Abstract
Management strategies for pregnancies with abnormal adherence/invasion of the placenta (placenta accreta spectrum, PAS) vary between centers. Expectant management (EM), defined as leaving the placenta in situ after the delivery of the baby, until its complete decomposition and elimination, has become a potential option for PAS disorders in selected cases, in which the risk of Caesarean hysterectomy is very high. However, expectant management has its own risks and complications. The aim of this study was to describe the rates of subsequent hysterectomy (HT) in patients that underwent EM for the treatment of PAS disorders. We reviewed the literature on the subject and found 12 studies reporting cases of HT after initial intended EM. The studies included 1918 pregnant women diagnosed with PAS, of whom 518 (27.1%) underwent EM. Out of these, 121 (33.2%) required subsequent HT in the 12 months following delivery. The rates of HT after initial EM were very different between the studies, ranging from 0 to 85.7%, reflecting the different characteristics of the patients and different institutional management protocols. Prospective multicenter studies, in which the inclusion criteria and management strategies would be uniform, are needed to better understand the role EM might play in the treatment of PAS disorders.
1. Introduction
In 1937, Irving and Hertig were the first to publish a cohort study including 20 cases of placenta accreta and a review on the previous 86 cases reported in the literature at that time [1]. All the cases were described as abnormal adherence of the placenta to the uterine wall, completely or partially, with the absence of decidua basalis in the area of adhesion. Among the possible etiologic factors mentioned were the manual removal of the placenta or uterine curettage, as only one case out of the 20 included in the cohort had a previous Caesarean section. In 1966, Luke et al. described placenta accreta as a spectrum of abnormal placentation with various degrees of invasion, from placenta vera or creta to placenta increta or percreta [2]. In 2018, the International Federation of Obstetrics and Gynecology (FIGO) proposed new terminology that included all three grades of abnormal placentation under the acronym of PAS (placenta accreta spectrum) [3], subsequently proposing a new clinical classification of PAS in three grades [4].
The incidence of PAS disorders in the general population of pregnant women is estimated at 1.7/10,000 pregnancies [5,6] and has been growing steadily over time, probably in relation to the increasing rates of Caesarean delivery [7,8,9].
Ultrasound is an excellent tool for the prenatal diagnosis of PAS [10] and, although some guidelines do not recommend a generalized screening program [11], the previous obstetrical history, with a clear recognition of the risk factors, and the systematic ultrasound evaluation of high-risk patients play an important role in antenatal diagnosis and allow planned intervention with a multidisciplinary approach.
There are three important stages in the care of pregnant women at increased risk of PAS: the recognition of the risk factors, establishing an accurate prenatal diagnosis, and referral to a tertiary center with expertise for follow-up and delivery [12]. The diagnosis of PAS is confirmed clinically, during CS, when, after the delivery of the baby, the placenta does not detach. In this situation, when an elective Caesarean hysterectomy (CSHT) can be safely performed, the obstetrician starts the procedure. This is considered the gold standard of treatment by many. There are some cases, however, in which the risk of bleeding and/or vesical-ureteral injuries are high, and the obstetrician can decide to leave the placenta in situ, ligate the umbilical cord close to placental insertion, and close the uterus and abdominal wall for a delayed hysterectomy (DHT), which is planned for 4–6 weeks later, or for expectant management (EM). Delayed hysterectomy, a hybrid strategy, is aimed at minimizing blood loss and avoiding visceral lesions, especially of the bladder and ureters [13]. Expectant management represents a real conservative option and is achieved by leaving the placenta in situ until its complete decomposition and elimination, with the intention of preventing heavy bleeding and reducing the risk of severe maternal complications related to post-Caesarean hemostasis hysterectomy [14]. Expectant management is preferred when intraoperative findings suggest an unacceptably high risk of bleeding or urinary tract injuries, or when the patient desires future fertility [14,15,16]. Another conservative management strategy consists in placental-myometrial en bloc resection and repair, described by Palacios et al. [17], or triple P procedure, described by Chandraharan et al., as an alternative to peri-partum hysterectomy or conservative management by retaining the placenta [18]. Interventional radiology techniques, such as pelvic artery embolization, can sometimes be used as adjuvants to any of these strategies in order to limit blood loss [19].
