BRCA Genes and Related Cancers: A Meta-Analysis from Epidemiological Cohort Studies
Abstract
:1. Introduction
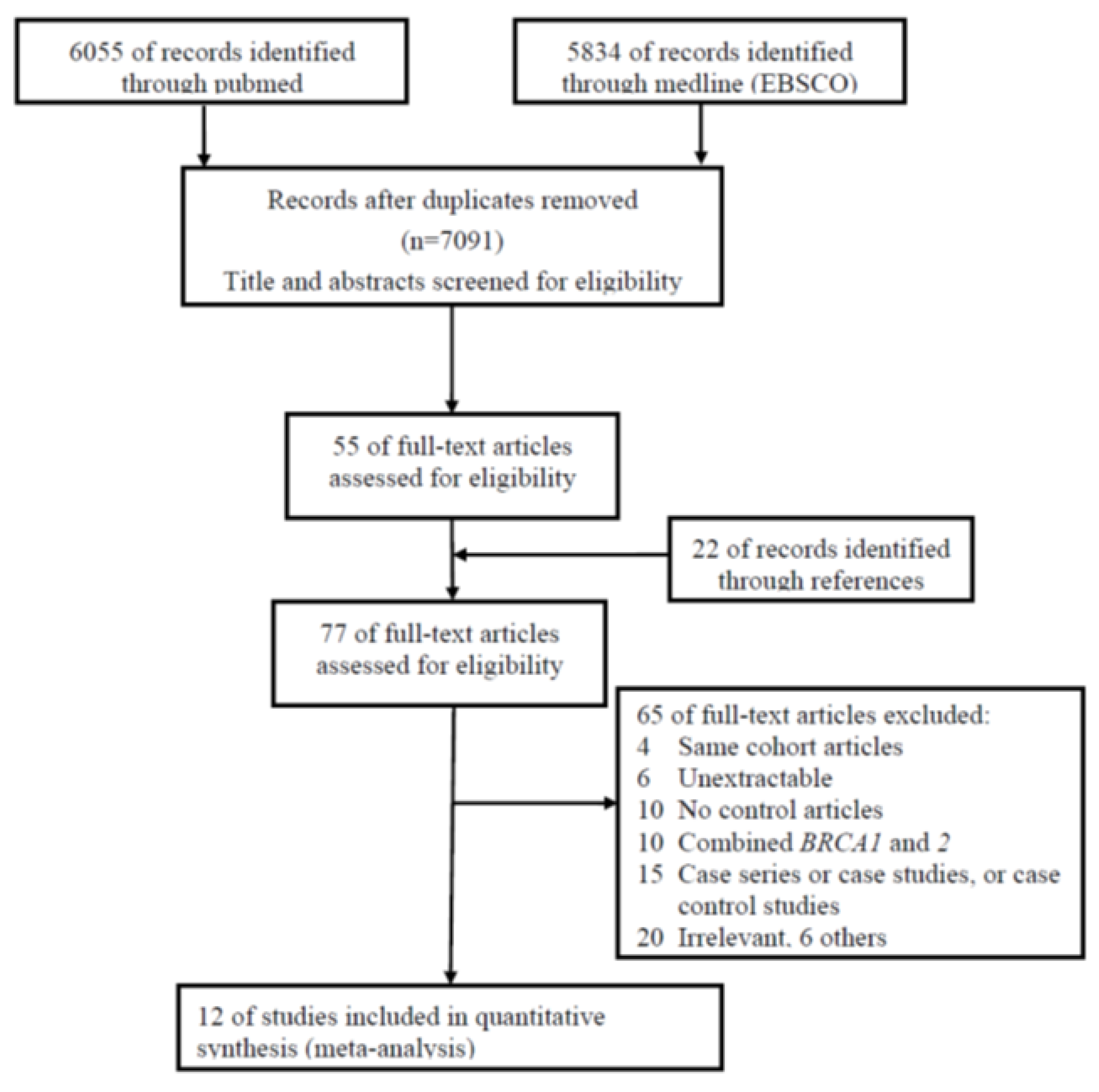
2. Materials and Methods
2.1. Search Strategy and Data Abstraction
2.2. Statistics
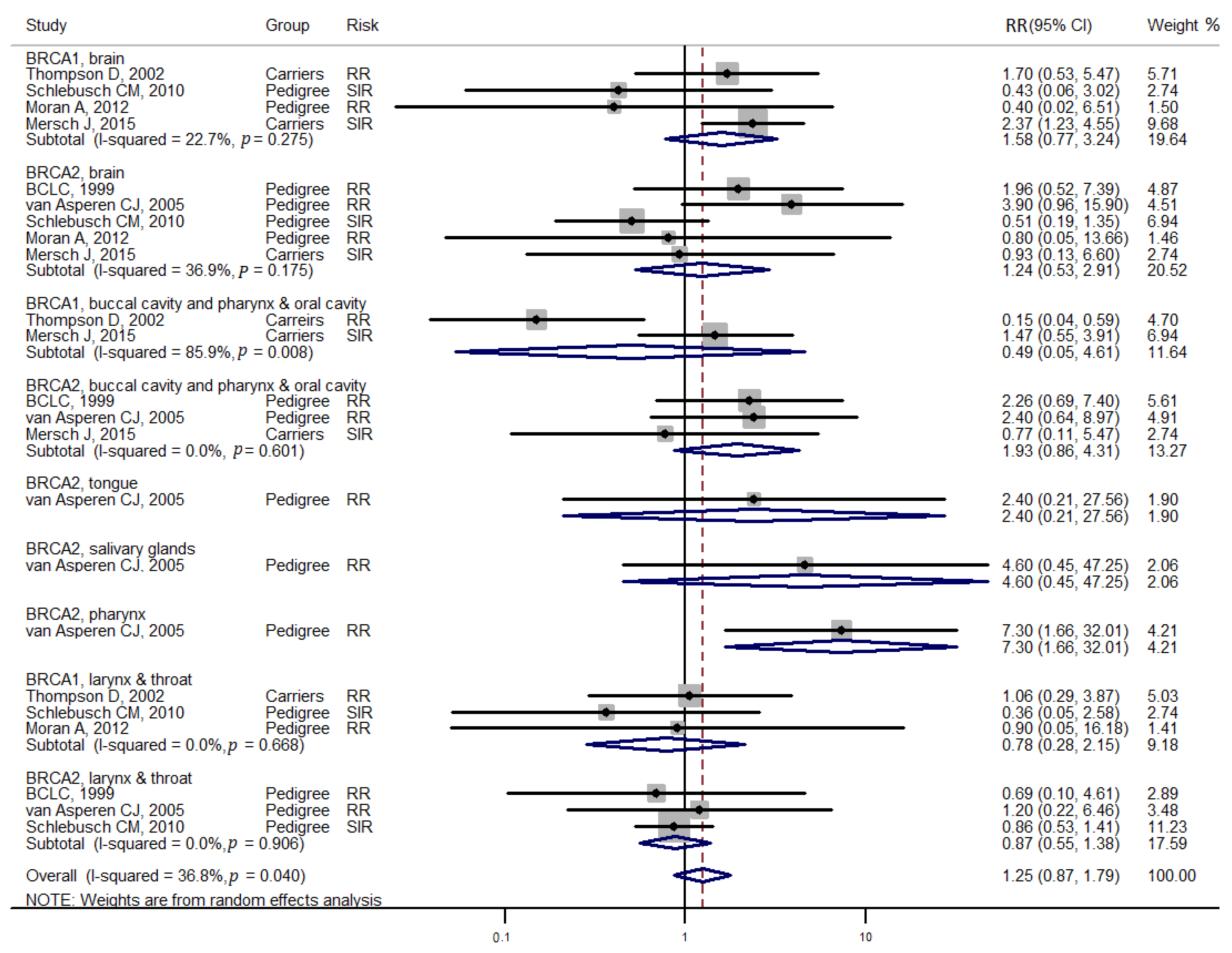
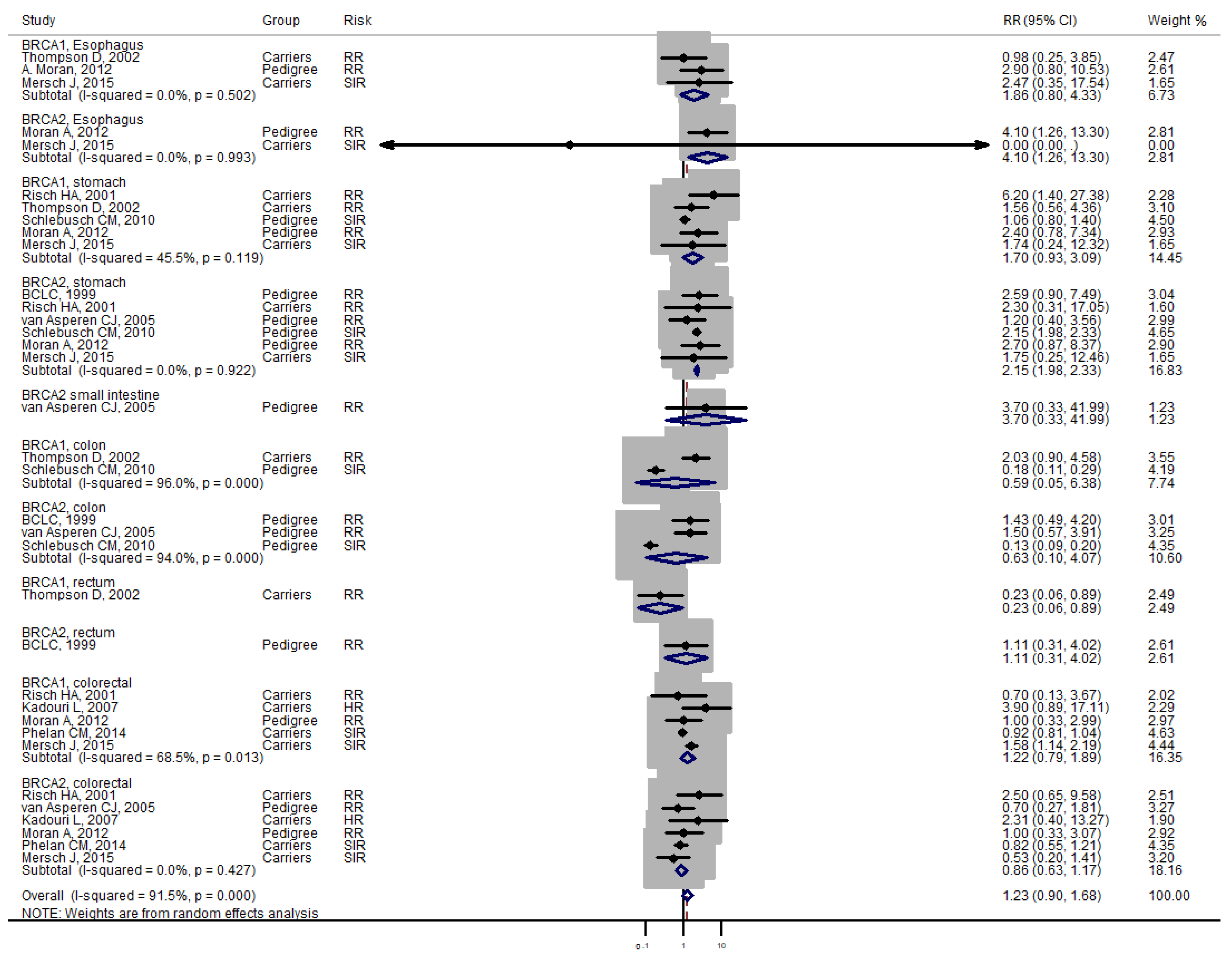
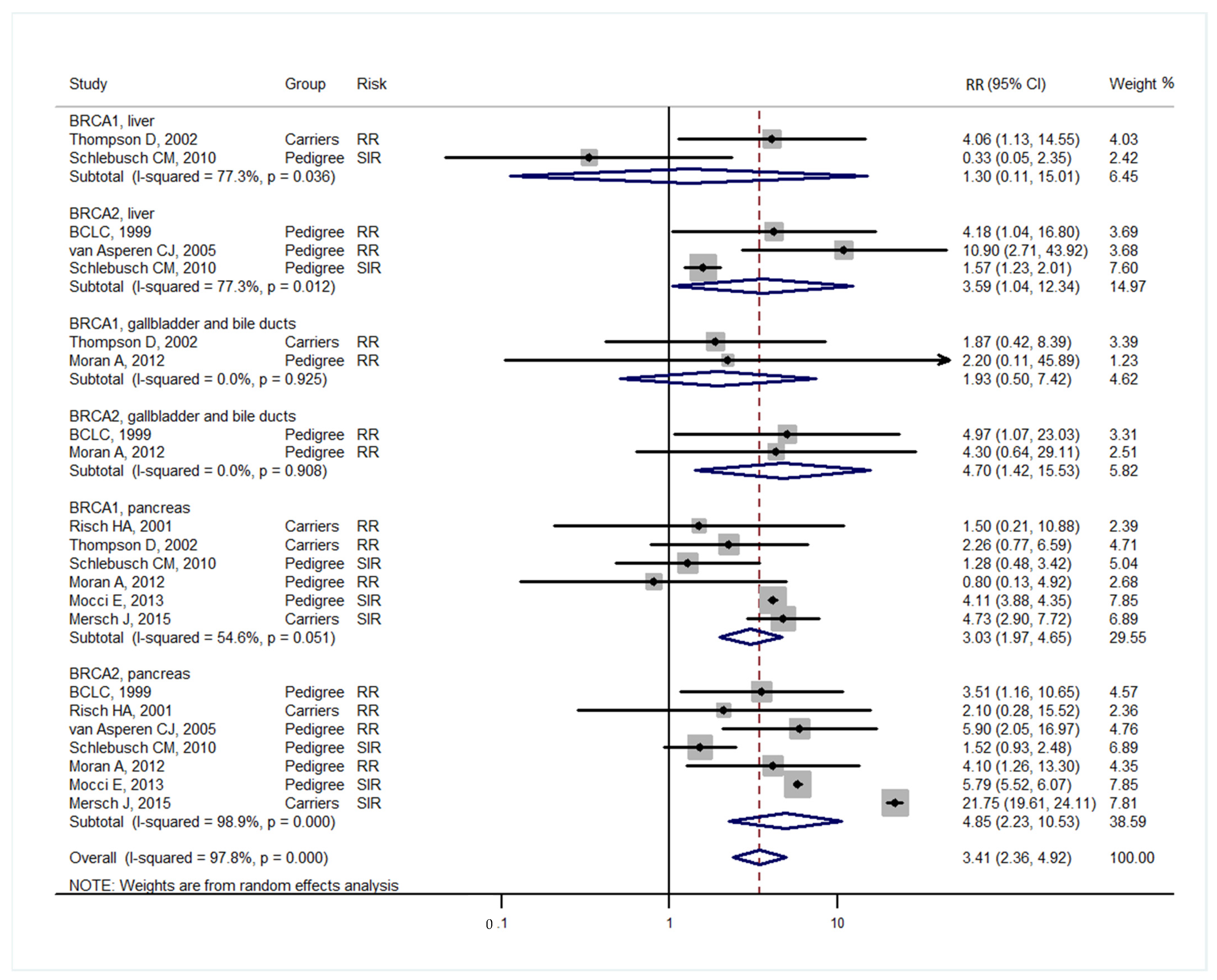
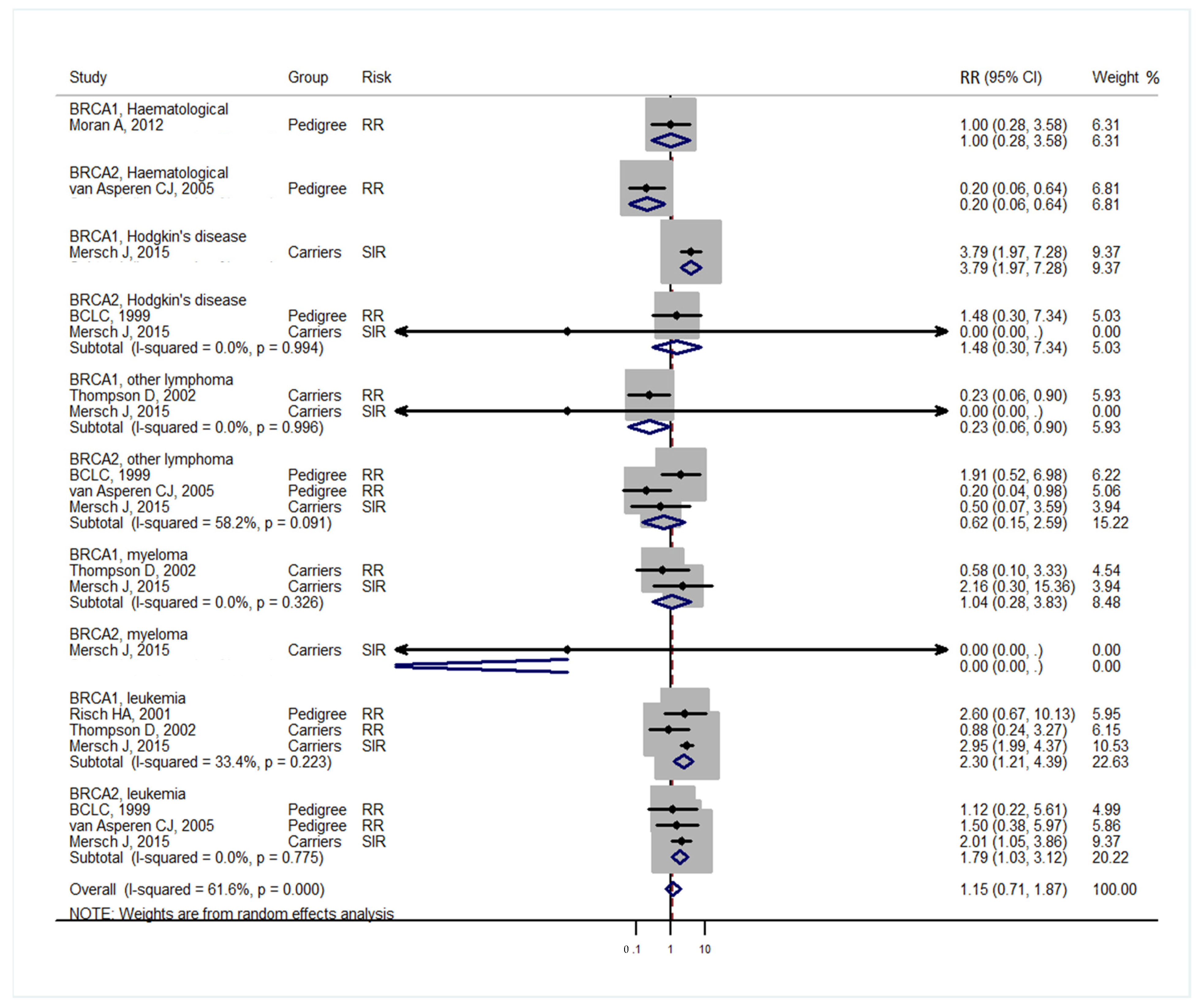
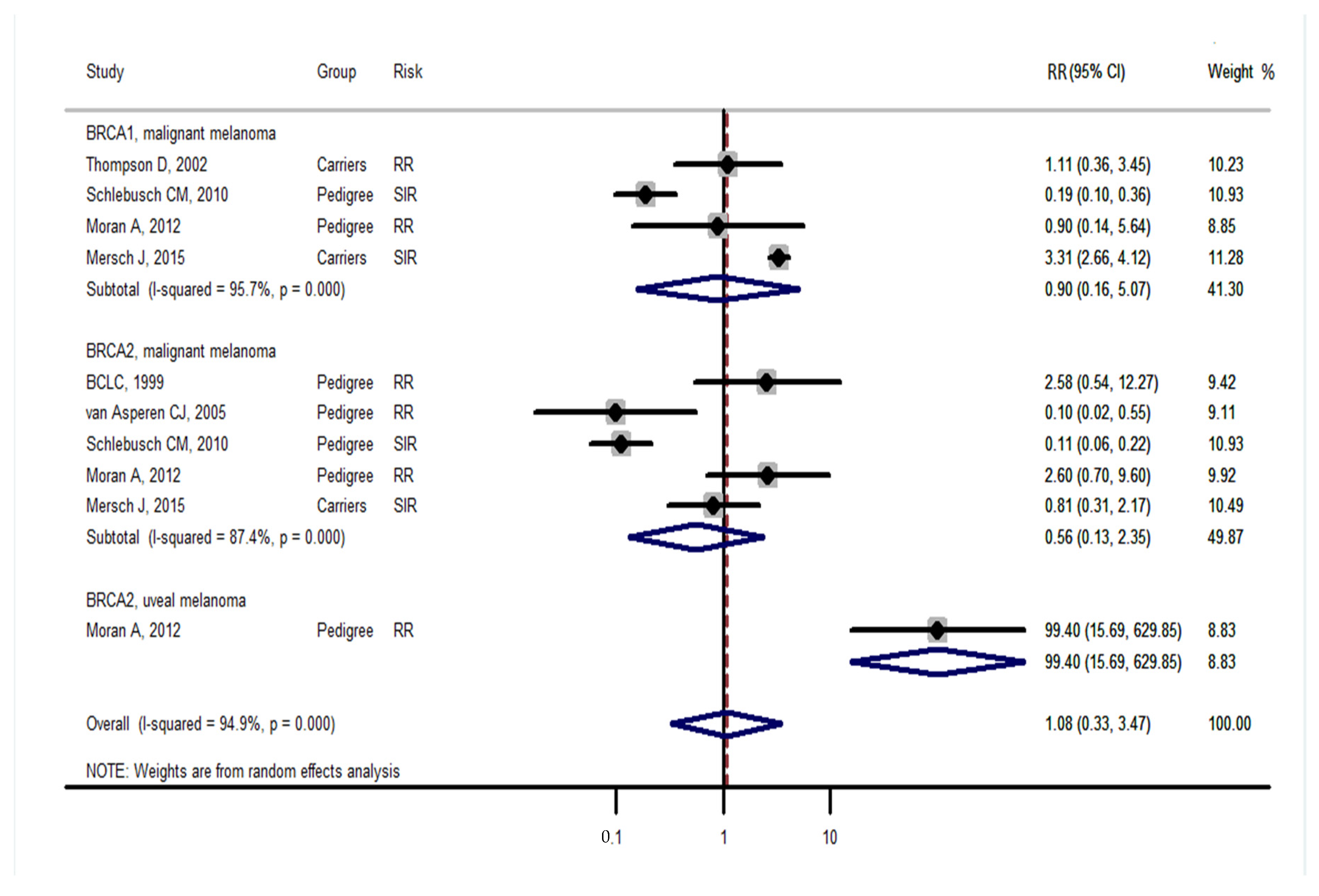
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nacson, J.; Krais, J.J.; Bernhardy, A.J.; Clausen, E.; Feng, W.; Wang, Y.; Nicolas, E.; Cai, K.Q.; Tricarico, R.; Hua, X.; et al. BRCA1 Mutation-Specific Responses to 53BP1 Loss-Induced Homologous Recombination and PARP Inhibitor Resistance. Cell Rep. 2018, 24, 3513–3527.e3517. [Google Scholar] [CrossRef] [PubMed]
- Couzin, J. The twists and turns in BRCA’s path. Science 2003, 302, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Easton, D.F. Cancer Incidence in BRCA1 mutation carriers. J. Natl. Cancer Inst. 2002, 94, 1358–1365. [Google Scholar] [CrossRef]
- Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar] [CrossRef]
- Mohamad, H.B.; Apffelstaedt, J.P. Counseling for male BRCA mutation carriers: A review. Breast 2008, 17, 441–450. [Google Scholar] [CrossRef]
- Easton, D.F.; Steele, L.; Fields, P.; Ormiston, W.; Averill, D.; Daly, P.A.; McManus, R.; Neuhausen, S.L.; Ford, D.; Wooster, R.; et al. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13. Am. J. Hum. Genet. 1997, 61, 120–128. