Abstract
Point-of-care ultrasound (PoCUS) has become an indispensable standard in emergency medicine. Emergency medicine ultrasound (EMUS) is the application of bedside PoCUS by the attending emergency physician to assist in the diagnosis and management of many time-sensitive health emergencies. In many ways, using PoCUS is not only the mere application of technology, but also a fusion of already existing examiner skills and technology in the context of a patient encounter. EMUS practice can be defined using distinct anatomy-based applications. The type of applications and their complexity usually depend on local needs and resources, and practice patterns can vary significantly among regions, countries, or even continents. A different approach suggests defining EMUS in categories such as resuscitative, diagnostic, procedural guidance, symptom- or sign-based, and therapeutic. Because EMUS is practiced in a constantly evolving emergency medical setting where no two patient encounters are identical, the concept of EMUS should also be practiced in a fluid, constantly adapting manner driven by the physician treating the patient. Many recent advances in ultrasound technology have received little or no attention from the EMUS community, and several important technical advances and research findings have not been translated into routine clinical practice. The authors believe that four main areas have great potential for the future growth and development of EMUS and are worth integrating: 1. In recent years, many articles have been published on novel ultrasound applications. Only a small percentage has found its way into routine use. We will discuss two important examples: trauma ultrasound that goes beyond e-FAST and EMUS lung ultrasound for suspected pulmonary embolism. 2. The more ultrasound equipment becomes financially affordable; the more ultrasound should be incorporated into the physical examination. This merging and possibly even replacement of aspects of the classical physical exam by technology will likely outperform the isolated use of stethoscope, percussion, and auscultation. 3. The knowledge of pathophysiological processes in acute illness and ultrasound findings should be merged in clinical practice. The translation of this knowledge into practical concepts will allow us to better manage many presentations, such as hypotension or the dyspnea of unclear etiology. 4. Technical innovations such as elastography; CEUS; highly sensitive color Doppler such as M-flow, vector flow, or other novel technology; artificial intelligence; cloud-based POCUS functions; and augmented reality devices such as smart glasses should become standard in emergencies over time.
1. Introduction
1.1. “You Have to Know the Past to Understand the Present”—Carl Sagan
Traditional diagnostic ultrasound was developed in the 20th century [1]. After some crucial technological advances in the 1960s and 1970s, two different approaches to diagnostic ultrasound began to emerge [2]. Some medical systems began using ultrasound (US) imaging services that were usually embedded in the specialty of radiology, with few other specialties developing a significant and consultative ultrasound practice. Over time, and with increasing ultrasound use in those particular regions, radiologists and even cardiologists started to employ technicians to obtain images, especially once it became a real-time imaging tool and required learning hands-on skills. However, this approach came to the detriment of large numbers of physicians being able to develop adequate hands-on skills in ultrasound, and it likely caused this type of practice model to function as a consultative imaging test. These exams were seldom performed by the treating physician and hence had to use very standardized approaches for imaging certain areas of the body. The physician requesting the test was rarely present during the exam. Hence, opportunities to ad hoc modify or tailor exams in real time or change or broaden the clinical questions of an exam were very limited. Obviously, exams that would require serial imaging and in patients with very fluid and dynamic clinical presentations (one of the best aspects of real-time ultrasound) are not feasible with this approach.
In parallel to these developments, other countries created a very different approach to diagnostic ultrasound, which was likely due to profound differences in medical practice and health care delivery. In this setting, the treating physician was encouraged to perform their own ultrasounds, and the concept of the “technician” was not embraced. In the mid-20th century, this parallel approach was the domain of internists, gynecologists, surgeons, but also cardiologists, and radiologists [3,4,5,6]. Diagnostic ultrasounds were performed by the treating physician skilled in hands-on image acquisition. This included the clinical interpretation of the findings. Over time, many other specialists joined the ranks of ultrasound-practicing physicians in countries where this differing concept was practiced [7,8,9,10]. Naturally, right from the beginning, ultrasound examinations were often already embedded in the clinical examination, so the performing physician was able to adapt and modify the exam ad hoc. One could make the argument that this was the earliest phase of “clinical ultrasound” and to some degree already included the concept of “point-of-care” sonography. Needless to say, more and more physicians recognized ultrasound’s diagnostic capabilities and began to practice physician-performed ultrasound. For instance, by the 1990s, diagnostic ultrasound was so common in some countries using this practice model that many primary care physicians, internists, or obstetrics/gynecologists had sonography equipment in their private offices and incorporated ultrasound scans as part of their patients’ clinical visits.
Physician-performed diagnostic ultrasound became so common in some health care systems that occasionally, efforts were undertaken to evaluate pathways to avoid the overuse of diagnostic ultrasound [11]. As a remarkable contrast, systems that mainly practiced technician-based ultrasound and limited ultrasound hands-on practice to much fewer specialties seemed to experience a relative decrease in overall diagnostic ultrasound performance. Compared to computed tomography or MRI, diagnostic ultrasound started to lose its share of imaging studies. For instance, a study by Liebeskind et al. based in a large tertiary care radiology department evaluated the causes for a decline in diagnostic ultrasound referrals by primary care physicians. The lack of knowledge of the diagnostic capacity of ultrasound among referring physicians was identified as the major factor [12]. This worldwide dichotomy of diagnostic ultrasound practice patterns is fascinating and still present. It is, in our view, an intriguing curiosity about medical practice in the 20th and early 21st centuries.
However, driven by technological advancements in the early 1980s, a third practice model for diagnostic ultrasound emerged. Surgeons, and eventually emergency medicine physicians of the new specialty of emergency medicine, began to use focused sonography in acute trauma patients in the shock room [13,14]. This was possible because of newer equipment allowing for more mobile and smaller machines with expanded processor capacity. These machines could be positioned next to the patient, moved by physicians to different exam spaces, and allowed for dedicated real-time imaging in acutely ill patients at their bedside. In the shock room, this method was initially limited to the rapid detection of trauma-related hemoperitoneum [15]. However, true portable and hand-held ultrasound machines were already utilized by 1978 and were employed by specialists outside the shock room in the 1980s [16]. Focused point-of-care exams were included in these evaluations. For instance, a two-dimensional echocardiography study by Schwarz and Meltzer in 1988 reported an impressive 96% accuracy in diagnosing cardiac wall motion abnormalities in patients hospitalized for acute ischemic cardiac events who experienced recurring chest pain episodes during admission. This study was conducted with a handheld portable device that weighed 3.3 pounds, had a 2 h battery life, and eventually had the capacity to print images. Bedside cardiology, obstetrics, and even residual bladder volume evaluations at the point of care were performed [17].
During those early years, the newly formed specialty of emergency medicine (EM) quickly recognized the diagnostic potential of ad hoc bedside and portable sonography [18], and one could argue that the need to define new methods of physician-performed ultrasound at the point of care was greatest in countries practicing a technician-based ultrasound model. Ultrasound, with its ability to answer pressing clinical questions in real time, especially in unstable or undifferentiated patients with acute complaints, made it the perfect diagnostic candidate in these settings. Initially, use was focused on identifying causes of hemodynamic instability in medical and trauma patients, procedure guidance, or patients presenting with undifferentiated abdominal, back, and chest pain [19,20]. Gradually, EM physicians expanded their use of sonographic point of care. Soon, the term emergency medicine ultrasound (EMUS) was used to describe exams that were exclusively performed by physicians, similar to the above-described physician-based ultrasound concept but using a laser-focused diagnostic approach. Emergency physicians (EPs) in both types of practice models (technician-performed vs. physician-performed ultrasound) started to include EMUS in their scope of practice. Not surprisingly, EPs began introducing the EMUS approach into systems where US was traditionally physician-performed, and physicians of other specialties who already practiced or understood the “point of care” approach (and likely already tailored their own exams to clinical presentations and point of care) had very different implementation experiences and hurdles to overcome compared to EPs practicing in technician-dependent systems with a paucity of specialists performing their own exams and unfamiliarity with this ultrasound concept [21].
