Abstract
Background and Objectives: Acinetobacter baumannii (A. baumannii), particularly carbapenem-resistant A. baumannii (CRAB), represents a grave concern in healthcare settings and is associated with high mortality. This study aimed to conduct molecular, mutational, and phylogenetic analyses of specific genes in CRAB and evaluate the synergistic effects of selected antimicrobial combinations. Materials and Methods: Phenotypic characterization was performed on six CRAB strains by using the Modified Hodge Test (MHT) and IMP-EDTA Double-Disc Synergy Test (IMP-EDTA DDST). Carbapenemase- and metallo-beta-lactamase-encoding genes were amplified by using Polymerase Chain Reaction. Phylogenetic analysis using the MEGA 11 tool was used to determine the evolutionary relatedness of these genes. Mutational analysis was performed by using I-Mutant, MUPro, and PHD-SNP bioinformatics tools to predict mutations in the carbapenemase-encoding genes. Microdilution checkerboard titration assessed the synergistic effects of antimicrobial combinations (azithromycin–meropenem, rifampicin–meropenem, meropenem–colistin, and azithromycin–colistin) on these CRAB isolates. Results: The phenotypic characterization of six CRAB isolates revealed positive results for MHT and IMP-EDTA DDST. The molecular characterization revealed that carbapenemase- and MBL-encoding genes were present in all isolates with varying frequencies, including blaOXA-51 (100%) and blaIMP (0%). The sequence analysis revealed high evolutionary relatedness to sequences in the NCBI database. The mutational analysis identified 16 mutations, of which 1 mutation (P116L) in the blaOXA-58 gene predicted a change in the protein product, potentially contributing to carbapenem resistance. The checkerboard titration method did not reveal any synergism among the tested antimicrobial combinations against CRAB. Conclusion: This study’s findings underscore the significant challenges posed by CRAB isolates harboring multiple resistant genes in treatment. This highlights the urgent need for novel antimicrobial agents, a crucial step towards reducing mortality rates not only in Pakistan but also globally.
1. Introduction
Acinetobacter baumannii (A. baumannii) is notorious for causing severe hospital-acquired infections, resulting in high mortality rates [,,]. The ongoing battle against infectious diseases continues as medication resistance rapidly emerges, especially among Gram-negative bacteria []. Carbapenem-resistant A. baumannii was first reported in 1991 in the United States, and since then, A. baumannii species have developed significant multidrug resistance (MDR) [].
Exploring alternative chemotherapeutic drugs effective in treating multidrug-resistant bacteria, including A. baumannii (MDR-AB), has become a significant concern in public health. This highlights the importance of researching new and potentially beneficial compounds []. Clinicians actively seek alternative treatments to traditional antimicrobial agents due to the global increase in multidrug-resistant Gram-negative bacterial infections. Antimicrobials like polymyxins (colistin) are now considered viable therapeutic options to combat the shortage of new antimicrobial agents. However, it is recommended to avoid using colistin as monotherapy in cases of A. baumannii to prevent antimicrobial resistance development [,,].
Antimicrobial resistance in A. baumannii arises from various mechanisms, including beta-lactamase production, enzymatic alteration of efflux pumps, the presence of aminoglycosides, deficiencies in permeability, and modifications to specific target sites []. Furthermore, the exploration of different mutations in resistance genes in integrons, plasmids, and transposons also contributes to the increasing resistance capability of A. baumannii []. More importantly, this resistance is thought to develop from acquired and intrinsic oxacillinases, particularly blaOXA-23 and blaOXA-51 [,], with blaOXA-23 being the most widespread cause of developing carbapenem resistance globally []. Studies indicate that insertion sequences like ISAbaf are crucial to developing resistance to carbapenem antimicrobials in A. baumannii. These insertion sequences are found upstream in the promoter region of genes associated with carbapenem resistance and contribute to enhanced expression of resistance genes [,]. Therefore, our objective was to develop a thorough understanding of resistance to carbapenems in A. baumannii by identifying mutations in these genes and whether or not they exert a deleterious effect on gene expression, inducing a subsequent change in the protein product.
The literature suggests that combining two or more antimicrobials could be a promising strategy for tackling antimicrobial resistance. These combinations of antimicrobial agents have the potential to enhance susceptibility against pathogenic bacteria, making them appealing and valuable choices for patient treatment []. Multiple studies have investigated the synergism of antimicrobial therapies against MDR-AB in various regions [,,,], but few studies have been observed in Pakistani scenarios. Combination therapy demonstrates the potential for broad-spectrum activity and enhanced bactericidal effects against bacterial strains [].Hence, another aim was to assess the synergistic effects of various combination therapies against CRAB in Lahore, Pakistan. This study aimed to provide medical professionals with information about viable antimicrobial combinations to understand better how to treat patients with MDR-AB infections.
2. Materials and Methods
This study was conducted at the Institute of Molecular Biology and Biotechnology, University of Lahore, and the Institute of Microbiology, University of Veterinary and Animal Sciences, Lahore, from April 2021 to April 2022. The protocol of this study was approved by the ethical review committee of University of Lahore (Reg: DMB-02173003). This study is an extension of our previously published work []. Six carbapenemase- and metallo-beta-lactamase-producing CRAB strains, namely, S10, S67, S84, S96, S97, and S98, which are known to be resistant to the majority of antimicrobials except colistin, were selected. These strains also harbor specific carbapenemase and metallo-beta-lactamase genes, as shown in Table 1.

Table 1.
Antimicrobial resistance and resistance genes detected in CRAB.