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, Y.C.; Li, C.Y.; Lee, Y.L.; Chen, B.L. BRCA1 and BRCA2 Gene Mutations and Lung Cancer Risk: A Meta-Analysis. Medicina 2020, 56, 212. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; McBride, A.; Yun, S.; Bhattacharjee, S.; Slack, M.; Martin, J.R.; Jeter, J.; Abraham, I. BRCA1 and BRCA2 Gene Mutations and Colorectal Cancer Risk: Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 1178–1189. [Google Scholar] [CrossRef]
- Cullinane, C.M.; Creavin, B.; O’Connell, E.P.; Kelly, L.; O’Sullivan, M.J.; Corrigan, M.A.; Redmond, H.P. Risk of colorectal cancer associated with BRCA1 and/or BRCA2 mutation carriers: Systematic review and meta-analysis. Br. J. Surg. 2020, 107, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Brose, M.S.; Rebbeck, T.R.; Calzone, K.A.; Stopfer, J.E.; Nathanson, K.L.; Weber, B.L. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J. Natl. Cancer Inst. 2002, 94, 1365–1372. [Google Scholar] [CrossRef]
- Ford, D.; Easton, D.F.; Bishop, D.T.; Narod, S.A.; Goldgar, D.E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 1994, 343, 692–695. [Google Scholar] [CrossRef]
- Noh, J.M.; Choi, D.H.; Baek, H.; Nam, S.J.; Lee, J.E.; Kim, J.W.; Ki, C.S.; Park, W.; Huh, S.J. Associations between BRCA Mutations in High-Risk Breast Cancer Patients and Familial Cancers Other than Breast or Ovary. J. Breast Cancer 2012, 15, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Beiner, M.E.; Finch, A.; Rosen, B.; Lubinski, J.; Moller, P.; Ghadirian, P.; Lynch, H.T.; Friedman, E.; Sun, P.; Narod, S.A.; et al. The risk of endometrial cancer in women with BRCA1 and BRCA2 mutations. A prospective study. Gynecol. Oncol. 2007, 104, 7–10. [Google Scholar] [CrossRef]
