Abstract
Background and Objectives: Hepatocellular carcinoma (HCC) is the leading cause of liver cancer worldwide and has a high mortality rate. Its incidence has increased due to metabolic-associated liver disease (MAFLD) epidemics. Liver transplantation and surgery remain the most resolute measures. Despite the optimistic use of multi-kinase inhibitors, namely sorafenib, the co-existence of chronic liver disease made the response rate low in these patients. Immune checkpoint inhibitors (ICIs) have become a promising hope for certain advanced solid tumors and, also, for advanced HCC. Unfortunately, a large cohort of patients with HCC fail to respond to immunotherapy. Materials and Methods: We conducted a narrative search on the main medical databases for original articles, reviews, meta-analyses, randomized clinical trials, and case series using the following keywords and acronyms and their associations: hepatocellular carcinoma, immunotherapy, checkpoint inhibitors, gut microbiota, and fecal microbiota transplantation. Results: ICIs are a promising and sufficiently safe treatment option for HCC. In detail, they have significantly improved survival and prognosis in these patients vs. sorafenib. Although there are several highlighted mechanisms of resistance, the gut microbiota signature can be used both as a response biomarker and as an effect enhancer. Practically, probiotic dose-finding and fecal microbiota transplantation are the weapons that can be used to increase ICI’s treatment-response-reducing resistance mechanisms. Conclusion: Immunotherapy has been a significant step-up in HCC treatment, and gut microbiota modulation is an effective liaison to increase its efficacy.
1. Introduction
Hepatocellular carcinoma (HCC) is the primary tumor of the liver, often developing in the context of chronic liver disease. It has a global prevalence among cancers, making it the third and seventh most common malignancy in men and women, respectively. Interestingly, HCC is the sixth most commonly occurring cancer worldwide and, due to its constantly increasing incidence, has become the third leading cause of cancer-related death among general populations and the most common cause of death in patients with cirrhosis [1,2]. The incidence of HCC is rapidly growing despite the decreased incidence of chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) falling, mainly because of the new metabolic pandemic affecting our Westernized societies, re-assembled by metabolically associated fatty liver disease (MAFLD) that comprised the definition of nonalcoholic fatty liver disease (NAFLD) [3,4].
Thus, both the increased prevalence and mortality of HCC have been pushing researchers towards better therapeutic approaches. In fact, HCC treatment is complex because of the scarce knowledge of genome mutations and variegated pathophysiology. The standard of care for HCC remains orthotopic liver transplantation and/or surgical resection at the early neoplasm stage [5]. However, we must recognize that there is a shortage of available organs for transplantation and that a high percentage of HCC patients are not eligible for surgical resection as they are not at early cancer stages and, more importantly, they suffer from chronic liver disease leading to advanced organ dysfunction [6,7]. In detail, the most widely adopted HCC staging classification is the updated Barcelona Clinic Liver Cancer (BCLC) [8]. The definitive curative therapies for HCC remain surgical resection and liver transplantation, which can be performed only in patients at very early (0) and early (A) stages. However, because of similar survival times, less invasiveness and, last but not least, a lower economic burden compared to surgical options, percutaneous ablative therapies (namely, radiofrequency ablation (RFA) and microwave ablation (MWA)) are considered the first treatment approach in both of these stages [9,10]. In fact, these techniques have an effective local antitumor effect, but the response rate is relatively weak and might not lead to tumor growth control. Indeed, there is a high local recurrence rate of HCC [11].
It is important to mention that the vast majority of HCC patients (namely, about 65–70%) are still diagnosed in the intermediate (B) or advanced (C) tumoral stages. Therefore, they are ineligible for radical therapies. These patients are considered for transarterial or systemic therapies. The latter show effective results but, at the current status, are non-curative or “palliative”. Precisely, they yield a lower 5-year survival rate. In particular, according to BCLC tumor staging and management, transarterial chemoembolization (TACE) is recommended as first-line therapy for unresectable intermediate-stage HCC (stage B) [12]. More recently, other radiological locoregional therapies have been considered, and other transarterial techniques (namely, transarterial radioembolization (TARE) with yttrium-90) have been suggested as a safe and effective alternative treatment options for HCC patients with a liver-prevalently located disease but not able to tolerate systemic therapies [13].
Despite its poor side effect profile and scarce improvement in overall survival (OS) (namely, less than 3 months vs. placebo), the multi-kinase inhibitor sorafenib has been used as the first-line therapy for Child-Pugh A liver cirrhosis and unresectable/metastatic HCC [7]. Two trials, Sorafenib HCC Assessment Randomized Protocol (SHARP) and Asia Pacific (AP), led to its Food and Drug administration (FDA) approval in 2007 [14,15]. The OS rate of sorafenib is much higher in patients with chronic HCV hepatitis than in those with other etiologies [16]. Subsequently, Lenvatinib was approved as an alternative to sorafenib because it was non-inferior to it [17]. Multi-target tyrosine inhibitors (regorafenib and cabozantinib) [18] and vascular endothelial growth factor (VEGF) receptor inhibitors (ramucirumab) are single-agent second-line treatments for patients failing to respond to sorafenib [19,20]. The combination of atezolizumab and bevacizumab is now regarded as the standard first-line treatment for patients with advanced HCC due to the significant and clinically meaningful improvements in terms of OS, progression-free survival (PFS), objective response rate (ORR) [8,21], and complete response rate (CRR) compared with sorafenib monotherapy [22]. Indeed, the combination of tremelimumab and durvalumab has been reported to be superior to sorafenib in patients with advanced or unresectable HCC, adding another first-line treatment option [23].
Recently, immune checkpoint inhibitors (ICIs) have emerged as alternatives for patients with adequate performance status. In fact, HCC cells have a deep immune system surveillance and escape behavior [24]. In 2017, the FDA approved nivolumab as an add-on treatment for patients failing to respond to sorafenib. Therefore, pembrolizumab was approved. These two immunotherapies belong to the group of programmed cell death protein-1 (PD-1) inhibitors. In addition, a combination of ipilimumab [a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor] and nivolumab was also approved by the FDA. These trials showed one-by-one that ICIs are superior to sorafenib in terms of OS and PFS [25]. Despite these significant improvements in immunotherapy vs. the standard of care for HCC treatment, almost 60% of these patients do not respond to ICIs. In detail, because of several restricted selection parameters, only 10–20% of HCC patients are eligible for first-line ICI therapy. Moreover, this eligible percentage is reduced to less than 10% in the second-line treatment. Therefore, only a small number of HCC patients could actually benefit from immunotherapy. Thus, there is an urgent need for effective predictive serological and/or tissue biomarkers to identify patients likely to benefit from immunotherapy in the context of “personalized “therapy choice. This would reduce the economic impact of treatments’ costs on our healthcare systems. The use of effective biomarkers would also help to avoid ICI-associated adverse events in patients pre-identified as non-responders [26,27].