2.1. Isolation and Phenotypic Characterization of CRAB
These strains were isolated from patient samples collected from various laboratories and tertiary care facilities in Lahore, Pakistan. Biotyping was conducted through colonial morphology testing, Gram staining, biochemical assays, and API 20 E Analysis []. The antimicrobial resistance profiles of the A. baumannii isolates were assessed by using the Kirby–Bauer Method []. A panel of 13 antimicrobials, including ceftazidime, cefepime, piperacillin/tazobactam, doxycycline, gentamicin, tobramycin, imipenem, meropenem, ciprofloxacin, levofloxacin, amikacin, trimethoprim–sulfamethoxazole, and colistin, was employed to assess their susceptibility patterns. The MIC of colistin was determined by the broth dilution method, with interpretation guided by the Clinical and Laboratory Standards Institute [] protocols []. Carbapenemase-producing A. baumannii strains were detected by using the MHT [], while MBL-producing strains were identified through the Double-Disc Synergy Test (DDST) utilizing imipenem-ethylenediamine tetra acetic acid (IPM-EDTA) [].
2.2. Molecular Detection of Resistant Genes
A standardized DNA extraction kit (Thermo Scientific Purification Kit for Genomic DNA; Gene JET Cat#K-0721, Waltham, MA, USA) was used to extract DNA from freshly grown A. baumannii. Carbapenemase-encoding genes, including blaOXA-24, blaOXA-23, blaOXA-58, and blaOXA-51, and MBL-encoding genes, including blaNDM-1, blaVIM, and blaIMP, were amplified by using forward and reverse primers used for PCR as published in our previous study []. The details of the primers used for PCR are also shown in Supplementary Table S1. The amplicons were sequenced, analyzed by BioEdit, and submitted to NCBI GenBank for accession numbers.
2.3. Phylogenetic Analysis of Carbapenemase- and MBL-Encoding Genes of CRAB
The Molecular Evolutionary Genetic Analysis version 11 (MEGA 11) tool was utilized for the phylogenetic analysis of carbapenemase-encoding genes, including the blaOXA-23, blaOXA-24, blaOXA-58, and blaOXA-51 genes. The software application aligned the study sequences with those reported in the National Center of Biotechnology Information (NCBI) by using the Clustal W-tool within MEGA 11. The initial phylogenetic tree was constructed by using a neighbor-joining (NJ) algorithm on a matrix of pairwise distances estimated through a maximum composite likelihood approach. In the phylogenetic tree, the terminal nodes represented sequences with their accession numbers connected through divergent points or internal nodes, where the genetic distances between sequences were illustrated by branch length.
2.4. Mutational Analysis of Carbapenemase-Encoding Genes of CRAB
A Basic Local Alignment Search Tool (BLAST) was used to convert nucleotide sequences into protein sequences, and the Fast Adaptive Shrinkage Threshold Algorithm (FASTA) sequences of proteins were retrieved. Nucleotide sequences of carbapenemase-encoding genes (i.e., the blaOXA-23, blaOXA-24, blaOXA-58, and blaOXA-51 genes) were converted into protein sequences. For mutational analysis, the I-Mutant [], MUPro [], and PHD-SNP [] tools were applied. These tools were utilized to predict whether the mutations could lead to gene expression changes in protein structure and function, thereby contributing to carbapenem resistance. We classified gene expression as “Changed” if two or all three software tools predicted a potential alteration, indicating damage. Mupro and I-MUTANT assessed changes in protein stability, while PhD-SNP predicted associations with the disease based on gene expression. The effects of mutations were categorized as “Decrease”, “Increase”, or “Neutral”. In the context of I-Mutant and MUPro, an “Increase” signified that the mutation was stable and likely to impact gene expression, interpreted as a “Change” in expression. Conversely, “Decrease” indicated instability and a lower probability of altering gene expression, interpreted as “No Change”. For PHD-SNP, a stable mutation was denoted as “Disease”, indicating a “Change” in expression, whereas an unstable mutation was labeled as “Neutral”, representing “No Change” [].
2.5. Combination Synergy Testing
The synergistic effects of various antimicrobial combinations were determined by using the microdilution checkerboard titration method []. A 96-well microplate was used to assess the synergistic effects of the antimicrobial combinations azithromycin–meropenem, rifampicin–meropenem, meropenem–colistin, and azithromycin–colistin. A table showing antibiotic concentration ranges used for the microdilution checkerboard titration method is also shown in Supplementary Table S2. All six carbapenemase- and metallo-beta-lactamase-producing CRAB strains, namely, S10, S67, S84, S96, S97, and S98, with specific genetic makeup as shown in Table 1, were exposed to these combinations. These combinations were chosen for the study based on the previous literature indicating their potential efficacy against carbapenem-resistant A. baumannii (CRAB) infections. The MICs of these individual antimicrobials were determined in the range of 0.25 µg/mL to 256 µg/mL by the microdilution technique following the CLSI guidelines []. The MIC of colistin of ≥4 µg/mL was considered resistant, and an MIC of meropenem of ≥8 µg/mL was considered resistant. No susceptibility breakpoints were available for azithromycin and rifampicin against A. baumannii within the CLSI guidelines. However, in this case, the CLSI criteria for staphylococci were used to determine resistance, where an MIC of ≥4 µg/mL for rifampicin was considered resistant []. Additionally, the CLSI criteria for Enterobacterales were used to determine resistance to azithromycin, with an MIC of ≥32 µg/mL considered resistant, as outlined by Humphries et al. in 2021 []. The strain ATCC 25922 of Escherichia coli was used to ensure quality control.
