Abstract
Our study focuses on free energy calculations of SARS-CoV-2 spike protein receptor binding motives (RBMs) from wild type and variants of concern (VOCs), with emphasis on SARS-CoV-2 Omicron. Our computational analysis underlines the occurrence of positive selection processes that specify Omicron host adaption and bring changes on the molecular level into context with clinically relevant observations. Our free energy calculation studies regarding the interaction of Omicron’s RBM with human angiotensin converting enzyme 2 (hACE2) indicate weaker binding to the receptor than Alpha’s or Delta’s RBMs. Upon weaker binding, fewer viruses are predicted to be generated in time per infected cell, resulting in a delayed induction of danger signals as a trade-off. Along with delayed immunogenicity and pathogenicity, more viruses may be produced in the upper respiratory tract, explaining enhanced transmissibility. Since in interdependence on the human leukocyte antigen type (HLA type), more SARS-CoV-2 Omicron viruses are assumed to be required to initiate inflammatory immune responses, and because of pre-existing partial immunity through previous infections and/or vaccinations, which mostly guard the lower respiratory tract, overall disease severity is expected to be reduced.
1. Amino Acid Sequence Alignments Point to a Shift in RBM Characteristics
Within the receptor binding domain (RBD; aa319 to aa541) of the wild type (wt) SARS-CoV-2 spike protein, the amino acid sequence stretch aa437 to aa508 encompasses the receptor binding motif (RBM) [1]. Amino acid residue exchanges have been observed at distinct RBM positions with all variants of concern (VOCs) [2]. The newly reported Omicron VOC carries ten exchanged amino acids (Figure 1) in its RBM, of which four (K440, S446, K478, and A484) are also found in SARS-CoV-1-, in bat-, and/or in civet-derived RBMs at the respective positions and through which Omicron’s RBM can be distinguished from that of SARS-CoV-2’s wt [3].
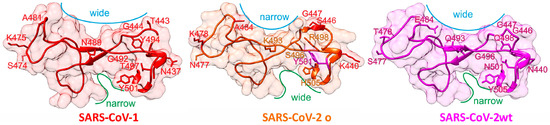
Figure 1.
Structure comparisons of SARS-CoV receptor binding motives. Mutated amino acid residues in SARS-CoV-2 o and their counterparts in SARS-CoV-1 or SARS-CoV-2 wt (labeled) are shown as stick models. For further explanations see text.
Figure 1 shows 3D structures of SARS-CoV-1 [4] and SARS-CoV-2 wt [1] from X-ray data, whereas the RBM structure of SARS-CoV-2 o has been modeled by AlphaFold [5]. Although the overall topology of the SARS-CoV-2 RBMs are quite comparable, structure details which result from distinct amino acid exchanges yield local surface differences, such as a narrower distance between A484 and G447 in Omicron’s RBM compared with that between E484 and G447 in wt’s RBM (blue lines in Figure 1). In contrast, the cleft which is located next to amino acid residue 505 is narrower in wt’s RBM as compared with that of Omicron (green lines in Figure 1). Both surface alterations indicate a somewhat different interaction geometry with the hACE2 receptor. From the remaining six exchanged amino acid residues, five (N477, K493, S496, R498, and H505) are unique to Omicron, which further differentiates Omicron’s from wt’s SARS-CoV-2 spike protein when comparing the here-assembled seven RBMs (Table 1). Importantly, Omicron encodes for Y501 which was found to strengthen binding in alpha, beta, and gamma VOCs [6]. Of note, bat RBMs (BM48-31 and Rp3) do not bind to hACE2 [7] whereas SARS-CoV-1 binds to hACE2 with lower affinity than does SARS-CoV-2 wt [8]. For assignments of virus variants and strains to SARS-CoV-2 phylogeny tree locations and phylogeography, see Supplement (Figure S1).

Table 1.
Amino acid sequence alignments of coronavirus spike protein receptor binding motifs.
