Abstract
Background and Objectives: The relationship between three-dimensional (3D) scanning-derived body surface measurements and biomarkers in patients with coronary artery disease (CAD) were assessed. Methods and Methods: The recruitment of 98 patients with CAD confirmed by cardiac catheterization and 98 non-CAD patients were performed between March 2016 and December 2017. A health questionnaire on basic information, life style variables, and past medical and family history was completed. 3D body surface measurements and biomarkers were obtained. Differences between the two groups were assessed and multivariable analysis performed. Results: It was found that chest width (odds ratio [OR] 0.761, 95% confidence interval [CI] = 0.586–0.987, p = 0.0399), right arm length (OR 0.743, 95% CI = 0.632–0.875, p = 0.0004), waist circumference (OR 1.119, 95% CI = 1.035–1.21, p = 0.0048), leptin (OR 1.443, 95% CI = 1.184–1.76, p = 0.0003), adiponectin (OR 0.978, 95% CI = 0.963–0.994, p = 0.006), and interleukin 6 (OR 1.181, 95% CI = 1.021–1.366, p = 0.0254) were significantly associated with CAD. The combination of biomarker scores and body measurement scores had the greatest area under the curve and best association with CAD (area under the curve of 0.8049 and 95% CI = 0.7440–0.8657). Conclusions: Our study suggests that 3D derived body surface measurements in combination with leptin, adiponectin, and interleukin 6 levels may direct us to those at risk of CAD, allowing a non-invasive approach to identifying high-risk patients.
1. Introduction
Cardiovascular disease, in particular coronary artery disease (CAD), is one of the main causes of morbidity and mortality in the world [1]. Obesity is described as an independent risk factor for CAD [2] and is generally defined by an excess of body fat with the most commonly used anthropometric index being the body mass index (BMI) [3]. Obese individuals with the same amount of total body fat can have markedly distinct risk factor profiles [4], with abdominal fat having strong associations with CAD, mortality [5,6,7,8], and type 2 diabetes [9].
Visceral adipose tissue is an important endocrine organ, responsible for secreting hormones involved in a range of processes, e.g., control of sensitivity to insulin and inflammatory process mediators, and vascular hemostasis [10,11]. Biomarkers which play a role in insulin resistance and inflammation have been found to be associated with cardiovascular diseases. Leptin is an important link between obesity and the development of cardiovascular disease partially due to its effects on arterial pressure, formation of arterial thrombosis, aggregation of platelets, and on inflammatory vascular response [12]. Low adiponectin levels have also been found to be an independent risk factor for CAD [13]. The inflammatory biomarker C-reactive protein is positively correlated to the risk of cardiovascular events [14]. Interleukin 6 (IL6) and interleukin 8 have also been shown to play an important role in atherogenesis and atherosclerotic plaque destabilization [15,16]. It has also been demonstrated that the induction of the cytokine transforming growth factor beta-1 is associated with myocardial infarction [17].
A noninvasive three-dimensional (3D) scanning technology has been developed to obtain anthropometric measurements with many advantages over traditional methods, such as computed tomography scanners, X-rays, and bioelectrical impedance [18]. The aim of our study is to explore the association between CAD, biomarkers, and body measures with the use of 3D body scanning, providing more information to be used in clinical practice, epidemiological studies, and preventative medicine.
2. Materials and Methods
2.1. Study Subjects
From March 2016 to December 2017, a total of 98 patients found to have CAD as confirmed by cardiac catheterization exam at Chang Gung Memorial Hospital, Keelung, were recruited into our study CAD group. The same number of 98 sex- and age-matched patients presenting to our Department of Health Promotion and Examination were enrolled into the control group. Informed consent was obtained from all participants. This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Chang Gung Medical Foundation (201405148B0).