- Schlebusch, C.M.; Dreyer, G.; Sluiter, M.D.; Yawitch, T.M.; van den Berg, H.J.; van Rensburg, E.J. Cancer prevalence in 129 breast-ovarian cancer families tested for BRCA1 and BRCA2 mutations. S. Afr. Med. J. 2010, 100, 113–117. [Google Scholar] [CrossRef]
- Mocci, E.; Milne, R.L.; Mendez-Villamil, E.Y.; Hopper, J.L.; John, E.M.; Andrulis, I.L.; Chung, W.K.; Daly, M.; Buys, S.S.; Malats, N.; et al. Risk of pancreatic cancer in breast cancer families from the breast cancer family registry. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 803–811. [Google Scholar] [CrossRef]
- Phelan, C.M.; Iqbal, J.; Lynch, H.T.; Lubinski, J.; Gronwald, J.; Moller, P.; Ghadirian, P.; Foulkes, W.D.; Armel, S.; Eisen, A.; et al. Incidence of colorectal cancer in BRCA1 and BRCA2 mutation carriers: Results from a follow-up study. Br. J. Cancer 2014, 110, 530–534. [Google Scholar] [CrossRef]
- Bancroft, E.K.; Page, E.C.; Castro, E.; Lilja, H.; Vickers, A.; Sjoberg, D.; Assel, M.; Foster, C.S.; Mitchell, G.; Drew, K.; et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: Results from the initial screening round of the IMPACT study. Eur. Urol. 2014, 66, 489–499. [Google Scholar] [CrossRef]
- Mersch, J.; Jackson, M.A.; Park, M.; Nebgen, D.; Peterson, S.K.; Singletary, C.; Arun, B.K.; Litton, J.K. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 2015, 121, 269–275. [Google Scholar] [CrossRef]
