Abstract
Biliary and pancreatic cancers occur silently in the initial stage and become unresectable within a short time. When these diseases become symptomatic, biliary obstruction, either with or without infection, occurs frequently due to the anatomy associated with these cancers. The endoscopic management of these patients has changed, both with time and with improvements in medical devices. In this review, we present updated and integrated concepts for the endoscopic management of malignant biliary stricture. Endoscopic biliary drainage had been indicated in malignant biliary obstruction, but the concept of endoscopic management has changed with time. Although routine endoscopic stenting should not be performed in resectable malignant distal biliary obstruction (MDBO) patients, endoscopic biliary drainage is the treatment of choice for palliation in unresectable MDBO patients. Self-expanding metal stents (SEMS) have better stent patency and lower costs compared with plastic stents (PS). For malignant hilum obstruction, PS and uncovered SEMS yield similar short-term outcomes, while a covered stent is not usually used due to a potential unintentional obstruction of contralateral ducts.
1. Introduction
Percutaneous transhepatic biliary drainage was introduced ~40 years ago [1] for the treatment of obstructive jaundice; however, the more convenient endoscopic trans-papillary drainage, which was introduced in 1981 [2,3], is more commonly used in clinical practice today. The most common malignancy diseases that can cause obstructive jaundice are cholangiocarcinoma, pancreatic cancer [4], and ampullary neoplasms. Cholangiocarcinoma, which arises from the epithelial cells of the intrahepatic or extrahepatic bile ducts, can be divided into intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC). The incidence of ECC has increased in the USA [5], whereas ICC incidence has increased in both sexes in Europe [6]. At the same time, the pancreatic cancer burden has also increased in recent years [7], making endoscopic management of malignant obstructive jaundice an important issue. Due to the lack of effective treatment choice, the need for endoscopic/radiologic approaches has increased and the clinical condition may be changed due to emerging evidence in targeted therapies of cholangiocarcinoma in the following years [8].
Differences in the level of obstruction of the biliary system allow for a further division of these kinds of problems into resectable or unresectable hilum or distal biliary obstructions. Computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography (MRCP), and even endoscopic ultrasound sonography (EUS) should be used to evaluate the stage and primary source of a malignancy [9,10].
2. Resectable Malignancy
Treatment plans for malignancies with different primary origins differ from one another when resectable biliary obstructive malignancy diseases are discussed. In general, routine endoscopic stenting has no obvious clinical benefits for patients with malignant distal biliary obstruction (MDBO) [11,12,13,14]. Nevertheless, most endoscopists worldwide still perform routine endoscopic retrograde cholangiopancreatography (ERCP) and even biliary stenting prior to surgical approaches.
For cholangiocarcinoma, the Bismuth classifications (Figure 1) have been used for decades as a treatment guide [15]. ERCP is useful for confirmation of the obstruction level and for histological proof via either brushing cytology or biopsy, although these have limited sensitivity [16,17]. Mother–baby cholangioscopy [18,19,20] and single-operator SpyGlass cholangioscopy [21] plus targeted biopsy can improve the sensitivity for detecting a biliary malignancy to 89–100% and 66.2% and the specificity to 87–96% and 97.0%, respectively. For Bismuth type I lesions without lymph node and distant metastasis, surgical resection should be considered a first approach.
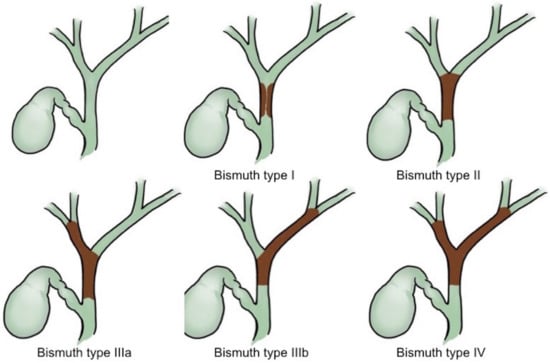
Figure 1.
Bismuth classifications of cholangiocarcinoma.
For hilum cholangiocarcinoma without distant metastasis, resectable tumors present the possibility of resection of the involved intra- and extrahepatic bile ducts as well as the associated hepatic lobes and caudate lobe. Some recent reports have shown excellent results after surgical resection in patients with Bismuth III and IV disease [22,23], but the surgical management of these patients is still under debate.