Ultrasound is a technology that not only captures real time images, but is also able to visualize the real-time status of the living person, and the “life” of the patient [22]. One of the most profound examples is likely parents (as well as the sonographer) being able to see an unborn child’s movements and heartbeat [22]. However, it is also a technique that requires two human beings to interact in very close proximity. Naturally, this interaction includes many layers of additional interpersonal communication allowing the sonographer physician to capture a more holistic view of the patient, to talk to their body, and to understand the uniqueness of the person using their intuition, improvisation, and their capability to react to individuality [22]. Therefore, it makes great sense that the attending physician performs the examination themselves. This approach has the advantage that all the intangible layers of information will not be lost. In our opinion, the ultrasound-guided dialogue with the living body is a simple, but also the best way, to penetrate into the depths of the hidden pathologies of our patients, which are revealed ever so gradually over the course of the examination.
In the present, PoCUS and EMUS, as we practice it, is an established bedside diagnostic or interventional type of sonography performed and interpreted by adequately trained and directly for patient-caring physicians. It is documented with stored images and reports, just like any other diagnostic imaging modality, and its comprehensiveness and completeness are adapted to the clinical question and the individual patient. A large number of clinical studies, systematic reviews, and position and consensus papers form the basis for the many recommendations by experts and professional societies that the entire range of PoCUS can, should, and must be used in emergency patients, and that underline its usefulness. The following examples are a selection of the state of the art of EMUS documented with relevant citations out of the abundant studies available. The list of indications for EMUS has increased significantly in recent years, and its benefits for improving the clinical evaluation of patients and the performance of interventions have been proven many times over [23,24,25,26,27,28,29,30,31,32,33,34,35]. There is also a lot of evidence that EMUS speeds up consultation processes [36,37,38]. PoCUS is also often used as part of clinical pathways, either as a pure ultrasound algorithm or in combination with clinical scores or assessments and serves as a guide for further action [39,40]. There are also many indications of the superiority of PoCUS versus the traditional approach and other technologies [19,24,25,31,41,42,43]. Unfortunately, we do not have enough studies that show a benefit of PoCUS and EMUS on the clinical outcome of patients. Nevertheless, there are some reports on their effects on mortality and hospital length of stay or stay in the emergency room [40,44,45,46]
Finally, it should be mentioned that, to our knowledge, no studies have shown a negative effect on the outcome of patients treated with PoCUS.
The major advantages are the speed and mobility not only of the equipment but also the examiners. Furthermore, it can be used in almost all specialties employing diagnostic ultrasound and it can be adapted to individual requirements. It is crucial that internationally recognized standards in education and training programs are available to guide EMUS and PoCUS practice. As already mentioned, many well-established medical societies that are representing specialties with increasing PoCUS or EMUS use have published guidelines and definitions of their ultrasound practice [47,48,49,50,51,52,53,54,55,56,57,58,59]. Other societies, mostly from regions and specialties that do not subscribe to the physician-performed sonography concept, have also attempted to establish definitions of PoCUS and EMUS. These are, of course, valuable attempts by medical societies and specialists practicing within the diagnostic ultrasound realm, but given that there is often extremely limited personal experience of EMUS and PoCUS among these experts, those contributions need to be analyzed with great caution and under the lenses of actual practical experience [49].
In this manuscript, we, as EMUS and PoCUS experts, will focus on definitions of EMUS and PoCUS, as well as current and evolving EMUS concepts.
1.2. Current EMUS Concepts
The concept of EMUS is based on the fact that specific clinical questions in emergencies can be answered with the help of sonographic findings and that therapeutic or other consequences can be derived from them. For this purpose, two types of examinations are distinguished: basic, core, or primary; and advanced or secondary exams. The criteria for this distinction are as follows:
- −
- Incomplete to complete coverage of the problem by the sonographic question/s (simple or complex, a single question or multiple yes–no questions, directly or indirectly translatable into the clinical question);
- −
- Degree of difficulty in technical execution;
- −
- Whether rare or common;
- −
- Whether of clinical importance or less relevant;
- −
- Availability of probe and special software (for example, echocardiography).
Because there are different ideas in the emergency community regarding the inclusion and weighting of these five criteria, there is a lack of consensus about what should be considered an indication and assigned to which category (basic or advanced).
Individual EMUS applications are grouped together. The most cited common classification in the literature is one of following five groups by the American College of Emergency Medicine (Table 1) [51]:

Table 1.
Scope of emergency ultrasound [51].
- Resuscitative;
- Diagnostic;
- Procedural guidance;
- Symptom- or sign-based;
- Therapeutic.
2. Evolving EMUS Concepts
Fundamental and critical considerations of the physical examination, especially concerning the use of the stethoscope, a deeper understanding of physical and pathophysiological processes with correlation to sonographic possibilities, technical innovations, and advances in the field of education and training (competence-centered) lead to new concepts and types of applications awaiting their introduction and integration into the practice of emergency medicine.
3. Examples of New EMUS Application Possibilities and Concepts
In recent years, many articles have been published on novel applications. They concern nearly all organs and systems of the human body. Only a small part has found its way into routine use. We would like to present two examples: E-FAST, as one of the first indications for EMUS still with potential for development, and pulmonary embolism as a neglected indication. In our opinion, E-FAST needs to be redefined. First, we should go back to the original idea of E-FAST, i.e., to detect major internal bleeding in the trunk and a pneumothorax in unstable patients in less than 2 min. In stable patients, we have more time and can integrate the many technical advances and new indications of ultrasonography into the E-FAST exam. These new applications will overcome the stagnation of E-FAST, which has hampered further development for over 20 years. The pulmonary embolism as a second example shows another problem. The diagnosis of pulmonary embolism (PE) poses a major challenge for emergency departments. The mortality is still high despite the large availability of computed tomographic pulmonary angiography (CTPA), and the risks and problems with CTPA such as radiation exposure, allergic reactions, high costs, and binding of resources, which this technique entails, may not be underestimated. Lung ultrasound, still considered impossible in the 2012 edition of Harrison’s principles of internal medicine, has been used in German-speaking countries for over 30 years for the diagnosis of PE but not in other countries. These two examples are intended to show that PoCUS is still changing and that development has not stood still.
3.1. To New Shores in Trauma
The original idea behind the focused assessment with sonography for trauma (FAST) was to quickly detect a possible underlying bleeding into the peritoneal, pleural, or pericardial cavity in unstable trauma patients. With the addition of pneumothorax, the extended FAST (E-FAST) was introduced. Soon, FAST and later E-FAST were expanded to include stable trauma patients and other indications, such as ruptured extrauterine pregnancy or undifferentiated shock [60]. Thanks to improved technology, new modalities, and the growing scientific knowledge of integrating ultrasound into the management of trauma patients, it is now possible to detect even small fluid collections in the peritoneal space, injuries to solid organs with high-sensitivity color Doppler (Figure 1) or contrast-enhanced ultrasound (CEUS) (Figure 2), retroperitoneal hematoma (Figure 3), and free air [61,62]. Many other indications were added over time, such as fractures of the ribs, sternum, and extremity bones (Figure 4), and other musculoskeletal problems and vascular extremity injuries [63,64,65]. Particularly worth mentioning are dislocations of the shoulder (Figure 5), with a rapid reduction in wait time due to not needing to wait for an X-ray to exclude a fracture [37].