Each drug was diluted by using a two-fold dilution method []. The fractional inhibitory concentration index (FICI) was calculated as the sum of the fractional inhibitory concentration (FIC) of drug A and the FIC of drug B.
FIC of Drug A = MIC of drug A in combination/MIC of drug A alone
FIC of Drug B = MIC of drug B in combination/MIC of drug B alone
The results were interpreted based on the following criteria []:
- An FIC index ≤ 0.5 indicates synergy
- An FIC index within 0.5–1 indicates partial synergy
- An FIC index ≥ 1–<4 indicates indifference
- An FIC index ≥ 4 indicates antagonism.
3. Results
3.1. Phenotypic Characterization
Six A. baumannii isolates were identified by using API 20E. Carbapenemase production was confirmed in all six isolates by the MHT, while MBL production was detected in all isolates by using the IPM-EDTA DDST. All isolates exhibited resistance to cefepime, ceftazidime, piperacillin/tazobactam, ciprofloxacin, levofloxacin, gentamicin, amikacin, and tobramycin. However, two (33.3%) isolates showed susceptibility to doxycycline, and three (50%) isolates were susceptible to trimethoprim–sulfamethoxazole. Colistin showed effectiveness against all isolates, with none of the latter exhibiting resistance. The antimicrobial susceptibility results are summarized in Table 1.
3.2. Molecular Characterization of MBL- and Carbapenemase-Encoding Genes of CRAB
The blaOXA-51 gene is a naturally occurring gene unique to A. baumannii species, and it was detected in all six isolates. Other genes, including blaOXA-51, blaOXA-58, blaOXA-24, blaOXA-23, blaNDM-1, and blaVIM, were detected with variable frequency among the isolates of CRAB, as mentioned in Table 1. None of the isolates tested positive for blaIMP.
The DNA sequencing analysis of carbapenemase- and MBL-encoding genes of A. baumannii was conducted by using BioEdit, resulting in sequences for the blaOXA-24, blaOXA-23, blaOXA-58, blaOXA-51, and blaNDM-1 genes as described in our previous publication [].
3.3. Phylogenetic Studies
A phylogenetic analysis was conducted on the DNA sequenced genes, including blaOXA-23, blaOXA-24, blaOXA-51, blaOXA-58, and blaNDM-1, from our CRAB isolates, and the results were depicted as phylogenetic trees. The respective phylogenetic trees included GenBank accession numbers for our study strains. The tree for query strain S67 OXA-24 gene in Figure 1 showed 97% evolutionary relatedness to the NCBI databases. In Figure 2, the phylogenetic representation of study strain S96 OXA-58 displayed 100% evolutionary resemblance with NCBI databases. Similarly, in Figure 3, the phylogenetic analysis of study strain S97 OXA-58 exhibited 96% evolutionary resemblance with the NCBI database. Study strain S98 OXA-58 demonstrated 100% evolutionary relatedness with the NCBI database in Figure 4. Figure 5 illustrates 99% evolutionary relatedness with the NCBI database for study strain S10 OXA-23. In Figure 6 and Figure 7, study strain S84 NDM-1 and S10 OXA-51 showed 99% and 100% evolutionary closeness with the NCBI database, respectively.
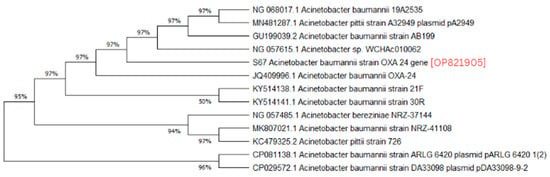
Figure 1.
Phylogenetic analysis of blaOXA-24 gene.

Figure 2.
Phylogenetic analysis of blaOXA-58 gene present in study strain 96.

Figure 3.
Phylogenetic analysis of blaOXA-58 gene present in study strain 97.

Figure 4.
Phylogenetic analysis of blaOXA-58 gene present in study strain 98.
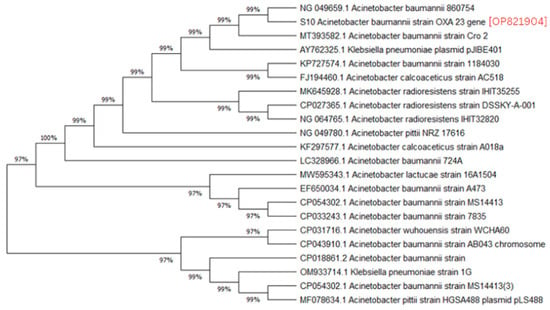
Figure 5.
Phylogenetic analysis of blaOXA-23 gene.

Figure 6.
Phylogenetic analysis of blaNDM-1 gene.
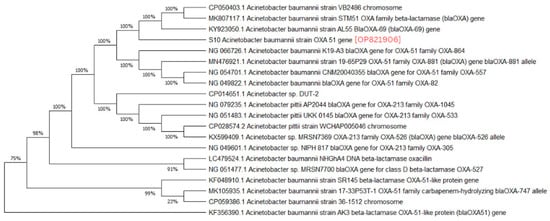
Figure 7.
Phylogenetic analysis of blaOXA-51 gene.