Endoscopic stenting is the mainstream endoscopic management approach for malignant distal biliary obstruction of cholangiocarcinoma or pancreatic cancers, but most clinical studies [11,24] and meta-analyses [12,13,14] have failed to show any benefits of routine pre-operative endoscopic stenting in MDBO patients. However, although this stenting should be avoided in resectable MDBO, an interesting study showed that more than 80% of doctors in academic centers in the USA still perform this drainage prior to surgery [25]. In pancreatic cancer patients with asymptomatic obstructive jaundice, the recommendation of the Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy (ASGE) is against routine preoperative ERCP [9].
In ampullary neoplasms, EUS and intraductal ultrasound sonography (IDUS) can assess the depth of invasion as well as the intraductal extension. Importantly, surgical ampullectomy can reduce medical expenses yet have similar clinical outcomes for those that are achieved via pancreaticoduodenectomy [26] for ampullary neoplasms that are limited to the ampulla of Vater. Endoscopic ampullectomy [27] can be performed for adenoma and early ampullary adenocarcinoma by experienced endoscopists in well-equipped endoscopy centers.
3. Unresectable Malignancy
3.1. Malignant Distal Biliary Obstruction
Endoscopic biliary drainage with biliary stent placement is the treatment of choice for palliation in patients with distal malignant biliary obstruction that is caused by unresectable neoplasms, with a success rate of up to 95% [28,29,30] and with a lower morbidity compared to surgery [31]. Endoscopic drainage has a low risk of complications, but it is associated with a high probability of recurrent biliary obstruction [32] when compared with surgical bypass. After an endobiliary radiofrequency ablation (RFA) system was applied to biliary malignant obstructions, series intra-luminal RFA and self-expanding metal stents (SEMS) became a safe treatment choice with better intervention-free survival compared to SEMS alone [33,34,35]. Percutaneous trans-hepatic cholangiography and drainage (PTCD) should be reserved for unsuccessful endoscopic procedures in patients with MDBO.
In some situations, PTCD has been used as part of a rendezvous endoscopic approach. A comparison in 2016 between EUS-guided and PTCD rendezvous drainage after the failure of primary ERCP in MDBO showed that EUS rendezvous had a significantly lower success rate than the PTCD rendezvous [36]. However, successful EUS rendezvous offered a significantly shorter post-procedure hospital stay and fewer follow-up biliary interventions. As technology improves and skills in EUS-guided biliary drainage gradually mature, EUS-guided biliary drainage will produce better outcomes than that of conventional ERCP biliary drainage in high-tech endoscopy centers [37,38,39].
3.2. Stent Selection
Different stent types are available for the treatment of MDBO. SEMS (Figure 2) have a larger luminal diameter than plastic stents (Figure 3), and they were designed to overcome the limitations of occlusion and stent patency that are associated with plastic stents [40]. SEMS offer a better stent patency of 3.6–9.1 months compared to 1.8–5.5 months for plastic stents [41,42,43,44] in MDBO, but the median patient survival times are similar with either stent type [45]. SEMS are more expensive than plastic stents, but SEMS lower the total medical expenses [41,46] due to the reduced frequency of re-interventions. Different types of metal stents are now available, and covered stents show superior stent patency over uncovered metal stents in the treatment of MDBO [47]. Some previous case studies showed no significant differences in stent patency between covered and uncovered SEMS because covered stents had more drawbacks due either to stent migration or the occurrence of acute cholecystitis [48,49,50]. Recent studies have revealed that fully covered SEMS (FCSEMS) that are used for MDBO treatment have low stent migration rates and trigger few cholecystitis events [51]. A prospective study of unresectable pancreatic cancer with obstructive jaundice demonstrated a significantly longer survival time and no stent dysfunction for covered SEMS with an anti-migration system than it did without an anti-migration system [52,53]. These studies suggest that covered SEMS are a valuable and cost-effective option for MDBO treatment because of their increased patency, less tumor in-growth, and easy removal [53,54]. However, several recent large studies [55,56,57] and a recent meta-analysis from Canada [58] have revealed no clear benefit of FCSEMS over uncovered SEMS (UCSEMS) in terms of stent patency and complications. Conversely, one multi-center study from Italy showed increased stent migration and even earlier stent occlusion with FCSEMS [59], while two other studies revealed no stent patency benefit between partially covered SEMS (PCSEMS) and UCSEMS [60,61]. Most studies to date on MDBO have shown no significant differences in stent patency between FCSEMS, PCSMES, or UCSEMS. Notably, while FCSEMS is used by many well-recognized endoscopists, its superiority is still under debate.