2.2. Anthropometrical Parameters
Three-dimensional body surface measurements were collected using a whole-body 3D laser scanner according to previously published methods. In addition to body weight, body height, and BMI, 35 measurements from four anatomical regions were made. The trunk region included the chest profile area, chest circumference, chest width (CW), waist profile area, waist circumference (WC), waist width, trunk volume, and trunk surface area. The head and neck region included the head surface area, head volume, head circumference, and neck circumference. The hip to the lower limb region included the hip profile area, hip circumference, hip width, left and right leg volume, left and right leg surface area, left and right calf circumference, left and right thigh circumference, and left and right leg length. The upper limb region included the left and right arm volume, left and right arm surface area, left and right arm length (RAL), left and right upper arm circumference, and left and right forearm circumference. The 3D laser scanning machine (LT3DCam) was built by Logistic Technology Company (LTC, Hsinchu, Taiwan), and was proven to have a high standard of accuracy due to the objective and comprehensive ways of measuring the human body surface. The standard procedure of measuring required the subject to remove all outer clothes except for underwear in preparation for scanning (women with bras in addition to pants) and to stand still on the stage for scanning (a total scanning time is about 10 s) [19]. The software system collected, realigned, constructed, and measured a subject’s whole-body digital stature and selected information. The measurement error of the 3D scanner in measuring the human body surface was checked; the error in the x- and y-axis was approximately 1 mm (1.2%), and in the z-axis it was less than 0.1 mm (0.2%) [20].
2.3. Data Collection
Upon recruitment, a questionnaire was given to acquire information on the following: date of birth; sex; occupation; education; marital status; history of cigarette smoking, alcohol drinking, and betel nut chewing; personal medical history (including hypertension, diabetes, heart disease, chronic kidney disease, liver cirrhosis, and chronic hepatitis). A medical chart review confirmed the answers provided. For those with no history of diabetes, a fasting blood glucose level was obtained. Diabetes was defined according to American Diabetes Association guidelines. For those without a history of hypertension, blood pressure was measured with a mercury sphygmomanometer on the left arm after the patient had been resting for 20 min in a seated position. Hypertension was defined according to the 2017 Hypertension Clinical Practice Guidelines (systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or the use of antihypertensive medication) [21].
2.4. Laboratory Analysis
Venous blood was sampled overnight. Assays for high-sensitivity C-reactive protein were carried out in the Department of Laboratory Medicine, Keelung Chang Gung Memorial Hospital. Biomarkers including IL6, IL8, leptin, adiponectin, and transforming growth factor beta-1 were measured using commercially available enzyme-linked immunosorbent assays (Boster Biological Technology, Pleasanton, CA, USA).
2.5. Statistics
Two independent sample t-tests were used to compare differences between the continuous variables of the groups, and results were presented as the mean ± standard deviation (SD). The χ2 test was used to differentiate between the distribution of categorical variables, and results were expressed using frequencies and percentages between the groups. The 3D body surface measurements were screened using a two-sample t-test by comparing differences between CAD patients and controls. To avoid collinearity in the regression analysis, one body measurement with the lowest p value was selected from each anatomic dimension for subsequent multivariable analysis. A logistic regression model was used to determine the strength of the association between the selected body measurements and the presence of CAD. In addition to the forced-in sociodemographic variables, a backward model selection with p < 0.1 was used to determine variables, including lifestyle variables, to be retained in the regression model. The modulating effect was examined by comparing models with and without biomarkers while calculating the strength of association (odds ratio [OR]) between the body measurement combinations and CAD. In order to find associations with CAD in individual patients, biochemical and body shape variables that significantly differed between the non-CAD and CAD groups were further analyzed. This was done by calculating optimal cutoff values for continuous variables using a receiver operating characteristic (ROC) analysis. The statistical software used for the analyses in this study was SPSS 25.0 (IBM Corporation, Armonk, NY, USA).