The aim of our study was to review the recent literature published on the method of EM in treating PAS and to describe the risk of subsequent hysterectomy when this approach is used. We also aimed to underline the possible advantages and disadvantages of the different modalities employed in the treatment of PAS.
2. Materials and Methods
We undertook an analysis of the reviews and single/multicenter studies that report maternal outcomes, and specifically, the rate of subsequent HT, when EM is used for the treatment of PAS disorders. PubMed was searched for studies published from the beginning of records in relation to treatment of PAS disorders. We then selected those dealing with expectant management and that were published in the last 15 years. We searched for the terms “expectant”, “expectative”, or “conservative” and “placenta accreta”, “percreta”, and “increta”. The search retrieved 468 entries. We selected the studies and literature reviews that included more than three reported cases.
3. Results
We found 12 studies that met our search criteria, published between 2010 and 2022, that included cases of PAS managed expectantly. Of the twelve studies (Table 1), nine had a retrospective design, while three had a prospective approach to enrolling patients. The 12 studies included 1918 cases of pregnant women diagnosed with PAS disorders, of whom 518 (27.1%) were expectantly managed, which was defined as “leaving the placenta in situ”. Of those initially managed by EM, 121 (33.2%) required subsequent hysterectomy up to 12 months following delivery. Table 1 summarizes the main findings of the studies.

Table 1.
Studies in which expectant management was intended.
We found large variations in the reported rates of subsequent HT between the studies in the centers where EM was used for the treatment of PAS. Most studies were retrospective, inherently presenting specific biases. Patients with different severities of PAS were included: in some of the studies, there were differences in the severity of PAS based on the prenatal diagnosis (percreta, accreta/increta), while many of the studies did not report a PAS grade. In some of the studies (Table 1), there was an intention to remove all the placenta, or parts of it, before EM was offered. We also noticed from the analyzed studies that during the process of EM, other strategies, such as interventional radiology techniques were added to improve success.
4. Discussion
There is a lack of consensus regarding the optimal strategy for the management of PAS disorders [16], with a wide variation around the globe [32,33]. When PAS is suspected before delivery, the patient/couple should be counseled and involved in the decisions over treatment. A question that needs to be answered when offering the option of EM when treating PAS is related to the risks of complications and need for a subsequent HT. Furthermore, women managed expectantly should be informed that they require long-term follow-up, with multiple hospital visits for blood tests and ultrasounds (Figure 1) and immediate access to health care facilities [34].
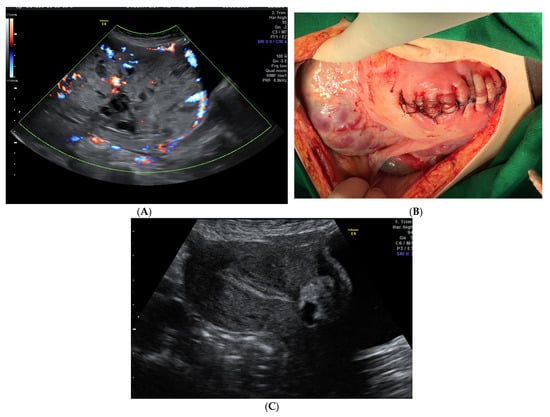
Figure 1.
(A). Ultrasound appearance of the placenta at 35 weeks of pregnancy. (B). Placenta left in situ and corporeal-fundal uterine suture at delivery. (C). Transabdominal ultrasound examination 27 weeks after delivery. On the anterior uterine wall, the small placenta is noted, and the endometrium is linear. The patient had a history of CS and, at 20 weeks, she was diagnosed with placenta previa with a high suspicion of anterior abnormal invasion to the urinary bladder. She had no vaginal bleeding. Planned Caesarean section was performed at 36 weeks of gestation, with a vertical mid-line incision chosen for the abdomen and a fundal incision of the uterus to avoid the upper pole of the placenta. After the delivery of the baby, the decision to leave the placenta in situ was taken to avoid significant bleeding and bladder injury. After the ligature of the umbilical cord close to its placental insertion, the uterine wall was sutured (B). Close monitoring was offered and, at 27 weeks after delivery, the placenta was almost fully evacuated (C) and the patient had normal menstruation.