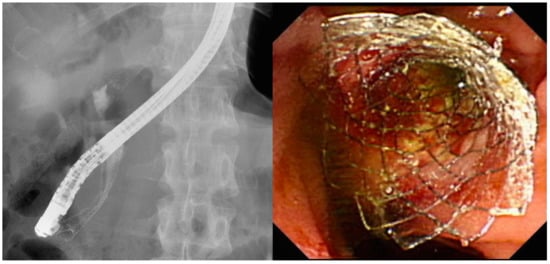
Figure 2.
Self-expanding metal stents for use in pancreatic cancers with malignant distal biliary obstruction.
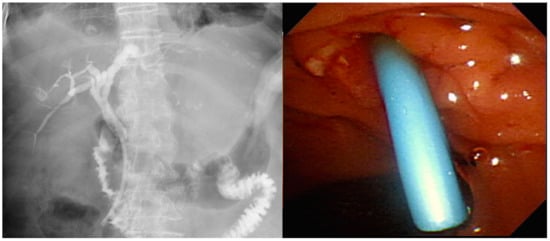
Figure 3.
Plastic stent for use in pancreatic cancers with malignant distal biliary obstruction.
4. Malignant Hilar Biliary Obstruction
EUS is a valuable diagnostic modality for the staging of hilum cholangiocarcinomas, particularly for the evaluation of unresectable perihilar cholangiocarcinoma for liver transplantation [62]. Percutaneous laparoscopic biopsy and even EUS-fine-needle aspiration (EUS-FNA) of the primary tumor are not recommended in patients who are candidates for liver transplantation because of the high risk of peritoneal dissemination following these procedures [63]. Endoscopic biliary stenting for malignant obstructive jaundice offers symptomatic improvements [64,65] and has been widely accepted as an effective palliation treatment [31,66], but no widespread consensus has yet been reached regarding the optimal stent types and placement procedures for stent insertion [67]. Liver volume drainage in excess of 50% should be achieved using multiple stenting to improve survival [68]. Selection of the drainage method and stent requires the use of CT, MRI, or MRCP.
4.1. Bilateral or Unilateral Drainage
Some evidence indicates that in cases of bilateral biliary opacification, bilateral drainage offers a better survival benefit when compared to unilateral drainage [69,70]. After adjustment for the Bismuth stage, two parallel SEMS should be deployed for advanced cholangiocarcinomas such as Bismuth types III and IV obstructions [71]. PTCD is considered an alternative choice if primary endoscopic drainage fails, and some evidence has shown that PTCD is an even better choice for advanced hilum obstruction, which occurs in Bismuth types III and IV patients with unresectable malignant obstructions [71,72,73,74].
The evidence is still insufficient and no clear consensus has been reached regarding the benefits of unilateral versus bilateral drainage for hilar malignant obstruction, although the bilateral approach can be used by most experts using the SEMS stent-within-stent placement [75,76] with the newly designed Y-shaped devices, and this shows promising results [77,78]. These procedures can be performed using parallel [79] (Figure 4a) or stent-in-stent [80] (Figure 4b) deployment of SEMS, but not enough evidence is available to identify which method is preferable. Previous data have shown that draining 25% of the liver volume was sufficient to relieve jaundice [81]; however, a recent study indicated that patients who had liver volume drainage of >50% experienced greater jaundice relief than those who had a lower volume drained [68]. However, because the right lobe of the liver covers 55–60% of the liver volume, while the left and caudate lobes cover 30–35% and 10% of the liver volume, respectively [82], draining >50% of the liver volume usually requires the use of bilateral stenting or multi-segmental stenting, depending on the individual patient’s anatomy.
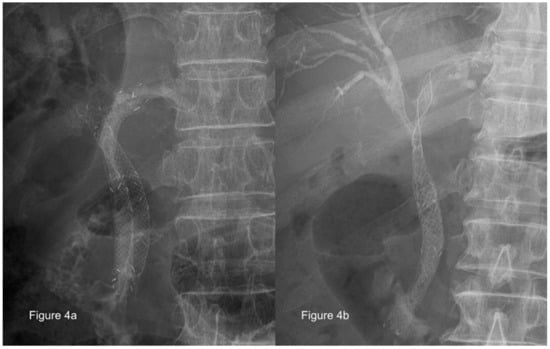
Figure 4.
