDR S. Typhi.
2. Materials and Methods
2.1. Sampling
Blood samples from the suspected typhoid patients in tertiary care hospitals in Kohat, Islamabad, and Rawalpindi were collected and cultured. Informed patient consent was obtained, and ethical approval was granted by the KUST research ethics committee (Ref. No. KUST/Ethical Committee/16-14).
2.2. Isolation and Identification of S. Typhi
Clinical samples were transported in broth media, and subcultured on Bismuth Sulfite medium. Biochemical and serological assays were performed. fliC-d specific gene of S. Typhi was amplified as described [9].
2.3. Antibiogram Assay
The antibiotic susceptibility of isolates was performed as described earlier [9]. Briefly, ampicillin (AMP), chloramphenicol (C), co-trimoxazole (SXT), ciprofloxacin (CIP), levofloxacin (LEV), azithromycin (AZM), imipenem (IMP), ceftriaxone (CRO), aztreonam (ATM), cefepime (FEP), cefixime (CFM), cefoperazone (CFP), cefoxitin (FOX), and nalidixic acid (NA) antibiotic discs (Oxoid, UK) were used. Isolates were lawned equivalent to 0.5 McFarland turbidity standard on Mueller–Hinten agar and incubated overnight at 37 °C. Zone of inhibition was measured and interpreted for each antibiotic [10].
2.4. Molecular Detection of Drug Resistance Genes
After screening of the phenotypic resistance profile, DNA was extracted from selected isolates and processed for parA, parC, parE, gyrA, gyrB (fluoroquinolone resistance genes), qnrA, qnrB, qnrC, qnrS, aac(6’)-ib-cr) (plasmid genes), dfrA7, dfrA14, cat (cotrimoxazole and chloramphenicol resistance genes), blaOXA, blaSHV1, blaCTX15, and blaTEM (ESBL genes) were screened by using PCR assay as reported [9]. After phenotypic and molecular antibiotic susceptibility, isolates were considered multidrug resistant (MDR), which exhibited resistance to ampicillin, trimethoprim-sulfamethoxazole, and chloramphenicol, while XDR were those isolates that were resistant to ampicillin, trimethoprim-sulfamethoxazole, third-generation cephalosporins, and fluoroquinolones as per the CDC report [11].
2.5. Protein Extraction and Quantification
MDR (n = 3) and XDR (n = 3) S. Typhi isolates were selected for protein extraction and quantification [12]. The fresh BHI broth culture was incubated at 37 °C till attainment of optical density (OD) up to 0.9 at 600 nm. One ml bacterial culture with an OD of 0.9 was centrifuged, and the supernatant was discarded. Urea thiourea was added to the pellet and mixed gently. The cell lysate was centrifuged at 12,000 rpm. The supernatant was processed for protein quantification as described by Bradford [13].
2.6. SDS PAGE, and Trypsin In-Gel Digestion
A 10% resolving gel and 4% stacking SDS-PAGE gel were prepared. Equal proteins from each sample were loaded on the gel. Samples were resolved, and the gel was stained for 1 h in Coomassie Blue. The gel was then de-stained in 10% acetic acid for an overnight shaking incubation, and sliced into small bands. The sliced gel bands were processed for trypsin in-gel digestion as described earlier [14].
2.7. Peptide Sequencing Using Nanoflow LC-LTQ-Orbitrap MS Analysis and Mascot Search
A hybrid ion trap mass spectrometer (LC-LTQ-Orbitrap Velos, Thermo Scientific, Carlsbad, CA, USA) was used for the protein’s analysis. The adsorbed peptides were electrically blasted into the mass spectrometer using a laser-pulled tip of a capillary column with silica-based particles (Model P-2000, Sutter Instruments, Novato, CA, USA). The solvent B amount was added. The ten most significant ions from the full MS scan (m/z 350–1500) were chosen for the MS/MS analysis. The repetition duration for dynamic exclusion was 24 s, while the exclusion duration was 12 s. MDR and XDR biological replicates were processed by LC-MS/MS.