Among the emerging biomarkers of both treatment response and adverse event prediction, the human gut microbiota is gaining more and more favorable evidence [28,29]. The human gut microbiota is a complex ecosystem encompassing more than 50 bacterial species shared by every individual. Indeed, it also includes viruses, protozoa, fungi, archaea, and yeasts [30]. Its functions range from nutrient absorption and digestion to metabolism modulation and immune system regulation [31]. The latter is of particular interest in the context of carcinogenesis and the re-establishment of immune surveillance in cancerous conditions [32].
We aimed to review literature data on HCC treatment options and, in particular, the impact of ICIs, their use limitations, and the gut microbiota’s role as a response biomarker and, perhaps, an enhancer.
2. Methods: Literature Search
In particular, we conducted a PubMed and Medline search for original articles, reviews, meta-analyses, and case series using the following keywords, their acronyms, and their associations: hepatocellular carcinoma, immunotherapy, checkpoint inhibitors, gut microbiota, and fecal microbiota transplantation. When appropriate, preliminary pieces of evidence from abstracts belonging to main national and international gastroenterological meetings (e.g., United European Gastroenterology Week, Digestive Disease Week) were also included. The items found from the above-mentioned sources were reviewed by two of the authors (L.A. and M.M.) according to PRISMA guidelines [33]. The last MEDLINE search was dated 28 February 2023. Finally, a narrative review was performed.
2.1. Hepatocellular Carcinoma Treatment
2.1.1. HCC Treatment Fundaments
In brief, clinical guidelines for the standard of care of HCC patients include: curative therapies (e.g., radiofrequency or microwave ablation, liver resection, and transplantation) for early-stage cancers; transarterial chemoembolization (TACE) for intermediate-stage cancers; and, the main item treated in this review of literature, systemic pharmacologic treatments reserved for advanced tumors. The latter can be divided into the first and second lines [34,35]. Interestingly, the median survival time is higher than 6 years for resection/ablation procedures and 10 years for transplantation. At intermediate HCC progression stages, there is a median survival time of 20–30 months. The latter is limited to 10–16 months for advanced HCC staging [22,36]. Thus, the advanced tumor stages are fertile ground for research trying to develop systemic therapy approaches for these patients.
The SHARP trial and consequent approval of sorafenib use in advanced HCCs have been cutting-edge moments in hepatology. The main success was the recognition of overall survival (OS) benefits with sorafenib use. ICIs have overcome these limits. Certainly, systemic therapy is for those patients not eligible for locoregional/curative therapy but with adequate performance status and stable liver function. Preliminarily, patients should be screened for viral hepatitis and predictive markers of response, as stated by the American Society of Clinical Oncology (ASCO) [21,37]. Endpoints of HCC therapies are overall survival and other surrogate endpoints (e.g., response rate and progression-free survival (PFS)) [21,38], following the RECIST (the Response Evaluation Criteria in Solid Tumors) and, thereafter, the modified RECIST (mRECIST) guidelines [21,39]. Importantly, sorafenib obtained a significant survival extension but at a low objective response rate (ORR), according to standard RECIST criteria.
2.1.2. Immune Checkpoints and Hepatocellular Carcinoma: The Origin of the New Frontier of Immunotherapy
Immune checkpoints are expressed in various cell types, such as natural killer (NK) and dendritic cells (DCs), tumor-associated macrophages (TAMs), monocytes, and myeloid-derived suppressor cells (MDSCs) [40]. Immune checkpoints are proteins that can inhibit immune cell function, leading to a reduction in wide-field tissue damage. Despite that, as already demonstrated in the literature, tumor cells may disrupt the immune resistance mechanism [41]. In human cancers, the most studied immune checkpoints are: cytotoxic T-lymphocyte associated protein 4 (CTLA-4), programmed death cell protein 1 (PD1)/ligands (PDL1), which showed to have an interesting role in HCC treatment [42]; lymphocyte activation gene 3 (LAG-3), T-cell membrane protein 3 (TIM-3), and B- and T-lymphocyte attenuator (BTLA). The last three molecules do not fall within the field of interest of this review [43].
HCC appeared to be a good environment for immune checkpoint inhibitors (ICIs) treatment due to the high intrahepatic lymphocyte expression of PD-1 in chronic liver diseases. In detail, these diseases are associated with a greater expression of PD-1L in Kupffer cells [44]. In further detail, PD-1 is a receptor expressed on activated T cells, B cells, NK cells, MDSC, and DCs. Mainly, it can inhibit the immune system via tyrosine phosphatase SHP-2, preventing autoimmunity [45]. The ligand is PD-L1, which is expressed by somatic cells in a pro-inflammatory setup; it also suppresses T-cell migration, proliferation, and the release of cytotoxic cytokines (Figure 1A) [46]. Therefore, it is of extreme importance for signaling in liver tumors: It is driven by cancer cells, which constantly express PD-L1 and consequently activate PD-1 in tumor-infiltrating lymphocytes (TILs), evading immune surveillance [47]. Interesting, several studies demonstrated the association between high expression of PD-L1 in liver cancer cells and poor prognosis in HCC [48,49]. The latter was mainly due to tumor recurrence aggressiveness [50,51].
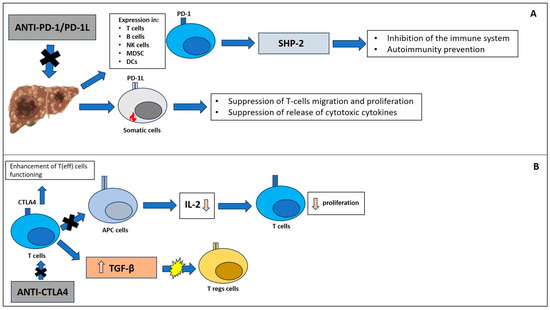
Figure 1.
Immune checkpoint pathways: (A) PD-1/PD-1L and CTLA4 (B).
CTLA4 is a negative regulator of the immune response. Molecularly, it is an intracellular protein within T-cells and translocates to the cell surface when the T-cell receptor is binding CD28. Furthermore, surface CTLA4 binds CD80 and CD86, blocking the linkage of these to CD28. This results in inhibition of T cell proliferation and activation [52]. Importantly, its role in tumorigenesis is associated with the inhibition of interaction between T cells and antigen-presenting cells. Thus, there is a reduction in cytokine production (e.g., IL-2) and T cell proliferation [53]. On the other hand, CTLA-4 may stimulate the expression of immune regulatory cytokines such as transforming growth factor-β (TGF-β). Therefore, CTLA4 has a role in T-reg activation and differentiation because its receptor is constitutively expressed on these cells (Figure 1B). Further, when it is blocked, antitumor activity and autoimmunity are impaired [54]. Therapeutically, the anti-CTLA-4 antibodies can block CTLA4 on Tregs together while enhancing T(eff) cell functioning [55].