Parallel or stent-in-stent deployment of self-expanding metal stents for use in treatment of malignant hilar biliary obstruction (Courtesy of Dr. Nai-Jen Liu). (a) Parallel deployment; (b) stent-in-stent deployment
4.2. Stent Selection
Plastic stents and uncovered SEMS yield similar short-term outcomes in patients with malignant hilar strictures due to stent migration in SEMS [83]. SEMS provide a longer biliary patency when compared with plastic stents; thus, they are the choice of most endoscopists [64,69,74,84]. Covered stents are not usually used in patients with malignant hilar biliary obstruction due to the possibility of unintentional obstruction of the contralateral and/or side branch ducts.
4.3. Rescue Management
EUS-guided biliary drainage via EUS-guided hepaticogastrostomy (EUS-HGS) is a good alternative for draining malignant hilar biliary obstruction following the failure of an initial ERCP. An EUS-guided rendezvous procedure with the conventional ERCP access [85,86] is typically used in most situations, except for concurrent duodenal obstruction. EUS-HGS [87,88] is the preferred treatment option if either a guidewire cannot pass through the duodenum or the presence of a concurrent duodenal obstruction is found. Another alternative approach to EUS-HGS is EUS-guided choledochoduodenostomy (EUS-CDS) [89], which offers management methods other than PTCD. Due to the high rate of complications, EUS-guided biliary drainage has served as an alternative approach over the last two years; however, some encouraging results have shown a similar safety profile and procedure success rate and even better stent patency during that same timeframe [36,37,90].
5. Summary
In cases of malignant biliary obstruction, CT, MRI, MRCP, and EUS should be used for tumor staging and further drainage planning. For resectable malignant biliary obstruction lesions, routine endoscopic drainage or ERCP are not advised, except in cases of concurrent infection. Endoscopic drainage is the treatment of choice for unresectable biliary obstructions. PTCD and EUS-guided biliary drainage can serve as alternative approach methods. EUS-guided biliary drainage can provide equal clinical benefits without increasing complications in a high-tech endoscopy center. In addition, PTCD plays an important role in community hospitals, where the ERCP and/or EUS approaches are not available.
For MDBO, single-stent insertion is adequate, and SEMS have better patency than plastic stents; however, the superiority of FCSEMS, PCSEMS, and UCSEMS remains under debate. For malignant hilar biliary obstructions, drainage of >50% of the liver volume should be achieved by either bilateral stenting or multi-segmental stenting, and SEMS’ longer biliary patency when compared with plastic stents should be considered. The general approaches to malignant biliary obstruction are summarized in the flow chart presented in Figure 5.
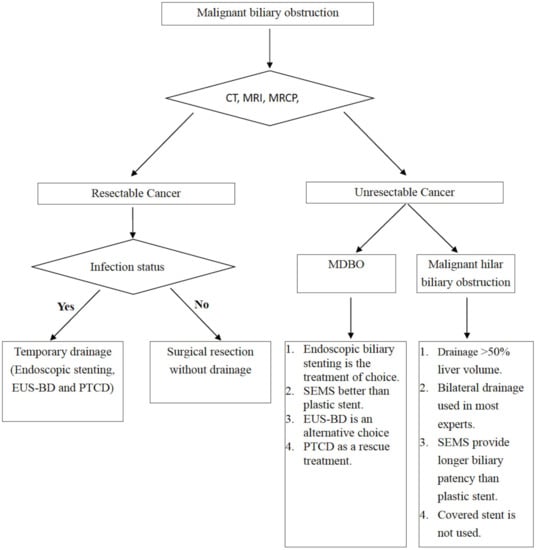
Figure 5.
Flow chart for the management of malignant biliary obstruction.
Author Contributions
C.-C.W. and M.-C.T. contributed to the conception and design; C.-C.W., W.-W.S., and T.-W.Y. contributed to the analysis and interpretation of previous literature; C.-C.W. and W.-W.S. contributed to drafting the manuscript; Yang TW and M.-C.T. contributed to critical revision of the manuscript; and M.-C.T. contributed to supervision. All authors have read and agreed to the published version of the manuscript.
Funding
Chung Shan Medical University Hospital research program, Taichung, Taiwan (CSH-2013-C-032).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
ASGE: American Society for Gastrointestinal Endoscopy; CT: computed tomography; ECC: extrahepatic cholangiocarcinoma; ERCP: endoscopic retrograde cholangiopancreatography; EUS: endoscopic ultrasound sonography; EUS-FNA: endoscopic ultrasound sonography–fine-needle aspiration; EUS-BD: endoscopic ultrasound-guided biliary drainage; FCSEMS: fully covered self-expanding metal stent; ICC: intrahepatic cholangiocarcinoma; IDUS: intraductal ultrasound sonography; MDBO: malignant distal biliary obstruction; MRCP: magnetic resonance cholangiopancreatography; MRI: magnetic resonance imaging; PCSEMS: partially covered self-expanding metal stent; PS: plastic stent; PTCD: percutaneous trans-hepatic cholangiography and drainage; SEMS: self-expanding metal stent; UCSEMS: uncovered self-expanding metal stent.