Mascot v 2.3.02 and MaxQuant v1.2 [15] were used to examine the raw M.S. files from the Orbitrap Velos (Computational Systems Biochemistry, Martinsried, Germany). The protein sequence database for S. Typhi (CT18) was retrieved from the NCBI database and archived before being used in the current study. The peptide tolerance was set to 4.5 ppm, while the MS/MS fragment tolerance was set to 0.8Da in MaxQuant. The incidence of false discovery might be as high as 1%. The differentially regulated protein’s ratio was derived by dividing the XDR protein by the MDR S. Typhi protein; if the ratio of XDR to MDR was greater than two, the protein was considered up-regulated; if it was less than 0.5, the protein was considered down-regulated. Three biological replicate experiments were incorporated.
2.8. Data Analysis
Proteins with an average fold change ≥2 and ≤0.5 and a p-value of ≤0.05 were statistically considered significant differentially regulated proteins using the student’s t-test (Microsoft, Redmond, DC, USA).
2.9. Bioinformatics Analysis
In the bioinformatics study, the drug resistance gene sequences were first BLAST and tabulated. The FASTA sequences of significant up-regulated and down-regulated proteins were obtained from Uniport (https://www.uniprot.org/, accessed on 28 June 2023), and archived for further use. For the protein-protein interactions, the STRING v11.5 database was used to upload the up- and down-regulated proteins list, and their interactions were documented [16]. A web browser identified the indicated regulated proteins’ subcellular localization as “Cellugo prediction” (http://cello.life.nctu.edu.tw/cello2go/, accessed on 28 June 2023). For antigenicity predictions, the VaxiJen v2.0 web server (https://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html, accessed on 28 June 2023) has parameters: a 0.4 threshold value, bacteria as an organism, and sequence output [17]. Then, to CARD (https://card.mcmaster.ca, accessed on 28 June 2023), the FASTA sequences of the up- and down-regulated proteins were uploaded to search for the resistome, and their relevance to resistance was already recognized [18].
3. Results and Discussion
3.1. Phenotypic and Genotypic Antibiotic Susceptibility Assay for S. Typhi
S. Typhi was identified using biochemical and PCR-based assays. MDR and XDR S. Typhi isolates were confirmed through phenotypic and genotypic antibiotic susceptibility methods (Table 1).

Table 1.
Antibiotic susceptibility profile of MDR and XDR S. Typhi isolates processed for proteome analysis.
Previously XDR S. Typhi were reported from Pakistan, which were resistant to most of the available antibiotics, thus limiting choice of therapeutic antibiotics [1,3,9]. In the current study, XDR and MDR isolates were selected to process for differential proteomics.
3.2. Differential Proteomics of XDR vs. MDR S. Typhi
Proteomics tools are commonly employed for the detection of biomarkers, the search for vaccine candidates, and for therapeutic drug targets. Several studies have reported the importance of proteome analysis in the molecular pathogenesis of S. Typhi [5,6,9].
After MDR and XDR S. Typhi confirmation, the protein lysate was resolved on SDS PAGE and stained. Each gel band was sliced and processed for LC-LTQ-Orbitrap MS analysis (Figure 1). A total of 1267 proteins were identified. The differential proteome data are plotted on a volcano plot. Two-fold (horizontal) and p < 0.05 cut-offs are indicated by dotted vertical lines (Figure 2).
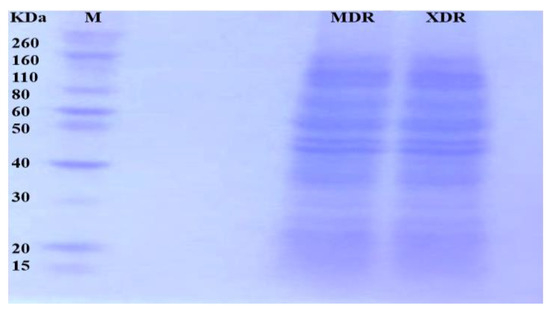
Figure 1.
Proteins from MDR and XDR S. Typhi isolates: Proteins were extracted, quantified, and loaded on 10% SDS-PAGE. The gel bands were excised for LC MS/MS analysis.