- van Asperen, C.J.; Brohet, R.M.; Meijers-Heijboer, E.J.; Hoogerbrugge, N.; Verhoef, S.; Vasen, H.F.; Ausems, M.G.; Menko, F.H.; Gomez Garcia, E.B.; Klijn, J.G.; et al. Cancer risks in BRCA2 families: Estimates for sites other than breast and ovary. J. Med. Genet. 2005, 42, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; O’Hara, C.; Khan, S.; Shack, L.; Woodward, E.; Maher, E.R.; Lalloo, F.; Evans, D.G. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam. Cancer 2012, 11, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kadouri, L.; Hubert, A.; Rotenberg, Y.; Hamburger, T.; Sagi, M.; Nechushtan, C.; Abeliovich, D.; Peretz, T. Cancer risks in carriers of the BRCA1/2 Ashkenazi founder mutations. J. Med. Genet. 2007, 44, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Risch, H.A.; McLaughlin, J.R.; Cole, D.E.; Rosen, B.; Bradley, L.; Kwan, E.; Jack, E.; Vesprini, D.J.; Kuperstein, G.; Abrahamson, J.L.; et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am. J. Hum. Genet. 2001, 68, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Lu, T.; Pan, J.; Guo, L.; Pang, Y.; Liu, P. Association between BRCA mutations and endometrial carcinoma: A systematic review with meta-analysis. Arch. Gynecol. Obstet. 2021, 303, 1569–1579. [Google Scholar] [CrossRef]