References
- Pinchuk, L.; Magnanini, F.; Nardi, G.; Bosolino, A.; Rubio, H. Percutaneous transhepatic biliary drainage in obstructive jaundice. Acta Gastroenterol. Latinoam 1981, 11, 279–284. [Google Scholar]
- Manegold, B.C. Obstructive jaundice of benign and malignant origin: Endoscopy in diagnosis and therapy. Langenbecks Arch. Chir. 1981, 355, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Safrany, L.; Schott, B.; Krause, S.; Balint, T.; Portocarrero, G. Endoscopic transpapillary bile duct drainage in malignant obstructive jaundice. Dtsch. Med. Wochenschr. 1982, 107, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Roque, J.; Ho, S.H.; Goh, K.L. Preoperative drainage for malignant biliary strictures: Is it time for self-expanding metallic stents? Clin. Endosc. 2015, 48, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.L.; Ilyas, J.A.; Duan, Z.; Green, L.K.; Younes, M.; El-Serag, H.B.; Davila, J.A. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig. Dis. Sci. 2014, 59, 3103–3110. [Google Scholar] [CrossRef]
- Bertuccio, P.; Bosetti, C.; Levi, F.; Decarli, A.; Negri, E.; La Vecchia, C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann. Oncol. 2013, 24, 1667–1674. [Google Scholar] [CrossRef]
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef]
- Simile, M.M.; Bagella, P.; Vidili, G.; Spanu, A.; Manetti, R.; Seddaiu, M.A.; Babudieri, S.; Madeddu, G.; Serra, P.A.; Altana, M.; et al. Targeted Therapies in Cholangiocarcinoma: Emerging Evidence from Clinical Trials. Medicina 2019, 55, 42. [Google Scholar] [CrossRef]
- American Society for Gastrointestinal Endoscopy Standards of Practice Committee; Anderson, M.A.; Appalaneni, V.; Ben-Menachem, T.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; Fisher, L.R.; et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest. Endosc. 2013, 77, 167–174. [Google Scholar] [CrossRef]
- De Angelis, C.; Marietti, M.; Bruno, M.; Pellicano, R.; Rizzetto, M. Endoscopic ultrasound in common bile duct dilatation with normal liver enzymes. World J. Gastrointest. Endosc. 2015, 7, 799–805. [Google Scholar] [CrossRef]
- Van der Gaag, N.A.; Rauws, E.A.; van Eijck, C.H.; Bruno, M.J.; van der Harst, E.; Kubben, F.J.; Gerritsen, J.J.; Greve, J.W.; Gerhards, M.F.; de Hingh, I.H.; et al. Preoperative biliary drainage for cancer of the head of the pancreas. N. Engl. J. Med. 2010, 362, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Velanovich, V.; Kheibek, T.; Khan, M. Relationship of postoperative complications from preoperative biliary stents after pancreaticoduodenectomy. A new cohort analysis and meta-analysis of modern studies. JOP 2009, 10, 24–29. [Google Scholar] [PubMed]
- Qiu, Y.D.; Bai, J.L.; Xu, F.G.; Ding, Y.T. Effect of preoperative biliary drainage on malignant obstructive jaundice: A meta-analysis. World J. Gastroenterol. 2011, 17, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sewnath, M.E.; Karsten, T.M.; Prins, M.H.; Rauws, E.J.; Obertop, H.; Gouma, D.J. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann. Surg. 2002, 236, 17–27. [Google Scholar] [CrossRef]
- Bismuth, H.; Nakache, R.; Diamond, T. Management strategies in resection for hilar cholangiocarcinoma. Ann. Surg. 1992, 215, 31–38. [Google Scholar] [CrossRef]
- De Bellis, M.; Sherman, S.; Fogel, E.L.; Cramer, H.; Chappo, J.; McHenry, L., Jr.; Watkins, J.L.; Lehman, G.A. Tissue sampling at ERCP in suspected malignant biliary strictures (Part 1). Gastrointest. Endosc. 2002, 56, 552–561. [Google Scholar] [CrossRef]
- Fogel, E.L.; deBellis, M.; McHenry, L.; Watkins, J.L.; Chappo, J.; Cramer, H.; Schmidt, S.; Lazzell-Pannell, L.; Sherman, S.; Lehman, G.A. Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: A prospective study. Gastrointest. Endosc. 2006, 63, 71–77. [Google Scholar] [CrossRef]
