Abstract
Background: Guillain–Barré syndrome (GBS)—a rare condition characterized by acute-onset immune-mediated polyneuropathy—has been registered as a neurological manifestation of COVID-19, suggesting a possible link between these two conditions. Methods: We report a case series of patients with COVID-19-related GBS hospitalized in the Neurology Department of Colentina Clinical Hospital, Bucharest, Romania, between March 2020 and March 2021. Several variables were analyzed, such as the mean interval between the onset of COVID-19 symptoms and neurological ones, clinical features, treatment course, and outcome. Further on, we conducted a thorough literature review based on the PubMed and ScienceDirect scientific databases. Results: A total of 9 COVID-19 patients developed symptoms of GBS, out of which in 7, it manifested as an acute inflammatory demyelinating polyneuropathy (AIDP). Five patients presented respiratory failure, 2 requiring mechanical ventilation. All patients received a course of intravenous immunoglobulins, 2 additionally requiring plasma exchange. Upon discharge, all but 1 patient (who had not regained the ability to walk) had a positive outcome, and 1 died during admission. In the literature review, we analyzed the published sources at the time of writing. Conclusions: A link between COVID-19 and GBS might be possible; therefore, increased vigilance is required in the early identification of these cases for prompt diagnosis and treatment. Some notable differences such as an earlier onset of GBS symptoms, higher respiratory dysfunction, and higher mortality rates in COVID-19 patients have been observed between the presentation of GBS in the context of COVID-19 and GBS of other causes.
1. Introduction
Since its emergence in December 2019 in Wuhan, China, Coronavirus disease 2019 (COVID-19) has been spreading worldwide at an alarming pace, with 244,385,444 confirmed cases and 4,961,489 deaths at the time of writing, according to the World Health Organization [1]. As the name suggests, SARSV-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) predominantly affects the respiratory system, ranging from mild bilateral pneumonia to acute respiratory distress syndrome [2] due to an excessive increase in pro-inflammatory cytokine levels [3], after which in up to 97.7% of cases, pulmonary parenchyma alterations such as linear bands, ground-glass opacities, reticulations, bronchiolectasis, consolidations, bronchiectasis and volume loss may persist at least three months after the disease is detected by chest CT [4] or chest X-ray using a computational model [5]. However, numerous extra-respiratory manifestations (cardiac, gastrointestinal, hepatic, renal, neurological, olfactory, gustatory, ocular, cutaneous, and hematologic) have been reported as well [2,6]. In the acute phase, the most frequently occurring central nervous system manifestations of SARS-CoV-2 are headache and anosmia, whereas stroke and seizures are less commonly reported [7]. Regarding the peripheral nervous system, neuropathic pain accounted for between 7.24–42.8% of cases and is more frequently encountered [8], and Guillain–Barré syndrome (GBS) is much rarer [7].
Known as the most common cause of acute flaccid paralysis [8], GBS is a rare, heterogeneous condition characterized by acute-onset immune-mediated polyneuropathies. The underlying pathophysiologic mechanism is generally related to an abnormal immune response to previous infections (most typically, of the respiratory system), leading to autoantibody production responsible for peripheral nerve damage. These infectious agents can be either viral (e.g., human immunodeficiency virus, Cytomegalovirus, Epstein–Barr virus, Varicella-Zoster Virus, Herpes Simplex Virus, Hepatitis A, B, C, E Viruses, Chikungunya virus, Zika virus) or bacterial (e.g., Campylobacter jejuni, Mycoplasma pneumoniae, Haemophilus influenzae, Escherichia coli) [9].
GBS includes several variant subtypes from a clinical and electrophysiological standpoint. The most frequently occurring subvariants are acute inflammatory demyelinating polyradiculoneuropathy (AIDP) (85–90% cases), acute motor and sensory axonal neuropathy (AMSAN), acute motor axonal neuropathy (AMAN), and Miller–Fisher syndrome [10,11,12]. Several rarely occurring variants include Bickerstaff brainstem encephalitis, paraparetic GBS, acute pandysautonomia, purely sensitive GBS, facial diplegia, acute bulbar paralysis, and pharyngeal–cervical–brachial weakness [10,11,12].
Although a rarely occurring disease (1 to 2 cases per 100,000 per year) [13,14], some reports point towards what appears to be an increasing incidence in 2020, which coincides with the peak of the novel coronavirus outbreak [15,16]. In January 2020, Zhao et al. reported the first known case of GBS occurring in a 61-year-old woman infected with SARS-CoV-2 [17]. Furthermore, a study performed by Fragiel et al. on an Italian cohort over two months reported that GBS was more common among COVID-19 patients than among non-COVID 19 ones [18]. Since then, several similar cases have emerged, suggesting a possible link between the two conditions. Therefore, our report aims to outline whether the increased frequency of cases is a simple coincidence or there may be a possible link between GBS in COVID-19 patients.