3.4. Mutational Analysis of blaOXA Genes of CRAB Isolates
By using I-Mutant, MUPro, and PHD-SNP software, we observed 16 mutations in the carbapenemase-encoding genes (the blaOXA-24, blaOXA-51, and blaOXA-58 genes) of the CRAB isolates, as shown in Table 2. The blaOXA-58 gene of study strain S96 had the maximum of five mutations (A60L, I59M, P116L, S121Q, and F167P); the blaOXA-51 gene of study strain S10 had two mutations (E11Q and K50N); the blaOXA-58 gene of study strain S97 had four mutations (Q2K, G4S, I61M, and A62L); the blaOXA-58 gene of study strain S98 had four mutations (A60L, I59M, S121K, and V181I); and the blaOXA-24 gene of study strain S67 had only one mutation (M69L). No mutations were observed in the blaOXA-23 gene. Table 2 also presents the amino acid changes observed at specific positions in the protein sequences of these carbapenemase-encoding genes. Table 3 predicted the impact of these 16 mutations on the genetic expression of antimicrobial resistance in the carbapenemase-encoding genes (blaOXA-24, blaOXA-51, and blaOXA-58 genes). Out of 16 mutations, only 1 (P116L) was predicted to cause a “Change” in gene expression, indicating a damaging effect. The remaining 15 mutations showed conflicting predictions regarding their impact on protein stability and subsequent gene expression, suggesting a non-damaging effect, as shown in Table 3.

Table 2.
Mutations reported in carbapenemase-encoding genes of CRAB isolates.

Table 3.
Mutational analysis of carbapenemase-encoding genes of CRAB isolates.
3.5. Synergistic Effects of Antimicrobial Agents
The MIC results of all the antimicrobial agents, when used alone against all six carbapenemase and metallo-beta-lactamase producing CRAB strains, i.e., S10, S67, S84, S96, S97, and S98, with known genes, exhibited resistance to meropenem, azithromycin, and rifampicin, as described in Table 4. The MICs of colistin, rifampin, meropenem, and azithromycin were 2 µg/mL, 128 µg/mL, 64 µg/mL, and >256 µg/mL. Although the azithromycin MIC was >256, we used 256 for the FICI calculations. The checkerboard investigation with all four combinations of antimicrobials, i.e., azithromycin–meropenem, rifampicin–meropenem, meropenem–colistin, and azithromycin–colistin, showed an “indifference” result with FIC index ≥1–<4, as demonstrated in Table 5. No synergistic, partially synergistic, or antagonistic interactions were observed among the examined antimicrobial combinations.

Table 4.
MICs of antimicrobials against selected CRAB.

Table 5.
The checkerboard test results of carbapenem-resistant A. baumannii.
4. Discussion
The study was conducted to predict the mutations in antibiotic resistance genes in CRAB and to determine the in vitro effectiveness of the different antibiotic combinations against resistant A. baumanii. The data on these aspects of A. baumanii are scarce in Pakistan. Through molecular analysis, we detected MBL- and carbapenemase-encoding genes in the CRAB isolates, focusing on seven genes: blaOXA-51, blaOXA-23, blaOXA-24, blaOXA-58, blaVIM, blaNDM-1, and blaIMP. Our results align with previous findings. A study in the UK found high prevalence of the blaNDM-1, blaIMP, blaOXA-51, and blaOXA-23 genes in 112 A. baumannii samples from a Lahore tertiary care setting []. Another UK study showed high prevalence of blaOXA-23 and blaNDM-1 genes, with lower prevalence of blaVIM and blaIMP genes []. Similarly, a study from Pakistan reported blaOXA-51, blaOXA-23, and blaNDM-1 as the predominant genes in their isolates []. Previous studies have highlighted specific gene combinations contributing to A. baumannii antimicrobial resistance, such as blaOXA-51, blaOXA-23, and blaVIM, which were also common in our study [,,]. While blaOXA-23 and blaOXA-51 are commonly related to carbapenem resistance in A. baumannii, recent studies have shown a notable presence of blaNDM-1 and blaVIM, consistently with our findings [,,,].
Evolutionary relatedness among the isolates is crucial to understanding the multidrug-resistant patterns of A. baumannii and preventing carbapenem resistance in the community []. In our study, we observed a high level of evolutionary relatedness of MBL- and carbapenemase-encoding genes to the NCBI Database, with the blaOXA-51 and blaOXA-58 genes showing 100% resemblance in their sequences. In contrast, the blaOXA-23, blaOXA-24, and blaNDM-1 genes exhibited over 95% resemblance to the NCBI database. These results align with a study that found a similar high carbapenemase gene-relatedness in isolates from South Africa, a third-world country []. Another study conducted a phylogenetic analysis on A. baumannii isolates with efflux pump activity contributing to multidrug resistance. They found that all their isolates were blaOXA-51-positive and that approximately 75% exhibited efflux pump expression as a resistance mechanism. Their research identified two gene mutations, namely, the parC gene mutation and the gyrA gene mutation, responsible for inducing efflux pump expression in A. baumannii strains [].
We performed mutational analysis to predict the mutations in our CRAB isolates and indirectly their potential impact on gene expression. According to our criteria, we discovered 16 mutations responsible for carbapenem resistance in our isolates; however, only one mutation predicted a notable impact on gene expression. The mutation was reported in the blaOXA-58 gene present in study strain 96 in our study, and it displayed increased stability for the mutated gene, as predicted by software. According to our criteria, the mutations reported for blaOXA-24 and blaOXA-51 were regarded as having no harmful effect on gene expression. Exploring mutations in the isolates of A. baumannii is a relatively novel area in research. A recent study from China reported mutations in A. baumannii isolates causing resistance against colistin, which is used as a “last resort” pharmacotherapy after carbapenem resistance develops in susceptible patients []. They reported two key mutations responsible for colistin resistance in selected isolates, thereby strengthening the argument that mutations at a genetic level drive the development of antimicrobial resistance in A. baumannii. The amino acid substitutions in the blaOXA-51 gene drive most of the carbapenemase activity in A. baumannii species. A study conducted in Hong Kong identified three key mutations that contributed to enhanced catalytic activity [].