2.1.3. Immunotherapy and HCC
Since 2008, the main treatment for hepatocellular carcinoma (HCC) has been the oral multi-kinase inhibitor sorafenib. It has proven to prolong the overall survival time by 2.8 months [14]. During the last few years, they have used several combinations of sorafenib and similar molecules, such as lenvatinib (an inhibitor of VEGF receptors 1–3, FGF receptors 1–4, and the PDGF receptor α). These studies showed non-inferiority in overall survival vs. untreated advanced hepatocellular carcinoma patients [17]. Recently, a lot of interest has focused on immune-checkpoint inhibitors targeting CTLA4 [56,57] and PD1/PDL1 [58], respectively.
More in detail, two different molecules (namely, PD1 inhibitors), already approved for patients with advanced or metastatic melanoma and metastatic refractory non-small cell lung cancer [59,60], have shown a promising efficacy profile for HCC treatment. Nivolumab (anti-PD-1) was approved in 2016 and demonstrated to reach an objective response rate (ORR) of about 20% [61]. Importantly, the latter is fourfold bigger than those of sorafenib. Moreover, in a phase III multicenter trial, nivolumab was compared with sorafenib as a first-line treatment in patients with advanced HCC. Although the OS was not statistically significant (median OS of 16.4 vs. 14.7 months for nivolumab and sorafenib, respectively), nivolumab was shown to be safe and to have clinical activity improvement. Therefore, this drug may be considered as a first-line treatment for patients in whom TKI and anti-angiogenetic drugs are contraindicated [62]. Pembrolizumab (anti-PD-1) was approved in 2018 after a phase 2 multicenter trial demonstrated it to be able to increase the oncologic response in patients with advanced hepatocellular carcinoma previously treated with sorafenib (ORR 17%, 44% with stable disease, 33% with progressive disease) [63]. Similar results were obtained in a phase III trial comparing pembrolizumab vs. placebo as second-line therapy for sorafenib-pretreated patients. However, in this investigation, OS was not statistically significant (13.9 months vs. 10.6 months, pembrolizumab vs. placebo group, respectively). Indeed, the ORR was stable at 17% [64].
To date, there are several ongoing clinical trials (namely, phase I/II) investigating other PD-L1 inhibitors (namely, avelumab, atezolizumab, and durvalumab) used as monotherapy or in combination with other ICIs [15,16]. In particular, a multicenter non-randomized phase 2 trial showed promising results when these PD-L1 inhibitors were used as first- and second-line treatment of advanced HCC with the combination of camrelizumab (anti-PD1) and apatinib (VEGFR-2 tyrosine kinase inhibitor). In this case, the ORR was 34.3% when PD-L1 inhibitors were administered as first-line therapy and 22.5% when administered as second-line therapy. Interestingly, the PFS was 5.7 months and 5.5 months, respectively [30,31]. Indeed, the most important breakthrough in systemic therapy for advanced HCC has been represented by the combination of atezolizumab (anti-PDL1)/bevacizumab (anti-VEGFA). This is the updated first-line treatment for hepatocellular carcinoma due to the proven greater OSS (19.2 months) [14] and PFS (6.8 months), respectively [15,16] (Table 1).

Table 1.
Clinical trials on HCC and checkpoint inhibitors.
Unfortunately, despite these very promising findings, a significant portion of HCC patients show no significant benefits from immune-therapy administration. These contrasting results can be explained by different tumor biology, characteristics, and etiologies [22,65]. For instance, it is interesting to report findings from an exploratory analysis of the IMbrave150 trial. The latter showed the combination therapy of atezolizumab and bevacizumab having an ORR of 27% in NASH-HCC vs. 35% in HCC of other etiologies [66]. More interestingly, immune therapy shows a significantly different outcome for patients with viral vs. non-viral HCC origins. In fact, a recent meta-analysis confirmed that ICIs are less effective in patients with non-viral-derived HCC [67]. Indeed, this evidence is not completely in agreement. Two further meta-analyses reported no difference in ORR for viral vs. non-viral HCC [68,69].
The second class of molecules currently under investigation for HCC treatment is the CTLA-4 inhibitors (namely, tremelimumab and ipilimumab). Tremelimumab was initially studied in a phase II clinical trial as monotherapy for patients with HCV-related cirrhosis and secondary HCC, demonstrating an ORR of 17.6%. Time to progression was 6.48 months with a good safety profile [70]. Subsequently, another clinical trial studied the combination of tremelimumab with locoregional treatment (namely, TACE or radiofrequency ablation (RIA)), showing a higher ORR of 26.3%. This could be explained by the accumulation of intra-tumoral CD8+ T cells in treated patients. Collaterally, a reduction in HCV viral load was observed [71]. More recently, the phase III HIMALAYA trial demonstrated the greater efficacy of the combination of durvalumab (anti-PD-L1) and tremelimumab (STRIDE) vs. sorafenib as first line therapy for patients with advanced HCC. In fact, STRIDE showed a 36-month OS rate of 30.7% vs. 20.2% for sorafenib. This finding was accompanied by a manageable safety profile [72]. Thus, STRIDE has been recently approved by the US FDA for HCC treatment. This step paves the way for a new scenario for first-line treatment of advanced HCC [73,74].
Currently, there is another randomized, multicenter phase III trial using the combination of nivolumab and ipilimumab vs. sorafenib or lenvatinib as first-line treatment for HCC. This trial follows a previous study showing this combination therapy to have an acceptable safety profile and an interesting ORR of 31% vs. 14% for nivolumab monotherapy [75]. However, ICI use in HCC shows a significant rate of non-responders. There are several mechanisms explaining this finding. They are shown in Table 2.

Table 2.
Mechanisms involved in resistance to ICIs.
2.1.4. Fecal Microbiota Transplantation and Immunotherapy in HCC: Beyond a Simple Cancer-Intestinal Bacteria Association
The rising knowledge on gut microbiota functions, in general, and the contribution of gut “dysbiosis” in the loss of barrier function, in particular, leading to altered “gut–liver axis” and gut–liver immune system dysfunction, are the basis for future treatment aimed to block fibrosis progression in liver cirrhosis and to treat and destroy HCC cells [88,89].