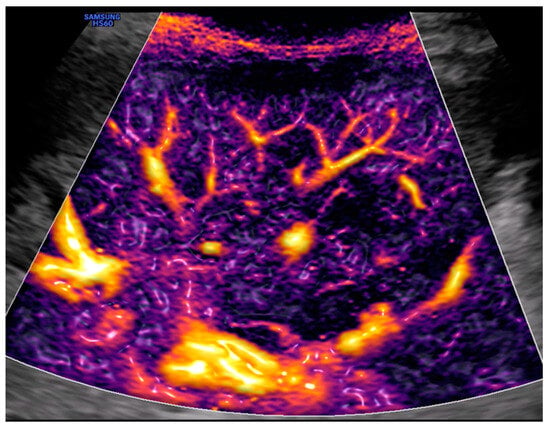
Figure 1.
Left upper quadrant view: spleen and Doppler with M-Flow technology. Image courtesy of J. Osterwalder.
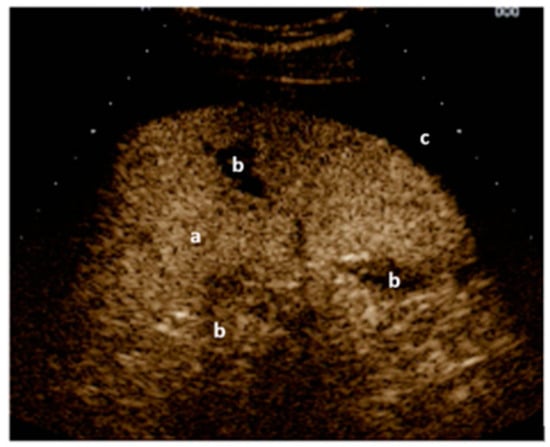
Figure 2.
Left upper quadrant view: CEUS of the spleen (a) with black gaps (b) within the spleen (lacerations/ruptures) and around the spleen (free fluid/blood) (c). Image courtesy of J. Osterwalder.
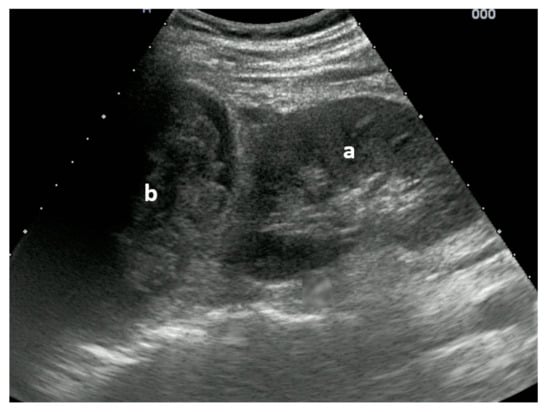
Figure 3.
Right upper quadrant view: (a) kidney with traumatic adrenal gland hemorrhage and retroperitoneal hematoma (b). Image courtesy of J. Osterwalder.
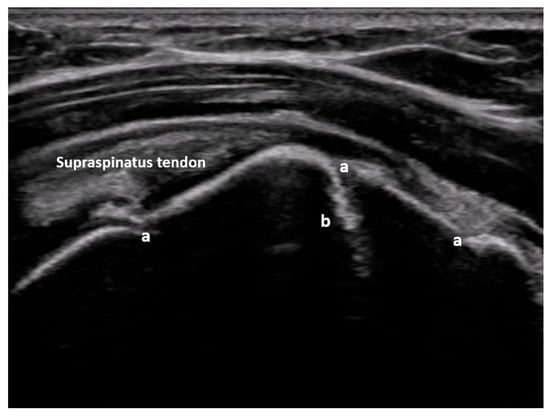
Figure 4.
Non-displaced major tuberosity fracture: lateral sagittal view at the level of the major tuberosity with corticalis interruptions (a) and chimney sign (b). Image courtesy of J. Osterwalder.
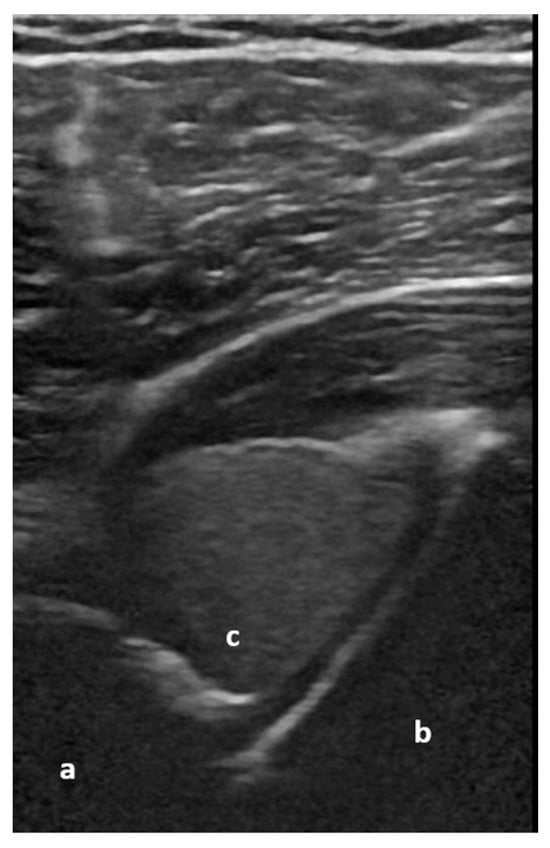
Figure 5.
Dorsal transverse view of an anteriorly dislocated humeral head (a), glenoid (b), and hemarthrosis (c). Image courtesy of J. Osterwalder.
There is also a full range of other sonographic examination options available to the emergency physician, which help to work through the primary survey, such as false or main stem endotracheal intubations, pulmonary contusions, hemodynamic evaluation, cerebral hypertension, and cerebral perfusion in traumatic brain injury (TBI) [66,67,68]. Of particular benefit is the hemodynamic monitoring for fluid therapy, the use of vasopressors, and the guidance of invasive procedures [69].
In summary, in addition to the traditional E-FAST examination in unstable patients and an expanded type of E-FAST for stable patients, we see many more fields of application of PoCUS for trauma patients:
- Expanding E-FAST in stable patients to include additional views for abdominal FAST including the retroperitoneum, evidence of solid organ injury, and free air;
- PoCUS other than traditional E-FAST such as ABCDE during the primary trauma survey;
- The monitoring of hemodynamics including cerebral perfusion in TBI to guide fluid and vasopressor treatment;
- Search for musculoskeletal and soft-tissue or vascular injuries;
- Guidance with invasive procedures.
Pulmonary Embolism
The gold standard for the diagnosis of pulmonary embolism is computed tomography pulmonary angiogram (CTPA). For many years, we have known that lung ultrasound (LUS) can diagnose pulmonary emboli that cause peripheral lung changes. To do so, at least two triangular (Figure 6A,B) or round hypoechogenic subpleural consolidations lacking central perfusion and usually measuring 1–2 cm in size must be detected [70].
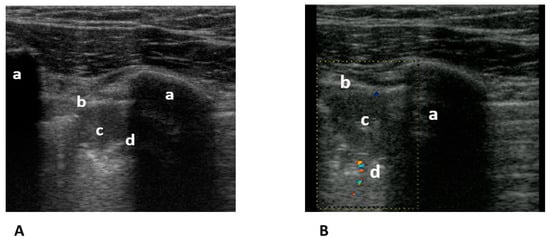
Figure 6.
(A) Transthoracic longitudinal view: ribs (a), pleural line (b), and subpleural triangular consolidation (c) with central bronchus reflex (d). (B) Transthoracic longitudinal view: rib (a) pleural line (b), and second subpleural triangular consolidation (c) with obstructed vessel (d). Image courtesy of J. Osterwalder.