Additionally, we conducted antimicrobial susceptibility testing and discovered that most of our isolates were resistant to various antimicrobials. Only a limited number of isolates exhibited susceptibility to doxycycline and trimethoprim–sulfamethoxazole, while all isolates demonstrated susceptibility to colistin. Our results align with a systematic review with low resistance to doxycycline and colistin. []. Similarly, a study from India found that A. baumannii isolates exhibited susceptibility to colistin, followed by tetracyclines, in antimicrobial susceptibility testing, with most isolates demonstrating resistance to carbapenems, corroborating our findings [].
Multiple pathways can contribute to the development of resistance against a specific class of antimicrobials []. Therefore, we explored the synergistic effects of various antimicrobial combinations. However, we did not observe any synergy, partial synergy, or antagonism with any combination. All the combinations, such as azithromycin–meropenem, rifampicin–meropenem, meropenem–colistin, and azithromycin–colistin, showed indifference results against all the strains (6/6, 100%). A meta-analysis of similar studies conducted in 2018 found considerable synergy rates [] with the same combinations used in our study, contrasting with our findings. Another study in São Paulo reported that synergistic effects were observed for these combinations in colistin-susceptible isolates [], which again contrasts with our study. A study conducted in India found a significant synergistic effect of 72% when combining colistin and meropenem against A. baumannii [].
Therefore, multiple studies evaluating the synergistic effects of antimicrobial combinations reported synergism, which differed from our study, possibly due to the presence of multiple resistant genes (bla OXA-23, bla OXA-24, bla OXA-51, bla OXA-58, bla NDM-1, and bla VIM) with varying frequencies co-harboring in CRAB isolates. This high resistance in A. baumannii strains in our study, primarily attributed to key resistance genes, has created an urgent need to explore more suitable therapeutic agents. The inefficacy of antimicrobials against drug-resistant bacteria has sparked renewed interest in silver nanoparticles. Research has begun to investigate silver nanoparticles and other inorganic nanoparticles [,], which may offer innovative approaches in the face of declining antimicrobial effectiveness []. However, there is minimal research on this topic in Pakistan. Further research should be conducted in Pakistan to explore the efficacy of nanoparticles, both independently and in synergy with other antimicrobials, against CRAB.
There were several fundamental limitations in this study. The sample size was small. Additionally, the study was conducted by using samples from a tertiary care facility in Lahore, which may not represent the national population due to potential variations in genetic mutations among A. baumannii species across different regions of Pakistan. Thirdly, the study included six bacterial isolates, which may not fully encompass the diverse antibacterial phenotypic characteristics of CRAB. We only conducted phylogenetic and mutational analysis on a limited number of genes instead of performing whole-genome sequencing and analysis. Fourthly, our study only explored four antimicrobial combinations when investigating synergistic effects due to constraints such as resource limitations and the need to prioritize the most promising options based on the existing literature. Therefore, it is recommended to test more combinations, including the tetracycline group (minocycline, doxycycline, and tigecycline) and novel agents like durlobactam/sulbactam with carbapenems and colistin, against CRAB, which might produce promising results. It is further recommended to globally explore these new combinations and then formulate antibiotic stewardship policies and treatment guidelines, emphasizing the need for continuous surveillance accordingly. Future research should prioritize the whole-genome sequencing of A. baumannii species to understand the mutations contributing to carbapenem resistance comprehensively. Additionally, further studies should involve mutational analysis on isolates that better represent the population to elucidate trends in carbapenem resistance throughout Pakistan. Given the innovative nature of mutational analysis for carbapenem resistance in A. baumannii species, the scope of this research should be expanded globally, particularly in endemic regions. Mutational analysis was performed by using predictive tools, so it is recommended to incorporate experimental validation in future studies to supplement and confirm the predictions made by these computational tools.
5. Conclusions
In conclusion, our study demonstrated the presence of specific genes (blaOXA-23, blaOXA-24, blaOXA-51, blaOXA-58, blaNDM-1, and blaVIM) in A. baumannii conferring resistance to potent antimicrobials. We identified that CRAB strains carrying these genes exhibit genetic relatedness to strains found worldwide, emphasizing the need for robust preventive measures. Additionally, our research study highlighted the broad antimicrobial resistance profile of CRAB strains, with limited efficacy in combination therapies, likely due to the coexistence of multiple resistance genes. These findings suggest several future research directions, including prioritizing comprehensive whole-genome sequencing to understand carbapenem resistance in A. baumannii more comprehensively. We recommend expanding mutational analyses across diverse isolates in Pakistan to better represent regional resistance trends. Additionally, there is a need to globally explore and validate new antimicrobial combinations, particularly novel agents like durlobactam/sulbactam with carbapenems and colistin, to inform effective antibiotic stewardship policies and treatment guidelines. These initiatives aim to address current limitations in antimicrobial efficacy and enhance treatment strategies against CRAB infections.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina60071086/s1, Supplementary Table S1: Primers used in this study; Supplementary Table S2: Antimicrobial concentrations used for checkerboard assay.