- Matanes, E.; Volodarsky-Perel, A.; Eisenberg, N.; Rottenstreich, M.; Yasmeen, A.; Mitric, C.; Lau, S.; Salvador, S.; Gotlieb, W.H.; Kogan, L. Endometrial Cancer in Germline BRCA Mutation Carriers: A Systematic Review and Meta-analysis. J. Minim. Invasive. Gynecol. 2021, 28, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Alkhushaym, N.; Fallatah, S.; Althagafi, A.; Aljadeed, R.; Alsowaida, Y.; Jeter, J.; Martin, J.R.; Babiker, H.M.; McBride, A.; et al. The association of BRCA1 and BRCA2 mutations with prostate cancer risk, frequency, and mortality: A meta-analysis. Prostate 2019, 79, 880–895. [Google Scholar] [CrossRef]
- Eccles, D.M.; Mitchell, G.; Monteiro, A.N.; Schmutzler, R.; Couch, F.J.; Spurdle, A.B.; Gomez-Garcia, E.B.; Group, E.C.W. BRCA1 and BRCA2 genetic testing-pitfalls and recommendations for managing variants of uncertain clinical significance. Ann. Oncol. 2015, 26, 2057–2065. [Google Scholar] [CrossRef]
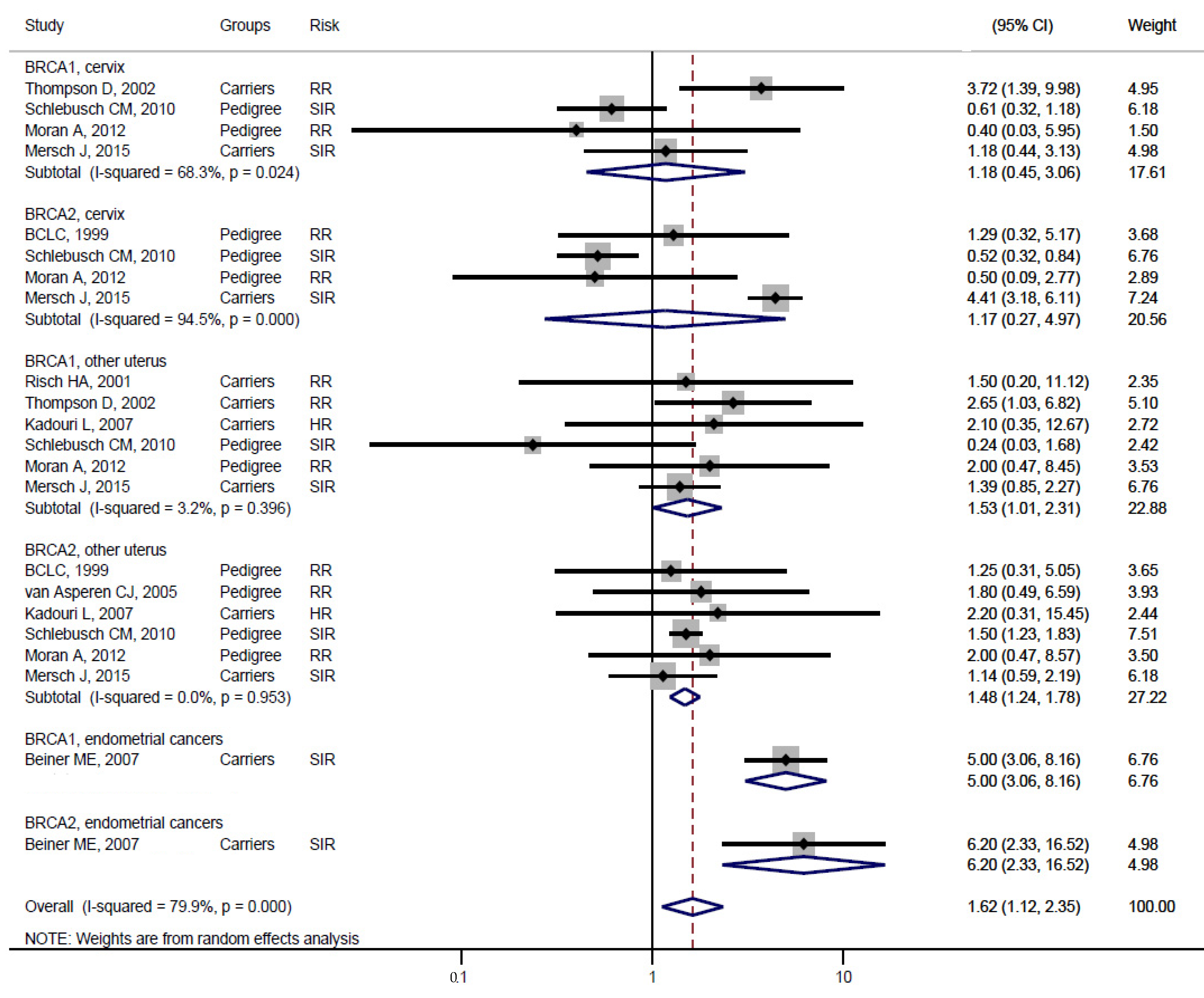
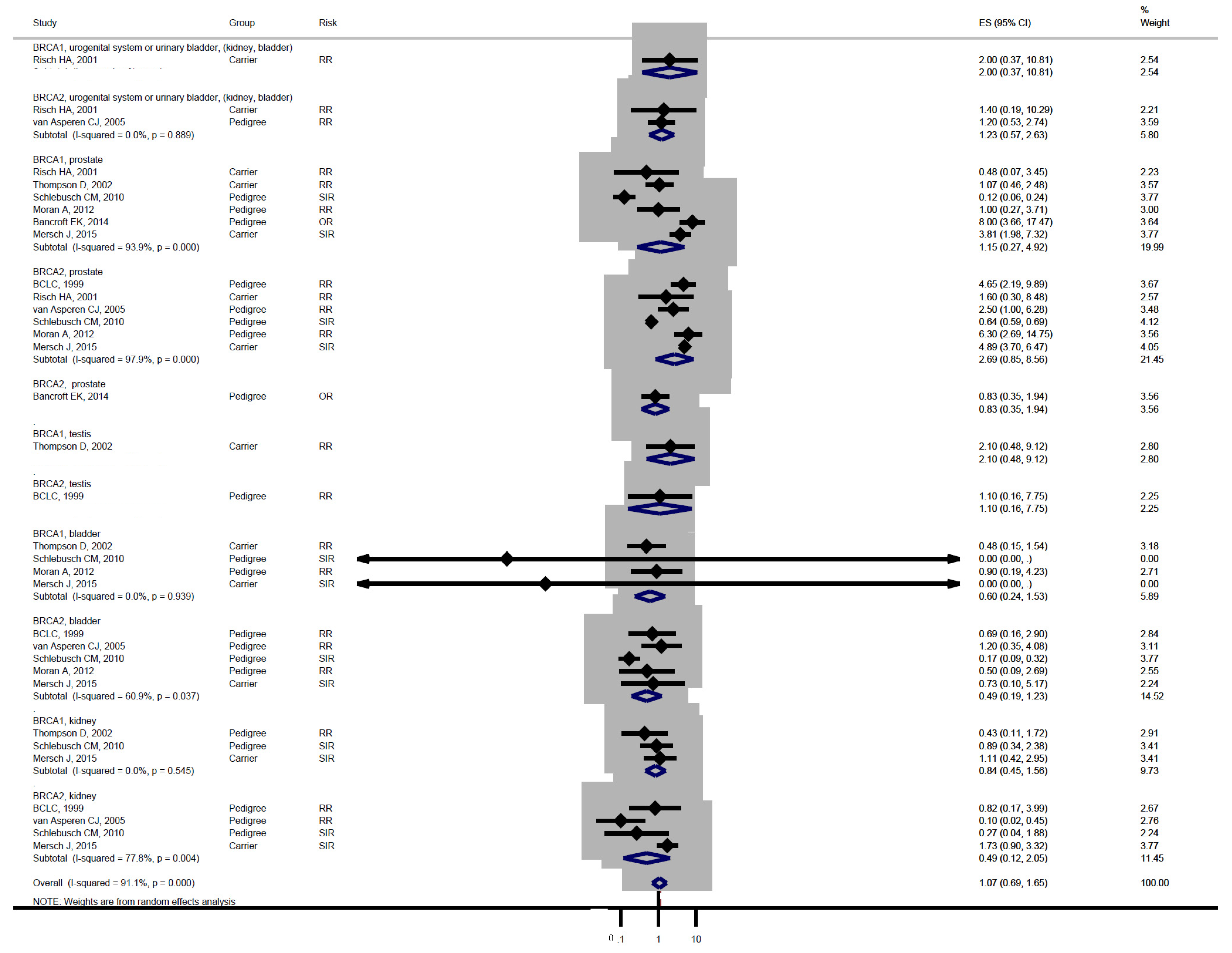
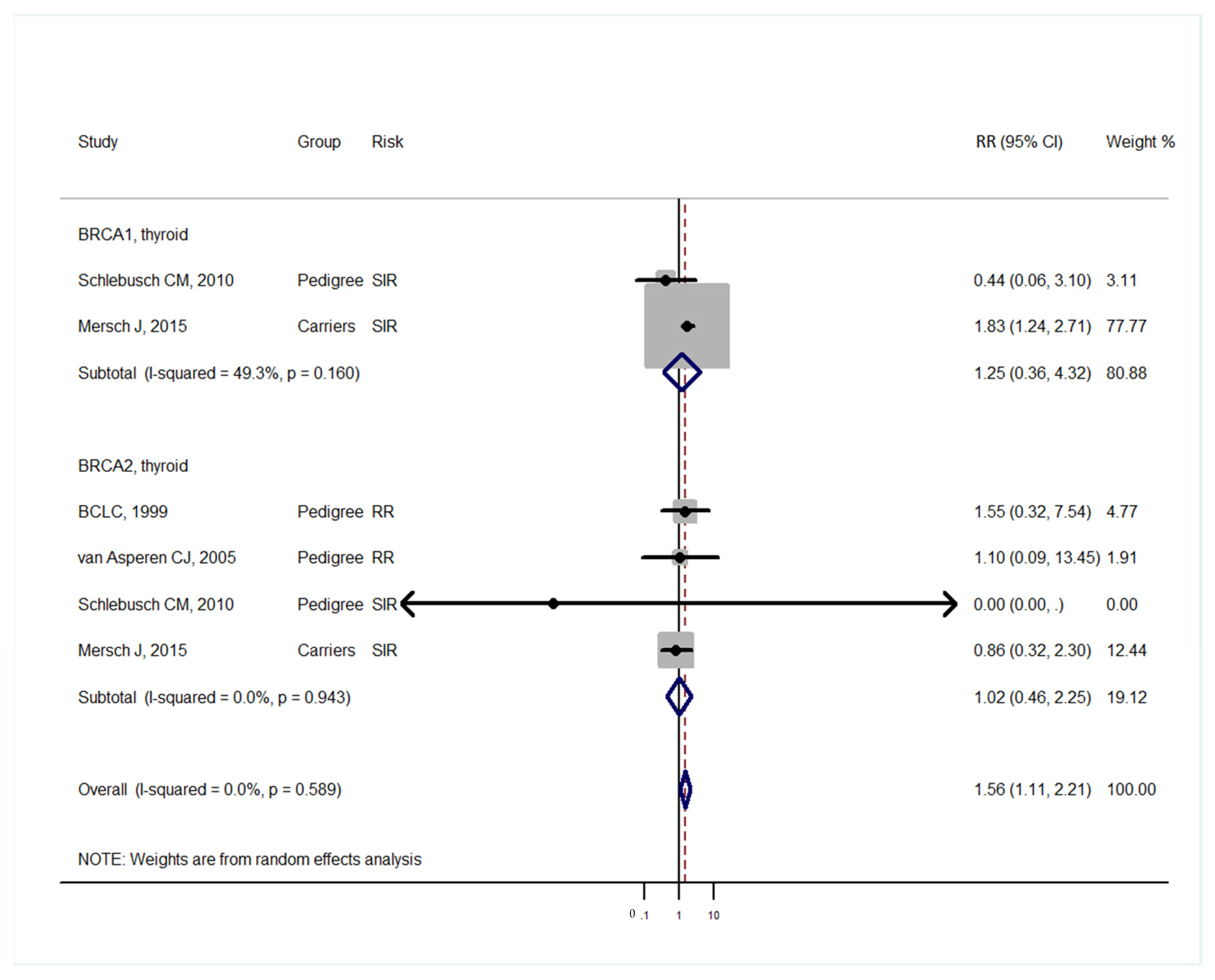
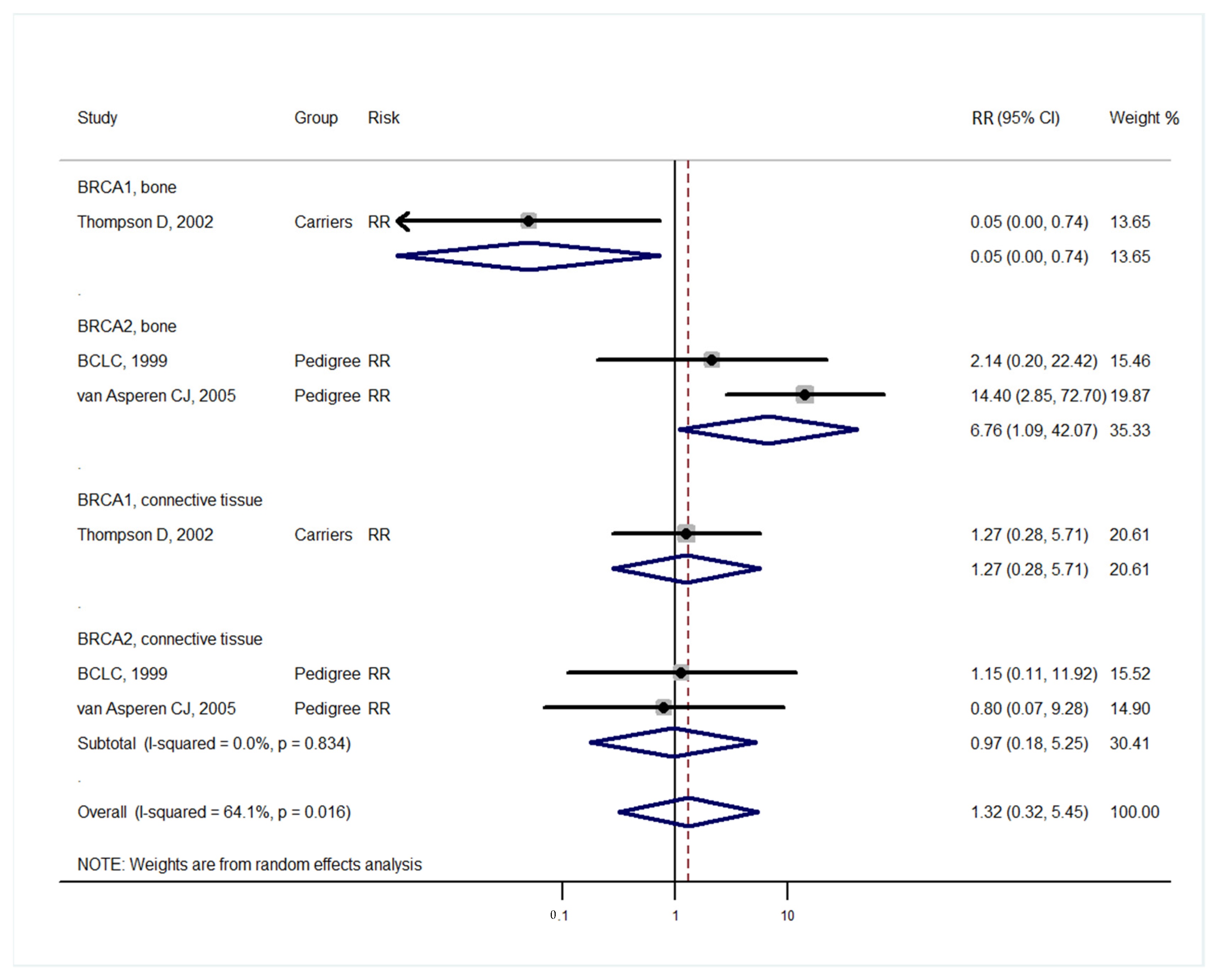

| Studies | Study Design | BRCA Carrier | Mean Age (Range) | Sex Distribution | Control | Median Follow-Up | Country (Population) |
|---|---|---|---|---|---|---|---|
| BCLC, 1999 [4] | Retrospective cohort | Kindred BRCA2 3728 Pedigree studies | - | - | Cancer incidence in five continents | - | BCLC, Western Europe, the US and Canada |
| Risch HA, 2001 [22] | Cohort studies | Direct sequencing | BRCA1 51.2 BRCA2 57.5 | Female only | Relatives of cases not carrying mutation | - | Toronto |