2. Materials and Methods
We report a case series of nine new cases of COVID-19-related GBS among patients diagnosed and treated in the Neurology Department of Colentina Clinical Hospital, Bucharest, Romania, between March 2020 and March 2021. The patients’ characteristics, such as demographic ones, clinical manifestations, results of paraclinical investigations, and outcomes, were retrospectively extracted from the archived charts of the patients as well as the electronic internal hospital system, anonymized and stored in an Excell table. All categorical variables were stated as numbers and percentages. Mean and median values and the distribution of patient characteristics were calculated with SPSS Version 25.0 (SPSS Inc., Chicago, IL, USA).
In addition, we conducted a thorough literature review in May 2021 based on the PubMed and ScienceDirect scientific databases, using the terms “COVID-19 and GBS”, “Guillain–Barré syndrome following COVID-19 infection”, and “GBS as a manifestation of COVID-19 infection” as search elements. All full-length articles written in English, including patients diagnosed with GBS and COVID-19 available at the moment of writing, were included, and an analysis of the clinical and paraclinical characteristics was made. As a result, 30 articles were included: 1 multi-center observational study, 6 case series, 1 scientific letter, 3 letters to the editor, and 19 case reports (Figure 1). We acknowledge that many other cases of GBS and COVID-19 patients could have been omitted due to their inclusion in more general studies on neurological manifestations in COVID-19 patients, which we did not encompass in our report.
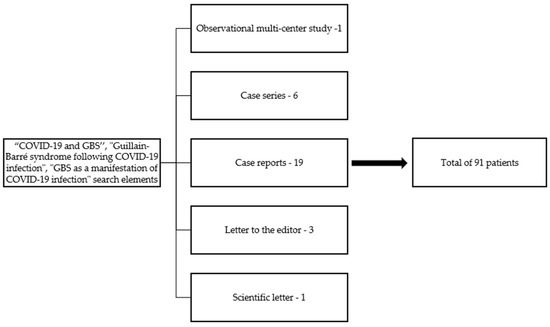
Figure 1.
Article selection.
3. Results
Nine patients (seven males and two females) with a median age of 56 years (varying from 39 to 67) were included in this case series, with an incidence of 1.42% (9 out of 631 hospitalized patients with the co-occurrence of COVID-19 and neurological manifestations). All patients had at least one positive nasopharyngeal swab RT-PCR test for SARS-CoV-2 during hospitalization. Unfortunately, SARS-CoV-2 sequencing to determine which SARS-CoV-2 variant each patient had was not possible due to the laboratory possibilities. However, we presume that most of our patients had the Alpha variant according to the reports of the National Institute of Public Health from Romania in that period [19]. The most common COVID-19 symptoms were fever, cough, and myalgia. The interval between the onset of COVID-19 symptoms and that of neurological ones ranged from 1 to 21 days (median interval = 9.5 days), suggesting a predominantly para-infectious mechanism of appearance of GBS. The period between the onset of neurological symptoms and hospitalization ranged from 24 h to 14 days. Typical GBS symptoms upon admission consisted in ascending lower limb weakness (7 cases), general areflexia (5 cases), and paresthesia (7 cases).
3.1. Presentation
3.1.1. Clinical Presentation
The characteristics of the clinical presentation of GBS are presented in Table 1. The median mMRC (Modified Medical Research Council) score was 2 for the lower limbs and 3.5 for the upper limbs, typical for the ascending pattern of GBS. Two patients developed paraplegia during hospitalization. Five patients presented cranial nerve involvement such as facial palsy, nystagmus, dysarthria, and mixed dysphagia. While hyporeflexia and areflexia were predominant, there was one case of hyperreflexia (without any history of upper motor neuron lesion). Regarding dysautonomia, one patient suffered from paralytic ileus, while two presented urinary and fecal incontinence. In terms of GBS variant distribution, there was a notable predominance of the demyelinating form (AIDP) among our patients (7 cases—77.7%), with only two cases of AMAN (22.3%). Signs of respiratory failure were present among five of our patients, with two of them requiring mechanical ventilation.

Table 1.
Patient clinical characteristics.