3. Results
A total of 98 patients were recruited into each of the CAD group and control group over a period between March 2016 and December 2017. Baseline characteristics for the study participants are shown in Table 1. Both groups were matched for age and sex, with 76.53% of patients being male and 72.45% equal to or greater than 50 years of age in both CAD and control groups. More patients in the CAD group had a lower educational level (73.47% vs. 37.76%, p = 0.001). Among lifestyle variables, the CAD group had more smokers (56.12% vs. 36.73%, p = 0.0099)), more patients that did not consume coffee (54.08% vs. 35.71%, p = 0.0097), and more that did not exercise (52.04% vs. 37.76%, p = 0.044). As regards to risk factors, more patients in the CAD group had hypertension (50% vs. 1.02%, p < 0.0001) and diabetes (36.73% vs. 3.06%, p < 0.0001), and fewer patients in the CAD group had hyperlipidemia (20.41% vs. 39.8%, p = 0.0031).

Table 1.
Characteristics of study participants.
Various biomarkers showed some differences between the CAD and control groups. (Table 2) Levels of HsCRP, IL6, IL8, and leptin were higher and adiponectin lower in the CAD group.

Table 2.
Distribution of biomarkers between study groups.
The 3D body surface scanning measurement results are shown in Table 3. The majority of measurements showed significant difference between the two groups, mainly with the CAD group having larger body measurements than controls. Associations between body measurements and biomarkers are presented in Table 4.

Table 3.
Comparison of body measurements between study groups.

Table 4.
Association between body measurements and biomarkers.
Multiple Logistic Regression and ROC Analysis
The associations between different body measurements and biomarkers on the occurrence of CAD were further assessed using a multiple logistic regression model adjusted for sex, age, education, exercise, smoking, alcohol drinking and coffee consumption, hypertension, diabetes, and hyperlipidemia. It was found that CW (OR 0.761, 95% CI = 0.586–0.987, p = 0.0399), RAL (OR 0.743, 95% CI = 0.632–0.875, p = 0.0004), WC (OR 1.119, 95% CI = 1.035–1.21, p = 0.0048), leptin (OR 1.443, 95% CI = 1.184–1.76, p = 0.0003), adiponectin (OR 0.978, 95% CI = 0.963–0.994, p = 0.006), and IL6 (OR 1.181, 95% CI = 1.021–1.366, p = 0.0254 were significantly associated with CAD (Table 5).

Table 5.
Multiple logistic regression analysis of developing CAD.
The biomarker score, body measurement score, biomarker and body measurement score ware calculated based on the estimated values generated by the Table 5 model, and the scores were adjusted by risk factors. Receiver operating characteristic (ROC) curve analyses were adopted to estimate the predictive values of biomarker score, body measurement score and biomarkers combined with body measurements score for the occurrence of CAD. It was found that the combination of biomarker scores and body measurement scores had the greatest area under the curve and best association with CAD as shown in Figure 1 and Table 6. (Area under the curve of 0.8056, 95% CI = 0.7450–0.8662, p < 0.0001).
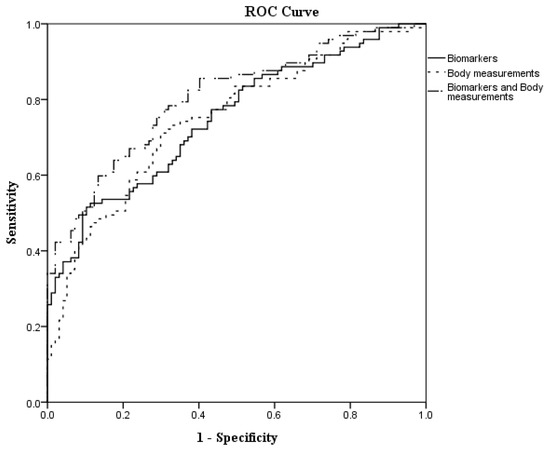
Figure 1.
The receiver operating characteristic (ROC) curves to predict CAD.

Table 6.
Area under the receiver operating characteristic curve (AUROC) and 95% confidence interval (CI) for the different scores.
