Abstract
Background and Objectives: Germline DNA damage response (DDR) gene mutations correlate with increased prostate cancer (PCa) risk and a more aggressive form of the disease. DDR mutation testing is recommended for metastatic PCa cases, while eligible information about the mutations’ burden in the early-stage localized PCa is still limited. This study is aimed at the prospective detection of DDR pathway mutations in cases with localized PCa and correlation with clinical, histopathological, and radiological data. A comparison to the previously assessed cohort of the advanced PCa was performed. Materials and Methods: Germline DDR gene mutations were assessed prospectively in DNA samples from 139 patients, using a five-gene panel (BRCA1, BRCA2, ATM, CHEK2, and NBN) targeted next-generation sequencing. Results: This study revealed an almost three-fold higher risk of localized PCa among mutation carriers as compared to non-carriers (OR 2.84 and 95% CI: 0.75–20.23, p = 0.16). The prevalence of germline DDR gene mutations in PCa cases was 16.8% (18/107) and they were detected only in cases with PI-RADS 4/5 lesions. BRCA1/BRCA2/ATM mutation carriers were 2.6 times more likely to have a higher (>1) cISUP grade group compared to those with a CHEK2 mutation (p = 0.27). However, the number of cISUP > 1-grade patients with a CHEK2 mutation was significantly higher in advanced PCa than in localized PCa: 66.67% vs. 23.08% (p = 0.047). Conclusions: The results of our study suggest the potential of genetic screening for selected DDR gene mutations for early identification of cases at risk of aggressive PCa.
1. Introduction
Prostate cancer (PCa) is one of the most common problems faced by the male population in the oncology field. Based on the data from the European Cancer Information System, PCa is the most frequently occurring cancer in men; it was responsible for 23.2% of new cancer cases in men in 2020 [1]. Low-risk localized PCa patients may benefit from active surveillance or can be treated by surgery or radiotherapy, usually resulting in complete remission. Intermediate and high-risk localized PCa patients might suffer from disease progression regardless of the primary treatment. In some patients with inherited specific genetic mutations, PCa may manifest in a more severe course, and resistance to conventional treatment develops earlier [2,3]. The transition of next-generation sequencing (NGS) from research to clinical practice revealed new possibilities in the detection of PCa-specific mutations. Due to progress in genomic technologies, mutation status can be verified not only in tumor tissue but also in human body liquids, and it is more widely used in genetic counseling. Blood-based testing of PCa-specific mutations in metastatic PCa patients became increasingly promising, while considering these alterations’ detection for localized PCa is still limited and not widely adopted in clinical practice.
Despite various genetic and epigenetic alterations detectable in PCa, inheritance of PCa risk is mainly attributed to genetic alterations of the DNA damage response (DDR) pathway [4]. This molecular pathway is responsible for the maintenance of the genomic integrity of the cell, and the main players of this pathway are proteins encoded by BRCA1, BRCA2, ATM, ATR, TP53, CHEK1, CHEK2, and some other genes. The proteins of the DDR pathway sense DNA damage, induce cell cycle arrest and DNA repair, and protect cells from deleterious genetic alteration accumulation. Inactivation of the DDR pathway leads to genomic instability, uncontrolled cell growth, and malignization. Strong enrichment of mutations in the genes of the DDR pathway is detectable in PCa tissue, especially in metastatic cases [5,6], while a systemic review of the largest PCa studies [7] suggests even higher median prevalence of these mutations in the germline profile of PCa. BRCA1, BRCA2, ATM, CHEK2, and NBN genes are some of the most important alterations in the PCa genetic evaluation process and are frequently included in extensive PCa gene panels.