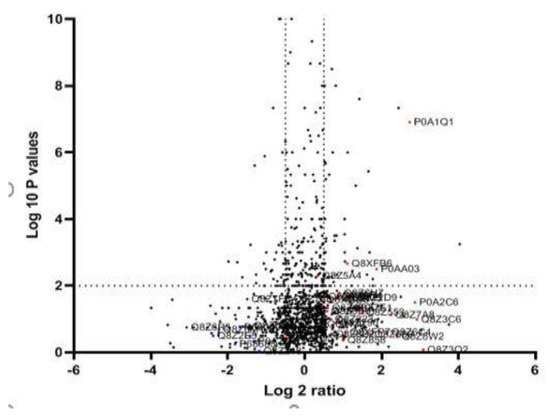
Figure 2.
Quantitative proteomic data of XDR and MDR S. Typhi: The logarithmic values of the abundance ratios are reported on the x-axis of a protein volcano plot. The negative logarithmic p-values calculated from the t-test on data from three biological replicates are plotted on the y-axis. Two-fold (horizontal) and p < 0.05 cut-offs are indicated by dotted lines (vertical). LC-MS/MS investigations may not be able to differentiate proteins with homologous sequences unless distinct peptides are found.
The heat map cluster matrix between XDR and MDR S. Typhi was created to present the protein expression changes (fold change). The column represents the sample type, while the rows represent proteins (Figure 3).
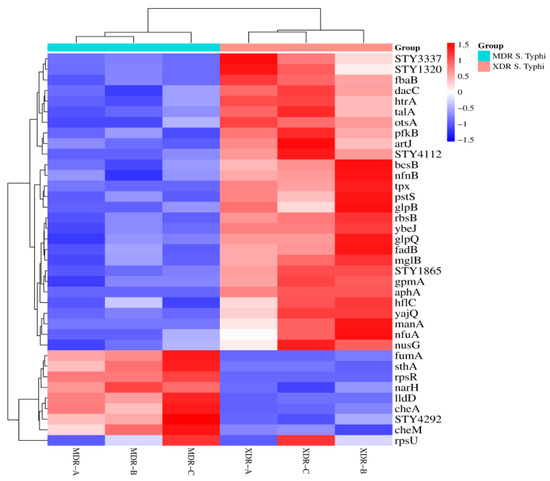
Figure 3.
Heat map of XDR and MDR S. Typhi: A matrix is created to present the protein expression changes (fold change) in MDR (n = 3) vs. XDR (n = 3) S. Typhi isolates. The Euclidean distance method was used. Branching nodes of the heat map represent the inter-cluster dissimilarity. An online resource (https://www.bioinformatics.com.cn/plot_basic_cluster_heatmap_plot_024_en, accessed on 5 January 2024) was used to build a cluster heat map.
After data analysis, 28 proteins were significantly up-regulated, while 9 were down-regulated in XDR S. Typhi. 28 proteins, including RbsB, STY3337, Tpx, STY1865, PfkB, AphA, ArtJ, YbeJ, MglB, GpmA, YajQ, STY4112, FadB, GlpB, HflC, NfnB, OtsA, GlpQ, NusG, TalA, STY1320, BcsB, NfuA, PstS, FbaB, HtrA, DacC, and ManA, were up-regulated in XDR S. Typhi. There were 9 proteins (CheM, SthA, LldD, CheA, NarH, RpsR, FumA, STY4292, and RpsU) significantly down-regulated in XDR S. Typhi (Table 2).

Table 2.
Differentially regulated proteins of XDR S. Typhi with gene names, functions, subcellular location (threshold 0.001), antigenicity (VaxiJen, threshold 0.4), and resistome match.
In the up-regulated proteins, RbsB is a component of the ABC transporter complex (RbsABC) that deals with ribose import, and the PstS protein deals with phosphate import. These periplasmic proteins were up-regulated at high fructose concentrations in Streptomyces lividans [19]. The up-regulation of RbsB and PstS might have a role in the emergence of the XDR S. Typhi phenotype.
Tpx, HtrA, and ArtJ were stress-response proteins that were up-regulated. Tpx is a thiol peroxidase and plays a role in stress response and metabolism [20]. HtrA is a heat shock protein; the deletion of hfq resulted in increased HtrA protein levels [19]. MglB is a chemotaxis-related up-regulated protein. In S. Typhimurium, the protein MglB is also overexpressed and engaged in chemotaxis [20]. The three proteins that have been up-regulated serve a variety of purposes, including those of coenzymes (AphA and DacC) and virulence proteins (VirP) (HlfC). AphA is an acid phosphatase that causes the dephosphorylation of several metabolic molecules. The protein DacC facilitates the production of peptidoglycans. In S. Typhimurium, the protein HlfC is part of a hfq operon and is essential for virulence [21].