The pooled sensitivity of 82% (95% CI 72–88) and specificity of 95% (95% CI 79–95) of LUS for pulmonary embolism in a systematic review and meta-analysis are lower than those of CTPA. The sensitivities vary between 42 and 98% [71]. This wide range is probably the result of different diagnostic criteria for PE, patient selection, and the experience of the examiners. Nevertheless, together with compression ultrasonography of the leg veins and right heart echocardiography (triple sonography), the sensitivity could be increased to 90%, albeit at the cost of a decrease in specificity to 86% [72]. An inhomogeneous meta-analysis on this topic resulted in a sensitivity of 91% and specificity of 81% [73]. However, the reference variable was CTPA in only two studies and clinically derived criteria in five studies. In this context, one paper is interesting. The authors suggested that triple sonography saved 56% of CTPAs because of alternative diagnoses [74]. Despite these encouraging results and the great advantages over CTPAs without radiation exposure, contraindications, the risk of anaphylaxis, high costs, and long waiting times, this type of lung sonography is only offered routinely in a few emergency departments around the world. We believe that it is time to use ultrasound as a diagnostic rule-in tool for PE and thus save some patients and crowded emergency departments from many CTs.
3.2. From the Stethoscope to PoCUS
Over 200 years ago, René-Théophile Hyacinthe Laennec invented the stethoscope. Originally, intended only for the heart, it was not until later that the auscultation of the lungs, intestines, and vessels was added. The stethoscope is probably the most widely used diagnostic instrument, but today it is also probably the most poorly used and thus has questionable diagnostic value in terms of its broad impact [75]. Whether the digital stethoscope with a smartphone app will lead to a turnaround in skill and revival is highly questionable. In contrast, PoCUS use, whether in the form of hand-held or small mobile devices, is recommended by many institutions, including the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) [58]. A plethora of studies have shown that US is superior to standard clinical examination and common exam tools, such as the stethoscope and chest and abdominal X-ray, for diagnosing cardiac, pulmonary, and abdominal problems [24,36,41,43,76,77,78].
However, this view is still disputed by some [79]. Contradicting this opinion is the fact that most physicians are poor at using the stethoscope, and the training requires considerable time and effort. Therefore, it makes sense that the paradigm will shift from “stop listening to look” [80]. However, we should not only look at the heart, lungs, abdomen, and vessels, where we have been listening so far, but also exploit the full potential of ultrasound and integrate palpation and percussion, thus redefining the physical examination of the body. Who does not know from experience that when looking for the boundary of lung/diaphragm, apex of the heart, or inferior border of the liver, one is often wrong with percussion/palpation [81]? A myriad of studies exist on this topic, with only one important paper on musculoskeletal indications mentioned here [25]. We believe that PoCUS using a portable or hand-held device could replace significant segments of auscultation exams wherever it is financially viable and should largely replace percussion and palpation. By doing so, we believe that time will be saved, and the use of other unnecessary imaging such as X-ray, CT, or MRI; cost; infrastructure; and human resources can be decreased. While the shift from stethoscope to PoCUS seems more valid than ever, it determining how to integrate it in limited resources regions appears challenging. Although studies are heterogenous and refer to different environments and settings, the development of a tailored curriculum according to the local needs and disease burden, as well as the use of tele-ultrasound training in conjunction with on-site training by POCUS experts are potential solutions in implementing this shift [82]. These are all key concerns in emergency medicine.
This paradigm shift might take place first in well-resourced countries and might begin with student education. The prerequisite is that many medical students already use ultrasound in anatomy, physiology, and pathophysiology courses and are introduced to the basics of its practical application. Clinical examination courses should be supplemented with PoCUS courses for cardiac, pulmonary, abdominal, and vascular examinations. Continuing education in emergency medicine could then focus on specific aspects of emergency medicine. However, physicians in specialty training must also be taught to use handheld or small mobile devices. This not only requires appropriate courses. Even more important is competence-based supervision in everyday practice, which has been largely neglected. This training and its integration into medical education should contain clearly defined learning objectives, accompanied by a structured assessment. The key elements may include the workplace-based supervision of image acquisition and interpretation, formed on relevant clinical indications, integrated in a structured approach within a logical manner and finally incorporated in medical decision making. Additional or alternative means of competence-based supervision can be applied remotely, either with on-site or web-based case simulation. New concepts and ideas are urgently needed to realize this complex and central requirement [83].
In regions with limited resources, in place of traditional imaging modalities which are usually based in a hospital-setting and require trained diagnosticians, PoCUS seems to be a feasible alternative. Instead of expensive medium- and high-end devices, investments should be made in small mobile and handheld devices that cost a fraction of the costs of cart-based systems or existing devices, and most importantly can be used in austere environments by the treating physician [84].
3.3. PoCUS-Ultrasound Visualization of Pathophysiological Processes and Correlation of Lung Ultrasound Artifacts with Pathological Lung Changes
Important topics in emergency medicine, such as fluid therapy and pulmonary edema and viral pneumonias, benefit from the correlation of new or deeper knowledge in US physics and pathophysiology. From this, new or improved diagnostic ultrasound concepts are emerging. For example, it has been increasingly recognized that the increase in cardiac output with fluid depends substantially on venous return [85]. Venous return can be easily and adequately described by the Doppler spectrum of large veins, particularly the hepatic vein [86]. Thus, the concepts of volume responsiveness can be combined with volume tolerance and intolerance [87]. In the field of lung sonography, the main concern is the so-called B-lines as vertical US artifacts, which occur at the interface of the pleura and the ventilated lung. Currently, a recent position paper of the World Federation for Ultrasound in Medicine and Biology (WFUMB) makes a clear distinction between artifacts that result from increased water retention in the lung and other parenchymal processes that do not lead to consolidation, such as interstitial lung pathologies [34,88].
3.4. Technical Innovations
Elastography: It has matured technically and has been utilized for over 20 years in medical imaging, mostly though in ultrasound-advanced regions of the world. However, it allows for multiple applications in emergency medicine such as trauma, degenerative musculoskeletal diseases, and pulmonary diseases that result in increased lung density without leading to consolidation [89].
Advanced color Doppler technology or other flow technologies: Because of very high sensitivities, it is now possible to visualize even small peripheral blood vessels, with certain devices even in three dimensions. In our opinion, it allows for the detection of hemorrhage, hematomas, and infarcts in solid organs, and in these cases, it can even replace contrast-enhanced ultrasound (CEUS). Among other things, this revolutionizes E-FAST [90,91,92].
Artificial intelligence (AI): An emergency physician needs significant training and supervision to obtain sufficient ultrasound expertise. Here, AI may be able to help via the incorporation of AI-assisted diagnosis into devices. In particular, deep learning technology and convolutional neural networks are promising. Potential applications include lung artifact sonography and quantitative echocardiography for determining ejection fraction (EF), wall motion abnormalities, volume flow, and valve dysfunction [93].
Cloud-based PoCUS: This is a new type of computing platform that has the advantages of low cost, high reusability, high performance, and easy expansion. This technology is already widely used in all fields of medicine for diagnostics and therapy. Thus, a large volume of imaging can be generated using the characteristics of real-time imaging. Using 5G technology, mobile terminal devices such as smartphones or tablets, which are central elements of the EMUS, allow for fast real-time transmissions. Cloud-based PoCUS has huge potential with many versatile possibilities [94].
Smart Glasses: Smart glasses are wearable computer glasses that add information to the visual field of the wearer but also can change their optical properties at runtime. These tools are already used in surveillance, security, and navigation, just to name a few areas of application. Augmented reality or virtual reality settings can be achieved. Smart glasses incorporated into PoCUS are already commercially available (Figure 7) and replace hand movements by the operator to adjust machine settings, triggering commands by eye-tracking and eye movements instead of using hands. Other emerging research suggests potential applications in procedural guidance. Here, pilot studies have shown potential promise in ultrasound-guided vascular access via a decrease in the number of cannulation attempts, procedure time, and procedure-related complications rates. These studies used glasses that project real-time ultrasound images over the visual field during the procedure and reduced head movements during interventions [95]. Of course, this technology is emerging and existing literature is still limited.