Most guidelines suggest DDR pathway mutation screening for familial and metastatic PCa, aiming at personalized therapy with poly-ADP ribose polymerase inhibitors (PARPi) [8]. According to the systemic review median prevalence rate for germline DDR gene mutations in general (unselected) PCa is higher than in the metastatic disease (18.6% vs 11.6%), suggesting a higher burden of these mutations than expected [7]. However, quite a few studies analyzed the DDR gene mutation rate in localized or locally advanced PCa and detected a prevalence of 1.44–9.5% [9,10,11,12,13]. In PCa, germline DDR mutations seem to have a higher prevalence than somatic ones and, due to the low penetrance of some mutations, remain undetected in families until manifestation in aggressive forms of cancer [7]. It was shown that patients with inherited pathogenic mutations of several DDR genes are at increased risk of developing more aggressive forms of the disease, while BRCA2 mutations are directly associated with poor survival in metastatic PCa [14,15,16]. Metastatic PCa patients with mutant DDR genes already benefit from targeted therapies with PARPi, while patients with the localized disease could be evaluated for the risk of early recurrence or take advantage of personalized treatment options in the future; however, such observations need further investigation. Early detection of DDR mutation carriers is also vital for family members’ consultation due to the high risk of aggressive breast, ovarian, and some other tumors [17]. Since at least a quarter of PCa patients identified with germline mutations lack a cancer-related family history, and the mutations possibly evolve de novo [18], it is important to develop algorithms for the meaningful selection of PCa patients for germline mutation testing.
Our prospective, single-center cohort study aimed to assess the prevalence of germline DDR gene mutations (BRCA1, BRCA2, ATM, CHEK2, and NBN) in patients with localized PCa that was diagnosed based on positive findings of multiparametric magnetic resonance and ultrasound imaging (mpMRI/UG) fusion-guided targeted biopsy. Associations between mutation status and clinical, histopathological, and radiological data were analyzed and a comparison to the previously assessed advanced PCa cohort [19] was performed.
2. Materials and Methods
2.1. Patient Cohort
Between 2019 and 2023, a total of 150 patients with suspected PCa were enrolled in this study at the National Cancer Institute (Vilnius, Lithuania). Prostate mpMRI was performed on all study patients and was reported using the Prostate Imaging Reporting & Data System version 2.1 (PI-RADSv2.1) [20]. A positive mpMRI scan was characterized by the presence of PI-RADS lesions with a score ≥ 3. Patients with a positive prostate mpMRI scan, in accordance with their medical history, clinical data, and/or elevated (>3.0 ng/mL) prostate-specific antigen (PSA) level, underwent mpMRI/US fusion-guided targeted biopsy. PSA density was defined as total PSA (ng/mL) divided by mpMRI calculated prostate volume (mL). Biopsy results were evaluated based on the International Society of Urological Pathology (cISUP) grading system. Low-risk localized, intermediate-risk localized, and high-risk localized/locally advanced diseases were defined by the European Association of Urology (EAU) risk stratification groups for biochemical recurrence [21]. This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the Regional Bioethics Committee (05.11.2019/No: 2019/11-1166-654) and written informed consent was obtained from all participants.
2.2. Sample Collection and NGS
Blood samples were collected prospectively into EDTA blood collection tubes according to the standardized clinical procedures. The collected buffy coat was frozen and stored at a temperature of −80 °C. The DNA extraction was performed by using a GeneJET Genomic DNA Purification kit (Thermo Fisher Scientific, Vilnius, Lithuania), following the manufacturer’s instructions. DNA concentration and purity were determined using the NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) as well as Qubit™ dsDNA BR Assay Kit on a Qubit™ 2.0 Fluorimeter (Invitrogen, TFS, Eugene, OR, USA) and stored at −20 °C until use. Targeted DNA sequencing was performed on the Ion Torrent™ Ion S5™ system, and for the library preparation, Ion AmpliSeq™ Library Kit 2.0 and custom On-Demand Panel (consisting of BRCA1, BRCA2, CHEK2, ATM, and NBN genes) (from Life Technologies (LT), Carlsbad, CA, USA) were used under conditions provided by the manufacturer’s protocol. Sequencing results were analyzed in the Ion ReporterTM Software (version 5.20.2.0) system (Life Technologies, Carlsbad, CA, USA), verified manually in the Integrative Genomics Viewer (IGV, version 2.6.3) tool (Broad Institute, Cambridge, MA, USA), and compared to the hg19 reference human genome sequence. The pathogenic and likely pathogenic mutations were confirmed if the mutation was listed in the clinical variant base ClinVar, as well as visualized on the Integrative Genomics Viewer 2.4.8 tool.