3.1.2. Paraclinical Investigations
Further explorations such as blood tests, cerebrospinal fluid (CSF) analysis, and antiganglioside antibody tests were performed to confirm the diagnosis of GBS Lumbar puncture in seven out of the nine cases. CSF fluid evaluation revealed cyto-albuminologic dissociation in all but one of the tested cases. Serum ganglioside antibodies were evaluated for a single patient and were negative. Blood tests were performed on admission, during hospitalization, and shortly before discharge. Their mean values are listed in Table 2. Inflammatory (e.g., C reactive protein (CRP), interleukin-6 (IL-6), ferritin, fibrinogen), and coagulation (international normalized ratio (INR), D-dimers) markers were elevated among most patients. Thoracic CT scans were performed to evaluate the severity of the COVID-19 infection. The median pulmonary parenchymal surface affected by reticulonodular infiltration (indicative of SARS-CoV-2-related pneumonia) was 20%. Due to exceptional conditions, electrophysiological investigations could not be performed, as our department was the first one declared and dedicated only to COVID-19 patients, and specific paraclinical investigations could not be accessed.

Table 2.
Mean blood test results.
3.1.3. Treatment
All patients received a course of intravenous immunoglobulins (0.4 g/kg per day for five days), two requiring plasma exchange (before the course of intravenous immunoglobulins). The SARS-CoV-2 infection was managed with antivirals such as remdesivir or favipiravir (five patients) and immunosuppressants such as tocilizumab (one patient) [20] and hydroxychloroquine (one patient) [21] according to local guidelines, as directed by the infectionist. Additional therapy with dexamethasone was administered to patients with clinically significant pulmonary involvement (four cases). All patients received low molecular weight heparin (LMWH), depending on patient particularities (eight received prophylactic doses, and one received therapeutic doses).
3.1.4. Outcome
Eight patients were discharged within 2 to 52 days (median interval = 12 days), with a single case of exitus due to acute respiratory failure by day two of hospitalization. All subjects showed motor and sensory function improvement in variable degrees. Upon discharge, only one patient had not regained the ability to walk. Cranial nerve involvement also improved, except for a single case of persisting horizontal nystagmus. By the time of discharge, all patients tested negative for SARS-CoV-2. The first positive and the first negative RT-PCR interval ranged from 1 to 22 days (median interval = 14 days). None of the patients developed signs of COVID-19-related long-term complications such as chronic respiratory dysfunction, neutropenia, or thrombocytopenia (Table 3).

Table 3.
Diagnosis, treatment, outcome.
3.2. Literature Review
Regarding the existing literature that reported the association between COVID-19 and GBS: 19 case reports, 6 case series, 3 scientific letters, 1 scientific letter, and 1 observational multi-center study—all comprising a total of 91 patients were included at the moment of the article writing. Out of the 91 reported patients, a male predominance (70.32%) was encountered as in GBS of other etiologies [11]. Their ages ranged from 17 to 84 years (median age = 57 years). Most reported studies used RT-PCR as the primary diagnostic tool for detecting SARS-CoV-2 infection (91.9%). One case, reported by Esteban Molina et al., had a negative RT-PCR test, which was later considered a false negative result, while Svačina et al. reported an equivocal RT-PCR result [22,23]. Three patients were diagnosed with COVID-19 using thoracic computer tomography and one via serologic tests.
The most notable COVID-19 symptoms were fever, cough, dyspnea, and myalgia. The interval between the onset of COVID-19 symptoms and that of neurological ones varied from 12 h to 36 days (median = 11 days). On the other hand, the most frequently occurring neurological symptoms were progressive tetraparesis (55 patients—60.4%), areflexia (61 patients—67.03%), paresthesia (39 patients—42.85%), hypoesthesia (32 patients—35.16%), and facial weakness (17 patients—18.68%). Respiratory failure was noted in 24 cases (26.37%), 18 requiring mechanical ventilation, the description being provided in Table 4.

Table 4.
Clinical features synopsis in literature.
Several investigations have been performed, including the cerebrospinal fluid examination (CSF), electromyographic studies, MRI, and serum antiganglioside antibodies assessment. Out of the 91 cases, 49 underwent CSF evaluation during hospitalization. Thirty-two patients presented cyto-albuminologic dissociation, a finding that may suggest a more rapidly installing pattern of CSF fluid abnormalities in the COVID-19 related GBS, as opposed to non-COVID-19 induced GBS, in which only up to 50% of cases or less have modified CSF results within the first two weeks of disease and 75% after the first three weeks [10]. Nerve conduction studies were performed on 81 patients. Their descriptions are listed in Table 5.

Table 5.
Diagnosis, treatment, and outcome synopsis in literature.
MRI was reported for 24 patients, which revealed leptomeningeal enhancement in one case, facial nerve enhancement in another, and caudal nerve roots enhancement in two subjects. Serum antiganglioside antibodies were evaluated in 15 subjects, out of which four were positive. GBS variants were reported in 77 out of the 91 cases, and their distribution follows—56 AIDP cases (72.52%), seven AMAN cases (7.7%), seven AMSAN cases (7.7%), one case of polyneuritis cranialis (1.1%), four cases of Miller–Fisher syndrome (4.4%), and two cases of axonal-demyelinating sensorimotor polyradiculoneuropathy (2.2%).