Figure 7.
Smart glasses. Image courtesy of J. Osterwalder.
Future research assessing the additional use of established ultrasound technologies such as elastography or high-sensitive flow will likely emerge when these technologies will be more accessible for point of care settings. AI applications in PoCUS are already on a rapidly increasing trajectory and will likely present one of the fastest growth areas in PoCUS. The speed of advancement in AI technology and effects of AI on medical imaging practice will need to be monitored with the same strict ethical guidance as other AI applications in our daily life, such as driving, design, entertainment, food production, etc.
Future research will improve our understanding of AI’s indications and potential limitations, and how to best implement these exciting new tools.
4. Conclusions
Emergency sonography is the application of bedside PoCUS by the attending physician to assist in solutions to as many time-sensitive problems as possible in emergency patient evaluation and management encounters. To date, EMUS encompasses five domains (resuscitative, diagnostic, procedural guidance, symptom- or sign-based, and therapeutic) and two applications are distinguished: basic and advanced. These five domains have been described by many publications and are detailed in several emergency medicine society’s guidelines and position statements [83,96].
Enormous technological advances and scientific findings from the practical application research of PoCUS in traumatic and non-traumatic situations open many new possibilities that are waiting to be introduced and integrated into emergency medicine:
- There are new indications that concern all organs and systems of the human body. Only a small part has found its way into routine use. The following are two important examples: ultrasound in trauma patients, which should have gone beyond E-FAST long ago, and pulmonary embolism, which is already a standard among experts.
- As soon as the financial barriers fall, the change from stethoscope, percussion, and mostly palpation, to sonoscope should be implemented as much as possible. That is, the training of clinical examination of medical students must be revolutionized and, wherever possible and appropriate, the already trained physicians must be retrained—a Herculean task.
- A profound insight into the pathophysiological processes and an understanding of the physical phenomena of ultrasound should be combined. This knowledge must be translated into concepts to better handle hemodynamics, especially volume management, and artifact sonography of the lung (b-lines and elastography to determine lung density).
- Technical innovations, such as elastography, advanced color Doppler, CEUS and other flow technology, artificial intelligence, cloud-based PoCUS and smart glasses, await their use in emergencies.
Author Contributions
Conceptualization J.O.; writing—original draft preparation, J.O.; writing—review, and editing B.H. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
E.P has received a speaker honorarium from GE. J.O and B.H declare no conflict of interest.
References
- Dietrich, C.F.; Bolondi, L.; Duck, F.; Evans, D.H.; Ewertsen, C.; Fraser, A.G.; Gilja, O.H.; Jenssen, C.; Merz, E.; Nolsoe, C.; et al. History of Ultrasound in Medicine from its birth to date (2022), on occasion of the 50 Years Anniversary of EFSUMB. A publication of the European Federation of Societies for Ultrasound In Medicine and Biology (EFSUMB), designed to record the historical development of medical ultrasound. Med. Ultrason. 2022, 24, 434–450. [Google Scholar] [PubMed]
- Hoffmann, B. The future is not the sonoscope. J. Ultrasound Med. 2003, 22, 997–998, author reply 998–1000. [Google Scholar] [PubMed]
- Available online: https://www.ultraschallmuseum.de/index.php?link=120 (accessed on 3 October 2023).
- Rettenmaier, G. Sonografischer Oberbauchstatus. Aussagefähigkeit und Indikationen der Ultraschall-Schnittbilduntersuchung des Oberbauchs [Sonographic status of upper abdomen. Diagnostic significance and indications of the ultrasonic sectional examination of the upper abdomen]. Internist 1976, 17, 549–564. [Google Scholar] [PubMed]
- Kristensen, J.K.; Buemann, B.; Kühl, E. Ultrasonic scanning in the diagnosis of splenic haematomas. Acta Chir. Scand. 1971, 137, 653–657. [Google Scholar] [PubMed]
- Edler, I.; Lindström, K. The history of echocardiography. Ultrasound Med. Biol. 2004, 30, 1565–1644. [Google Scholar] [CrossRef]
- Li, L.; Yong, R.J.; Kaye, A.D.; Urman, R.D. Perioperative Point of Care Ultrasound (POCUS) for Anesthesiologists: An Overview. Curr. Pain. Headache Rep. 2020, 24, 20. [Google Scholar] [CrossRef]
- Sorensen, B.; Hunskaar, S. Point-of-care ultrasound in primary care: A systematic review of generalist performed point-of-care ultrasound in unselected populations. Ultrasound J. 2019, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Simard, R. Ultrasound Imaging of Orthopedic Injuries. Emerg. Med. Clin. N. Am. 2020, 38, 243–265. [Google Scholar] [CrossRef]
- Breakey, N.; Osterwalder, J.; Mathis, G.; Lehmann, B.; Sauter, T.C. Point of care ultrasound for rapid assessment and treatment of palliative care patients in acute medical settings. Eur. J. Intern. Med. 2020, 81, 7–14. [Google Scholar] [CrossRef]
- Krug, B.; Stützer, H.; Wolters, U.; Mauch, C.; Staib, P.; Töx, U.; Steffen, H.M.; Warm, M.; Ghafur, S.; Araba, F.; et al. Entwicklung der sonographischen Diagnostik in einer Radiologischen Universitätsklinik von 1994 bis 2001 [Development of ultrasonography in a radiological university department from 1994 to 2001]. Rofo 2002, 174, 767–775. [Google Scholar] [CrossRef]
- Liebeskind, M.E.; Arger, P.H.; Liebeskind, A.; Maston, K.; Langlotz, C. Using. sonography to examine adult patients at an academic medical center: Have usage patterns changed with the expansion of managed care? AJR Am. J. Roentgenol. 2002, 179, 1395–1399. [Google Scholar] [CrossRef]
- Strittmatter, B.; Lausen, M.; Salm, R.; Kohlberger, E. Die Wertigkeit der Ultraschalldiagnostik beim stumpfen Bauch- und Thoraxtrauma. Langenbecks Arch. Chiv. 1988, 373, 202–205. [Google Scholar] [CrossRef]
- Jehle, D.; Guarino, J.; Karamanoukian, H. Emergency department ultrasound in the evaluation of blunt abdominal trauma. Am. J. Emerg. Med. 1993, 11, 342–346. [Google Scholar] [CrossRef]
- Rozycki, G.S.; Shackford, S.R. Ultrasound, what every trauma surgeon should know. J. Trauma 1996, 40, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ob-ultrasound.net/history2.html (accessed on 3 October 2023).
- Schwarz, K.Q.; Meltzer, R.S. Experience rounding with a hand-held two-dimensional cardiac ultrasound device. Am. J. Cardiol. 1988, 62, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Kendall, J.L.; Hoffenberg, S.R.; Smith, R.S. History of emergency and critical care ultrasound: The evolution of a new imaging paradigm. Crit. Care Med. 2007, 35 (Suppl. 5), S126–S130. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.reliasmedia.com/articles/68664-society-for-academic-medicine-ultrasound-position-statement (accessed on 3 October 2023).