The treatment options for PAS and their advantages and disadvantages are presented in Table 2.

Table 2.
Analysis of different management decisions in PAS disorders.
Our study aimed to review the outcomes of EM for PAS, especially in relation to the need for subsequent hysterectomy. Overall, in the studies included in our paper, 33.2% of women with PAS disorders who underwent EM required HT. The rate of success of EM observed in our study (67.8%) is similar to the figures published recently in a review by Sentilhes et al. [33], who found that the uterine preservation rate with EM was 78%, and that the rate of severe maternal morbidity was about 6%. A summary of other reviews published in the literature on the subject is presented in Table 3.

Table 3.
Review studies on expectant management in PAS.
The overall need for HT in those who underwent initial expectant management was 37.5% in the reviews included. Again, the rates of reported HT were different between the studies. The reviews reported the complications that can arise during EM as hemorrhage, infection, fistulas and sepsis, coagulopathy, and even maternal death. There is a lack of consensus on how expectant management for treating PAS is defined in the literature, who should benefit from this approach, and how follow-ups should be arranged. The primary and secondary outcomes reported by different studies are very heterogenous (obtaining statistical power to test a hypothesis requires a large number of participants, or PAS disorders are relatively rare). Our paper reflects these large differences between studies. Another issue regarding the expectant management of PAS disorders is the lack of histological confirmation of the diagnosis. When a hysterectomy is performed for PAS, specimens can be examined fresh by a senior pathologist, together with the lead obstetrician, to establish the existence of an abnormal adhesion, complete or focal. Biopsies are taken from the most suspicious areas. Correct diagnosis and reporting allow a better correlation with the US aspects and a possible reassessment of future management [39]. Currently, the diagnosis of placenta with abnormal adhesion is dominated by clinics, which leads to overdiagnosis [40]. Histopathology confirmation should become the gold standard for diagnosing PAS [39]. A correct histopathological diagnosis and the adoption of FIGO classification criteria also allow a reassessment of the epidemiological data [40]. Unfortunately, the histological diagnosis cannot be obtained in case of the expectant management of PAS [41].
Our study has certain limitations that we acknowledge. We did not have the required data to undertake a systematic review related to the subject; thus, there may have been subjectivity bias. We included studies published in the English and French languages, potentially missing reports from different settings in low- or middle-income countries. Nevertheless, our study could be of benefit to doctors counseling women with PAS regarding options for management and their associated risk. It is surprising that the results of the EM were so scattered and so different between the centers. Future prospective multicenter studies are required to better understand the role of expectant management in the treatment of PAS. These studies should be controlled and include centers with similar management protocols. The most convincing results would be achieved through a randomized controlled trial, in which EM could be compared with other strategies, if feasible. Regarding counseling, a more pragmatic and approach, focused on providing patients with information, would be for centers treating PAS disorders to audit their own data and to offer counseling based on these data.
5. Conclusions
Although Caesarean hysterectomy is recommended by most authorities as the gold standard, the expectant management of PAS disorders is increasingly used with the goals of avoiding severe maternal morbidity, or even mortality, associated with surgery, as well as preserving fertility. There is a growing body of evidence showing that expectant management, in selected and carefully monitored cases, could be successful. In our study, approximately 1 in 3 women required a HT after expectant management. When complications occur during follow-up for EM, they can potentially be treated with fewer risks, by multidisciplinary teams, in dedicated centers. There are limited data on expectant management in placenta percreta. The application of expectant management in PAS disorders has the disadvantage of the lack of histopathological diagnosis.
We acknowledge that PAS disorders are iatrogenic pathologies and, despite encouraging success, expectant management is still a debated subject. A safer reduction in the rate of primary Caesarean delivery would reduce the incidence of and risks associated with PAS disorders. Pregnant women, especially in countries with a high rate of CSs, doctors, and society at large, should be aware of the potential complications associated with CS.