4. Discussion
Our study results show that lower educational level, no coffee consumption, physical inactivity, low adiponectin, high leptin, and high IL6 levels were associated with CAD. In terms of 3D body measurements, compared to the traditional BMI assessment, smaller CW and RAL with higher WC were also associated with CAD. In addition, the combination of biomarker scores and body measurement scores had the highest predictive value for CAD as shown with ROC analysis. These findings have given us a novel method for assessing the risk of those who may have CAD.
Inflammation contributes to CAD, among which IL6 plays an important role in atherogenesis and atherosclerotic plaque destabilization. IL6 is associated with vascular endothelial injury and tissue fibrosis, promotes angiogenesis, and increases vascular permeability [22]. Once IL6 levels are abnormally elevated, a series of pathological changes occurs including inflammatory injury, plaque formation and rupture, and thrombosis. Chronic exposure to IL6 also disturbs insulin action and body fat. Yet, despite having proinflammatory properties, IL6 also plays an important role in anti-inflammation. Enhanced fat oxidation occurs when IL6 is increased acutely, leading to improved insulin-stimulated glucose uptake with anti-inflammatory effects. With chronic secretion under obese conditions, these effects are not seen, probably due to the development of IL6 resistance [23]. In our study, it was found that IL6 was associated with CAD.
Adipose tissue is associated with CAD, abdominal adiposity causes development of adipose cells that are enlarged and dysfunctional [24]. These dysfunctional adipose tissues secrete pro-inflammatory biomarkers including prostaglandins, C-reactive protein, and cytokines such as interleukins and leptin with a decrease in adiponectin levels [25,26]. Leptin can cause vascular smooth muscle hypertrophy and oxidative stress, and stimulates vascular inflammation which may then lead to the development of type 2 diabetes mellitus, hypertension, atherosclerosis, and CAD [27]. Some studies have shown that increased leptin levels in plasma are associated with adverse outcomes in heart failure and CAD [28]. In CAD patients, higher serum leptin levels were significantly related to an increasing number of stenotic coronary arteries and arterial stiffness [29]. Another adipokine, adiponectin, also has important effects on the cardiovascular system. Its levels are negatively correlated with metabolic and cardiovascular disorders [30], with low levels having been shown to be an independent risk factor for cardiovascular disease [31,32]. In contrast to leptin, adiponectin levels are directly correlated with insulin sensitivity and inversely correlated with adiposity [33,34,35]. Certainly, as shown in our study population, the above mentioned adipokines were found to be associated with CAD.
By using a more accurate 3D body scanning method, we found that higher WC, lower CW and lower RAL were also associated with CAD. WC, which reflects abdominal obesity, has been suggested to be superior to BMI for CAD risk prediction [36], and this was similarly seen in our study. In addition to the important role it has in CAD, leptin has also been found to affect bone metabolism via both direct and indirect mechanisms [37]. Studies have shown that leptin resistance or insulin resistance as found in obesity may lead to poorer bone health [38,39]. Increased adiposity can also lead to decreased bone mass, affecting cortical bone more than trabecular bone [40,41]. These mechanisms may help explain the findings of shorter RAL associated with CAD in our study. Interestingly, CW was associated with CAD in our population. The thoracic cavity, when intact and closed, constrains the heart and lungs to a limited space, such that intrathoracic pressure changes throughout respiratory phases can have varying effects on cardiac function. Thus, one with a smaller chest width may have impaired pulmonary function or motion capacity of organs in the chest in addition to limitation of circulation flow rates. It has been shown that small whole heart volume predicts cardiovascular events in patients with stable chest pain [42]. During normal breathing, chest wall motion is determined by the displacement from respiration and the displacement by heart activity. There has been an interest in how chest wall motion provides information on the cardiorespiratory system with the design of different chest wall models [43]. A smaller CW may therefore also be an indicator that there is restricted cardiopulmonary displacement from cardiovascular impairment. Dynamic lung and chest wall compliance can be measured by the pressure–volume curve [44]. In fact, it has been documented that abdominal obesity preferentially depresses chest wall compliance resulting in a marked decrease in functional residual capacity and expiratory reserve volume [45]. This may very well explain the link between the high WC and lower CW we see associated with CAD in our population.