- American College of Emergency Physicians. ACEP emergency ultrasound guidelines—2001. Ann Emerg Med. 2001, 38, 470–481. [Google Scholar] [CrossRef]
- Seitz, K.; Braun, B. Sonografie kompetent Von der Indikation zur Interpretation; Georg Thieme Verlag: New York, NY, USA, 2016. [Google Scholar]
- Maio, G. Medicine and the holistic understanding of the human being: Ultrasound examination as dialog. Ultraschall Med. 2014, 35, 98–107. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Nazerian, P.; Castagno, D.; Tozzetti, C.; Tizzani, P.; Tizzani, M.; Porrino, G.; Ferreri, E.; Busso, V.; et al. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: A randomized controlled trial. Eur. J. Heart Fail. 2019, 21, 754–766. [Google Scholar] [CrossRef]
- Maw, A.M.; Hassanin, A.; Ho, P.M.; McInnes, M.D.F.; Moss, A.; Juarez-Colunga, E.; Soni, N.J.; Miglioranza, M.H.; Platz, E.; DeSanto, K.; et al. Diagnostic Accuracy of Point-of-Care Lung Ultrasonography and Chest Radiography in Adults With Symptoms Suggestive of Acute Decompensated Heart Failure: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e190703. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Albano, D.; Allen, G.; Bazzocchi, A.; Bignotti, B.; Chianca, V.; Facal de Castro, F.; Drakonaki, E.E.; Gallardo, E.; Gielen, J.; et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur. Radiol. 2018, 28, 5338–5351. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yoshida, T.; Noma, H.; Nomura, T.; Suzuki, A.; Mihara, T. Diagnostic accuracy of point-of-care ultrasound for shock: A systematic review and meta-analysis. Crit. Care. 2023, 27, 200. [Google Scholar] [CrossRef]
- Wu, J.; Ge, L.; Wang, X.; Jin, Y. Role of point-of-care ultrasound (POCUS) in the diagnosis of an abscess in paediatric skin and soft tissue infections: A systematic review and meta-analysis. Med. Ultrason. 2022, 24, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X. Sonography of Dermatologic Emergencies. J. Ultrasound Med. 2017, 36, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, F.H.J.; Buise, M.P.; Claassen, J.J.F.; Dierick-van Daele, A.T.M.; Bouwman, A.R.A. Comparison of ultrasound guidance with palpation and direct for peripheral vein cannulation in adult patients: A systematic review and meta-analysis. Br. J. Anaesth. 2018, 121, 358–366. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Adriaensen, M.; Albano, D.; Alcala-Galiano, A.; Allen, G.; Aparisi Gómez, M.P.; Aringhieri, G.; Bazzocchi, A.; Beggs, I.; Chianca, V.; et al. Clinical indications for image guided interventional procedures in the musculoskeletal system: A Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)-part III, nerves of the upper limb. Eur. Radiol. 2020, 30, 1498–1506. [Google Scholar] [CrossRef]
- Brousseau, A. Parent DMBET 3: Advantages of ultrasound-assisted lumbar puncture. Emerg. Med. J. 2016, 33, 163–165. [Google Scholar] [CrossRef]
- Labovitz, A.J.; Noble, V.E.; Bierig, M.; Goldstein, S.A.; Jones, R.; Kort, S.; Porter, T.R.; Spencer, K.T.; Tayal, V.S.; Wei, K. Focused cardiac ultrasound in the emergent setting: A consensus statement of the American Society of Echocardiography and American College of Emergency Physicians. J. Am. Soc. Echocardiogr. 2010, 23, 1225–1230. [Google Scholar] [CrossRef]
- Rubano, E.; Mehta, N.; Caputo, W.; Paladino, L.; Sinert, R. Systematic review: Emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad. Emerg. Med. 2013, 20, 128–138. [Google Scholar] [CrossRef]
- Demi, L.; Wolfram, F.; Klersy, C.; De Silvestri, A.; Ferretti, V.V.; Muller, M.; Miller, D.; Feletti, F.; Wełnicki, M.; Buda, N.; et al. New International Guidelines and Consensus on the Use of Lung Ultrasound. J. Ultrasound Med. 2023, 42, 309–344. [Google Scholar] [CrossRef]
- Haji-Hassan, M.; Lenghel, L.M.; Bolboacă, S.D. Hand-Held Ultrasound of the Lung: A Systematic Review. Diagnostics 2021, 11, 1381. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, M.; Scorpiniti, M.; Gigli, C.; Nazerian, P.; Vanni, S.; Innocenti, F.; Stefanone, V.T.; Savinelli, C.; Coppa, A.; Bigiarini, S.; et al. Point-of-Care Ultrasonography for Evaluation of Acute Dyspnea in the ED. Chest 2017, 151, 1295–1301. [Google Scholar] [CrossRef]
- Gottlieb, M.; Patel, D.; Marks, A.; Peksa, G.D. Ultrasound for the diagnosis of shoulder dislocation and reduction: A systematic review and meta-analysis. Acad. Emerg. Med. 2022, 29, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Peksa, G.D.; Pandurangadu, A.V.; Nakitende, D.; Takhar, S.; Seethala, R.R. Utilization of ultrasound for the evaluation of small bowel obstruction: A systematic review and meta-analysis. Am. J. Emerg. Med. 2018, 36, 234–242. [Google Scholar] [CrossRef]
- Barr, L.; Hatch, N.; Roque, P.J.; Wu, T.S. Basic ultrasound-guided procedures. Crit. Care Clin. 2014, 30, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.V.; Szigetváry, C.; Szabó, L.; Dembrovszky, F.; Rottler, M.; Ocskay, K.; Madzsar, S.; Hegyi, P.; Molnár, Z. Point-of-care ultrasound improves clinical outcomes in patients with acute onset dyspnea: A systematic review and meta-analysis. Intern. Emerg. Med. 2023, 18, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Goldstein, I.; Mourgeon, E.; Cluzel, P.; Grenier, P.; Rouby, J.J. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004, 100, 9–15. [Google Scholar] [CrossRef]
- Kimura, B.J. Point-of-care cardiac ultrasound techniques in the physical examination: Better at the bedside. Heart 2017, 103, 987–994. [Google Scholar] [CrossRef]
- Chan, K.K.; Joo, D.A.; McRae, A.D.; Takwoingi, Y.; Premji, Z.A.; Lang, E.; Wakai, A. Chest ultrasonography versus supine chest radiography for diagnosis of pneumothorax in trauma patients in the emergency department. Cochrane Database Syst. Rev. 2020, 7, CD013031. [Google Scholar] [CrossRef]
- Huang, C.T.; Chang, C.H.; Chen, J.Y.; Ling, D.A.; Lee, A.F.; Wang, P.H.; Wu, C.K.; Ko, Y.C.; Hsiao, Y.T.; Lien, W.C.; et al. The effect of point-of-care ultrasound on length of stay and mortality in patients with chest pain/dyspnea. Ultraschall Med. 2023, 44, 389–394. [Google Scholar] [CrossRef]
- Wang, P.H.; Chen, J.Y.; Ling, D.A.; Lee, A.F.; Ko, Y.C.; Lien, W.C.; Huang, C.H. Earlier point-of-care ultrasound, shorter length of stay in patients with acute flank pain. Scand. J. Trauma Resusc. Emerg. Med. 2022, 30, 29. [Google Scholar] [CrossRef] [PubMed]
- Guner, N.G.; Yurumez, Y.; Yucel, M.; Alacam, M.; Guner, S.T.; Ercan, B. Effects of Point-of-care Ultrasonography on the Diagnostic Process of Patients Admitted to the Emergency Department with Chest Pain: A Randomised Controlled Trial. J. Coll. Physicians Surg. Pak. 2020, 30, 1262–1268. [Google Scholar] [PubMed]
- Gottlieb, M.; Holladay, D.; Peksa, G.D. Ultrasound-assisted Lumbar Punctures: A Systematic Review and Meta-Analysis. Acad. Emerg. Med. 2019, 26, 85–96. [Google Scholar] [CrossRef]
- Bhatt, M.; Braun, C.; Patel, P.; Patel, P.; Begum, H.; Wiercioch, W.; Varghese, J.; Wooldridge, D.; Alturkmani, H.J.; Thomas, M.; et al. Diagnosis of deep vein thrombosis of the lower extremity: A systematic review and meta-analysis of test accuracy. Blood Adv. 2020, 4, 1250–1264. [Google Scholar] [CrossRef]
- Patel, M.D.; Sill, A.P.; Dahiya, N.; Chen, F.; Eversman, W.G.; Kriegshauser, J.S.; Young, S.W. Performance of an algorithm for diagnosing acute cholecystitis using clinical and sonographic parameters. Abdom. Radiol. 2022, 47, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Reyes, D.; Acevedo-Cardona, A.O.; Gómez-González, J.F.; Echeverry-Piedrahita, D.R.; Aguirre-Flórez, M.; Giraldo-Diaconeasa, A. Point-of-care ultrasound in cardiorespiratory arrest (POCUS-CA): Narrative review article. Ultrasound J. 2021, 13, 46. [Google Scholar] [CrossRef]
- American College of Emergency Physicians. Policy Statement. Definition of Clinical Ultrasonography. Approved April. 2023. Available online: https://www.acep.org/siteassets/new-pdfs/policy-statements/ultrasound-guidelines--emergency-point-of-care-and-clinical-ultrasound-guidelines-in-medicine.pdf (accessed on 3 October 2023).