The vast majority of cases were treated with a course of intravenous immunoglobulins (74 patients—81.31%). Ten underwent plasma exchange (10.98%), one received both therapeutic options, one patient received only corticosteroids, and three received no treatment. In terms of outcome, there were five cases of exitus (5.49%), while the remaining 74 reported cases displayed variable degrees of improvement.
4. Discussion
According to our case series that included nine patients with GBS and COVID-19, the occurrence of GBS might be correlated to COVID-19. This could be potentially associated with the high incidence of respiratory failure, as in our patients (five out of nine and two requiring mechanical ventilation), which was higher than that reported in the literature (26.37%), suggesting a possible correlation between GBS secondary to COVID-19 and an increased risk of respiratory dysfunction versus GBS of other causes (25%) [13,15,28]. In addition, it would have been interesting to determine if the degree of respiratory failure differs depending on the SARS-CoV-2 variant since it is generally acknowledged that the Omicron variant induces less severe pulmonary involvement and consequently less respiratory failure, for which additional studies are needed [51]. In such a way, it would be easier to understand if the respiratory failure is related only to GBS or if the SARS-CoV-2 infection also has an additional contribution to it.
In addition, the fact that the interval between COVID-19 onset and GBS symptoms onset in our case series as well as in other reports was less than two weeks [27,32,35,37,39,40] might indicate that GBS following COVID-19 occurs slightly earlier than GBS following other infections, in which symptoms arise two to four weeks after infection [13,23]. These figures also suggest a predominantly para-infectious mechanism of appearance, considering the interval between the onset of COVID-19 symptoms and neurological ones (1 to 21 days)—slightly shorter than literature reports [18,20]. Potential mechanisms involved in this para-infectious pattern, also noted by several other authors [27,52], can be attributed either to a highly pro-inflammatory state (frequently occurring in COVID-19) leading to a dysimmune reaction, as seen in Zika Virus–GBS-related cases, or to direct viral nerve destruction [53,54]. Moreover, the significant response to intravenous immunoglobulins might again indicate an underlying immune-mediated mechanism of COVID-19-related GBS rather than direct peripheral nerve damage. On the other hand, the mortality rate may be higher among COVID-19-related GBS patients than in non-COVID-19 GBS cases, less than 3% [48,55].
Another curiosity observed in our case series was the presence of hyperreflexia in one patient, which is rarely encountered in GBS [13].
Regarding similitudes between GBS in COVID-19 patients and GBS of other causes, the demyelinating pattern, in other words, AIDP on electrophysiologic studies, was predominant in our case series as well as in other reports [47,50], similarly to non-COVID–19-related GBS [10], analogous to the male predominance [11].
Limitations
Due to circuit restrictions related to the SARS-CoV-2 pandemic, our clinic has drastically limited access to diagnostic tests for SARS-CoV-2 infected patients, such as electrophysiologic studies, causing difficulties in distinguishing GBS from COVID 19-related respiratory complications and other conditions such as critical illness neuropathy. Moreover, the prevalence of GBS related to COVID-19 is yet to be determined, and given the impaired access to medical services associated with the COVID-19 pandemic, mild cases of GBS are at risk of being overlooked. Thus, further studies should be performed to establish the prevalence and understand the underlying mechanisms of this co-occurrence. Additionally, physicians should maintain a high level of suspicion when dealing with GBS cases and screen them for SARS-CoV-2 infection, given that COVID-19-related respiratory symptoms may be mild or even absent in many instances.
The increased incidence of Guillain–Barré Syndrome cases and the causal association with COVID-19 is not enough for this relationship to be established with certainty, as long as other known and described associations were not excluded, as investigating for other diseases or co-infections like syphilis, hepatitis, tuberculosis, Lyme disease, influenza, systemic lupus erythematosus, etc., was not possible due to restrictions and special conditions, even though clinical manifestations were clear and the differential diagnosis did not need to be made exhaustively.
5. Conclusions
Considering recent worldwide case reports, there might be a link between GBS and COVID-19. Our report reiterated the numerous similarities between GBS related to COVID-10 and GBS of other causes, such as the predominance of demyelinating forms, male predominance, and the favorable response to intravenous immunoglobulins. The differences reside in an earlier onset of GBS symptoms, higher respiratory dysfunction, and higher mortality rates in COVID-19 patients compared to GBS due to other causes, making prompt diagnosis and treatment crucial in managing such cases.