- Fukuda, Y.; Tsuyuguchi, T.; Sakai, Y.; Tsuchiya, S.; Saisyo, H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest. Endosc. 2005, 62, 374–382. [Google Scholar] [CrossRef]
- Shah, R.J.; Langer, D.A.; Antillon, M.R.; Chen, Y.K. Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin. Gastroenterol. Hepatol. 2006, 4, 219–225. [Google Scholar] [CrossRef]
- Osanai, M.; Itoi, T.; Igarashi, Y.; Tanaka, K.; Kida, M.; Maguchi, H.; Yasuda, K.; Okano, N.; Imaizumi, H.; Itokawa, F. Peroral video cholangioscopy to evaluate indeterminate bile duct lesions and preoperative mucosal cancerous extension: A prospective multicenter study. Endoscopy 2013, 45, 635–642. [Google Scholar] [CrossRef]
- Navaneethan, U.; Hasan, M.K.; Lourdusamy, V.; Njei, B.; Varadarajulu, S.; Hawes, R.H. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: A systematic review. Gastrointest. Endosc. 2015, 82, 608–614e602. [Google Scholar] [CrossRef] [PubMed]
- Zaydfudim, V.M.; Clark, C.J.; Kendrick, M.L.; Que, F.G.; Reid-Lombardo, K.M.; Donohue, J.H.; Farnell, M.B.; Nagorney, D.M. Correlation of staging systems to survival in patients with resected hilar cholangiocarcinoma. Am. J. Surg. 2013, 206, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.W.; Hu, B.S.; Chu, Y.J.; Tan, Y.C.; Ji, X.; Chen, K.; Ding, X.M.; Zhang, A.; Chen, F.; Dong, J.H. One-stage resection for Bismuth type IV hilar cholangiocarcinoma with high hilar resection and parenchyma-preserving strategies: A cohort study. World J. Surg. 2013, 37, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Committee ASoP; Eloubeidi, M.A.; Decker, G.A.; Chandrasekhara, V.; Chathadi, K.V.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; Jue, T.L.; et al. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest. Endosc. 2016, 83, 17–28. [Google Scholar] [CrossRef]
- Yang, D.; Perbtani, Y.B.; An, Q.; Agarwal, M.; Riverso, M.; Chakraborty, J.; Brar, T.S.; Westerveld, D.; Zhang, H.; Chauhan, S.S.; et al. Survey study on the practice patterns in the endoscopic management of malignant distal biliary obstruction. Endosc. Int. Open 2017, 5, E754–E762. [Google Scholar] [CrossRef]
- Quirk, D.M.; Rattner, D.W.; Fernandez-del Castillo, C.; Warshaw, A.L.; Brugge, W.R. The use of endoscopic ultrasonography to reduce the cost of treating ampullary tumors. Gastrointest. Endosc. 1997, 46, 334–337. [Google Scholar] [CrossRef]
- Hernandez, L.V.; Catalano, M.F. Endoscopic papillectomy. Curr. Opin. Gastroenterol. 2008, 24, 617–622. [Google Scholar] [CrossRef]
- Isayama, H.; Nakai, Y.; Kawakubo, K.; Kogure, H.; Hamada, T.; Togawa, O.; Sasahira, N.; Hirano, K.; Tsujino, T.; Koike, K. Endoscopic retrograde cholangiopancreatography for distal malignant biliary stricture. Gastrointest. Endosc. Clin. N. Am. 2012, 22, 479–490. [Google Scholar] [CrossRef]
- Lee, J.H. Self-expandable metal stents for malignant distal biliary strictures. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 463–480. [Google Scholar] [CrossRef]
- Kahaleh, M.; Tokar, J.; Conaway, M.R.; Brock, A.; Le, T.; Adams, R.B.; Yeaton, P. Efficacy and complications of covered Wallstents in malignant distal biliary obstruction. Gastrointest. Endosc. 2005, 61, 528–533. [Google Scholar] [CrossRef]
- Taylor, M.C.; McLeod, R.S.; Langer, B. Biliary stenting versus bypass surgery for the palliation of malignant distal bile duct obstruction: A meta-analysis. Liver Transpl. 2000, 6, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.C.; Morris, E.; Leyden, J.; MacMathuna, P. Malignant distal biliary obstruction: A systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat. Rev. 2007, 33, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.N.; Jeong, S.; Choi, H.J.; Cho, J.H.; Cheon, Y.K.; Park, S.W.; Kim, Y.S.; Lee, D.H.; Moon, J.H. The safety of newly developed automatic temperature-controlled endobiliary radiofrequency ablation system for malignant biliary strictures: A prospective multicenter study. J. Gastroenterol. Hepatol. 2019, 34, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Mizandari, M.; Kumar, J.; Pai, M.; Chikovani, T.; Azrumelashvili, T.; Reccia, I.; Habib, N. Interventional radiofrequency ablation: A promising therapeutic modality in the management of malignant biliary and pancreatic duct obstruction. J. Cancer 2018, 9, 629–637. [Google Scholar] [CrossRef]