Faced with CAD being such an important cause of death worldwide, we sought to explore its associations with the more accurate method of 3D-derived body measurements and biomarkers in an attempt to gain more mechanistic insight.
Limitations
As our study design was of a cross-sectional study, we are unable to infer causality from the results. The 3D body measurements were performed only at one point in time with no repeated estimations or data on changes over time. Our study population was of Chinese adults in a hospital setting so the results might not be applied to other ethnicities, age groups, or populations in the community. Therefore, it may be necessary to clarify these conclusions in further longitudinal studies and in a wider population.
5. Conclusions
In our study, 3D anthropometrics provide incremental information regarding associations of body surface measurements with CAD. It has been shown that shorter RAL and CW, and longer WC measurements combined with lower adiponectin and higher leptin and IL6 levels were associated with CAD. Although the precise mechanisms are far from clear, by combining non-invasive 3D body surface measurements together with biomarkers, we may in future be able to explore a different mechanistic approach to CAD, and non-invasively identify those with this condition in clinical practice, in addition to providing more information in epidemiological studies and preventative medicine.
Author Contributions
N.-I.Y.: planned, conducted study; collected, managed data, performed statistical analyses and prepared the manuscript. L.-T.K.: provided clinical expertise, assisted in data collection and interpreted data. C.-C.L.: assisted in compiling the database and data analyses. M.-K.T.: provided clinical expertise, interpretation of data. I.-W.W.: provided clinical expertise, interpretation of data. S.-W.C.: provided clinical expertise, interpretation of data. K.-H.H.: responsible for conceptualization, review and supervision of the study. All authors were responsible for drafting. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Chang Gung Medical Foundation (CMRPG2F0081, CMRPG2F0082, CMRPG2F0083, CMRPD3F0021, CMRPD3F0022, CMRPD3F0023, NARPD3J0021, NARPD3J0022 and NARPD3L0061), the Ministry of Science and Technology, Taiwan (108-2410-H-182-008-MY2 and 110-2410-H-182-013-MY3) and the Wang Jhan-Yang Public Trust Fund (WJY 2020-HR-01, WJY 2021-HR-01, WJY 2022-HR-01, WYJ 2021-AP-01 and WYJ 2022-AP-01).
Institutional Review Board Statement
This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Chang Gung Medical Foundation (201405148B0). The Ethical Committee approval date was 13 October 2015.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Due to ethical restrictions, the data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to thank all the patients who agreed to participate in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moran, A.E.; Forouzanfar, M.H.; Roth, G.A.; Mensah, G.A.; Ezzati, M.; Murray, C.J.; Naghavi, M. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: The Global Burden of Disease 2010 study. Circulation 2014, 129, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [PubMed]
- Keys, A.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; Taylor, H.L. Indices of relative weight and obesity. Int. J. Epidemiol. 2014, 43, 655–665. [Google Scholar] [CrossRef]
- Després, J.P.; Moorjani, S.; Lupien, P.J.; Tremblay, A.; Nadeau, A.; Bouchard, C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 1990, 10, 497–511. [Google Scholar] [CrossRef]
- Kissebah, A.H.; Vydelingum, N.; Murray, R.; Evans, D.J.; Hartz, A.J.; Kalkhoff, R.K.; Adams, P.W. Relation of body fat distribution to metabolic complications of obesity. J. Clin. Endocrinol. Metab. 1982, 54, 254–260. [Google Scholar] [CrossRef]