Specifically, the mechanistic knowledge of the microbiome-HCC harmful “game” derives from preclinical animal models. In rodents, the activation of TLR4 signaling after gram-negative lipopolysaccharide (LPS) exposure and, also, the direct detrimental effect of microbially produced secondary bile acids within the liver “promote” carcinogenesis [90]. Therefore, Dapito et al. showed that the depletion of the microbiota “protected “against fibrosis and cancer development in mice [91]. Similarly, neomycin protected against HCC development upon diethylnitrosamine/carbon tetrachloride (DEN/CCl4) animal administration [92]. Secondary bile acids, namely deoxycholic acid (DCA), contribute to liver inflammation via the promotion of the senescence-associated secretory phenotype (SASP). This pathway is crucial in metabolic (namely, obesity)-associated HCC development [80]. In addition, DCA is able to induce NASH-associated HCC via mTOR activation [93]. Conversely, antibiotic treatment led to a reduction of secondary bile acid and, consensually, an increase in the primary bile acid pool, resulting in increased anti-tumor immunity expansion [94].
Thus, the dreamt bacteriotherapy can restore “gut eubiosis,” re-establishing physiological intestinal permeability and reducing the passage of pathogen-associated molecular patterns (PAMPs) such as endotoxin and the related chronic inflammation within the liver. The latter effect can re-establish immune surveillance towards hepatocytes accumulating mutations, namely potential tumor cells (e.g., HCC and its “brothers “) [75]. We can hypothesize that bacteriotherapy would be as effective as it was earlier, namely during the first stages of chronic liver disease [95].
Gut microbiota modulation is feasible through diet, probiotics, prebiotics, and antibiotics [96]. The latter have obtained some promising results in terms of efficacy in preventing HCC development, but these are not devoid of toxic and resistance-development side effects [83,84]. Probiotic administration in HCC patients has shown safer and efficacy-proven results from the few available trials. In a randomized clinical trial, probiotics’ capability to re-establish gut barrier function in F3-F4 HCC subjects undergoing surgery was proven [97]. Furthermore, using the probiotic BIFICO throughout the preoperative phase of HCC patients’, the authors found these bugs able to accelerate postoperative liver function recovery [98]. Indeed, the use of probiotics in liver cancer patients needs more evidence and must take into consideration the issue of dose- and time-finding. Moreover, we cannot exclude the development of potentially pathogenic strains. Thus, a new direct and more effective way to restore gut eubiosis and potentially increase ICI efficacy is fecal microbiota transplantation (FMT).
In FMT, fecal healthy donors are carefully selected through strict exclusion criteria (e.g., malnutrition, obesity) in order to exclude the risk of disease transmission such as in the obesity case [99]. Technically, fecal material is collected from the donor, suspended in a saline solution, and mixed in a blender. It results in liquefied stool that is filtered through a strainer in order to remove fibers [100]. Therefore, fecal material is ready to be delivered via endoscopy (e.g., colonoscopy or nasojejunal tube), enema, or colonic transendoscopic enteral tubing. Oral capsules have shown similar efficacy as colonoscopy-administered procedures. However, the frequency of doses and optimal overall duration of the capsule-administration regimen are still under investigation [101]. To date, FMT has been approved for the treatment of recurrent Clostridium difficile infection by 2014, with an effectiveness of about 90% [102].
There are promising studies on its use in cancer conditions, but not in HCC patients yet. Baruch et al. reported the first-in-human clinical trials in melanoma patients. FMT was significantly associated with an immune system switch towards immune surveillance, as described by changes in gene expression profiles in both the gut lamina propria and the tumor neighborhood [103].
2.1.5. Gut Microbiome and Immunotherapy: A “Navigator” for HCC Treatment
The biopsy-sparing diagnostic approach to HCC has led to a non-invasive prognostic biomarker need. Furthermore, the ICIs arrival has recalled these biomarkers. In addition, despite the promising results obtained with ICIs’ use, a significant rate of patients do not take benefit from immunotherapy. Thus, there is a strong need for “precise “predictive markers in HCC patients [54,65]. Gut microbiota profiling seems a promising HCC treatment-response non-invasive biomarker [104,105].
Zheng et al. have studied dynamic variation and features of fecal gut microbiota during anti-PD1 immunotherapy (namely, Camrelizumab) in HCC after progression on sorafenib. They evaluated differences among responders and non-responders’ fecal samples at baseline, 1 week after treatment, and every 3 weeks until disease progression. Interestingly, fecal samples of responders have higher taxa richness and higher gene counts than those of non-responders. More intriguingly, the inter-group dissimilarity became significantly higher than the intra-group differentiation as early as 6 weeks after treatment imitation.
At baseline, in both responders and non-responders, Bacteroidetes were the most abundant, followed by Firmicutes and Proteobacteria. Typically, this microbial composition remained relatively stable at the phylum level in responders. On the other hand, Proteobacteria concentrations already increased after 3 weeks of treatment. They became predominant at week 12 in non-responders. Proteobacteria’s increased abundance was explained by the prevalence of Escherichia coli. Conversely, the most abundant proteobacterial member in responders was Klebsiella pneumoniae. Finally, at the linear discriminant analysis-effect size algorithm, 20 responder-enriched and 15 non-responder-enriched species were identified. In the responder group, there were 4 identified Lactobacillus species (namely, L. oris, L. mucosae, L. gasseri, and L. vaginalis). Bifidobacterium dentium, Streptococcus thermophilus, Coprococcus comes, Bacteroides cellulosilyticus, Subdoligranulum sp. Lachnospiraceae bacterium 7 1 58FAA, Ruminococcus obeum, Ruminococcus bromii, and Akkermansia muciniphila were also observed in treatment responders [106].
Chung et al. examined the gut microbiome of 8 advanced HCC patients (of whom 6 had chronic hepatitis B). Fecal samples were collected before the first administration of nivolumab and then, at the time of disease progression, during treatment. In responders, fecal samples were collected after 5–7 months of treatment. Responders have a higher Shannon index and a different phylogenetic diversity at the beta diversity analysis, when compared to non-responders. In detail, Dialister pneumosintes, Escherichia coli, Lactobacillus reteri, Streptococcus mutans, Enterococcus faecium, Streptococcus gordonii, Veillonella atypica, Granulicatella sp., and Trchuris trichiura were specifically prevalent in non-responders. Citrobacter freundii, Azospirillum sp., and Enterococcus durans were prevalent in responders. Moreover, an altered Firmicutes/Bacteroidetes ratio (<0.5 or >1.5) and a low Prevotella/Bacteroides ratio were significantly correlated with the non-responder profile. Conversely, the presence of Akkermansia species was observed in responders [107].