Author Contributions
Data collection and Manuscript writing, S.I.; Study design and Conceptualization, F.A.; Technical supervision and Review, M.N.; Assistance in the experiment, A.S.; Data analysis and Review, H.E.; Assistance in mutational analysis, T.A.; Assistance in manuscript writing, O.U.R. and E.F.; Technical guidance and Review, A.A.A. and T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The protocol of this study was approved by University of Lahore’s ethical review committee (Reg: DMB-02173003).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Munoz-Price, L.S.; Weinstein, R.A. Acinetobacter infection. N. Engl. J. Med. 2008, 358, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef]
- Cheikh, H.B.; Domingues, S.; Silveira, E.; Kadri, Y.; Rosário, N.; Mastouri, M.; Da Silva, G.J. Molecular characterization of carbapenemases of clinical Acinetobacter baumannii–calcoaceticus complex isolates from a University Hospital in Tunisia. 3 Biotech 2018, 8, 297. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Terbtothakun, P.; Voravuthikunchai, S.P.; Chusri, S. Evaluation of the synergistic antibacterial effects of fosfomycin in combination with selected antibiotics against carbapenem–resistant Acinetobacter baumannii. Pharmaceuticals 2021, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Lolans, K.; Rice, T.W.; Munoz-Price, L.S.; Quinn, J.P. Multicity outbreak of carbapenem-resistant Acinetobacter baumannii isolates producing the carbapenemase OXA-40. Antimicrob. Agents Chemother. 2006, 50, 2941–2945. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.C.; Oliveira, M.S.; Perdigao-Neto, L.V.; Rocha, C.K.D.; Guimaraes, T.; Rizek, C.; Levin, A.S.; Costa, S.F. Antimicrobial combinations against pan-resistant Acinetobacter baumannii isolates with different resistance mechanisms. PLoS ONE 2016, 11, e0151270. [Google Scholar] [CrossRef] [PubMed]
- Ustundag, G.; Oncel, E.K.; Sahin, A.; Keles, Y.E.; Aksay, A.K.; Ciftdogan, D.Y. Colistin treatment for multidrug-resistant gram-negative infections in children: Caution required for nephrotoxicity. Med. Bull. Sisli Etfal Hosp. 2022, 56, 427–434. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 guidance on the treatment of antimicrobial resistant gram-negative infections. Clin. Infect. Dis. 2023, ciad428. [Google Scholar] [CrossRef]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef]
- Geisinger, E.; Huo, W.; Hernandez-Bird, J.; Isberg, R.R. Acinetobacter baumannii: Envelope determinants that control drug resistance, virulence, and surface variability. Annu. Rev. Microbiol. 2019, 73, 481–506. [Google Scholar] [CrossRef]
- Pournajaf, A.; Rajabnia, R.; Razavi, S.; Solgi, S.; Ardebili, A.; Yaghoubi, S.; Khodabandeh, M.; Yahyapour, Y.; Emadi, B.; Irajian, G. Molecular characterization of carbapenem-resistant Acinetobacter baumannii isolated from pediatric burns patients in an Iranian hospital. Trop. J. Pharm. Res. 2018, 17, 135–141. [Google Scholar] [CrossRef]
- Vrancianu, C.O.; Gheorghe, I.; Czobor, I.B.; Chifiriuc, M.C. Antibiotic resistance profiles, molecular mechanisms and innovative treatment strategies of Acinetobacter baumannii. Microorganisms 2020, 8, 935. [Google Scholar] [CrossRef]
- Al-Kadmy, I.M.; Ibrahim, S.A.; Al-Saryi, N.; Aziz, S.N.; Besinis, A.; Hetta, H.F. Prevalence of genes involved in colistin resistance in Acinetobacter baumannii: First report from Iraq. Microb. Drug Resist. 2020, 26, 616–622. [Google Scholar] [CrossRef]
- Di Venanzio, G.; Flores-Mireles, A.L.; Calix, J.J.; Haurat, M.F.; Scott, N.E.; Palmer, L.D.; Potter, R.F.; Hibbing, M.E.; Friedman, L.; Wang, B. Urinary tract colonization is enhanced by a plasmid that regulates uropathogenic Acinetobacter baumannii chromosomal genes. Nat. Commun. 2019, 10, 2763. [Google Scholar] [CrossRef]
- Khurshid, M.; Rasool, M.H.; Siddique, M.H.; Azeem, F.; Naeem, M.; Sohail, M.; Sarfraz, M.; Saqalein, M.; Taj, Z.; Nisar, M.A. Molecular mechanisms of antibiotic co-resistance among carbapenem resistant Acinetobacter baumannii. J. Infect. Dev. Ctries. 2019, 13, 899–905. [Google Scholar] [CrossRef]
- Bellio, P.; Segatore, B.; Mancini, A.; Di Pietro, L.; Bottoni, C.; Sabatini, A.; Brisdelli, F.; Piovano, M.; Nicoletti, M.; Amicosante, G. Interaction between lichen secondary metabolites and antibiotics against clinical isolates methicillin-resistant Staphylococcus aureus strains. Phytomedicine 2015, 22, 223–230. [Google Scholar] [CrossRef]
- Nageeb, W.; Metwally, L.; Kamel, M.; Zakaria, S. In vitro antimicrobial synergy studies of carbapenem-resistant Acinetobacter baumannii isolated from intensive care units of a tertiary care hospital in Egypt. J. Infect. Public Health 2015, 8, 593–602. [Google Scholar] [CrossRef]