- Frankel, H.L.; Kirkpatrick, A.W.; Elbarbary, M.; Blaivas, M.; Desai, H.; Evans, D.; Summerfield, D.T.; Slonim, A.; Breitkreutz, R.; Price, S.; et al. Guidelines for the Appropriate Use of Bedside General and Cardiac Ultrasonography in the Evaluation of Critically Ill Patients-Part I: General Ultrasonography. Crit. Care Med. 2015, 43, 2479–2502. [Google Scholar] [CrossRef]
- Levitov, A.; Frankel, H.L.; Blaivas, M.; Kirkpatrick, A.W.; Su, E.; Evans, D.; Summerfield, D.T.; Slonim, A.; Breitkreutz, R.; Price, S.; et al. Guidelines for the Appropriate Use of Bedside General and Cardiac Ultrasonography in the Evaluation of Critically Ill Patients-Part II: Cardiac Ultrasonography. Crit. Care Med. 2016, 44, 1206–1227. [Google Scholar] [CrossRef]
- Statement on ultrasound examinations by surgeons. Committee on Emerging Surgical Technology and Education, American College of Surgeons. Bull. Am. Coll. Surg. 1998, 83, 37–40. [Google Scholar]
- Dietrich, C.F.; Goudie, A.; Chiorean, L.; Cui, X.W.; Gilja, O.H.; Dong, Y.; Abramowicz, J.S.; Vinayak, S.; Westerway, S.C.; Nolsøe, C.P.; et al. Point of Care Ultrasound: A WFUMB Position Paper. Ultrasound Med. Biol. 2017, 43, 49–58. [Google Scholar] [CrossRef]
- IFEM Point-of-Care Ultrasound Curriculum Guidance. 2014. Available online: http://www.ifem.cc/Resources/PoliciesandGuidelines.aspx (accessed on 3 October 2023).
- Lewis, D.; Rang, L.; Kim, D.; Robichaud, L.; Kwan, C.; Pham, C.; Shefrin, A.; Ritcey, B.; Atkinson, P.; Woo, M.; et al. Recommendations for the use of point-of-care ultrasound (POCUS) by emergency physicians in Canada. CJEM 2019, 21, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Jarman, R.D.; McDermott, C.; Colclough, A.; Bøtker, M.; Knudsen, L.; Harris, T.; Albaroudi, B.; Albaroudi, O.; Haddad, M.; Darke, R.; et al. EFSUMB Clinical Practice Guidelines for Point-of-Care Ultrasound: Part One (Common Heart and Pulmonary Applications) LONG VERSION. Ultraschall Med. 2023, 44, e1–e24. [Google Scholar] [CrossRef]
- Patel, M.D.; Horrow, M.M.; Kamaya, A.; Frates, M.C.; Dahiya, N.; Golding, L.; Chong, W.K.; Gerena, M.; Ghate, S.; Glanc, P.; et al. Mapping the ultrasound landscape to define point-of-care ultrasound and diagnostic ultrasound: A proposal from the society of radiologists in ultrasound and ACR commission in ultrasound. J. Am. Radiol. 2021, 18, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Jehle, D.; Heller, M. Ultrasonography in Trauma: The FAST Exam; American College of Emergency Physicians: Dallas, TX, USA, 2003. [Google Scholar]
- Osterwalder, J.; Mathis, G.; Hoffmann, B. New Perspectives for Modern Trauma Management—Lessons Learned from 25 Years FAST and 15 Years E-FAST. Ultraschall Med. 2019, 40, 560–583. [Google Scholar] [CrossRef]
- Sutarjono, B.; Kessel, M.; Alexander, D.; Grewal, E. Is it time to re-think FAST? A systematic review and meta-analysis of Contrast-Enhanced Ultrasound (CEUS) and conventional ultrasound for initial assessment of abdominal trauma. BMC Emerg. Med. 2023, 23, 8. [Google Scholar] [CrossRef]
- Ackermann, O. Fraktursonografie; Springer Nature: Berlin, Germany, 2019. [Google Scholar]
- Chen, K.C.; Lin, A.C.; Chong, C.F.; Wang, T.L. An overview of point-of-care ultrasound for soft tissue and musculoskeletal applications in the emergency department. J. Intensive Care 2016, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Gaitini, D.; Razi, N.B.; Ghersin, E.; Ofer, A.; Soudack, M. Sonographic evaluation of vascular injuries. J. Ultrasound Med. 2008, 27, 95–107. [Google Scholar] [CrossRef]
- Ramsingh, D.; Mangunta, V.R. The Use of Point-of-Care Ultrasonography in Trauma Anesthesia. Anesthesiol. Clin. 2019, 37, 93–106. [Google Scholar] [CrossRef]
- Fernando, S.M.; Tran, A.; Cheng, W.; Rochwerg, B.; Taljaard, M.; Kyeremanteng, K.; English, S.W.; Sekhon, M.S.; Griesdale, D.E.G.; Dowlatshahi, D.; et al. Diagnosis of elevated intracranial pressure in critically ill adults: Systematic review and meta-analysis. BMJ 2019, 366, l4225. [Google Scholar] [CrossRef]
- Shin, S.S.; Huisman, T.A.G.M.; Hwang, M. Ultrasound Imaging for Traumatic Brain Injury. J. Ultrasound Med. 2018, 37, 1857–1867. [Google Scholar] [CrossRef]
- Polyzogopoulou, E.; Velliou, M.; Verras, C.; Ventoulis, I.; Parissis, J.; Osterwalder, J.; Hoffmann, B. Point-of-Care Ultrasound: A Multimodal Tool for the Management of Sepsis in the Emergency Department. Medicina 2023, 59, 1180. [Google Scholar] [CrossRef] [PubMed]
- Mathis, G. Vaskuläre Lungenkonsolidierungen: Lungenembolie und Lungeninfarkt. In Bildatlas der Lungensonographie Hrsg. Gebhard Mathis; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Chen, W.; Xu, K.; Li, Y.; Hao, M.; Yang, Y.; Liu, X.; Huang, X.; Huang, Y.; Ye, Q. Clinical value of thoracic ultrasonography in the diagnosis of pulmonary embolism: A systematic review and meta-analysis. Med. Ultrason. 2022, 24, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Nazerian, P.; Vanni, S.; Volpicelli, G.; Gigli, C.; Zanobetti, M.; Bartolucci, M.; Ciavattone, A.; Lamorte, A.; Veltri, A.; Fabbri, A.; et al. Accuracy of point-of-care multiorgan ultrasonography for the diagnosis of pulmonary embolism. Chest 2014, 145, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Kagima, J.; Stolbrink, M.; Masheti, S.; Mbaiyani, C.; Munubi, A.; Joekes, E.; Mortimer, K.; Rylance, J.; Morton, B. Diagnostic accuracy of combined thoracic and cardiac sonography for the diagnosis of pulmonary embolism: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0235940. [Google Scholar] [CrossRef] [PubMed]