Author Contributions
Conceptualization, A.P.I. and E.I.D.; methodology, A.P.I. and E.I.D.; software, A.P.I.; validation, A.P.I. and I.O.; formal analysis, A.P.I. and I.O.; investigation, A.P.I.; resources, E.I.D.; data curation, A.P.I. and I.O.; writing—original draft preparation, A.P.I.; writing—review and editing, I.O., E.I.D. and B.O.P.; visualization, A.P.I. and I.O.; supervision, E.I.D. and B.O.P.; project administration, E.I.D.; funding acquisition, B.O.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Carol Davila” University of Medicine and Pharmacy Bucharest, Romania through Contract no. 33PFE/30.12.2021 funded by the Ministry of Research and Innovation within PNCDI III, Program 1—Development of the National RD system, Subprogram 1.2—Institutional Performance—RDI excellence funding projects. The APC was funded by “Carol Davila” University of Medicine and Pharmacy Bucharest, Romania, Department of Research and Grants.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Ethics Committee of Colentina Clinical Hospital ((Nr.23/23.09.2021)).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the case series. According to our local ethical regulatory items, since our clinic is affiliated to the “Carol Davila” University of Medicine and Pharmacy from Bucharest, patients expressly consent for research activities when signing the informed consent upon admission—this is specified in the operational procedure regarding the access in scientific interest to the archived data—PO MED 01 Edition 1. Rev 0/09.09.2015 and the operational procedure regarding access to patient data, processing, and protection of data—PO MED 02 Edition 1. Rev 0/01.07.2019.
Data Availability Statement
The datasets generated during the analysis of this study are available from the corresponding author on reasonable request.
Acknowledgments
Thank you to the entire medical staff of the Neurology Department at Colentina Clinical Hospital for monitoring and taking care of COVID-19 patients.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization—WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 30 October 2021).
- Tsai, P.-H.; Lai, W.-Y.; Lin, Y.-Y.; Luo, Y.-H.; Chen, H.-K.; Chen, Y.-M.; Lai, Y.-C.; Kuo, L.-C.; Chen, S.-D.; Chang, K.-J.; et al. Clinical manifestation and disease progression in COVID-19 infection. J. Chin. Med. Assoc. 2020, 84, 3–8. [Google Scholar] [CrossRef]
- Salton, F.; Confalonieri, P.; Campisciano, G.; Cifaldi, R.; Rizzardi, C.; Generali, D.; Pozzan, R.; Tavano, S.; Bozzi, C.; Lapadula, G.; et al. Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS. J. Clin. Med. 2022, 11, 2951. [Google Scholar] [CrossRef]
- Baratella, E.; Ruaro, B.; Marrocchio, C.; Starvaggi, N.; Salton, F.; Giudici, F.; Quaia, E.; Confalonieri, M.; Cova, M.A. Interstitial Lung Disease at High Resolution CT after SARS-CoV-2-Related Acute Respiratory Distress Syndrome According to Pulmonary Segmental Anatomy. J. Clin. Med. 2021, 10, 3985. [Google Scholar] [CrossRef]
- Alqahtani, M.; Abbas, M.; Alqahtani, A.; Alshahrani, M.; Alkulib, A.; Alelyani, M.; Almarhaby, A.; Alsabaani, A. A Novel Computational Model for Detecting the Severity of Inflammation in Confirmed COVID-19 Patients Using Chest X-ray Images. Diagnostics 2021, 11, 855. [Google Scholar] [CrossRef]
- Ricchio, M.; Tassone, B.; Pelle, M.; Mazzitelli, M.; Serapide, F.; Fusco, P.; Lionello, R.; Cancelliere, A.; Procopio, G.; Lio, E.; et al. Characteristics, Management, and Outcomes of Elderly Patients with Diabetes in a Covid-19 Unit: Lessons Learned from a Pilot Study. Medicina 2021, 57, 341. [Google Scholar] [CrossRef]
- Whittaker, A.; Anson, M.; Harky, A. Neurological Manifestations of COVID-19: A systematic review and current update. Acta Neurol. Scand. 2020, 142, 14–22. [Google Scholar] [CrossRef]