| Thompson D, 2002 [3] | Cohort study | Direct sequencing BRCA 2245 | - | - | Cancer incidence in five continents | 26.12 years (P-Y) | BCLC, Western Europe, the US and Canada |
| van Asperen CJ, 2005 [19] | Retrospective cohort | Pathogenic BRCA2 mutation 50% prior probability (pedigree) | - | 1088 (56%)/803 (44%) | Dutch cancer incidence rates | - | Netherlands |
| Beiner ME, 2007 [13] | Prospective cohort | Direct sequencing BRCA1 619 BRCA2 236 Both 2 | 54.4 (45–70) | Female only | Cancer incidence in five continents | 3.3 y | University of Toronto, multicentre North American, Europe and Israel |
| Kadouri L, 2007 [21] | Cohort studies Ashkenazi | BRCA1 229 BRCA2 100 Excluded both mutations | - | Female only | Ashkenazi non-carrier | 52.8, 57 years (P-Y) | Hadassah Medical Centre (Jerusalem, Israel) |
| Schlebusch CM, 2010 [14] | Cohort study | Pedigrees | - | 3682 (all), 57% female | National Cancer Registry | - | Clinic at the University of Pretoria, South African families |
| Moran A, 2012 [20] | Prospective cohort | Pedigree included BRCA1 1,15 BRCA2 1526 | - | 1100/715 931/595 | Northwest of England (1975–2005) | 22.6 years (P-Y) | Regional Genetics Clinics at Manchester and Birmingham (Northwest and West Midlands in England) |
| Mocci E, 2013 [15] | Cohort study | Deleterious BRCA1 and BRCA2 mutations and pedigree BRCA1 11,946 BRCA2 7773 | - | 6490/5457 4271/3502 | Cancer incidence in five continents | - | Six centres in the United States (Northern California Breast Cancer Family Registry, New York site of the BCFR, Utah site of the BCFR, Philadelphia site of the BCFR), Canada (Ontario Familial Breast Cancer Family Registry) and Australia (Australian Breast Cancer Family Registry). |