- Dutta, A.K.; Basavaraju, U.; Sales, L.; Leeds, J.S. Radiofrequency ablation for management of malignant biliary obstruction: A single-center experience and review of the literature. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 779–784. [Google Scholar] [CrossRef]
- Bill, J.G.; Darcy, M.; Fujii-Lau, L.L.; Mullady, D.K.; Gaddam, S.; Murad, F.M.; Early, D.S.; Edmundowicz, S.A.; Kushnir, V.M. A comparison between endoscopic ultrasound-guided rendezvous and percutaneous biliary drainage after failed ERCP for malignant distal biliary obstruction. Endosc. Int. Open 2016, 4, E980–E985. [Google Scholar] [CrossRef]
- Park, J.K.; Woo, Y.S.; Noh, D.H.; Yang, J.I.; Bae, S.Y.; Yun, H.S.; Lee, J.K.; Lee, K.T.; Lee, K.H. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest. Endosc. 2018, 88, 277–282. [Google Scholar] [CrossRef]
- Paik, W.H.; Lee, T.H.; Park, D.H.; Choi, J.H.; Kim, S.O.; Jang, S.; Kim, D.U.; Shim, J.H.; Song, T.J.; Lee, S.S.; et al. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am. J. Gastroenterol. 2018, 113, 987–997. [Google Scholar] [CrossRef]
- Lou, X.; Yu, D.; Li, J.; Feng, S.; Sun, J.J. Efficacy of endoscopic ultrasound-guided and endoscopic retrograde cholangiopancreatography-guided biliary drainage for malignant biliary obstruction: A systematic review and meta-analysis. Minerva Med. 2019, 110, 564–574. [Google Scholar] [CrossRef]
- Jaganmohan, S.; Lee, J.H. Self-expandable metal stents in malignant biliary obstruction. Expert Rev. Gastroenterol. Hepatol. 2012, 6, 105–114. [Google Scholar] [CrossRef]
- Knyrim, K.; Wagner, H.J.; Pausch, J.; Vakil, N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy 1993, 25, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Prat, F.; Chapat, O.; Ducot, B.; Ponchon, T.; Pelletier, G.; Fritsch, J.; Choury, A.D.; Buffet, C. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest. Endosc. 1998, 47, 1–7. [Google Scholar] [CrossRef]
- Katsinelos, P.; Paikos, D.; Kountouras, J.; Chatzimavroudis, G.; Paroutoglou, G.; Moschos, I.; Gatopoulou, A.; Beltsis, A.; Zavos, C.; Papaziogas, B. Tannenbaum and metal stents in the palliative treatment of malignant distal bile duct obstruction: A comparative study of patency and cost effectiveness. Surg. Endosc. 2006, 20, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, C.; Linder, S. Covered metal versus plastic stents for malignant common bile duct stenosis: A prospective, randomized, controlled trial. Gastrointest. Endosc. 2006, 63, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.J.; Baron, T.H.; Gostout, C.J.; Petersen, B.T.; Farnell, M.B. Palliation of malignant extrahepatic biliary obstruction with plastic versus expandable metal stents: An evidence-based approach. Clin. Gastroenterol. Hepatol. 2004, 2, 273–285. [Google Scholar] [CrossRef]
- Aadam, A.A.; Evans, D.B.; Khan, A.; Oh, Y.; Dua, K. Efficacy and safety of self-expandable metal stents for biliary decompression in patients receiving neoadjuvant therapy for pancreatic cancer: A prospective study. Gastrointest. Endosc. 2012, 76, 67–75. [Google Scholar] [CrossRef]