- Shahrizaila, N.; Lehmann, H.C.; Kuwabara, S. Guillain-Barré syndrome. Lancet 2021, 397, 1214–1228. [Google Scholar] [CrossRef]
- Ho, T.; Hsieh, S.-T.; Nachamkin, I.; Willison, H.J.; Sheikh, K.; Kiehlbauch, J.; Flanigan, K.; McArthur, J.C.; Cornblath, D.R.; McKhann, G.M.; et al. Motor nerve terminal degeneration provides a potential mechanism for rapid recovery in acute motor axonal neuropathy after campylobacter infection. Neurology 1997, 48, 717–724. [Google Scholar] [CrossRef]
- Doets, A.Y.; Verboon, C.; Berg, B.V.D.; Harbo, T.; Cornblath, D.R.; Willison, H.J.; Islam, Z.; Attarian, S.; Barroso, F.A.; Bateman, K.; et al. Regional variation of Guillain-Barré syndrome. Brain 2018, 141, 2866–2877. [Google Scholar] [CrossRef] [Green Version]
- Leonhard, S.E.; Mandarakas, M.R.; Gondim, F.A.A.; Bateman, K.; Ferreira, M.L.B.; Cornblath, D.R.; Van Doorn, P.A.; Dourado, M.E.; Hughes, R.A.C.; Islam, B.; et al. Diagnosis and management of Guillain–Barré syndrome in ten steps. Nat. Rev. Neurol. 2019, 15, 671–683. [Google Scholar] [CrossRef]
- Ray, S.; Jain, P.C. Acute bulbar palsy plus syndrome: A rare variant of Guillain–Barre syndrome. J. Pediatr. Neurosci. 2016, 11, 322–323. [Google Scholar] [CrossRef] [Green Version]
- Yuki, N.; Hartung, H.-P. Guillain–Barré Syndrome. N. Engl. J. Med. 2012, 366, 2294–2304. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Baughman, A.L.; Wise, M.; Morgan, O.W. Population Incidence of Guillain-Barré Syndrome: A Systematic Review and Meta-Analysis. Neuroepidemiology 2011, 36, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Filosto, M.; Piccinelli, S.C.; Gazzina, S.; Foresti, C.; Frigeni, B.; Servalli, M.C.; Sessa, M.; Cosentino, G.; Marchioni, E.; Ravaglia, S.; et al. Guillain-Barré syndrome and COVID-19: An observational multicentre study from two Italian hotspot regions. J. Neurol. Neurosurg. Psychiatry 2020, 92, 751–756. [Google Scholar] [CrossRef]
- Alshekhlee, A.; Hussain, Z.; Sultan, B.; Katirji, B. Guillain-Barre syndrome: Incidence and mortality rates in US hospitals. Neurology 2008, 70, 1608–1613. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, D.; Zhou, H.; Liu, J.; Chen, S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol. 2020, 19, 383–384. [Google Scholar] [CrossRef]
- Fragiel, M.; Miró, Ò.; Llorens, P.; Jiménez, S.; Piñera, P.; Burillo, G.; Martín, A.; Martín-Sánchez, F.J.; Lamberechts, E.J.G.; Jacob, J.; et al. Incidence, clinical, risk factors and outcomes of Guillain-Barré in COVID-19. Ann. Neurol. 2020, 89, 598–603. [Google Scholar] [CrossRef]
- National Institute of Public Health, Romania. Available online: https://insp.gov.ro/centrul-national-de-supraveghere-si-control-al-bolilor-transmisibile-cnscbt/infectia-cu-noul-coronavirus-sars-cov-2/analiza-cazuri-confirmate-covid19/ (accessed on 17 July 2022).
- Davidescu, E.I.; Odajiu, I.; Ilie, M.D.; Bunea, T.; Sandu, G.; Stratan, L.; Iftode, N.; Aramă, V.; Popescu, B.O. Influence of tocilizumab on the outcome of patients with COVID-19. retrospective observational study. Farmacia 2020, 68, 792–799. [Google Scholar] [CrossRef]
- Davidescu, E.I.; Odajiu, I.; Ilie, M.D.; Bunea, T.; Sandu, G.; Stratan, L.; Aramă, V.; Popescu, B.O. Treatment with hydroxychloroquine in patients with covid-19. Experience of a neurology department. Farmacia 2020, 68, 597–605. [Google Scholar] [CrossRef]
- Molina, E.A.; Martínez, M.M.; Chueca, S.P.; López, C.A.; Sancho Val, I.; Sanjuan-Villarreal, T.A. Guillain-Barré syndrome associated with SARS-CoV-2 infection. Med. Intensiva 2020, 44, 513–514. [Google Scholar] [CrossRef]
- Svačina, M.K.R.; Kohle, F.; Sprenger, A.; Lehmann, H.C. Could symptom overlap of COVID-19 and Guillain–Barré syndrome mask an epidemiological association? J. Neurol. 2021, 268, 3595–3597. [Google Scholar] [CrossRef]