Two proteins (CheA and CheM) involved in chemotaxis (bacterial movement) were down-regulated. In a previous study, these proteins were variably expressed. In the down-regulated protein, RpsU is a growth protein, and, when the growth rate in bacteria decreased, its gene was reported as a constituently expressed [20].
Among the other down-regulated proteins, LldD, RpsR, and FumA, among others, were reported to be involved in metabolic pathways. L-lactate is converted to pyruvate by the L-lactate dehydrogenase protein (LldD) [22]. The RpsR protein stabilizes the 30S subunit by attaching to protein S6. In aerobic circumstances in E. coli, fumarate dehydrogenase protein (FumA) is down-regulated, whereas its gene, FumA, is reported to be up-regulated [23].
3.3. Prediction of Cellular Localization, Antigenicity, and Resistome Map of Differentially Regulated Proteins
Among the 28 up-regulated proteins of XDR S. Typhi, the localization prediction tool showed proteins of the cytoplasmic (n = 17), periplasmic (n = 14), and inner membrane (n = 9). We found that 23 proteins were predicted to be antigenic, and 5 were regarded as non-antigenic. Most of the proteins (n = 24) were matched with the resistome map. Among the down-regulated proteins, 7 were cytoplasmic and 4 were inner membrane. Similarly, 8 proteins were antigenic, and all the proteins matched the resistome map (Table 2).
The resistome map of the regulated proteins showed a relationship with predicted antibiotic resistance mechanisms. It is evident that AMR comes with a survival strategy that may alter bacterial growth. To compensate for the survival cost or to handle the stress posed by antibiotics, the bacteria tend to fine-tune nutrient demands [24]. The possible explanation for the altered XDR associated proteins might be due to selective antibiotic pressure to manage the toxic effects of antimicrobial drugs.
3.4. Annotation of the Biological Function of Identified Proteins
The up-regulated proteins were mostly involved in the metabolic pathways (n = 10), biosynthesis of secondary metabolites (n = 8), and ABC transporters (n = 5). In the down-regulated proteins, most were involved in the metabolic pathways (n = 4) (Table 3).

Table 3.
Functional annotation for the differentially regulated proteins of XDR S. Typhi.
The proteome profile of XDR S. Typhi reflects that the up-regulated and down-regulated proteins are involved in diverse functions, not limited to ABC transport, metabolism, respiration, and signal transduction.
3.5. Protein–Protein Interaction and Annotation by STRING
A total of 28 proteins that are up-regulated in XDR are included in the protein–protein interaction map. S. Typhi demonstrated that most proteins interacted with other proteins both directly (n = 13) and indirectly (n = 8). Among the down-regulated proteins, three showed direct interaction, while four exhibited indirect interaction with other proteins (Figure 4).
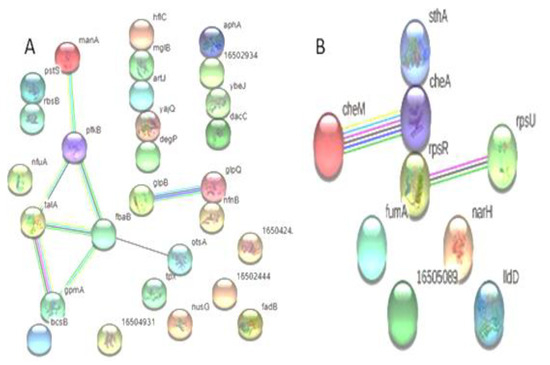
Figure 4.
Predicted interaction of up-regulated proteins (A) (n = 28) and down-regulated proteins (B) (n = 9) of S. Typhi: (A) Showing direct interaction of hflC, mglB, artJ, yajQ, degP, gloQ, nfnB, pstS, rbsB, aphA, 16502934, ybeJ, and dacC and indirect interaction of manA, pfkB, talA, fbaB, gpmA, otsA, glpB, and gloQ. (B) Showed the non-interaction of IIdD, narH, 16505089, fumA, and cheA and the direct interaction of rpsR, cheA, and sthA, and the indirect interaction of cheM with cheA; rpsR interacts with rpsU.