Author Contributions
Conceptualization, A.M.P. and G.P.; methodology, N.G., R.B. and G.I.; software, A.M.P.; validation, A.M.P. and G.P.; formal analysis, A.M.P.; investigation, G.P.; resources, A.M.P., R.B. and G.I.; data curation, G.P.; writing—original draft preparation, A.M.P.; writing—review and editing, N.G. visualization, A.M.P.; supervision, G.P.; administration, N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Irving, C.; Hertig, A.T. A study of placenta accreta. Surg. Gynecol. Obstet. 1937, 64, 178–200. [Google Scholar]
- Luke, R.K.; Sharpe, J.W.; Greene, R. Placenta accreta: The adherent or invasive placenta. Am. J. Obstet. Gynecol. 1966, 95, 660–668. [Google Scholar] [CrossRef]
- Jauniaux, E.; Ayres-De-Campos, D.; The FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Introduction. Int. J. Gynecol. Obstet. 2018, 140, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Ayres-De-Campos, D.; Langhoff-Roos, J.; Fox, K.A.; Collins, S.; Duncombe, G.; Klaritsch, P.; Chantraine, F.; Kingdom, J.; Grønbeck, L.; et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int. J. Gynecol. Obstet. 2019, 146, 20–24. [Google Scholar] [CrossRef]
- Silver, R.M. Abnormal placentation: Placenta previa, vasa previa, and placenta accreta. Obstet. Gynecol. 2015, 126, 654–658. [Google Scholar] [CrossRef]
- Fitzpatrick, K.E.; Sellers, S.; Spark, P.; Kurinczuk, J.J.; Brocklehurst, P.; Knight, M. Incidence and Risk Factors for Placenta Accreta/Increta/Percreta in the UK: A National Case-Control Study. PLoS ONE 2012, 7, e52893. [Google Scholar] [CrossRef]
- McKeogh, R.P.; D’Enrico, E. Placenta accrete. N. Engl. J. Med. 1951, 245, 159–165. [Google Scholar] [CrossRef]
- Belfort, M.A.; Publications Committee for Society for MFM. Placenta accreta. Am. J. Obstet. Gynecol. 2010, 203, 430–439. [Google Scholar] [CrossRef]
- Jauniaux, E.; Bhide, A. Prenatal ultrasound diagnosis and outcome of placenta previa accreta after cesarean delivery: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 217, 27–36. [Google Scholar] [CrossRef]
- D’Antonio, F.; Iacovella, C.; Bhide, A. Prenatal identification of invasive placentation using ultrasound: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2013, 42, 509–517. [Google Scholar] [CrossRef]
- RCOG. Placenta Praevia, Placenta Praevia Accreta and Vasa Praevia: Diagnosis and Management; Green top guideline; RCOG: London, UK, 2011. [Google Scholar]
- Jauniaux, E.; Bhide, A.; Kennedy, A.; Woodward, P.; Hubinont, C.; Collins, S.; The FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. Int. J. Gynecol. Obstet. 2018, 140, 274–280. [Google Scholar] [CrossRef]
- Zuckerwise, L.C.; Craig, A.M.; Newton, J.; Zhao, S.; Bennett, K.A.; Crispens, M.A. Outcomes following a clinical algorithm allowing for delayed hysterectomy in the management of severe placenta accreta spectrum. Am. J. Obstet. Gynecol. 2020, 222, 179.e1–179.e9. [Google Scholar] [CrossRef]
- Fox, K.A.; Shamshirsaz, A.A.; Carusi, D.; Secord, A.A.; Lee, P.; Turan, O.M.; Huls, C.; Abuhamad, A.; Simhan, H.; Barton, J.; et al. Conservative management of morbidly adherent placenta: Expert review. Am. J. Obstet. Gynecol. 2015, 213, 755–760. [Google Scholar] [CrossRef]
- Sentilhes, L.; Kayem, G.; Chandraharan, E.; Palacios-Jaraquemada, J.; Jauniaux, E.; The FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Conservative management. Int. J. Gynecol. Obstet. 2018, 140, 291–298. [Google Scholar] [CrossRef]