Mao et al. studied fecal samples from 65 patients affected by advanced HCC or biliary tract cancer (namely, 30:35) receiving anti-PD-1 therapies. Seventy-four taxa were significantly enriched in responders, compared to 40 taxa in non-responders. Within the first group, there was a higher abundance of Lachnospiraceae bacterium-GAM79, Alistipes sp. Marseille-P5997, Ruminococcus calidus, and Erysipelotichaceae bacterium-GAM147. In the non-responder’s group, there was a higher abundance of Veillonellaceae. Interestingly, immunotherapy-related adverse events correlated with the phylogenetic diversity of the gut microbiota. This finding can be explained by the immunotherapy-related colitis that was more likely associated with decreased gut microbiome diversity and relative abundance. Sixteen enriched taxa were identified in patients with diarrhea (namely, the Negativicutes class, Veillonellaceae family, and Dialister genus). Particularly, enrichment of Prevotellamassilia timonensis was observed in patients with severe diarrhea [108].
Ponziani et al. evaluated prospectively eleven patients with HCC treated with Tremelimumab and/or Durvalumab. Responders showed lower pretreatment fecal calprotectin, an increased relative abundance of Akkermansia, and a reduced relative abundance of Enterobacteriaceae vs. non-responders. Further, dynamic analysis of fecal calprotectin showed a temporal evolution opposite to the Akkermansia to Enterobacteriaceae ratio and gut microbiota alpha diversity [109].
Lee et al. analyzed baseline fecal samples of 94 patients receiving ICI treatment (nivolumab and pembrolizumab) for HCC (63.4% of those were HBV-related). Prevotella 9 was enriched in non-responders, whereas Lachnoclostridium, Lachnospiraceae, and Veillonella were predominant in responders. Furthermore, the evidence of Lachnoclostridium enrichment and Prevotella 9 depletion significantly predicted overall survival. The study included a validation cohort in which a better progression-free survival (PFS) and OS were observed in patients who had a preferable microbial signature, namely depleted Prevotella 9 and enriched Lachnoclostridium, vs. patients with a poor signature (namely, coexistence of enriched Prevotella 9 and depleted Lachnoclostridium) or a fair signature (namely, coexistent depletion or enrichment of these two taxa) [110].
Li et al. also evaluated the oral and gut microbiome profiles of 65 patients with HCC receiving ICIs. They found that Clostridiales/Ruminococcaceae were enriched in responders, and Bacteroidales were enriched in non-responders. Moreover, patients with a high Faecalibacterium abundance had a significantly prolonged PFS vs. those with a low abundance. On the other hand, patients with a high abundance of Bacteroidales had a shortened PFS vs. those with a low abundance [111]. The evidence on gut microbiota changes associated with the ICI treatment response are summarized in Table 3.

Table 3.
Gut microbiota changes associated with ICI treatment response profile.
3. Conclusions
HCC is a systemic cancer with growing prevalence and mortality. Despite the encouraging step-up in systemic therapy achieved with sorafenib and improved survival time, there was a big issue concerning tumor progression. The latter has not been solved by further systemic treatment options. More recently, HCC has become a target for novel immune-checkpoint inhibitors. These have shown superiority vs. the traditional multi-kinase inhibitor sorafenib in terms of survival rate and blockage of tumor progression. However, a significant proportion of treated HCC patients do not respond to ICIs. Another limitation of using ICIs is the small number of patients who can be enrolled in immune therapy. Finally, the occurrence of unfavorable side effects is responsible for the interruption of treatment.
Drug-resistance mechanisms, immune system response escape, and unfavorable immune system function within the liver can explain these pitfalls. These are hot topics in future HCC and immune-therapy research.
Beside alpha-fetoprotein, other biomarkers of treatment response have been studied and proposed. Among these, there is the gut microbiota, whose signature has shown interesting findings via new metagenomic methods. More in detail, gut dysbiosis seems to be associated with ICIs’ poor responses. In fact, immune system depression within the liver is associated with gut microbiota derangements. Moreover, certain “eu-” or “dysbiotic” microbiota is associated with a better or worse ICI response, respectively. This evidence is the basis for future lines of research: gut microbiota finger-printing before, during, and after ICI’s treatment can help predict patients’ eligibility, performance, and prognosis; gut dysbiosis modulation can help improve treatment response.
Thus, potential remodulation of dysbiosis via probiotics can improve patients’ outcomes under ICIs. However, this method of microbial modulation has several open issues, including the timing of probiotics’ administration, duration of administration, and side effect profile.
A more direct method to modulate the gut microbiota is FMT. However, data on FMT use in HCC patients are ongoing and call for researchers’ attention. In this regard, we have several concerns: What HCC patient should be treated, and for how long? What should be the safety profile of FMT? What is its interaction with ICIs?
Our attention is therefore focused on the future larger tracing of gut microbiota asset “per patient” in the context of personalized medicine, perhaps using the power of big data analysis provided by artificial intelligence (AI) in medicine.
Author Contributions
Conceptualization was performed by L.A., M.M. and E.S.; methodology was written and controlled by L.A., M.M. and E.S.; data validation was made by, L.A., M.E.A., G.S.B. and E.S.; formal analysis was made by F.P. and E.S.; investigation was conducted by M.M., M.E.A. and E.S.; data curation was performed by M.M., F.G. and G.G.M.S.; writing—original draft preparation was made by L.A., M.E.A. and E.S.; writing—review and editing was made by E.S. and G.S.B.; visualization of data was performed by L.A., F.G., F.P. and G.S.B.; supervision of the study was made by L.A. and E.S.; project administration was made by L.A., G.S.B. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mittal, S.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma: Consider the population. J. Clin. Gastroenterol. 2013, 47, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf; https://doi.org/10.3389/fonc.2020.00171 (accessed on 28 February 2023).