In detail: residue K478 has been designated the decisive amino acid exchange in Delta’s RBM [2]. K478 has been retained in Omicron, which, similar to residues K440, S446, and N477 (all three are rarely seen in other variants [9]), lends Omicron more “non-SARS-CoV-2 wt” characteristics, e.g., K478 matches with K465 in the RBM of SARS-CoV-1. When expressing A484, Omicron avoided the receptor binding weakening E484 residue found in alpha and in other VOCs [10]. The A484 matching residue from SARS-CoV-1’s RBM is A471 which is located adjacent to L472, one of the amino acid residues which is in direct contact with hACE2 and which has been assigned as important for species-specific binding [4]. Residue K493 in Omicron’s RBM is positioned where N479 is found in SARS-CoV-1’s RBM. This exchange in Omicron’s RBM was later corrected to R493. N479 of SARS-CoV-1’s RBM makes direct contact with hACE2 and is considered to be responsible for species-specific binding as well. An N479K exchange resulted in steric hindrance and in weakening of RBD-binding to hACE2 [11]. S496 and R498 are rare RBM mutations and adverse effects on binding can be estimated for R498 as opposed to Q498 on wt’s RBM because of charge repulsion [4]. Y501 was considered to strengthen binding to hACE2 considerably with respect to SARS-CoV-2 wt and was assumed to also increase virus replication rates [12,13,14]. Finally, H505 from SARS-CoV-2 Omicron is located where Y491 is placed in SARS-CoV-1. H505 replaces Y505 of SARS-CoV-2 wt’s RBD and of other VOCs, respectively [3]. Y505 is directly involved in binding to hACE2 and from the physicochemical properties of histidine vs. tyrosine one can conclude that the binding of Omicron’s RBD to hACE2 would not be positively affected by this exchange.
In sum, because of the multitude of amino acid exchanges and because of slightly altered surface geometry, the interaction of Omicron’s RBM with hACE2 is assumed to be weaker than that of Alpha’s or Delta’s RBM with hACE2.
2. Free Energy Calculations Indicate Weaker Receptor Binding of Omicron’s RBM
To substantiate our hypothesis of weaker interactions between Omicron’s RBM and hACE2 as compared with those of other VOCs, we performed free energy difference calculations (ΔΔG calculations) [15,16] for the SARS-CoV-2-derived RBMs (wt vs. Alpha or Delta or Omicron) when bound to hACE2 (Table 2). We then compared these binding differences to respective RBM-hDPP-IV interactions [17]. Since human DPP-IV is considered not to function as a receptor for SARS-CoV-2 in vivo [7], calculations of free energy differences of RBM-hDPP-IV complexes served as controls. Of note, the MERS virus uses hDPP-IV as a receptor and SARS-CoV-2 wt has been assumed to as well being able to bind to hDPP-IV [18]. For a description of experimental procedures, see the Supplement.

Table 2.
Spike protein receptor binding motif amino acid exchanges and changes of free energies with human ACE2 binding or human DPP-IV binding (a).
According to ΔΔG calculations on the respective amino acid exchanges and their contributions to receptor binding, one observes that with respect to the wt RBM, the Alpha RBM achieved slightly stronger binding to hACE2 when summing up all amino acid residue energy differences which arise from the respective single amino acid exchanges. The Delta RBM neither gained nor lost binding strength compared with wt RBM-hACE2 binding. Surprisingly, the Omicron RBM-hACE2 complex is energetically less favored (ΔΔG: +4.41 kJ/mol) than the complex between wt RBM and hACE2 which means that Omicron’s RBM binding to hACE2 is weakened with respect to hACE2 binding of either the wt, the Alpha, or the Delta RBM. Notably, the presence of N417, though located outside Omicron’s RBM, is known to reduce hACE2 binding [12,18] which correlates with our calculations where N417 affords an increase in free binding energy compared with K417 of SARS-CoV-2 wt’s RBM (ΔΔG +0.49 kJ/mol); see Supplementary Table S1.
By contrast, ΔΔG value differences of RBD-hDPP-IV binding of all VOCs showed that all their respective complexes were bound to hDPP-IV with weaker forces than wt (Table 2). Of note, N417 weakens binding to hDPP-IV even more (ΔΔG +0.64 kJ/mol) than that to hACE2; see Supplementary Table S2. This stands in agreement with the observation that SARS-CoV-2 wt uses hACE2 as in vivo entry into host cells rather than hDPP-IV [4]. Interestingly, Omicron RBM binding with hDPP-IV requests a smaller increase in free energy (ΔΔG: +4.05 kJ/mol) than that of Omicron RBM binding with hACE2 (ΔΔG: +4.90 kJ/mol) when taking the K417N exchange into account. It remains to be investigated whether such a free energy difference is large enough to cause Omicron to switch host receptors in vivo, and hence, to possibly alter tropism and to eventually produce different disease symptoms.