There is a growing body of literature on the role of protein–protein interactions in biological functions. Protein interactions are not limited to enzyme functions, metabolic reactions, cascade pathways, and negative and positive regulation [25]. In the present study, protein interaction prediction analysis showed direct interaction. These proteins are involved in several biological processes and might have a role in the resistance mechanisms of MDR and XDR S. Typhi.
Overall, the expression of these proteins might trigger the AMR mechanism that leads to increased resistance in S. Typhi. Further characterization of regulated proteins would be helpful to understand the XDR S. Typhi response and adaptation to available antibiotics, and will explore new avenues in the fight against global antimicrobial resistance.
4. Conclusions
The proteome profile of XDR S. Typhi identified 28 up-regulated and 9 down-regulated proteins. Functional annotation, interaction, localization, antigenicity, and resistome prediction further highlighted their possible role in the AMR mechanisms among XDR S. Typhi.
Author Contributions
N.Y.: conceptualization, investigation, methodology, formal analysis, writing—original draft; H.R. and M.Q.: conceptualization, project administration, supervision; Y.S.: validation; I.N. and N.K.: formal analysis, data curation; review and editing; K.J.A., M.A.A., F.M.A. and A.A.: funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taif University, Taif, Saudi Arabia (TU-DSPP-2024-107).
Institutional Review Board Statement
The KUST research ethical committee approved this study (Ref. No. KUST/Ethical Committee/16-14 dated 11 July 2016).
Informed Consent Statement
Written informed consent was taken from patients who participated in the study.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-107).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fatima, M.; Kumar, S.; Hussain, M.; Memon, N.M.; Vighio, A.; Syed, M.A.; Chaudhry, A.; Hussain, Z.; Baig, Z.I.; Baig, M.A.; et al. Morbidity and mortality associated with typhoid fever among hospitalized patients in Hyderabad District, Pakistan, 2017–2018: Retrospective record review. JMIR Public Health Surveill. 2021, 7, e27268. [Google Scholar] [CrossRef] [PubMed]
- Gajdacs, M.; Urban, E.; Stajer, A.; Barath, Z. Antimicrobial Resistance in the Context of the Sustainable Development Goals: A Brief Review. Eur. J. Investig. Health Psychol. Educ. 2021, 19, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.H.; Saleem, A.; Javed, S.O.; Ullah, I.; Rehman, M.U.; Islam, N.; Tahir, M.A.; Malik, T.; Hafeez, S.; Misbah, S. Rising XDR-Typhoid Fever Cases in Pakistan: Are We Heading Back to the Pre-antibiotic Era? Front. Public Health 2022, 9, 794868. [Google Scholar] [CrossRef]
- Alberto, F.; Maria, E.F.; Lieselotte, A.; Ana, R.; Karin, D.; Jorge, C.; Jose, M.; Guevara, M.R.; Felipe, C. Antibiotic-Resistant Salmonella typhi From Two Outbreaks: Few Ribotypes and IS200 Types Harbor Inc HI1 Plasmids. Microb. Drug Resist. 1997, 3, 339–343. [Google Scholar]
- Ansong, C.; Yoon, H.; Norbeck, A.D.; Gustin, J.K.; McDermott, J.E.; Mottaz, H.M.; Rue, J.; Adkins, J.N.; Heffron, F.; Smith, R.D. Proteomics analysis of the causative agent of typhoid fever. J. Proteome Res. 2008, 7, 546–557. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakraborty, K.; Nagaraja, T.; Basak, S.; Koley, H.; Dutta, S.; Mitra, U.; Das, S. An adhesion protein of Salmonella enterica serovar Typhi is required for pathogenesis and potential target for vaccine development. Proc. Natl. Acad. Sci. USA 2021, 108, 3348–3353. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, E.; Zeinalzadeh, E.; Taghizadeh, S.; Mehramouz, B.; Kamounah, F.S.; Khodadadi, E.; Ganbarov, K.; Yousefi, B.; Bastami, M.; Kafil, H.S. Proteomic Applications in Antimicrobial Resistance and Clinical Microbiology Studies. Infect. Drug Resist. 2020, 16, 1785–1806. [Google Scholar] [CrossRef]
- Safi, A.U.R.; Bendixen, E.; Rahman, H.; Khattak, B.; Wu, W.; Ullah, W.; Khan, N.; Ali, F.; Yasin, N.; Qasim, M. Molecular identification and differential proteomics of drug resistant Salmonella Typhi. Diagn. Microbiol. Infect. Dis. 2023, 105, 115883. [Google Scholar] [CrossRef]
- Yasin, N.; Rahman, H.; Sarwar, Y.; Qasim, M.; Nisa, I.; Ikram, A.; Zaman, G.; Khan, Z.; Mirza, M.R.; Khan, N.; et al. Salmonella Typhi from Northwest Pakistan: Molecular Strain Typing and Drug Resistance Signature. Microb. Drug Resist. 2022, 28, 120–126. [Google Scholar] [CrossRef]
- CLSI Document M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019.