- Esakoff, T.F.; Handler, S.J.; Granados, J.M.; Caughey, A.B. PAMUS: Placenta accreta management across the United States. J. Matern. Neonatal Med. 2012, 25, 761–765. [Google Scholar] [CrossRef]
- Palacios Jaraquemada, J.M.; Pesaresi, M.; Nassif, J.C.; Hermosid, S. Anterior placenta percreta: Surgical approach, hemostasis and uterine repair. Acta Obstet. Gynecol. Scand. 2004, 83, 738–744. [Google Scholar] [CrossRef]
- Chandraharan, E.; Rao, S.; Belli, A.-M.; Arulkumaran, S. The Triple-P procedure as a conservative surgical alternative to peripartum hysterectomy for placenta percreta. Int. J. Gynecol. Obstet. 2012, 117, 191–194. [Google Scholar] [CrossRef]
- Melber, D.J.; Berman, Z.T.; Jacobs, M.B.; Picel, A.C.; Conturie, C.L.; Zhang-Rutledge, K.; Binder, P.S.; Eskander, R.N.; Roberts, A.C.; McHale, M.T.; et al. Placenta Accreta Spectrum Treatment with Intraoperative Multivessel Embolization: The PASTIME protocol. Am. J. Obstet. Gynecol. 2021, 225, 442.e1–442.e10. [Google Scholar] [CrossRef]
- Marcellin, L.; Delorme, P.; Bonnet, M.P.; Grange, G.; Kayem, G.; Tsatsaris, V.; Goffinet, F. Placenta percreta is associated with more frequent severe maternal morbidity than placenta accreta. Am. J. Obstet. Gynecol. 2018, 219, 193.e1–193.e9. [Google Scholar] [CrossRef]
- de Marcillac, F.D.; Lecointre, L.; Guillaume, A.; Sananes, N.; Fritz, G.; Viville, B.; Boudier, E.; Nisand, I.; Gaudineau, A.; Langer, B.; et al. Maternal morbidity and mortality associated with conservative management for placenta morbidly adherent (accreta) diagnosed during pregnancy. Report of 15 cases and review of literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 45, 849–858. [Google Scholar] [CrossRef][Green Version]
- Sentilhes, L.; Ambroselli, C.; Kayem, G.; Provansal, M.; Fernandez, H.; Perrotin, F.; Winer, N.; Pierre, F.; Benachi, A.; Dreyfus, M.; et al. Maternal Outcome After Conservative Treatment of Placenta Accreta. Obstet. Gynecol. 2010, 115, 526–534. [Google Scholar] [CrossRef]
- Fitzpatrick, K.E.; Sellers, S.; Spark, P.; Kurinczuk, J.J.; Brocklehurst, P.; Knight, M. The management and outcomes of placenta accreta, increta, and percreta in the UK: A population-based descriptive study. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 62–71. [Google Scholar] [CrossRef]
- Sentilhes, L.; Seco, A.; Azria, E.; Beucher, G.; Bonnet, M.-P.; Branger, B.; Carbillon, L.; Chiesa, C.; Crenn-Hebert, C.; Dreyfus, M.; et al. Conservative management or cesarean hysterectomy for placenta accreta spectrum: The PACCRETA prospective study. Am. J. Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Bassetty, K.C.; Vijayaselvi, R.; Yadav, B.; David, L.S.; Beck, M.M. Placenta accrete spectrum: Management and outcomes in a tertiary centre in India: An observational cross-sectional study. Trop. Doct. 2021, 51, 398–403. [Google Scholar] [CrossRef] [PubMed]
- van Beekhuizen, H.J.; Stefanovic, V.; Schwickert, A.; Henrich, W.; Fox, K.A.; Gziri, M.M.; Sentilhes, L.; Gronbeck, L.; Chantraine, F.; Morel, O.; et al. A multicenter observational survey of management strategies in 442 pregnancies with suspected placenta accreta spectrum. Acta Obstet. Gynecol. Scand. 2021, 100 (Suppl. S1), 12–20. [Google Scholar] [CrossRef] [PubMed]