2.3. Statistical Analysis
Statistical analysis was performed using R Version 4.1.1 on R Studio version 2022.07.0 (R Core Team, Vienna, Austria). Oncoprint was created using ComplexHeatmap R package version 2.11.1 [22]. Mutation associations with clinical data were assessed by Fisher exact test, Chi-square test, or t-test where appropriate. The odds ratio (OR) was computed by analyzing two-by-two tables. The statistical significance of the OR was evaluated using Fisher exact tests. Results were considered statistically significant if the p-value was <0.05.
3. Results
3.1. Characteristics of Study Group
In a study cohort of 150 patients, 11 cases were excluded from further analysis due to clinical or sample quantity reasons. After mpMRI/US fusion-guided targeted biopsy, all patients (n = 139) were divided into two groups: 107 were histologically confirmed with localized PCa, and 32 patients without PCa diagnosis were assigned to the control group. Based on the presence or absence of pathogenic mutation in the analyzed genes, the patients were divided into mutation-positive–DDR(+) and mutation-negative–DDR(−) groups.
PCa patients (n = 107) revealed PI-RADS lesions ranging from 3 to 5, with a dominance of PI-RADS 4 lesions (67/107, 62.62%). Also, different cISUP grade groups were observed: the most prevalent cISUP grade group was 1 (68/107, 63.55%), followed by grade group 2 (24/107, 22.43%), grade group 3 (11/107, 10.28%), and grade group 4 (4/107, 3.74%). According to EAU risk groups [21], patients with PCa revealed low-risk disease as the most common: 58/107, 54.20%. Intermediate- and high-risk diseases were detected in 44 (41.12%) and 5 (4.68%) patients, respectively. The main clinicopathological characteristics and mpMRI features of PCa patients are shown in Table 1.

Table 1.
Clinicopathological characteristics and mpMRI features of localized PCa patients.
3.2. DDR Gene Mutations Rates
Out of 139 cases, 14.4% (n = 20) were identified with germline mutations of selected DDR genes (BRCA1, BRCA2, ATM, CHEK2, and NBN) in the blood cells (Figure 1). The mutation rate in the group of cases with PCa diagnosis was 16.8% (18/107), and 19 alterations in DDR genes were found, with one case showing multiple alterations in the CHEK2 gene: CHEK2 c.470T>C and CHEK2 c.1100delC (Figure 2). BRCA1 mutation was detected in one PCa patient (0.93%), BRCA2—3 (2.8%), ATM—1 (0.93%), and CHEK2—13 (12.15%). None of the cases were identified with NBN mutation.
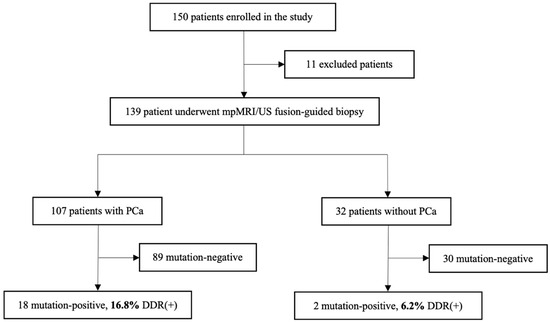
Figure 1.
Flowchart of the patient cohort included in the study. Abbreviations: mpMRI—multiparametric magnetic resonance imaging; US—ultrasound; DDR—DNA damage response; PCa—prostate cancer.
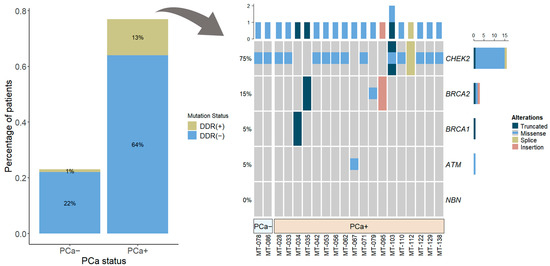
Figure 2.
Percentage of patients (n = 107) with and without DDR gene mutations: overall (barplot) and according to each selected gene (oncoprint). Abbreviations: DDR—DNA damage response; PCa+—prostate cancer, PCa−—controls.
In the control group of 32 cases without confirmed PCa diagnosis, only two CHEK2 gene mutations were detected (2/32; 6.2%). Comparison of localized PCa to control group revealed an almost three-fold higher risk of localized PCa among DDR gene mutation carriers as compared to non-carriers (OR 2.84 and 95% CI: 0.75–20.23, p = 0.16), and CHEK2 mutation was responsible for the doubling in risk of localized PCa (OR 1.95, 95% CI: 0.49–14.18, p = 0.51). Due to the small number of cases with DDR gene mutations, the OR comparison did not reach statistical significance.