As of yet, binding strength differences of SARS-CoV-2 VOCs’ spike proteins to hACE2 are estimated controversially, depending on molecular modelling and simulation approaches [19,20,21,22,23,24]. Experimental data on binding strengths of SARS-CoV-2 Omicron’s spike protein to hACE2 which so far have become available provide evidence that Omicron’s RBD interaction with hACE2 is weaker than that of Delta’s RBD [25,26,27], with one exception which states that binding strength of Delta’s or Omicron’s RBD to hACE2 was in similar ranges [28].
3. A Molecular Perspective on Transmissibility and Disease Outcome
Weaker binding of the spike protein to its receptor is assumed to slow down virus uptake into cells through cell surface membrane fusion and to direct virus uptake towards endocytosis-dependent cell entry. Less virus uptake per cell thus, elicits fewer danger signals, thereby retarding innate immune response [29] which over time might result in higher viral production in the upper respiratory tract. Also of interest, outside its RBM the Omicron spike protein carries the N679K, P681H, N679K, D614G exchanges [30,31] which may assist in enhancing the above mentioned mechanisms, and ultimately transmissibility [32,33]. Weaker RBM–hACE2 interaction stands in agreement with the postulated switch in Omicron’s cell entry mechanism towards a TMPRSS2-independent fusion and an associated major shift in replication properties [34], as efficient cell entry of viruses via endosomal uptake is not limited by receptor binding strength.
COVID-19 is considered a result of an overacting immune response mostly affecting the lower respiratory tract [35]. It is tempting to speculate whether Omicron’s assumed weaker RBM–hACE2 binding with respect to those of Alpha or Delta also contributed to clinical observations of less severe disease outcome upon SARS-CoV-2 Omicron infection compared with infections with other SARS-CoV-2 VOCs. A reduced entry/uptake of viral particles per cell and time which affects intracellular replication of SARS-CoV-2 in the lower respiratory tract will likely be associated with reduced and/or altered peptide loadings of HLA class I receptors and elicited CD8 T-cell mediated cytotoxicity. In parallel, reduced production of spike protein most likely leads to reduced presentation of antigen peptides by HLA class II on antigen presenting cells. Individuals with the same HLA type but altered peptide loads, e.g., because of virus variant-dependent proliferation differences, experience different signal strengths to the immune system. Different HLA-dependent signaling will also be observed between individuals who are infected with the same virus variant but are equipped with different HLA types which are present in respective ethnic populations [36]. Individual HLA polymorphisms seem to indeed have affected tropism and disease severity of COVID-19 [37]. According to our computational NetMHC predictions, a given HLA class I receptor configuration has greater impact on mitigating or enhancing disease severity as compared with virus strain variations (Table S3). Nevertheless, amino acid deletions, as exemplified by Omicron’s spike protein compared with wild type spike protein turn out to have stronger effects on differential HLA class peptide presentation (Table S4) than single point mutations (Table S5).
Reports of disease severity upon infections with SARS-CoV-2 Omicron, though at first anecdotal [38] or based on preliminary clinical studies [39,40,41], were validated by recent clinical studies from other parts of the world [42,43], confirming an overall less severe, rather mild, or even asymptomatic disease outcome, which again stands in agreement with results from in vitro studies [44] as well as with weaker binding of the virus to its receptor. Enhanced replication of SARS-CoV-2 Omicron in nasal epithelial cells has been shown and supports higher contagiousness whereas the reduced viral yields in human lung cells are in line with reduced disease severity [45]. Both observations are in agreement with weaker RBM–hACE2 binding of SARS-CoV-2 Omicron, as pointed out above.
From the molecular perspectives outlined here, it seems plausible that SARS-CoV-2 Omicron fulfilled some key criteria of a host-adapted virus variant with high contagion potential and less severe disease outcome [46]. Particularly after monospecific vaccine administration, SARS-CoV-2 Omicron might challenge a human’s post-immunized waning antibody/B-cell responses to induce a more general and perhaps long-lasting immunity by extending protective antibody repertoires and by simultaneously enhancing T-cell mediated immunity, as reported for Delta [47] and very recently for Omicron as well [48], thereby ultimately preparing an individual to defeat more pathogenic SARS-CoV-2 variants in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina58020226/s1, Methods; Figure S1: Phylogeny tree of SARS-CoV-2 strains; Table S1: Effects of SARS-CoV-2 RBD-exchanged amino acid residues on strengths of interaction with ACE2; Table S2: Effects of SARS-CoV-2 RBD-exchanged amino acid residues on strengths of interaction with DPP-IV; Table S3: Comparative Analysis of HLA class I peptides from spike proteins from Omicron variants and strains; Table S4: Determination of HLA peptide preferences depending on amino acids deleted in spike proteins from Omicron variants and strains; Table S5: HLA Peptide preferences depending on single point mutations in spike proteins of Omicron variants and strains.