- Pugliese, N.; Alfarone, L.; Arcari, I.; Giugliano, S.; Parigi, T.L.; Rescigno, M.; Lleo, A.; Aghemo, A. Clinical features and management issues of NAFLD-related HCC: What we know so far. Expert. Rev. Gastroenterol. Hepatol. 2023, 17, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.X.; Xie, S.; Guo, P.P.; Deng, Z.J.; Zhang, Z.Y.; Gao, W.; Zhang, W.G.; Zhong, J.H. Hepatocellular Carcinoma in Non-alcoholic Fatty Liver Disease: Current Progresses and Challenges. J. Clin. Transl. Hepatol. 2022, 10, 955–964. [Google Scholar] [CrossRef]
- Akateh, C.; Black, S.M.; Conteh, L.; Miller, E.D.; Noonan, A.; Elliott, E.; Pawlik, T.M.; Tsung, A.; Cloyd, J.M. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 3704–3721. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Sun, X.D.; Qiu, W.; Chen, Y.G.; Sun, D.W.; Lv, G.Y. Conversion therapy in liver transplantation for hepatocellular carcinoma: What’s new in the era of molecular and immune therapy? Hepatobiliary Pancreat. Dis. Int. 2023, 22, 7–13. [Google Scholar] [CrossRef]
- Yegin, E.G.; Oymaci, E.; Karatay, E.; Coker, A. Progress in surgical and nonsurgical approaches for hepatocellular carcinoma treatment. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 234–256. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Hasegawa, K.; Izumi, N.; Kudo, M.; Shimada, M.; Yamanaka, N.; Inomata, M.; Kaneko, S.; Nakayama, H.; Kawaguchi, Y.; et al. Surgery versus Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Randomized Controlled Trial (SURF Trial). Liver Cancer 2021, 11, 209–218. [Google Scholar] [CrossRef]
- Cucchetti, A.; Piscaglia, F.; Cescon, M.; Colecchia, A.; Ercolani, G.; Bolondi, L.; Pinna, A.D. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J. Hepatol. 2013, 59, 300–307. [Google Scholar] [CrossRef]
- Tiong, L.; Maddern, G.J. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br. J. Surg. 2011, 98, 1210–1224. [Google Scholar] [CrossRef]
- Granito, A.; Facciorusso, A.; Sacco, R.; Bartalena, L.; Mosconi, C.; Cea, U.V.; Cappelli, A.; Antonino, M.; Modestino, F.; Brandi, N.; et al. TRANS-TACE: Prognostic Role of the Transient Hypertransaminasemia after Conventional Chemoembolization for Hepatocellular Carcinoma. J. Pers. Med. 2021, 11, 1041. [Google Scholar] [CrossRef]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kudo, M.; Venook, A.P.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.H.; de Guevara, L.L.; et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J. Hepatol. 2016, 65, 1140–1147. [Google Scholar] [CrossRef]
- Jackson, R.; Psarelli, E.E.; Berhane, S.; Khan, H.; Johnson, P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. J. Clin. Oncol. 2017, 35, 622–628. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Shiri, P.; Ramezanpour, S.; Amani, A.M.; Dehaen, W. A patent review on efficient strategies for the total synthesis of pazopanib, regorafenib and lenvatinib as novel anti-angiogenesis receptor tyrosine kinase inhibitors for cancer therapy. Mol. Divers. 2022, 26, 2981–3002. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Takemura, N.; Yamashita, T.; Watadani, T.; Kaibori, M.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Aikata, H.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2021 Version (5th JSH-HCC Guidelines). Hepatol. Res. 2023, 24, 383–390. [Google Scholar] [CrossRef]
- Hui, R.W.; Mak, L.Y.; Cheung, T.T.; Lee, V.H.; Seto, W.K.; Yuen, M.F. Clinical practice guidelines and real-life practice on hepatocellular carcinoma: The Hong Kong perspective. Clin. Mol. Hepatol. 2022, 28, 217. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.K.; Qin, S.; Tai, D.W.; Lim, H.Y.; Yau, T.; et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients with Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Khanam, A.; Kottilil, S. New Therapeutics for HCC: Does Tumor Immune Microenvironment Matter? Int. J. Mol. Sci. 2022, 24, 437. [Google Scholar] [CrossRef]
- Mandlik, D.S.; Mandlik, S.K.; Choudhary, H.B. Immunotherapy for hepatocellular carcinoma: Current status and future perspectives. World J. Gastroenterol. 2023, 29, 1054–1075. [Google Scholar] [CrossRef]
- Brandi, N.; Renzulli, M. The Synergistic Effect of Interventional Locoregional Treatments and Immunotherapy for the Treatment of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 8598. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Aglitti, A.; Borzio, M.; Gambato, M.; Guarino, M.; Iavarone, M.; Lai, Q.; Levi Sandri, G.B.; Melandro, F.; Morisco, F.; et al. Overview of Immune Checkpoint Inhibitors Therapy for Hepatocellular Carcinoma; and The ITA.LI.CA Cohort Derived Estimate of Amenability Rate to Immune Checkpoint Inhibitors in Clinical Practice. Cancers 2019, 11, 1689. [Google Scholar] [CrossRef]
- Pan, A.; Truong, T.N.; Su, Y.H.; Dao, D.Y. Circulating Biomarkers for the Early Diagnosis and Management of Hepatocellular Carcinoma with Potential Application in Resource-Limited Settings. Diagnostics 2023, 13, 676. [Google Scholar] [CrossRef] [PubMed]
- Huo, R.; Chen, Y.; Li, J.; Xu, Q.; Guo, J.; Xu, H.; You, Y.; Zheng, C.; Chen, Y. Altered Gut Microbiota Composition and Its Potential Association in Patients with Advanced Hepatocellular Carcinoma. Curr. Oncol. 2023, 30, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Attaye, I.; Warmbrunn, M.V.; Boot, A.N.A.F.; van der Wolk, S.C.; Hutten, B.A.; Daams, J.G.; Herrema, H.; Nieuwdorp, M. A Systematic Review and Meta-analysis of Dietary Interventions Modulating Gut Microbiota and Cardiometabolic Dis-eases-Striving for New Standards in Microbiome Studies. Gastroenterology 2022, 162, 1911–1932. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Basilico, M.; Rinninella, E.; Carbone, F.; Schol, J.; Rasetti, C.; Abenavoli, L.; Santori, P. Probiotics and gut health. Minerva Gastroenterol. 2021, 67, 314–325. [Google Scholar] [CrossRef]