| Phelan CM, 2013 [16] | Prospective | BRCA deleterious mutation BRCA1 5481 BRCA2 1474 Both 60 | 47.3 (30–74) | Female only | Cancer incidence in five continents | 5.5 years (mean) | Five countries from Canada, the United States or Europe |
| Bancroft EK, 2014 [17] | Prospective cohort | 50% inheriting a mutation, pedigree BRCA1 791 BRCA2 731 | 60.1 59.8 | Male only | Family member test negative | ≥5 years | Multicentre, the Royal Marsden Hospital NHS Foundation Trust, St Mary’s Hospital, Manchester; The Princess Anne Hospital, Southampton; and Addenbrooke’s Hospital, Cambridge. |
| Mersch J, 2015 [18] | Prospective | BRCA deleterious mutation, variants included Excluded both mutations | Mean age last contact 49.36 (range, 17–90 years) | 584 (57.82)/29 (46.77) 426 (42.18)/33 (53.23) | United States Cancer Statistics: 1999–2010 Incidence and Mortality Web-Based Report | - | MD Anderson Cancer Centre |
| Studies | Representativeness of the BRCA Gene | Selection of Non-BRCA or General Population | Ascertainment of BRCA | Demonstration That Cancer Was Not Present Initially | Study Controls for Initial Age and/or an Additional Factor | Assessment of Outcome | Was Median Follow-Up 5 Years or More? | Adequacy of Follow-Up (>80%) | Total |
|---|---|---|---|---|---|---|---|---|---|
| BCLC, 1999 [4] | ★ | ★ | ★ | ★ | ★★ | — | — | ★ | 7 |