Author Contributions
Conceptualization, M.O.G. and H.-J.T.; methodology, M.O.G. and K.F.M.O.; software, K.F.M.O.; validation, M.O.G., K.F.M.O. and H.-J.T.; formal analysis, M.O.G., K.F.M.O. and H.-J.T.; investigation, M.O.G., K.F.M.O. and H.-J.T.; data curation, K.F.M.O.; writing—original draft preparation, M.O.G.; writing—review and editing, K.F.M.O. and H.-J.T.; visualization, K.F.M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We thank F. Steinbeck for excellent assistance with HLA isotype peptide loading calculations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Jhun, H.; Park, H.-Y.; Hisham, Y.; Song, C.-S.; Kim, S. SARS-CoV-2 Delta (B.1.617.2) Variant: A Unique T478K Mutation in Receptor Binding Motif (RBM) of Spike Gene. Immune Netw. 2021, 21, e32. [Google Scholar] [CrossRef] [PubMed]
- ECDC Threat Assessment Brief. Available online: https://www.ecdc.europa.eu/en/publications-data/threatassessment-brief-emergence-sars-cov-2-variant-b.1.1.529 (accessed on 30 November 2021).
- Li, F.; Li, W.; Farzan, M.; Harrison, S.C. Structure of SARS Coronavirus Spike Receptor-Binding Domain Complexed with Receptor. Science 2005, 390, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Hilton, S.K.; Ellis, D.; Crawford, K.H.D.; Dingens, A.S.; Navarro, M.J.; Bowen, J.E.; Tortorici, M.A.; Walls, A.C.; et al. Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding. Cell 2020, 182, 1295–1310. [Google Scholar] [CrossRef]
- Guruprasad, L. Human SARS CoV-2 spike protein mutations. Proteins 2020, 89, 569–576. [Google Scholar] [CrossRef]
- Cheng, M.H.; Krieger, J.M.; Kaynak, B.; Arditi, M.; Bahar, I. Impact of South African 501.V2 Variant on SARSCoV-2 Spike Infectivity and Neutralization: A Structure-based Computational Assessment. Bioinformatics, 2021; in press. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Sui, J.; Kuhn, J.H.; Moore, M.J.; Luo, S.; Wong, S.-K.; Huang, I.-C.; Xu, K.; Vasilieva, N.; et al. Receptor and viral determinants of SARS coronavirus adaptation to human ACE2. EMBO J. 2005, 24, 1634–1643. [Google Scholar] [CrossRef] [Green Version]
- Fratev, F. N501Y and K417N Mutations in the Spike Protein of SARS-CoV-2 Alter the Interactions with Both hACE2 and Human-Derived Antibody: A Free Energy of Perturbation Retrospective Study. J. Chem. Inf. Model 2021, 61, 6079–6084. [Google Scholar] [CrossRef]
- Rathnasinghe, R.; Jangra, S.; Cupic, A.; Martínez-Romero, C.; Mulder, L.C.F.; Kehrer, T.; Yildiz, S.; Choi, A.; Mena, I.; De Vrieze, J.; et al. The N501Y mutation in SARS-CoV-2 spike leads to morbidity in obese and aged mice and is neutralized by convalescent and postvaccination human sera. MedRxiv 2021. [Google Scholar] [CrossRef]
- Gu, H.; Chen, Q.; Yang, G.; He, L.; Fan, H.; Deng, Y.-Q.; Wang, Y.; Teng, Y.; Zhao, Z.; Cui, Y.; et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science 2020, 369, 1603–1607. [Google Scholar] [CrossRef]
- Opuni, K.F.M.; Solomon, S.; Metzen, F.; Frommholz, D.; Koy, C.; Röwer, C.; Glocker, M.O.; Illges, H.; Anderson, P.C. In silico Epitope Mapping of Glucose-6-Phosphate Isomerase: A Rheumatoid Arthritis Autoantigen. J. Proteom. Bioinform. 2017, 10, 60–72. [Google Scholar] [CrossRef]