- Pongpech, P.; Amornnopparattanakul, S.; Panapakdee, S.; Fungwithaya, S.; Nannha, P.; Dhiraputra, C.; Leelarasamee, A. Antibacterial activity of carbapenem-based combinations againts multidrug-resistant Acinetobacter baumannii. J. Med. Assoc. Thail. 2011, 93, 161. [Google Scholar]
- Choi, M.-J.; Yohannes, S.B.; Lee, S.-J.; Damte, D.; Reza, M.A.; Rhee, M.-H.; Kim, T.-H.; Park, S.-C. The in vitro Antibacterial Activity of Enrofloxacin-Trimethoprim Combination against Five Bacterial species. Pak. Vet. J. 2012, 32, 363–366. [Google Scholar]
- Ijaz, S.; Ansari, F.; Nawaz, M.; Anjum, A.A.; Rasool, K. Genetic Characterization of Carbapenem Resistant Acinetobacter baumannii in Tertiary care settings of Lahore, Pakistan. Adv. Life Sci. 2023, 10, 479–485. [Google Scholar]
- Whitman, W.B.; Rainey, F.; Kämpfer, P.; Trujillo, M.; Chun, J.; DeVos, P.; Hedlund, B.; Dedysh, S. Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley Online Library: Hoboken, NJ, USA, 2015; Volume 410. [Google Scholar]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 1–23. [Google Scholar]
- CLSI. Performance standards for antimicrobial susceptibility testing. In CLSI Document M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Amjad, A.; Mirza, I.A.; Abbasi, S.; Farwa, U.; Malik, N.; Zia, F. Modified Hodge test: A simple and effective test for detection of carbapenemase production. Iran. J. Microbiol. 2011, 3, 189. [Google Scholar]
- Yong, D.; Lee, K.; Yum, J.H.; Shin, H.B.; Rossolini, G.M.; Chong, Y. Imipenem-EDTA disk method for differentiation of metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 2002, 40, 3798–3801. [Google Scholar] [CrossRef]
- Capriotti, E.; Fariselli, P.; Casadio, R. I-Mutant2. 0: Predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005, 33, W306–W310. [Google Scholar] [CrossRef]
- Cheng, J.; Randall, A.; Baldi, P. Prediction of protein stability changes for single-site mutations using support vector machines. Proteins Struct. Funct. Bioinform. 2006, 62, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, E.; Calabrese, R.; Casadio, R. Predicting the insurgence of human genetic diseases associated to single point protein mutations with support vector machines and evolutionary information. Bioinformatics 2006, 22, 2729–2734. [Google Scholar] [CrossRef] [PubMed]
- Benamri, I.; Azzouzi, M.; Moussa, A.; Radouani, F. An in silico analysis of rpoB mutations to affect Chlamydia trachomatis sensitivity to rifamycin. J. Genet. Eng. Biotechnol. 2022, 20, 146. [Google Scholar] [CrossRef]
- Moody, J. Synergism testing: Broth microdilution checkerboard and broth macrodilution method. In Clinical Microbiology Procedures Handbook; Garcia, L.S., Isenberg, H., Eds.; ASM Press: Washington, DC, USA, 2004; pp. 1–23. [Google Scholar]
- Wayne, P. Performance standards for antimicrobial susceptibility testing. In CLSI Supplement M100; Clinical Laboratory Standard Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Almutairi, M.M. Synergistic activities of colistin combined with other antimicrobial agents against colistin-resistant Acinetobacter baumannii clinical isolates. PLoS ONE 2022, 17, e0270908. [Google Scholar] [CrossRef]
- Humphries, R.; Bobenchik, A.M.; Hindler, J.A.; Schuetz, A.N. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100. J. Clin. Microbiol. 2021, 59, e0021321. [Google Scholar] [CrossRef]
- Sopirala, M.M.; Mangino, J.E.; Gebreyes, W.A.; Biller, B.; Bannerman, T.; Balada-Llasat, J.-M.; Pancholi, P. Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 4678–4683. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N.; Ellington, M.J.; Coelho, J.M.; Turton, J.F.; Ward, M.E.; Brown, S.; Amyes, S.G.; Livermore, D.M. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 2006, 27, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Ellington, M.J.; Kistler, J.; Livermore, D.M.; Woodford, N. Multiplex PCR for rapid detection of genes encoding acquired metallo-β-lactamases. J. Antimicrob. Chemother. 2007, 59, 321–322. [Google Scholar] [CrossRef]
- Perry, J.D.; Naqvi, S.H.; Mirza, I.A.; Alizai, S.A.; Hussain, A.; Ghirardi, S.; Orenga, S.; Wilkinson, K.; Woodford, N.; Zhang, J. Prevalence of faecal carriage of Enterobacteriaceae with NDM-1 carbapenemase at military hospitals in Pakistan, and evaluation of two chromogenic media. J. Antimicrob. Chemother. 2011, 66, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Zeshan, B.; Qadri, M.M.A.; Ishaq, M.; Afzal, M.; Ahmed, N. Phenotypic and genotypic evaluation of antibiotic resistance of Acinetobacter baumannii bacteria isolated from surgical intensive care unit patients in Pakistan. Jundishapur J. Microbiol. 2021, 14, e113008. [Google Scholar] [CrossRef]