Comparison to the advanced PCa cohort from our previous study [19] revealed a higher rate of the DDR gene (BRCA1/BRCA2/ATM/CHEK2) mutations in the localized PCa than in the advanced disease (16.8% vs. 14.8%, p = 0.70).
3.3. Clinical Characteristics of DDR Mutation-Positive PCa
Analysis in the localized PCa group (n = 107) revealed that the DDR(+) cases (n = 18) were younger compared to DDR(−) cases (61.56 vs. 63.69 years, p = 0.27) and were presented with slightly lower PSA concentration and PSA density (Table 1). However, prostate volume was higher in the DDR(+) PCa group (55.77 vs. 44.16, p = 0.06) (Table 1). Importantly, BRCA1/BRCA2/ATM mutation carriers were markedly younger in the localized PCa cohort than in the advanced PCa group from our previous study [19]: 61.20 vs. 68.30 years; p = 0.06.
The localized PCa cases with DDR gene mutations were most frequently identified with PI-RADS 4 (12/18, 66.66%) lesions (Table 1), and the combined occurrence of any DDR gene mutation in PI-RADS 4/5 lesions reached 17.47%. The highest mutation rate was detected in cISUP grade group 4 and accounted for 25.00%. BRCA1/BRCA2/ATM mutation carriers (n = 5) revealed higher cISUP grade group scores (cISUP > 1 vs. cISUP = 1) compared to those with CHEK2 mutation (n = 13) and the overall group of patients with mutations: (n = 18)—60.00% vs. 23.08%, p = 0.27 and 60.00% vs. 33.33%, p = 0.34, respectively. There were no statistically significant differences in mutation frequency according to risk groups divided by low-risk and intermediate/high-risk disease (Table 1).
The DDR gene mutations rate was also high in the cISUP > 1-grade group scores among advanced PCa cases [19] and exceeded mutation prevalence in the localized cISUP > 1 disease (59.10% vs. 33.33%, p = 0.13), and the number of cISUP > 1-grade group patients with CHEK2 mutation was markedly higher in mCRPC as compared to localized PCa: 66.67% vs. 23.08% (p = 0.047) (Figure 3).
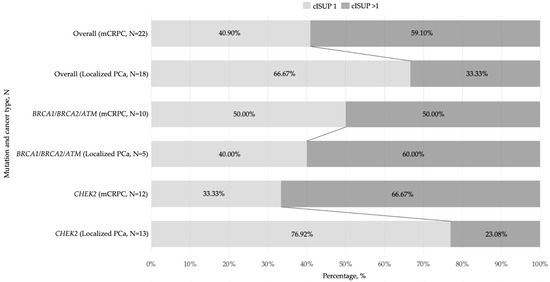
Figure 3.
Bar graphs showing a prevalence of various DDR(+) mutations by cISUP grade group in localized and advanced prostate cancer groups, N. Abbreviations: cISUP—International Society of Urological Pathology grade group; mCRPC—metastatic castration-resistant prostate cancer; PCa—prostate cancer.
4. Discussion
Currently, PCa in the localized setting is characterized by its heterogeneous nature, whereas standard treatment options are generally well-established and approved by various guidelines worldwide. Nonetheless, in this PCa stage, there remains a significant knowledge gap regarding the significance of germline DDR pathway mutations in the management of the disease. Until the development of castration resistance or the diagnosis of distant-spread diseases, the range of follow-up means or specific treatment possibilities for patients with the alterations remains limited, and the weight of various mutations on the disease aggressiveness remains obscure.
In this study, prospective five DDR gene mutation testing was performed in 139 men who underwent mpMRI/US fusion-guided targeted prostate biopsy. We detected a substantial rate of genetic alterations in histologically confirmed PCa patients, reaching a prevalence of 16.8% (18/107). In comparison, our previous study on advanced castration-resistant PCa (mCRPC) revealed a germline mutation prevalence of 14.8% in the same genes (BRCA1, BRCA2, ATM, and CHEK2) [19]. Our data support observations of previous studies [9,10,11,12,13], i.e., that mutation rates can be relatively high in localized PCa in comparison with metastatic disease. This observation suggests that DDR gene mutations are probably early events in the evolution of aggressive prostate tumors and emphasizes the significance of conducting germline testing from the early stages of PCa.