- Chatham, S.K.; Medalla, F.; Hughes, M. Emergence of Extensively Drug-Resistant Salmonella Typhi Infections Among Travelers to or from Pakistan—United States, 2016–2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 11–13. [Google Scholar] [CrossRef]
- Rahman, H.; Qasim, M.; Schultze, F.C.; Oellerich, M.; RAsif, A. Fetal calf serum heat inactivation and lipopolysaccharide contamination influence the human T lymphoblast proteome and phosphoproteome. Proteome Sci. 2011, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rahman, H.; Qasim, M.; Oellerich, M.; Asif, A.R. Identification of the Novel Interacting Partners of the Mammalian Target of Rapamycin Complex 1 in Human CCRF-CEM and HEK293 Cells. Int. J. Mol. Sci. 2014, 15, 4823–4836. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, R. Vaccine Design: Innovative Approaches and Novel Strategies. Expert Rev. Vaccines 2011, 10, 1385–1387. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic. Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Esteban, A.; Fernández-Abalos, J.M.; Santamaría, R.I. The high-affinity phosphate-binding protein PstS is accumulated under high fructose concentrations and mutation of the corresponding gene affects differentiation in Streptomyces lividans. Microbiology 2005, 151 Pt 8, 2583–2592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oshota, O.; Conway, M.; Fookes, M.; Schreiber, F.; Chaudhuri, R.R.; Yu, L.; Morgan, F.J.E.; Clare, S.; Choudhary, J.; Thomson, N.R.; et al. Transcriptome and proteome analysis of Salmonella enterica serovar Typhimurium systemic infection of wild type and immune-deficient mice. PLoS ONE 2017, 12, e0181365. [Google Scholar] [CrossRef]
- Sittka, A.; Pfeiffer, V.; Tedin, K.; Vogel, J. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol. Microbiol. 2007, 63, 193–217. [Google Scholar] [CrossRef]
- Lambert, B.; Dassanayake, M.; Oh, D.H.; Garrett, S.B.; Lee, S.Y.; Pettis, G.S. A novel phase variant of the cholera pathogen shows stress-adaptive cryptic transcriptomic signatures. BMC Genom. 2016, 17, 914. [Google Scholar] [CrossRef][Green Version]
- Cao, H.; Wei, D.; Yang, Y.; Shang, Y.; Li, G.; Zhou, Y.; Ma, Q.; Xu, Y. Systems-level understanding of ethanol-induced stresses and adaptation in E. coli. Sci. Rep. 2017, 7, 44150. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Charles, R.C.; Rollins, S.M.; Harris, J.B.; Bhuiyan, M.S.; Khanam, F.; Bukka, A.; Kalsy, A.; Porwollik, S.; Brooks, W.A.; et al. Analysis of Salmonella enterica serotype paratyphi A gene expression in the blood of bacteremic patients in Bangladesh. PLoS Neglected Trop. Dis. 2010, 4, e908. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, J.; Peng, W.; Wu, F.X.; Pan, Y. Protein–protein interactions: Detection, reliability assessment and applications. Brief. Bioinform. 2017, 18, 798–819. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).