- Larsson, B.; Svärdsudd, K.; Welin, L.; Wilhelmsen, L.; Björntorp, P.; Tibblin, G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br. Med. J. 1984, 288, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Donahue, R.P.; Abbott, R.D.; Bloom, E.; Reed, D.M.; Yano, K. Central obesity and coronary heart disease in men. Lancet 1987, 1, 821–824. [Google Scholar] [CrossRef]
- Ducimetiere, P.; Richard, J.; Cambien, F. The pattern of subcutaneous fat distribution in middle-aged men and the risk of coronary heart disease: The Paris Prospective Study. Int. J. Obes. 1986, 10, 229–240. [Google Scholar]
- Björntorp, P. Abdominal obesity and the development of noninsulin-dependent diabetes mellitus. Diabetes Metab. Rev. 1988, 4, 615–622. [Google Scholar] [CrossRef]
- Gruzdeva, O.V.; Akbasheva, O.E.; Dyleva, Y.A.; Antonova, L.V.; Matveeva, V.G.; Uchasova, E.G.; Fanaskova, E.V.; Karetnikova, V.N.; Ivanov, S.V.; Barbarash, O.L. Adipokine and Cytokine Profiles of Epicardial and Subcutaneous Adipose Tissue in Patients with Coronary Heart Disease. Bull Exp. Biol. Med. 2017, 163, 608–611. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Katritsis, D.; Raggi, P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 2014, 233, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ragino, Y.I.; Stakhneva, E.M.; Polonskaya, Y.V.; Kashtanova, E.V. The Role of Secretory Activity Molecules of Visceral Adipocytes in Abdominal Obesity in the Development of Cardiovascular Disease: A Review. Biomolecules 2020, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Hu, F.B.; Girman, C.J.; Rifai, N.; Manson, J.E.; Rexrode, K.M.; Rimm, E.B. Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atherosclerosis 2011, 219, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.L. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: Laboratory tests available to assess inflammation--performance and standardization: A background paper. Circulation 2004, 110, e572-6. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.; Frishman, W.H. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef]
- Gerszten, R.E.; Garcia-Zepeda, E.A.; Lim, Y.C.; Yoshida, M.; Ding, H.A.; Gimbrone, M.A., Jr.; Luster, A.D.; Luscinskas, F.W.; Rosenzweig, A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 1999, 398, 718–723. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-β Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6, 140. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Hsu, K.H.; Hwang, C.J.; Hu, P.M.; Lin, T.M.; Chiou, W.K. Waist-to-thigh ratio can also be a better indicator associated with type 2 diabetes than traditional anthropometrical measurements in Taiwan population. Ann. Epidemiol. 2006, 16, 321–331. [Google Scholar] [CrossRef]
- Snijder, M.B.; Visser, M.; Dekker, J.M.; Goodpaster, B.H.; Harris, T.B.; Kritchevsky, S.B.; De Rekeneire, N.; Kanaya, A.M.; Newman, A.B.; Tylavsky, F.A.; et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005, 48, 301–308. [Google Scholar] [CrossRef]
- Yu, C.Y.; Lo, Y.H.; Chiou, W.K. The 3D scanner for measuring body surface area: A simplified calculation in the Chinese adult. Appl. Ergon. 2003, 34, 273–278. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef]
- El-Kadre, L.J.; Tinoco, A.C. Interleukin-6 and obesity: The crosstalk between intestine, pancreas and liver. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Huth, C.; Pigeon, É.; Riou, M.; St-Onge, J.; Arguin, H.; Couillard, E.; Dubois, M.J.; Marette, A.; Tremblay, A.; Weisnagel, S.J.; et al. Fitness, adiposopathy, and adiposity are independent predictors of insulin sensitivity in middle-aged men without diabetes. J. Physiol. Biochem. 2016, 72, 435–444. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Khaza’ai, H.; Rahmat, A.; Patimah, I.; Abed, Y. Obesity can predict and promote systemic inflammation in healthy adults. Int. J. Cardiol. 2016, 215, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Is obesity an inflammatory condition? Nutrition 2001, 17, 953–966. [Google Scholar] [CrossRef]