- Leungtongkam, U.; Thummeepak, R.; Wongprachan, S.; Thongsuk, P.; Kitti, T.; Ketwong, K.; Runcharoen, C.; Chantratita, N.; Sitthisak, S. Dissemination of bla OXA-23, bla OXA-24, bla OXA-58, and bla NDM-1 Genes of Acinetobacter baumannii Isolates from Four Tertiary Hospitals in Thailand. Microb. Drug Resist. 2018, 24, 55–62. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Mathur, P.; Kapil, A.; Das, B.K.; Ray, P.; Gautam, V.; Sistla, S.; Parija, S.C.; Walia, K.; Ohri, V. Molecular characterization & epidemiology of carbapenem-resistant Acinetobacter baumannii collected across India. Indian J. Med. Res. 2019, 149, 240–246. [Google Scholar]
- Sharma, S.; Banerjee, T.; Yadav, G.; Kumar, A. Susceptibility profile of blaOXA-23 and metallo-β-lactamases co-harbouring isolates of carbapenem resistant Acinetobacter baumannii (CRAB) against standard drugs and combinations. Front. Cell. Infect. Microbiol. 2023, 12, 1068840. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, H.; Yao, Y.; Zhang, L.; Zhou, Z.; Leptihn, S.; Yu, Y.; Hua, X.; Fu, Y. Description of a rare pyomelanin-producing carbapenem-resistant Acinetobacter baumannii strain coharboring chromosomal OXA-23 and NDM-1. Microbiol. Spectr. 2022, 10, e02144-22. [Google Scholar] [CrossRef]
- Anane, Y.A.; Apalata, T.; Vasaikar, S.; Okuthe, G.E.; Songca, S. Molecular detection of carbapenemase-encoding genes in multidrug-resistant Acinetobacter baumannii clinical isolates in South Africa. Int. J. Microbiol. 2020, 2020, 7380740. [Google Scholar] [CrossRef]
- Ibrahim, M.E. Prevalence of Acinetobacter baumannii in Saudi Arabia: Risk factors, antimicrobial resistance patterns and mechanisms of carbapenem resistance. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Zander, E.; Stefanik, D.; Higgins, P.G.; Roca, I.; Vila, J.; McConnell, M.J.; Cisneros, J.M.; Seifert, H.; WP4, M.W.G. High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J. Antimicrob. Chemother. 2017, 72, 3277–3282. [Google Scholar] [CrossRef] [PubMed]
- Adjei, A.Y.; Vasaikar, S.D.; Apalata, T.; Okuthe, E.G.; Songca, S.P. Phylogenetic analysis of carbapenem-resistant Acinetobacter baumannii isolated from different sources using Multilocus Sequence Typing Scheme. Infect. Genet. Evol. 2021, 96, 105132. [Google Scholar] [CrossRef] [PubMed]
- Nogbou, N.-D.; Nkawane, G.M.; Ntshane, K.; Wairuri, C.K.; Phofa, D.T.; Mokgokong, K.K.; Ramashia, M.; Nchabeleng, M.; Obi, L.C.; Musyoki, A.M. Efflux pump activity and mutations driving multidrug resistance in Acinetobacter baumannii at a Tertiary Hospital in Pretoria, South Africa. Int. J. Microbiol. 2021, 2021, 9923816. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, H.; Jiang, Y.; Shao, L.; Yang, S.; Chen, D. New mutations involved in colistin resistance in Acinetobacter baumannii. Msphere 2020, 5, e00895-19. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-W.; Liu, C.-Y.; Wong, H.-Y.; Chan, W.-C.; Wong, K.-Y.; Chen, S. Specific Amino Acid Substitutions in OXA-51-Type β-Lactamase Enhance Catalytic Activity to a Level Comparable to Carbapenemase OXA-23 and OXA-24/40. Int. J. Mol. Sci. 2022, 23, 4496. [Google Scholar] [CrossRef]
- Moradi, J.; Hashemi, F.B.; Bahador, A. Antibiotic resistance of Acinetobacter baumannii in Iran: A systemic review of the published literature. Osong Public Health Res. Perspect. 2015, 6, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Chopra, D.; Wazahat, R.; Dhingra, S.; Dudeja, M. Antimicrobial susceptibility patterns of an emerging multidrug resistant nosocomial pathogen: Acinetobacter baumannii. Malays. J. Med. Sci. MJMS 2018, 25, 129. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-F.; Lan, C.-Y. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J. Clin. Cases WJCC 2014, 2, 787. [Google Scholar] [CrossRef]
- Jiang, Z.; He, X.; Li, J. Synergy effect of meropenem-based combinations against Acinetobacter baumannii: A systematic review and meta-analysis. Infect. Drug Resist. 2018, 11, 1083–1095. [Google Scholar] [CrossRef]
- Gunalan, A.; Sarumathi, D.; Sastry, A.S.; Ramanathan, V.; Rajaa, S.; Sistla, S. Effect of combined colistin and meropenem against meropenem resistant Acinetobacter baumannii and Pseudomonas aeruginosa by checkerboard method: A cross sectional analytical study. Indian J. Pharmacol. 2021, 53, 207–212. [Google Scholar] [PubMed]
- Wan, G.; Ruan, L.; Yin, Y.; Yang, T.; Ge, M.; Cheng, X. Effects of silver nanoparticles in combination with antibiotics on the resistant bacteria Acinetobacter baumannii. Int. J. Nanomed. 2016, 11, 3789–3800. [Google Scholar] [CrossRef] [PubMed]
- Temocin, F.; Erdinc, F.S.; Tulek, N.; Demirelli, M.; Ertem, G.; Kinikli, S.; Koksal, E. Synergistic effects of sulbactam in multi-drug-resistant Acinetobacter baumannii. Braz. J. Microbiol. 2015, 46, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Arafa, A.A.; Kandil, M.M. Antimicrobial Activity of Zinc Oxide Nanoparticles against ESBL Producing Klebsiella pneumoniae Isolated from Equines in Egypt. Pak. Vet. J. 2023, 44, 176–182. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).