- Eastmond, A.K.; Shetty, C.; Rizvi, S.M.H.A.; Sharaf, J.; Williams, K.D.; Tariq, M.; Acharekar, M.V.; Guerrero Saldivia, S.E.; Unnikrishnan, S.; Chavarria, Y.Y.; et al. A Systematic Review of the Gastrointestinal Microbiome: A Game Changer in Colorectal Cancer. Cureus 2022, 14, e28545. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, A.; Hartshorn, K.L. A Comprehensive Narrative Review on the History, Current Landscape, and Future Directions of Hepatocellular Carcinoma (HCC) Systemic Therapy. Cancers 2023, 15, 2506. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Hwang, J.P.; Feld, J.J.; Hammond, S.P.; Wang, S.H.; Alston-Johnson, D.E.; Cryer, D.R.; Hershman, D.L.; Loehrer, A.P.; Sabichi, A.L.; Symington, B.E.; et al. Hepatitis B Virus Screening and Management for Patients with Cancer Prior to Therapy: ASCO Provisional Clinical Opinion Update. J. Clin. Oncol. 2020, 38, 3698–3715. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2008, 10, 25–34. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Greten, T.F.; Sangro, B. Targets for immunotherapy of liver cancer. J. Hepatol. 2017, 68, 157–166. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Onuma, A.E.; Zhang, H.; Huang, H.; Williams, T.M.; Noonan, A.; Tsung, A. Immune Checkpoint Inhibitors in Hepatocellular Cancer: Current Understanding on Mechanisms of Resistance and Biomarkers of Response to Treatment. Gene. Expr. 2020, 20, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Prithviraj, P.; Anaka, M.; Bridle, K.R.; Crawford, D.H.G.; Dhungel, B.; Steel, J.C.; Jayachandran, A. Monitoring immune checkpoint regulators as predictive biomarkers in hepatocellular carcinoma. Front. Oncol. 2018, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.J.; Bao, J.J.; Wang, J.Z.; Wang, Y.; Jiang, M.; Xing, M.Y.; Zhang, W.G.; Qi, J.Y.; Roggendorf, M.; Lu, M.J.; et al. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J. Gastroenterol. 2011, 17, 3322–3329. [Google Scholar] [CrossRef]
- Baumeister, S.H.; Freeman, G.J.; Dranoff, G.; Sharpe, A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016, 34, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef]
- Ye, Y.; Zhou, L.; Xie, X.; Jiang, G.; Xie, H.; Zheng, S. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J. Surg. Oncol. 2009, 100, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, X.Y.; Qiu, S.J.; Yamato, I.; Sho, M.; Nakajima, Y.; Zhou, J.; Li, B.Z.; Shi, Y.H.; Xiao, Y.S.; et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin. Cancer Res. 2009, 15, 971–979. [Google Scholar] [CrossRef]
- Calderaro, J.; Rousseau, B.; Amaddeo, G.; Mercey, M.; Charpy, C.; Costentin, C.; Luciani, A.; Zafrani, E.S.; Laurent, A.; Azoulay, D.; et al. Programmed death ligand 1 expression in hepatocellular carcinoma: Relationship with clinical and pathological features. Hepatology 2016, 64, 2038–2046. [Google Scholar] [CrossRef]
- Jung, H.I.; Jeong, D.; Ji, S.; Ahn, T.S.; Bae, S.H.; Chin, S.; Chung, J.C.; Kim, H.C.; Lee, M.S.; Baek, M.J. Overexpression of PD-L1 and PD-L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res. Treat. 2017, 49, 246–254. [Google Scholar] [CrossRef]
- Chang, H.; Jung, W.; Kim, A.; Kim, H.K.; Kim, W.B.; Kim, J.H.; Kim, B.H. Expression and prognostic significance of programmed death protein 1 and programmed death ligand-1; and cytotoxic T lymphocyte-associated molecule-4 in hepatocellular carcinoma. APMIS 2017, 125, 690–698. [Google Scholar] [CrossRef]
- Chambers, C.A.; Kuhns, M.S.; Egen, J.G.; Allison, J.P. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef]
- Schneider, H.; Downey, J.; Smith, A.; Zinselmeyer, B.H.; Rush, C.; Brewer, J.M.; Wei, B.; Hogg, N.; Garside, P.; Rudd, C.E. Reversal of the TCR stop signal by CTLA-4. Science 2006, 313, 1972–1975. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef]
- Peggs, K.S.; Quezada, S.A.; Chambers, C.A.; Korman, A.J.; Allison, J.P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 2009, 206, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Kruger, S.; Ilmer, M.; Kobold, S.; Cadilha, B.L.; Endres, S.; Ormanns, S.; Schuebbe, G.; Renz, B.W.; D’Haese, J.G.; Schloesser, H.; et al. Advances in cancer immunotherapy 2019—Latest trends. J. Exp. Clin. Cancer Res. 2019, 38, 268. [Google Scholar] [CrossRef] [PubMed]
- Ngiow, S.F.; von Scheidt, B.; Akiba, H.; Yagita, H.; Teng, M.W.; Smyth, M.J. Anti-TIM3 antibody promotes T cell IFN-g mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011, 71, 3540–3551. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Merck & Co Inc. Keytruda (Pembrolizumab) [Package Insert]; Merck & Co Inc.: Whitehouse Station, NJ, USA, 2015. [Google Scholar]
- Bristol-Myers Squibb Company. Opdivo (Nivolumab) [Package Insert]; Bristol-Myers Squibb Company: Princeton, NJ, USA, 2015. [Google Scholar]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label; non-comparative; phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised; multicentre; open-label; phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised; open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized; Double-Blind; Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Khemlina, G.; Ikeda, S.; Kurzrock, R. The biology of Hepatocellular carcinoma: Implications for genomic and immune therapies. Mol. Cancer 2017, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Zhu, A.X.; Cheng, A.L.; Galle, P.R.; Ikeda, M.; Nicholas, A.; Verret, W.; Li, L.; Gaillard, V.E.; Lencioni, R.; et al. Exploratory analysis to examine the association between treatment response and overall survival (OS) in patients (pts) with unresectable hepatocellular carcinoma (HCC) treated with atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor). J. Clin. Oncol. 2021, 15, 4071. [Google Scholar] [CrossRef]
- Haber, P.K.; Puigvehí, M.; Castet, F.; Lourdusamy, V.; Montal, R.; Tabrizian, P.; Buckstein, M.; Kim, E.; Villanueva, A.; Schwartz, M.; et al. Evidence-Based Management of Hepatocellular Carcinoma: Systematic Review and Meta-analysis of Randomized Controlled Trials (2002–2020). Gastroenterology 2021, 161, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Danilova, L.; Lim, S.J.; Verma, R.; Xavier, S.; Leatherman, J.M.; Sztein, M.B.; Fertig, E.J.; Wang, H.; Jaffee, E.; et al. Viral status; immune microenvironment and immunological response to checkpoint inhibitors in hepatocellular carcinoma. J. Immunother. Cancer 2020, 8, e000394. [Google Scholar] [CrossRef]
- Ding, Z.; Dong, Z.; Chen, Z.; Hong, J.; Yan, L.; Li, H.; Yao, S.; Yan, Y.; Yang, Y.; Yang, C.; et al. Viral Status and Efficacy of Immunotherapy in Hepatocellular Carcinoma: A Systematic Review with Meta-Analysis. Front. Immunol. 2021, 12, 733530. [Google Scholar] [CrossRef]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef]
- Kudo, M. Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2022, 11, 592–596. [Google Scholar] [CrossRef]
- FDA approves tremelimumab in combination with durvalumab for unresectable hepatocellular carcinoma. Available online: https://rb.gy/pl2hji (accessed on 10 June 2023).