- Dehouck, Y.; Kwasigroch, J.M.; Rooman, M.; Gilis, D. BeAtMuSiC: Prediction of changes in protein–protein binding affinity on mutations. Nucleic Acids Res. 2013, 41, W333–W339. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Shi, X.; Jiang, L.; Zhang, S.; Wang, D.; Tong, P.; Guo, D.; Fu, L.; Cui, Y.; Liu, X.; et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013, 23, 986–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, Z.; Yang, L.; Lian, X.; Xie, Y.; Li, S.; Xin, S.; Cao, P.; Lu, J. The MERS-CoV Receptor DPP4 as a Candidate Binding Target of the SARS-CoV-2 Spike. Science 2020, 23, 101160. [Google Scholar] [CrossRef]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta Variant of SARS-CoV-2: A Comparative Computational Study of Spike protein. BioRxiv 2021. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, L.; Mo, M.; Li, Y.; Han, J.; Li, J.; Yang, Y.; Zhang, X.; Gong, C.; Lu, K.; et al. The effect of the multiple mutations in Omicron RBD on its binding to human ACE2 receptor and immune evasion: An investigation of molecular dynamics simulations. Signal Transduct. Target. Ther. 2022, 7. [Google Scholar] [CrossRef]
- Lupala, C.S.; Ye, Y.; Chen, H.; Su, X.-D.; Liu, H. Mutations in RBD of SARS-CoV-2 Omicron variant result stronger binding to human ACE2 protein. Biochem. Biophys. Res. Commun. 2022, 590, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Golcuk, M.; Yildiz, A.; Gur, M. The Omicron Variant Increases the Interactions of SARS-CoV-2 Spike Glycoprotein with ACE2. BioRxiv 2021. [Google Scholar] [CrossRef]
- Shah, M.; Woo, H.G. Omicron: A heavily mutated SARS-CoV-2 variant exhibits stronger binding to ACE2 and potently escape approved COVID-19 therapeutic antibodies. BioRxiv 2021. [Google Scholar] [CrossRef]
- Jawaid, M.Z.; Baidya, A.; Mahboubi-Ardakani, R.; Davis RLCox, D.L. Simulation of the omicron variant of SARS-CoV-2 shows broad antibody escape, weakened ACE2 binding, and modest increase in furin binding. BioRxiv 2021. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, L.; Mo, M.; Liu, T.; Wu, C.; Gong, C.; Lu, K.; Gong, L.; Zhu, W.; Xu, Z. SARS-CoV-2 Omicron RBD shows weaker binding affinity than the currently dominant Delta variant to human ACE2. Signal Transduct. Target. Ther. 2022, 7, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Li, L.; Liu, S.; Wang, Q.; Zhang, D.; Xu, Z.; Han, P.; Li, X.; Peng, Q.; Su, C.; et al. Receptor binding and complex structures of human ACE2 to spike RBD from Omicron and Delta SARS-CoV-2. Cell, 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Bertoglio, F.; Steinke, S.; Heine, P.A.; Ynga-Durand, M.A.; Zuo, F.; Du, L.; Korn, J.; Milošević, M.; Wenzel, E.V.; et al. Human serum from SARS-CoV-2 vaccinated and COVID-19 patients shows reduced binding to the RBD of SARS-CoV-2 Omicron variant. MedRxiv 2021. [Google Scholar] [CrossRef]
- Mannar, D.; Saville, J.W.; Zhu, X.; Srivastava, S.S.; Berezuk, A.M.; Tuttle, K.S.; Marquez, C.; Sekirov, I.; Subramaniam, S. SARS-CoV-2 Omicron Variant: ACE2 Binding, Cryo-EM Structure of Spike Protein-ACE2 Complex and Antibody Evasion. BioRxiv 2021. [Google Scholar] [CrossRef]
- Matzinger, P. The Danger Model: A Renewed Sense of Self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Tsueng, G.; Mullen, J.; Alkuzweny, M.; Cano, M.; Rush, B.; Haag, E.; Latif, A.A.; Zhou, X.; Qian, Z.; Andersen, K.G. Outbreak.info: A standardized, searchable platform to discover and explore COVID-19 resources and data. BioRxiv 2022. [Google Scholar] [CrossRef]
- World of Molecules. Available online: https://www.worldofmolecules.com/3D/B.1.1.529-variant.html (accessed on 30 November 2021).