- Koenig, S.; Chandra, S.; Alaverdian, A.; Dibello, C.; Mayo, P.H.; Narasimhan, M. Ultrasound assessment of pulmonary embolism in patients receiving CT pulmonary angiography. Chest 2014, 145, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Fily, R.A. Is it time for the sonoscope? if so, the let’s do it right. J. Ultrasound Med. 2003, 22, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Kobal, S.L.; Trento, L.; Baharami, S.; Tolstrup, K.; Naqvi, T.Z.; Cercek, B.; Neuman, Y.; Mirocha, J.; Kar, S.; Forrester, J.S.; et al. Comparison of effectiveness of hand carried ultrasound to bedside cardiovascular physical examination. Am. J. Cardiol. 2005, 96, 1002–1006. [Google Scholar] [CrossRef]
- Martindale, J.L.; Wakai, A.; Collins, S.P.; Levy, P.D.; Diercks, D.; Hiestand, B.C.; Fermann, G.F.; de Souza, I.; Sinert, R. Diagnosing acute heart failure in the emergency department: A systematic review and meta-analyis. Acad. Emerg. Med. 2016, 23, 223–242. [Google Scholar] [CrossRef]
- Alzahran, S.A.; Al-Salamah, M.A.; Al-Madani, W.H.; Elbarbay, M. Systematic review and meta-analysis for the use of ultrasound versus radiology in diagnosing of pneumonia. Crit. Ultrasound J. 2017, 9, 6. [Google Scholar] [CrossRef]
- Fuster, V. The stethoscope’s prognosis. Very much alive and very necessary. J. Am. Coll. Cardiol. 2016, 67, 1118–1119. [Google Scholar] [CrossRef]
- Solomon, S.D.; Saldana, F. Point-of-Care Ultrasound in medical education—Stop listening and look. N. Engl. J. Med. 2014, 370, 1083–1085. [Google Scholar] [CrossRef]
- Mouratev, G.; Howe, D.; Hoppmann, R.; Poston, M.B.; Reid, R.; Varnadoe, J.; Smith, S.; McCallum, B.; Rao, V.; DeMarco, P. Teaching medical students ultrasound to measure liver size: Comparison with experienced clinicians using physical examination alone. Teach. Learn. Med. 2013, 25, 84–88. [Google Scholar] [CrossRef]
- Tran, T.T.; Hlaing, M.; Krause, M. Point-of-Care Ultrasound: Applications in Low- and Middle-Income Countries. Curr. Anesthesiol. Rep. 2021, 11, 69–75. [Google Scholar] [CrossRef]
- Osterwalder, J.; Tabakovic, S.; Jenssen, C.; Dietrich, C.F.; Connolly, J.; Polyzogopoulou, E.; Cantisani, V.; Wüstner, M.; Jarman, B.; Hoffmann, B. Emergency Point-of-Care Ultrasound Stewardship—A Joint Position Paper by EuSEM and EFSUMB and Endorsed by IFEM and WFUMB. Ultraschall Med. 2023, 44, 379–388, Erratum in Ultraschall Med. 28 April 2023. [Google Scholar] [CrossRef]
- Burleson, S.L.; Pigott, D.C.; Gullett, J.P.; Greene, C.; Gibson, C.B.; Irvine, S.; Kaminstein, D. Point-of-care ultrasound in resource-limited settings: The PURLS fellowship. Ultrasound J. 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Persichini, R.; Lai, C.; Teboul, J.L.; Adda, I.; Guérin, L.; Monnet, X. Venous return and mean systemic filling pressure: Physiology and clinical applications. Crit. Care 2022, 26, 150. [Google Scholar] [CrossRef] [PubMed]
- Kenny, J.E. Assessing fluid intolerance with doppler ultrasonography: A physiological framework. Med. Sci. 2022, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, X.T.; Long, Y.; Liu, D.W. Monitoring changes in hepatic venous velocities flow after a fluid challenge can identify shock patients who lack fluid responsiveness. Chin. Med. J. 2017, 130, 1202–1210. [Google Scholar] [CrossRef]
- Mathis, G.; Horn, R.; Morf, S.; Prosch, H.; Rovida, S.; Soldati, G. WFUMB position paper on reverberation artefacts in lung ultrasound: B-lines or comet-tails? Med. Ultrason. 2021, 23, 70–73. [Google Scholar] [CrossRef]
- Quarato, C.M.I.; Venuti, M.; Dimitri, L.; Lacedonia, D.; Simeone, A.; Mirijello, A.; De Cosmo, S.; Maiello, E.; Taurchini, M.; Scioscia, G.; et al. Transthoracic ultrasound shear wave elastography for the study of subpleural lung lesions. Ultrasonography 2022, 41, 93–105. [Google Scholar] [CrossRef]
- Lu, C.; Merrill, C.; Medellin, A.; Novak, K.; Wilson, S.R. Bowel Ultrasound State of the Art: Grayscale and Doppler Ultrasound, Contrast Enhancement, and Elastography in Crohn Disease. J. Ultrasound Med. 2019, 38, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Michels, G.; Horn, R.; Helfen, A.; Hagendorff, A.; Jung, C.; Hoffmann, B.; Jaspers, N.; Kinkel, H.; Greim, C.A.; Knebel, F.; et al. Standardisierte Kontrastmittelsonographie (CEUS) in der klinischen Akut- und Notfallmedizin sowie Intensivmedizin (CEUS Akut): Konsensuspapier der DGIIN, DIVI, DGINA, DGAI, DGK, ÖGUM, SGUM und DEGUM [Standardized contrast-enhanced ultrasound (CEUS) in clinical acute and emergency medicine and critical care (CEUS Acute): Consensus statement of DGIIN, DIVI, DGINA, DGAI, DGK, ÖGUM, SGUM and DEGUM]. Med. Klin. Intensivmed. Notfmed. 2022, 117 (Suppl. S1), 1–23. Erratum in Med. Klin. Intensivmed. Notfmed. 2022, 117, 24. (In German) [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.U.; Eisenbrey, J.R.; Deganello, A.; Zahid, M.; Sharbidre, K.; Sidhu, P.; Robbin, M.L. Microvascular Flow Imaging: A State-of-the-Art Review of Clinical Use and Promise. Radiology 2022, 305, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, S.M.; Tsumura, R.; Hardin, J.W.; Hoffmann, B.; Zhang, Z.; Zhang, H.K. Anatomical Feature-Based Lung Ultrasound Image Quality Assessment Using Deep Convolutional Neural Network. IEEE Int. Ultrason. Symp. 2021, 2021, 9593662. [Google Scholar] [CrossRef]
- Libon, J.; Ng, C.; Bailey, A.; Hareendranathan, A.; Joseph, R.; Dulai, S. Remote diagnostic imaging using artificial intelligence for diagnosing hip dysplasia in infants: Results from a mixed-methods feasibility pilot study. Paediatr. Child. Health. 2023, 28, 285–290. [Google Scholar] [CrossRef]
- Jang, Y.E.; Cho, S.A.; Ji, S.H.; Kim, E.H.; Lee, J.H.; Kim, H.S.; Kim, J.T. Smart Glasses for Radial Arterial Catheterization in Pediatric Patients: A Randomized Clinical Trial. Anesthesiology 2021, 135, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Ultrasound Guidelines: Emergency, Point-of-Care and Clinical Ultrasound Guidelines in Medicine. Ann. Emerg. Med. 2017, 69, e27–e54. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).