| Risch HA, 2001 [22] | ★ | ★ | ★ | ★ | —— | — | — | — | 4 |
| Thompson D, 2002 [3] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | — | 8 |
| van Asperen CJ, 2005 [19] | ★ | ★ | ★ | ★ | ★★ | ★ | — | — | 7 |
| Beiner ME, 2007 [13] | ★ | ★ | ★ | ★ | ★— | — | — | — | 5 |
| Kadouri L, 2007 [21] | ★ | ★ | ★ | ★ | —— | — | ★ | — | 5 |
| Schlebusch CM, 2010 [14] | ★ | ★ | ★ | ★ | ★— | — | — | — | 5 |
| Moran A, 2012 [20] | ★ | ★ | ★ | ★ | ★ ★ | ★ | ★ | — | 8 |
| Mocci E, 2013 [15] | ★ | ★ | ★ | ★ | ★★ | — | — | — | 6 |
| Phelan CM, 2014 [16] | ★ | ★ | ★ | ★ | ★★ | — | ★ | — | 7 |
| Bancroft EK, 2014 [17] | ★ | ★ | ★ | ★ | —— | ★ | ★ | — | 6 |
| Mersch J, 2015 [18] | ★ | ★ | ★ | ★ | ★— | ★ | — | ★ | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Lee, Y.-L.; Li, C.-Y. BRCA Genes and Related Cancers: A Meta-Analysis from Epidemiological Cohort Studies. Medicina 2021, 57, 905. https://doi.org/10.3390/medicina57090905
Lee Y-C, Lee Y-L, Li C-Y. BRCA Genes and Related Cancers: A Meta-Analysis from Epidemiological Cohort Studies. Medicina. 2021; 57(9):905. https://doi.org/10.3390/medicina57090905
Chicago/Turabian StyleLee, Yen-Chien, Yen-Ling Lee, and Chung-Yi Li. 2021. "BRCA Genes and Related Cancers: A Meta-Analysis from Epidemiological Cohort Studies" Medicina 57, no. 9: 905. https://doi.org/10.3390/medicina57090905
APA StyleLee, Y.-C., Lee, Y.-L., & Li, C.-Y. (2021). BRCA Genes and Related Cancers: A Meta-Analysis from Epidemiological Cohort Studies. Medicina, 57(9), 905. https://doi.org/10.3390/medicina57090905