To our knowledge, limited data exist on the association of mpMRI lesions and the presence of DDR gene mutations in localized PCa studies, usually aiming at a radiological nodal status. When analyzing the prevalence of the DDR gene mutations based on the PI-RADSv2.1 scoring system, we found that the combined occurrence of any DDR gene mutation in PI-RADS 4/5 lesions reached 17.47%. Meanwhile, no DDR gene mutations were detected in cases with lower-category (PI-RADS ≤ 3) lesions. Although these results are arguable because of their clinical application, their potential benefit might be seen in the future, when different strategies of germline testing can be adopted in patients with suspicious PCa.
In our study, the DDR pathway mutations were highly specific to cases, and only two controls without confirmed PCa were identified with CHEK2 gene mutations. In our cohort, DDR gene mutations were associated with an almost three-fold, while CHEK2 mutations were associated with a two-fold, increase in localized PCa risk. Positive DDR mutation status may not only help to identify PCa but it also is associated with an aggressive course [14,15] and can help verify localized PCa cases that demand timely treatment.
In our cohort, CHEK2 alteration was found to be the most prevalent (12.15%) in localized PCa, and missense alteration c.470T>C was the predominant type of mutation (12/19). Most notably, Wang, Y. et al. [23], revealed that CHEK2 c.470T>C significantly increased the PCa risk: OR 1.80, 95% CI: 1.51–2.14, p < 0.0001. The significant association between CHEK2 mutations and PCa risk (OR 1.9, 95% CI: 1.6–2.2, p < 0.0001) was also found in a study by Cybulski, C. et al. [24]. Specifically, they observed that truncating CHEK2 variants was associated with higher risk when compared to missense mutations such as c.470T>C [24]. Our study results, in comparison with our previous study [19], revealed a predominance of CHEK2 mutations in high cISUP grade mCRPC, but not in localized PCa (p = 0.047). This encourages further studies of the impact of various CHEK2 mutation types on the aggressiveness of PCa.
The combination of alterations in BRCA1/BRCA2 and ATM genes is associated with more aggressive PCa and is widely investigated in extensive mCRPC trials with PARPi [25,26,27]. In our cohort, BRCA1/BRCA2 and ATM mutations accounted for a percentage of 4.67. The prevalence of BRCA1/BRCA2 and ATM mutations was found to be greater than the reported rates of low-risk localized PCa patients by Na, R. et al. [9], i.e., 1.44%, and is consistent with the findings in the cohort analyzed by Marshall, C.H. et al. [10]—5.4%. When comparing these alterations individually, with the European ancestry patients from the large study by Lee, D.J. et al. [13], we identified higher mutation rates in BRCA1 (0.93% vs. 0.77%), BRCA2 (2.8% vs. 1.0%), and ATM (0.93% vs. 0.51%). The Cancer Genome Atlas (TCGA) [5] analysis of 333 primary prostate tumors revealed BRCA2 and BRCA1 mutation rates that were quite similar to our study (3% and 1%, respectively), while ATM mutations were slightly more frequent than in our study (4% in TCGA vs. 1% in our study). Looking closer at this study on cBioPortal [28], when excluding somatic mutations in this particular cohort, the BRCA1 mutation rate was 1% and the BRCA2 was 1.5%, and there were no germline mutations noted in any of the other six genes (ATM, NBN, and CHEK2 from our study, and CDK12, FANCD2, and RAD51c from the TCGA study).
In our study, the DDR pathway alterations were less common in low-risk PCa patients than in intermediate- or high-risk diseases compared to non-carriers. DDR gene mutation carriers with BRCA1/BRCA2 and ATM alterations were 2.6 and 1.8 times more likely to have a higher (>1) cISUP grade group, compared to those with CHEK2 mutation and with all mutated cases, respectively. Taken together, our data and the data of other authors [2,3,14,15] suggest that these DDR gene mutations can significantly contribute to a more aggressive course of PCa.