- Bueso, T.; Montalvan, V.; Lee, J.; Gomez, J.; Ball, S.; Shoustari, A.; Julayanont, P.; Jumper, C. Guillain-Barre Syndrome and COVID-19: A case report. Clin. Neurol. Neurosurg. 2020, 200, 106413. [Google Scholar] [CrossRef]
- Kamel, W.A.; Ismail, I.I.; Al-Hashel, J.Y. Guillain-Barre Syndrome following COVID-19 Infection: First Case Report from Kuwait and Review of the Literature. Dubai Med. J. 2021, 4, 42–46. [Google Scholar] [CrossRef]
- Singh, R.; Shiza, S.T.; Saadat, R.; Dawe, M.; Rehman, U. Association of Guillain-Barre Syndrome with COVID-19: A Case Report and Literature Review. Cureus 2021, 13, e13828. [Google Scholar] [CrossRef]
- Sidig, A.; Abbasher, K.; Abbasher, H.; Abbasher, M.H.A. COVID-19 and Guillain-Barre Syndrome Case Report. J. Neurol. Neurobiol. 2020, 7, e21246. [Google Scholar]
- Webb, S.; Wallace, V.C.; Martin-Lopez, D.; Yogarajah, M. Guillain-Barré syndrome following COVID-19: A newly emerging post-infectious complication. BMJ Case Rep. 2020, 13, e236182. [Google Scholar] [CrossRef]
- Korem, S.; Gandhi, H.; Dayag, D.B. Guillain-Barré syndrome associated with COVID-19 disease. BMJ Case Rep. 2020, 13, e237215. [Google Scholar] [CrossRef]
- Almutairi, A.; Bin Abdulqader, S.; Alhameed, M.; Alit, S.; Alosaimi, B. Guillain-BarrE Syndrome Following COVID-19: A Case Report. J. Res. Med. Dent. Sci. 2021, 9, 7–10. [Google Scholar]
- Mantefardo, B.; Gube, A.A.; Awlachew, E.; Sisay, G. Novel Coronavirus (COVID-19)-Associated Guillain–Barre’ Syndrome: Case Report. Int. Med. Case Rep. J. 2021, 14, 251–253. [Google Scholar] [CrossRef]
- Camdessanche, J.-P.; Morel, J.; Pozzetto, B.; Paul, S.; Tholance, Y.; Botelho-Nevers, E. COVID-19 may induce Guillain–Barré syndrome. Rev. Neurol. 2020, 176, 516–518. [Google Scholar] [CrossRef]
- Sedaghat, Z.; Karimi, N. Guillain Barre syndrome associated with COVID-19 infection: A case report. J. Clin. Neurosci. 2020, 76, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Virani, A.; Rabold, E.; Hanson, T.; Haag, A.; Elrufay, R.; Cheema, T.; Balaan, M.; Bhanot, N. Guillain-Barré Syndrome associated with SARS-CoV-2 infection. IDCases 2020, 20, e00771. [Google Scholar] [CrossRef] [PubMed]
- Caamaño, D.S.J.; Beato, R.A. Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARS-CoV-2. J. Clin. Neurosci. 2020, 77, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Coen, M.; Jeanson, G.; Almeida, L.A.C.; Hübers, A.; Stierlin, F.; Najjar, I.; Ongaro, M.; Moulin, K.; Makrygianni, M.; Leemann, B.; et al. Guillain-Barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav. Immun. 2020, 87, 111–112. [Google Scholar] [CrossRef]
- Alberti, P.; Beretta, S.; Piatti, M.; Karantzoulis, A.; Piatti, M.L.; Santoro, P.; Viganò, M.; Giovannelli, G.; Pirro, F.; Montisano, D.A.; et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol. Neuroimmunol. Neuroinflamma. 2020, 7, e741. [Google Scholar] [CrossRef]
- Yakoby, J.; Litvak, I.; Yu, E. Guillain-Barré Syndrome after Novel Coronavirus Disease 2019. J. Emerg. Med. 2021, 61, e67–e70. [Google Scholar] [CrossRef]
- Diez-Porras, L.; Vergés, E.; Gil-López, F.J.; Vidal, M.J.; Massons, J.; Arboix, A. Guillain-Barré-Strohl syndrome and COVID-19: Case report and literature review. Neuromuscul. Disord. 2020, 30, 859–861. [Google Scholar] [CrossRef]
- Rajdev, K.; Victor, N.; Buckholtz, E.S.; Hariharan, P.; Saeed, M.A.; Hershberger, D.M.; Bista, S. A Case of Guillain-Barré Syndrome Associated With COVID-19. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620961198. [Google Scholar] [CrossRef]
- Rana, S.; Lima, A.A.; Chandra, R.; Valeriano, J.; Desai, T.; Freiberg, W.; Small, G. Novel Coronavirus (COVID-19)-Associated Guillain–Barré Syndrome: Case Report. J. Clin. Neuromuscul. Dis. 2020, 21, 240–242. [Google Scholar] [CrossRef]