- Cammarota, A.; Zanuso, V.; Manfredi, G.F.; Murphy, R.; Pinato, D.J.; Rimassa, L. Immunotherapy in hepatocellular carcinoma: How will it reshape treatment sequencing? Ther. Adv. Med. Oncol. 2023, 15, 17588359221148029. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J. Clin. Oncol. 2019, 37, 4012. [Google Scholar] [CrossRef]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017, 7, 1420–1435. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Correjon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef]
- Gao, J.; Shi, L.Z.; Zhao, H.; Chen, J.; Xiong, L.; He, Q.; Chen, T.; Roszik, J.; Bernatchez, C.; Woodman, S.E.; et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016, 167, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Galarreta, M.; Bresnahan, E.; Molina-Sánchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Mhainaut, M.; et al. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef]
- George, S.; Miao, D.; Demetri, G.D.; Adeegbe, D.; Rodig, S.J.; Shukla, S.; Lipschitz, M.; Amin-Mansour, A.; Raut, C.P.; Carter, S.L.; et al. Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma. Immunity 2017, 46, 197–204. [Google Scholar] [CrossRef]
- Shayan, G.; Srivastava, R.; Li, J.; Schmitt, N.; Kane, L.P.; Ferris, R.L. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology 2016, 6, e1261779. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, J.D.; et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef]
- Viehl, C.T.; Moore, T.T.; Liyanage, U.K.; Frey, D.M.; Ehlers, J.P.; Eberlein, T.J.; Goedegebuure, P.S.; Linehan, D.C. Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann. Surg. Oncol. 2006, 13, 1252–1258. [Google Scholar] [CrossRef]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Peggs, K.S.; Curran, M.A.; Allison, J.P. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Investig. 2006, 116, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2017, 168, 542. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Lupo, M.; Iegri, C.; Gasbarrini, A.; De Santis, A.; Tack, J. Intestinal permeability in non-alcoholic fatty liver disease: The gut-liver axis. Rev. Recent Clin. Trials 2014, 9, 141–147. [Google Scholar]
- Song, Q.; Zhang, X. The Role of Gut-Liver Axis in Gut Microbiome Dysbiosis Associated NAFLD and NAFLD-HCC. Biomedicines 2022, 10, 524. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Betrapally, N.S.; Gillevet, P.M. Decompensated Cirrhosis and Microbiome Interpretation. Nat. Cell Biol. 2015, 525, E1–E2. [Google Scholar] [CrossRef]
- Dapito, D.H.; Mencin, A.; Gwak, G.-Y.; Pradere, J.-P.; Jang, M.-K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of Hepatocellular Carcinoma by the Intestinal Microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-X.; Yan, H.-X.; Liu, Q.; Yang, W.; Wu, H.-P.; Dong, W.; Tang, L.; Lin, Y.; He, Y.-Q.; Zou, S.-S.; et al. Endotoxin Accumulation Prevents Carcinogen-induced Apoptosis and Promotes Liver Tumorigenesis in Rodents. Hepatology 2010, 52, 1322–1333. [Google Scholar] [CrossRef]
- Yamada, S.; Takashina, Y.; Watanabe, M.; Nagamine, R.; Saito, Y.; Kamada, N.; Saito, H. Bile Acid Metabolism Regulated by the Gut Microbiota Promotes Non-alcoholic Steatohepatitis-associated Hepatocellular Carcinoma in Mice. Oncotarget 2018, 9, 9925–9939. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut Microbiome–mediated Bile Acid Metabolism Regulates Liver Cancer via NKT Cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, M.; Beraza, N. The Role of the Microbiome in Liver Cancer. Cancers 2021, 13, 2330. [Google Scholar] [CrossRef]
- Das, B.K. Altered gut microbiota in hepatocellular carcinoma: Insights into the pathogenic mechanism and preclinical to clinical findings. APMIS 2022, 130, 719–740. [Google Scholar] [CrossRef]
- NIH. Influence of Probiotics Administration Before Liver Resection in Liver Disease (LIPROCES). U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/study/NCT02021253?term=Gut+microbes&cond=Hepatocellular+Carcinoma&draw=2&rank=5 (accessed on 28 February 2023).
- NIH. Clinical Study on BIFICO Accelerating Postoperative Liver Function Recovery in Patients with Hepatocellular Carcinoma. U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/study/NCT05178524?term=Gut+microbes&cond=Hepatocellular+Carcinoma&draw=2&rank=4 (accessed on 28 February 2023).
- Alang, N.; Kelly, C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2015, 2, ofv004. [Google Scholar] [CrossRef]
- Chen, D.; Wu, J.; Jin, D.; Wang, B.; Cao, H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int. J. Cancer 2019, 145, 2021–2031. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule- vs. Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for diagnosis; treatment; and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 2013, 108, 478–498. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Pallozzi, M.; Di Tommaso, N.; Maccauro, V.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R.; Pompili, M. Non-Invasive Biomarkers for Immunotherapy in Patients with Hepatocellular Carcinoma: Current Knowledge and Future Perspectives. Cancers 2022, 14, 4631. [Google Scholar] [CrossRef]
- Gok Yavuz, B.; Hasanov, E.; Lee, S.S.; Mohamed, Y.I.; Curran, M.A.; Koay, E.J.; Cristini, V.; Kaseb, A.O. Current Landscape and Future Directions of Biomarkers for Immunotherapy in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 1195–1207. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, T.; Tu, X.; Huang, Y.; Zhang, H.; Tan, D.; Jiang, W.; Cai, S.; Zhao, P.; Song, R.; et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 193. [Google Scholar] [CrossRef]
- Chung, M.W.; Kim, M.J.; Won, E.J.; Lee, Y.J.; Yun, Y.W.; Cho, S.B.; Joo, Y.E.; Hwang, J.E.; Bae, W.K.; Chung, I.J.; et al. Gut microbiome composition can predict the response to nivolumab in advanced hepatocellular carcinoma patients. World J. Gastroenterol. 2021, 27, 7340–7349. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wang, D.; Long, J.; Yang, X.; Lin, J.; Song, Y.; Xie, F.; Xun, Z.; Wang, Y.; Wang, Y.; et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. Immunother. Cancer 2021, 9, e003334. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; De Luca, A.; Picca, A.; Marzetti, E.; Petito, V.; Del Chierico, F.; Reddel, S.; Paroni Sterbini, F.; Sanguinetti, M.; Putignani, L.; et al. Gut Dysbiosis and Fecal Calprotectin Predict Response to Immune Checkpoint Inhibitors in Patients with Hepatocellular Carcinoma. Hepatol. Commun. 2022, 6, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Wu, C.; Hung, L.C.J.; Chi, C.T.; Lee, I.C.; Yu-Lun, K.; Chou, S.H.; Luo, J.C.; Hou, M.C.; Huang, Y.H. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor–treated unresectable hepatocellular carcinoma. J. Immunother. Cancer 2022, 10, e004779. [Google Scholar] [CrossRef]
- Li, L.; Ye, J. Characterization of gut microbiota in patients with primary hepatocellular carcinoma received immune checkpoint inhibitors: A Chinese population-based study. Medicine 2020, 99, e21788. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2017, 359, 91–97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).