- Lional, K.M.; Tagore, S.; Wright, A. Uterine conservation in placenta accrete spectrum (PAS) disorders: A retrospective case series. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G.; Devisme, L.; Coulon, C. Placenta du spectre accreta: Prise en charge et morbidité dans une maternité française de niveau 3. Gyn Ecol. Obstet. Fertil. Senol. 2020, 48, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, K.; Otani, T.; Kondoh, E.; Makino, S.; Tanaka, M.; Takeda, S.; the Perinatal Research Network Group in Japan. Retrospective multicenter study of leaving the placenta in situ for patients with placenta previa on a cesarean scar. Int. J. Gynecol. Obstet. 2018, 140, 345–351. [Google Scholar] [CrossRef]
- Kutuk, M.S.; Ak, M.; Ozgun, M. Leaving the placenta in situ versus conservative and radical surgery in the treatment of placenta accreta spectrum disorders. Int. J. Gynecol. Obstet. 2018, 140, 338–344. [Google Scholar] [CrossRef]
- Su, H.-W.; Yi, Y.-C.; Tseng, J.-J.; Chen, W.-C.; Chen, Y.-F.; Kung, H.-F.; Chou, M.-M. Maternal outcome after conservative management of abnormally invasive placenta. Taiwan J. Obstet. Gynecol. 2017, 56, 353–357. [Google Scholar] [CrossRef]
- Cal, M.; Ayres-De-Campos, D.; Jauniaux, E. International survey of practices used in the diagnosis and management of placenta accreta spectrum disorders. Int. J. Gynecol. Obstet. 2018, 140, 307–311. [Google Scholar] [CrossRef]
- Sentilhes, L.; Kayem, G.; Mattuizzi, A. Conservative approach: Intentional retention of the placenta. Best Prac. Res. Clin. Obstet. Gynaecol. 2021, 72, 52–66. [Google Scholar] [CrossRef]
- Gică, N.; Ragea, C.; Botezatu, R.; Peltecu, G.; Gică, C.; Panaitescu, A.M. Incidence of Emergency Peripartum Hysterectomy in a Tertiary Obstetrics Unit in Romania. Medicina 2022, 58, 111. [Google Scholar] [CrossRef]
- Clausen, C.; Lönn, L.; Langhoff-Roos, J. Management of placenta percreta: A review of published cases. Acta Obstet. Gynecol. Scand. 2014, 93, 138–143. [Google Scholar] [CrossRef]
- Pather, S.; Strockyj, S.; Richards, A.; Campbell, N.; De Vries, B.; Ogle, R. Maternal outcome after conservative management of placenta percreta at caesarean section: A report of three cases and a review of the literature. Aust. N. Z. J. Obstet. Gynaecol. 2014, 54, 84–87. [Google Scholar] [CrossRef]
- Bisschop, C.N.S.; Schaap, T.P.; Vogelvang, T.E.; Scholten, P.C. Invasive placentation and uterus preserving treatment modalities: A systematic review. Arch. Gynecol. Obstet. 2011, 284, 491–502. [Google Scholar] [CrossRef]
- Timmermans, S.; van Hof, A.C.; Duvekot, J.J. Conservative Management of Abnormally Invasive Placentation. Obstet. Gynecol. Surv. 2007, 62, 529–539. [Google Scholar] [CrossRef]
- Jauniaux, E.; Alfirevic, Z.; Bhide, A.G.; Belfort, M.A.; Burton, G.J.; Collins, S.; Dornan, S.; Jurkovic, D.; Kayem, G.; Kingdom, J.; et al. Placenta Praevia and Placenta Accreta: Diagnosis and Management. BJOG Int. J. Obstet. Gynaecol. 2019, 126, e1–e48. [Google Scholar] [CrossRef]
- Jauniaux, E.; Zheng, W.; Yan, J. Confirming the Diagnosis and Classifying Placenta Accreta Spectrum (PAS) Disorders: Minutes of 2020 Online International Workshop on PAS in Beijing. Matern. Med. 2021, 3, 229–231. [Google Scholar] [CrossRef]
- Panaitescu, A.M.; Ciobanu, A.M.; Gică, N.; Peltecu, G.; Botezatu, R. Diagnosis and Management of Cesarean Scar Pregnancy and Placenta Accreta Spectrum: Case Series and Review of the Literature. J. Ultrasound Med. 2021, 40, 1975–1986. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).