There are several limitations of this study. Although our findings revealed a high percentage of DDR gene mutation carriers among localized PCa patients, the sample size with DDR mutations remains relatively small and reduces the statistical power of the study. Also, only five genes were included in the analysis, and the NBN mutation was not detected at all. Several other DDR pathway genes are associated with genetic risk of PCa and may also be important in localized disease. In our analysis, we did not include the family cancer history of the study patients, because family histories were incomplete or inaccurate in a majority of the cases. In addition, we did not investigate how the mutation status may affect clinical outcomes, though this facet merits further studies.
The high prevalence of DDR alterations in localized PCa observed in our and other studies suggests that these mutations are an early event in prostate carcinogenesis. Since DDR deficiency may be associated with an aggressive cancer phenotype, knowledge of DDR gene status in localized stage disease may be critical, requiring more accurate decision-making in various clinical settings, such as choosing active surveillance over radical therapy or surgery over radiation, assessing the optimal timing of salvage radiotherapy after radical prostatectomy, and many other. In addition, DDR gene mutation testing in early-stage localized PCa can provide a better understanding of the molecular biology of PCa and optimize genetic testing strategies for familial cancer management. The impact on clinicopathological data and the correlation between mpMRI findings and mutation status revealed in our study provides additional arguments for wider DDR gene mutation testing in PCa.
5. Conclusions
Results of our study display a quite high rate of germline DDR mutations in localized PCa with certain implications on clinical outcomes suggesting a potential benefit of targeted genetic testing for the early identification of mutation carriers at risk of aggressive PCa.
Author Contributions
Conceptualization, S.J. and F.J.; formal analysis, I.V.; investigation, T.J., I.V. and R.S.; data curation, T.J., R.S., A.M., A.V. and A.U.; writing—original draft preparation, T.J., I.V. and R.S.; visualization, T.J., I.V. and R.S.; supervision, S.J. and F.J.; funding acquisition, S.J. and F.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Bioethics Committee (No: 2019/11-1166-654) (5 November 2019).
Informed Consent Statement
Written informed consent was obtained from all participants involved in this study.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We would like to thank the Future Biomedicine Charity Fund (Lithuania) for the donation of materials used in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cancer Burden Statistics and Trends across Europe|ECIS. Available online: https://ecis.jrc.ec.europa.eu/ (accessed on 30 October 2023).
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K.; Guy, M.; et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Leongamornlert, D.; Saunders, E.; Dadaev, T.; Tymrakiewicz, M.; Goh, C.; Jurgnauth-Little, S.; Kozarewa, I.; Fenwick, K.; Assiotis, I.; Barrowdale, D.; et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease. Br. J. Cancer 2014, 110, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Castro, E.; Aragón, M.I.; Cendón, Y.; Cattrini, C.; López-Casas, P.P.; Olmos, D. Genetic aberrations in DNA repair pathways: A cornerstone of precision oncology in prostate cancer. Br. J. Cancer 2021, 124, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Abeshouse, A.; Ahn, J.; Akbani, R.; Ally, A.; Amin, S.; Andry, C.D.; Annala, M.; Aprikian, A.; Armenia, J.; Arora, A.; et al. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.H.; Swift, S.L.; White, H.; Misso, K.; Kleijnen, J.; Quek, R.G.W. A systematic review of the prevalence of DNA damage response gene mutations in prostate cancer. Int. J. Oncol. 2019, 55, 597–616. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Gomella, L.G.; Petrylak, D.P. When and How to Use PARP Inhibitors in Prostate Cancer: A Systematic Review of the Literature with an Update on On-Going Trials. Eur. Urol. Oncol. 2020, 3, 594–611. [Google Scholar] [CrossRef]