- Mackenzie, N.; Lopez-Coronel, E.; Dau, A.; Maloof, D.; Mattar, S.; Garcia, J.T.; Fontecha, B.; Lanata, C.M.; Guillen-Burgos, H.F. Concomitant Guillain-Barre syndrome with COVID-19: A case report. BMC Neurol. 2021, 21, 135. [Google Scholar] [CrossRef]
- Yiu, A.C.; Hussain, A.; Okonkwo, U.A.; Villacorta-Lyew, R.; McMahon, M.J.; Blattner, M. Guillain–Barre Syndrome Associated with COVID-19 Pneumonia—The First Documented Case in a U.S. Military Intensive Care Unit. Mil. Med. 2021, usab158. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Han, S.C.; Kelly, S.; Tamimi, M.; Giglio, B.; Lewis, A. A Case Series of Guillain-Barré Syndrome After COVID-19 Infection in New York. Neurol. Clin. Pract. 2020, 11, e576–e578. [Google Scholar] [CrossRef] [PubMed]
- Okhovat, A.A.; Ansari, B.; Hemasian, H.; Haghi-Ashtiani, B.; Advani, S.; Ziaadini, B.; Abdi, S.; Sikaroudi, H.; Nafissi, S.; Fatehi, F. Guillain-Barre syndrome in patients with coronavirus disease-2019: Report of six cases and review of literature. Curr. J. Neurol. 2021, 19, 122–130. [Google Scholar] [CrossRef]
- Gutiérrez-Ortiz, C.; Méndez-Guerrero, A.; Rodrigo-Rey, S.; Pedro-Murillo, E.S.; Bermejo-Guerrero, L.; Gordo-Mañas, R.; de Aragón-Gómez, F.; Benito-León, J. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 2020, 95, e601–e605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foresti, C.; Servalli, M.C.; Frigeni, B.; Rifino, N.; Storti, B.; Gritti, P.; Fabretti, F.; Grazioli, L.; Sessa, M. COVID-19 provoking Guillain–Barré syndrome: The Bergamo case series. Eur. J. Neurol. 2020, 28, e84–e85. [Google Scholar] [CrossRef]
- Toscano, G.; Palmerini, F.; Ravaglia, S.; Ruiz, L.; Invernizzi, P.; Cuzzoni, M.G.; Franciotta, D.; Baldanti, F.; Daturi, R.; Postorino, P.; et al. Guillain–Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2574–2576. [Google Scholar] [CrossRef]
- Nanda, S.; Handa, R.; Prasad, A.; Anand, R.; Zutshi, D.; Dass, S.K.; Bedi, P.K.; Pahuja, A.; Shah, P.K.; Sharma, B. COVID-19 associated Guillain-Barre Syndrome: Contrasting tale of four patients from a tertiary care centre in India. Am. J. Emerg. Med. 2020, 39, 125–128. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Abdelhak, A.; Foschi, M.; Tumani, H.; Otto, M. Guillain–Barré syndrome spectrum associated with COVID-19: An up-to-date systematic review of 73 cases. J. Neurol. 2020, 268, 1133–1170. [Google Scholar] [CrossRef]
- Gu, B.; Yao, L.; Zhu, X.-Y.; Zou, T.; Feng, Y.-J.; Yan, J.-Y.; Zhang, J.-P.; Tang, P.-J.; Chen, C. Comparison of initial clinic characteristics of hospitalized patients in Suzhou City during the COVID-19 Omicron wave with ancestral variant wave. Ther. Adv. Respir. Dis. 2022, 16, 17534666221110346. [Google Scholar] [CrossRef]
- Abolmaali, M.; Heidari, M.; Zeinali, M.; Moghaddam, P.; Ghamsari, M.R.; Makiani, M.J.; Mirzaasgari, Z. Guillain–Barré syndrome as a parainfectious manifestation of SARS-CoV-2 infection: A case series. J. Clin. Neurosci. 2020, 83, 119–122. [Google Scholar] [CrossRef]
- Ottaviani, D.; Boso, F.; Tranquillini, E.; Gapeni, I.; Pedrotti, G.; Cozzio, S.; Guarrera, G.M.; Giometto, B. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): A case report from an Italian COVID-hospital. Neurol. Sci. 2020, 41, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Parra, B.; Lizarazo, J.; Jiménez-Arango, J.A.; Zea-Vera, A.F.; González-Manrique, G.; Vargas, J.; Angarita, J.A.; Zuñiga, G.; Lopez-Gonzalez, R.; Beltran, C.L.; et al. Guillain–Barré Syndrome Associated with Zika Virus Infection in Colombia. N. Engl. J. Med. 2016, 375, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.V.D.; Bunschoten, C.; van Doorn, P.A.; Jacobs, B.C. Mortality in Guillain-Barre syndrome. Neurology 2013, 80, 1650–1654. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).