- Smith, C.C.; Mocanu, M.M.; Davidson, S.M.; Wynne, A.M.; Simpkin, J.C.; Yellon, D.M. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br. J. Pharmacol. 2006, 149, 5–13. [Google Scholar] [CrossRef]
- Abel, E.D.; Litwin, S.E.; Sweeney, G. Cardiac remodeling in obesity. Physiol. Rev. 2008, 88, 389–419. [Google Scholar] [CrossRef]
- Tsai, J.P.; Wang, J.H.; Chen, M.L.; Yang, C.F.; Chen, Y.C.; Hsu, B.G. Association of serum leptin levels with central arterial stiffness in coronary artery disease patients. BMC Cardiovasc Disord. 2016, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 2012, 425, 560–564. [Google Scholar] [CrossRef]
- Koenig, W.; Khuseyinova, N.; Baumert, J.; Meisinger, C.; Löwel, H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: Results from the 18-year follow-up of a large cohort from southern Germany. J. Am. Coll Cardiol. 2006, 48, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Frystyk, J.; Berne, C.; Berglund, L.; Jensevik, K.; Flyvbjerg, A.; Zethelius, B. Serum adiponectin is a predictor of coronary heart disease: A population-based 10-year follow-up study in elderly men. J. Clin. Endocrinol. Metab. 2007, 92, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J.; Scalia, R.G.; Ma, X.L. Protective vascular and myocardial effects of adiponectin. Nat. Clin. Pract. Cardiovasc Med. 2009, 6, 27–35. [Google Scholar] [CrossRef]
- Zhu, W.; Cheng, K.K.; Vanhoutte, P.M.; Lam, K.S.; Xu, A. Vascular effects of adiponectin: Molecular mechanisms and potential therapeutic intervention. Clin. Sci. 2008, 114, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Huxley, R.R.; Wildman, R.P.; Woodward, M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J. Clin. Epidemiol. 2008, 61, 646–653. [Google Scholar] [CrossRef]
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2015, 64, 105–113. [Google Scholar] [CrossRef]
- Shin, D.; Kim, S.; Kim, K.H.; Lee, K.; Park, S.M. Association between insulin resistance and bone mass in men. J. Clin. Endocrinol. Metab. 2014, 99, 988–995. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, D.J.; Lee, Y.; Chung, Y.S. Insulin is inversely associated with bone mass, especially in the insulin-resistant population: The Korea and US National Health and Nutrition Examination Surveys. J. Clin. Endocrinol. Metab. 2014, 99, 1433–1441. [Google Scholar] [CrossRef][Green Version]
- Hong, X.; Arguelles, L.M.; Liu, X.; Tsai, H.J.; Hsu, Y.H.; Wang, B.; Zhang, S.; Li, Z.; Tang, G.; Liu, X.; et al. Percent fat mass is inversely associated with bone mass and hip geometry in rural Chinese adolescents. J. Bone Miner Res. 2010, 25, 1544–1554. [Google Scholar] [CrossRef]
- Pollock, N.K.; Laing, E.M.; Baile, C.A.; Hamrick, M.W.; Hall, D.B.; Lewis, R.D. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am. J. Clin. Nutr. 2007, 86, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Foldyna, B.; Zeleznik, R.; Eslami, P.; Mayrhofer, T.; Scholtz, J.E.; Ferencik, M.; Bittner, D.O.; Meyersohn, N.M.; Puchner, S.B.; Emami, H.; et al. Small whole heart volume predicts cardiovascular events in patients with stable chest pain: Insights from the PROMISE trial. Eur. Radiol. 2021, 31, 6200–6210. [Google Scholar] [CrossRef]
- Singh, A.; Rehman, S.U.; Yongchareon, S.; Chong, P.H.J. Modelling of chest wall motion for cardiorespiratory activity for Radar-Based NCVS Systems. Sensors 2020, 20, 5094. [Google Scholar] [CrossRef] [PubMed]
- Lutfi, M.F. The physiological basis and clinical significance of lung volume measurements. Multidiscip Respir. Med. 2017, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Nzekwu, M.-M.U. The effects of body mass index on lung volumes. Chest 2006, 130, 827–833. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).