- Na, R.; Zheng, S.L.; Han, M.; Yu, H.; Jiang, D.; Shah, S.; Ewing, C.M.; Zhang, L.; Novakovic, K.; Petkewicz, J.; et al. Germline Mutations in ATM and BRCA1/2 Distinguish Risk for Lethal and Indolent Prostate Cancer and are Associated with Early Age at Death. Eur. Urol. 2017, 71, 740–747. [Google Scholar] [CrossRef]
- Marshall, C.H.; Fu, W.; Wang, H.; Baras, A.S.; Lotan, T.L.; Antonarakis, E.S. Prevalence of DNA repair gene mutations in localized prostate cancer according to clinical and pathologic features: Association of Gleason score and tumor stage. Prostate Cancer Prostatic Dis. 2018, 22, 59–65. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, H.; Li, S.; Wiley, K.; Zheng, S.L.; LaDuca, H.; Gielzak, M.; Na, R.; Sarver, B.A.J.; Helfand, B.T.; et al. Rare Germline Pathogenic Mutations of DNA Repair Genes Are Most Strongly Associated with Grade Group 5 Prostate Cancer. Eur. Urol. Oncol. 2020, 3, 224–230. [Google Scholar] [CrossRef]
- Berchuck, J.E.; Zhang, Z.; Silver, R.; Kwak, L.; Xie, W.; Lee, G.-S.M.; Freedman, M.L.; Kibel, A.S.; Van Allen, E.M.; McKay, R.R.; et al. Impact of Pathogenic Germline DNA Damage Repair alterations on Response to Intense Neoadjuvant Androgen Deprivation Therapy in High-risk Localized Prostate Cancer. Eur. Urol. 2021, 80, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Hausler, R.; Le, A.N.; Kelly, G.; Powers, J.; Ding, J.; Feld, E.; Desai, H.; Morrison, C.; Doucette, A.; et al. Association of Inherited Mutations in DNA Repair Genes with Localized Prostate Cancer. Eur. Urol. 2022, 81, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.H.; Pritchard, C.C.; Montgomery, B.; Lin, D.W.; Nelson, P.S. Prostate Cancer Screening in a New Era of Genetics. Clin. Genitourin. Cancer 2017, 15, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Totaro, A.; Gandi, C.; Bientinesi, R.; Moretto, S.; Gavi, F.; Pierconti, F.; Iacovelli, R.; Bassi, P.; Sacco, E. Germline mutations in prostate cancer: A systematic review of the evidence for personalized medicine. Prostate Cancer Prostatic Dis. 2023, 26, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Romero-Laorden, N.; del Pozo, A.; Lozano, R.; Medina, A.; Puente, J.; Piulats, J.M.; Lorente, D.; Saez, M.I.; Morales-Barrera, R.; et al. Prorepair-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2019, 37, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S.; ESMO Guidelines Committee. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Nombela, P.; Lozano, R.; Aytes, A.; Mateo, J.; Olmos, D.; Castro, E. BRCA2 and Other DDR Genes in Prostate Cancer. Cancers 2019, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Januskevicius, T.; Sabaliauskaite, R.; Dabkeviciene, D.; Vaicekauskaite, I.; Kulikiene, I.; Sestokaite, A.; Vidrinskaite, A.; Bakavicius, A.; Jankevicius, F.; Ulys, A.; et al. Urinary DNA as a tool for germline and somatic mutation detection in castration-resistant prostate cancer patients. Biomedicines 2023, 11, 761. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhami, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; Santis, M.D.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, B.; Ye, D. CHEK2 Mutation and Risk of Prostate Cancer: A Systematic Review and Meta-Analysis. Int. J. Clin. Exp. Med. 2015, 8, 15708. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4658955 (accessed on 14 November 2023). [PubMed]
- Cybulski, C.; Wokolorczyk, D.; Kluzniak, W.; Jakubowska, A.; Gorski, B.; Gronwald, J.; Huzarski, T.; Kashyap, A.; Byrski, T.; Debniak, T.; et al. An Inherited NBN Mutation Is Associated with Poor Prognosis Prostate Cancer. Br. J. Cancer 2012, 108, 461–468. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Fizazi, K.; Piulats, J.M.; Reaume, M.N.; Ostler, P.; McDermott, R.; Gingerich, J.R.; Pintus, E.; Sridhar, S.S.; Bambury, R.M.; Emmenegger, U.; et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N. Engl. J. Med. 2023, 388, 719–732. [Google Scholar] [CrossRef]
- Bryce, A.H.; Piulats, J.M.; Reaume, P.J.; Ostler, R.S.; McDermott, J.R.; Gingerich, E.; Pintus, S.S.; Sridhar, W.; Abida, G.; Daugaard, A.; et al. Rucaparib for metastatic castration-resistant prostate cancer (mCRPC): TRITON3 interim overall survival and efficacy of rucaparib vs docetaxel or second-generation androgen pathway inhibitor therapy. J. Clin. Oncol. 2023, 41 (Suppl. S6), 18. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).