- Torjesen, I. COVID-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. Br. Med. J. 2021, 375, n2943. [Google Scholar] [CrossRef]
- Pulliam, J.R.C.; van Schalkwyk, C.; Govender, N.; von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. Br. Med. J. 2021. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; Logan, N.; de Lorenzo, G.; Furnon, W.; Scott, S.; Manali, M.; Szemiel, A.; et al. The hyper-transmissible SARS-CoV-2 Omicron variant exhibits significant antigenic change, vaccine escape and a switch in cell entry mechanism. MedRxiv 2022. [Google Scholar] [CrossRef]
- Olbei, M.; Hautefort, I.; Modos, D.; Treveil, A.; Poletti, M.; Gul, L.; Shannon-Lowe, C.D.; Korcsmaros, T. SARS-CoV-2 Causes a Different Cytokine Response Compared to Other Cytokine Storm-Causing Respiratory Viruses in Severely Ill Patients. Front. Immunol. 2021, 12, 629193. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Torsiello, E.; Spiezia, F.; Oliva, F.; Tingart, M.; Maffulli, N. Association between HLA genotypes and COVID-19 susceptibility, severity and progression: A comprehensive review of the literature. Eur. J. Med. Res. 2021, 26, 84. [Google Scholar] [CrossRef]
- Naemi, F.M.A.; Al-Adwani, S.; Al-Khatabi, H.; Al-Nazawi, A. Association between the HLA genotype and the severity of COVID-19 infection among South Asians. J. Med. Virol. 2021, 93, 4430–4437. [Google Scholar] [CrossRef] [PubMed]
- Abdool Karim, S.S.; Abdool Karim, Q. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Wolter, N.; Jassat, W.; Walaza, S. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. MedRxiv 2021. [Google Scholar] [CrossRef]
- Ferguson, N.; Ghani, A.; Hinsley, W.; Volz, E.; on behalf of the Imperial College COVID-19 Response Team. Report 50: Hospitalisation risk for Omicron cases in England 2021; Imperial College London: London, UK, 2021; Volume 2021, pp. 1–12. [Google Scholar] [CrossRef]
- Sheikh, A.; Kerr, S.; Woolhouse, M.; McMenamin, J.; Robertson, C. Severity of Omicron Variant of Concern and Vaccine Effectiveness Against Symptomatic Disease: National Cohort with Nested Test Negative Design Study in Scotland. Available online: https://www.pure.ed.ac.uk/ws/portalfiles/portal/245818096/Severity_of_Omicron_variant_of_concern_and_vaccine_effectiveness_against_symptomatic_disease.pdf (accessed on 23 December 2021).
- Lewnard, J.A.; Hong, V.X.; Patel, M.M.; Kahn, R.; Lipsitch, M.; Tartof, S.Y. Clinical outcomes among patients infected with Omicron (B.1.1.529) SARS-CoV-2 variant in southern California. MedRxiv 2022. [Google Scholar] [CrossRef]
- Wang, L.; Berger, N.A.; Kaelber, D.C.; Davis, P.B.; Volkow, N.D.; Xu, R. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of Omicron. MedRxiv 2022. [Google Scholar] [CrossRef]
- Chan Chi-wai, M. Available online: https://www.med.hku.hk/en/news/press/20211215-omicron-sars-cov-2-infection?utm_medium=social&utm_source=twitter&utm_campaign=press_release (accessed on 23 December 2021).
- Peacock, T.P.; Brown, J.C.; Zhou, J.; Thakur, N.; Newman, J.; Kugathasan, R.; Sukhova, K.; Kaforou, M.; Bailey, D.; Barclay, W.S. The SARS-CoV-2 variant, Omicron, shows enhanced replication in human primary nasal epithelial cells. BioRxiv 2021. [Google Scholar] [CrossRef]
- Telenti, A.; Arvin, A.; Corey, L. After the pandemic: Perspectives on the future trajectory of COVID-19. Nature 2021, 596, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.A.; McBride, S.K.; Winders, B.; Schoen, D.; Trautmann, L.; Curlin, M.E.; Tafesse, F.G. Antibody Response and Variant Cross-Neutralization After SARS-CoV-2 Breakthrough Infection. JAMA 2022, 327, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Karim, F.; Cele, S.; San, J.E.; Lustig, G.; Tegally, H.; Bernstein, M.; Ganga, Y.; Jule, Z.; Reedoy, K.; et al. Omicron infection enhances neutralizing immunity against the Delta variant. Medrxiv 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).