- Isayama, H.; Komatsu, Y.; Tsujino, T.; Sasahira, N.; Hirano, K.; Toda, N.; Nakai, Y.; Yamamoto, N.; Tada, M.; Yoshida, H.; et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut 2004, 53, 729–734. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, M.H.; Choi, J.S.; Lee, S.S.; Seo, D.W.; Kim, J.H.; Han, J.; Kim, J.C.; Choi, E.K.; Lee, S.K. Covered versus uncovered wallstent for malignant extrahepatic biliary obstruction: A cohort comparative analysis. Clin. Gastroenterol. Hepatol. 2006, 4, 790–796. [Google Scholar] [CrossRef]
- Yoon, W.J.; Lee, J.K.; Lee, K.H.; Lee, W.J.; Ryu, J.K.; Kim, Y.T.; Yoon, Y.B. A comparison of covered and uncovered Wallstents for the management of distal malignant biliary obstruction. Gastrointest. Endosc. 2006, 63, 996–1000. [Google Scholar] [CrossRef]
- Fumex, F.; Coumaros, D.; Napoleon, B.; Barthet, M.; Laugier, R.; Yzet, T.; Le Sidaner, A.; Desurmont, P.; Lamouliatte, H.; Letard, J.C.; et al. Similar performance but higher cholecystitis rate with covered biliary stents: Results from a prospective multicenter evaluation. Endoscopy 2006, 38, 787–792. [Google Scholar] [CrossRef]
- Petersen, B.T.; Kahaleh, M.; Kozarek, R.A.; Loren, D.; Gupta, K.; Kowalski, T.; Freeman, M.; Chen, Y.K.; Branch, M.S.; Edmundowicz, S.; et al. A multicenter, prospective study of a new fully covered expandable metal biliary stent for the palliative treatment of malignant bile duct obstruction. Gastroenterol. Res. Pract. 2013, 2013, 642428. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yamashita, Y.; Tanaka, K.; Konishi, H.; Yazumi, S.; Nakai, Y.; Nishiyama, O.; Uehara, H.; Mitoro, A.; Sanuki, T.; et al. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: A randomized multicenter trial. Am. J. Gastroenterol. 2013, 108, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Fukasawa, M.; Takano, S.; Kadokura, M.; Shindo, H.; Takahashi, E.; Hirose, S.; Kawakami, S.; Fukasawa, Y.; Sato, T.; et al. Partially covered metal stents have longer patency than uncovered and fully covered metal stents in the management of distal malignant biliary obstruction: A retrospective study. BMC Gastroenterol. 2017, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Kahaleh, M.; Talreja, J.P.; Loren, D.E.; Kowalski, T.E.; Poneros, J.M.; Degaetani, M.; Raijman, I.; Sejpal, D.V.; Patel, S.; Rosenkranz, L.; et al. Evaluation of a fully covered self-expanding metal stent with flared ends in malignant biliary obstruction: A multicenter study. J. Clin. Gastroenterol. 2013, 47, e96–e100. [Google Scholar] [CrossRef]
- Lee, B.S.; Ryu, J.K.; Jang, D.K.; Chung, K.H.; Yoon, W.J.; Kim, J.; Woo, S.M.; Lee, S.H.; Lee, W.J.; Kim, Y.T. Reintervention for occluded metal stent in malignant bile duct obstruction: A prospective randomized trial comparing covered and uncovered metal stent. J. Gastroenterol. Hepatol. 2016, 31, 1901–1907. [Google Scholar] [CrossRef]
- Lee, J.H.; Krishna, S.G.; Singh, A.; Ladha, H.S.; Slack, R.S.; Ramireddy, S.; Raju, G.S.; Davila, M.; Ross, W.A. Comparison of the utility of covered metal stents versus uncovered metal stents in the management of malignant biliary strictures in 749 patients. Gastrointest. Endosc. 2013, 78, 312–324. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Sun, P.; Yu, Q.; Wang, K.; Chang, W.; Song, Z.; Zheng, Q. Covered versus Uncovered Self-Expandable Metal Stents for Managing Malignant Distal Biliary Obstruction: A Meta-Analysis. PLoS ONE 2016, 11, e0149066. [Google Scholar] [CrossRef]
- Almadi, M.A.; Barkun, A.N.; Martel, M. No benefit of covered vs. uncovered self-expandable metal stents in patients with malignant distal biliary obstruction: A meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 27–37.e21. [Google Scholar] [CrossRef]
- Conio, M.; Mangiavillano, B.; Caruso, A.; Filiberti, R.A.; Baron, T.H.; De Luca, L.; Signorelli, S.; Crespi, M.; Marini, M.; Ravelli, P.; et al. Covered versus uncovered self-expandable metal stent for palliation of primary malignant extrahepatic biliary strictures: A randomized multicenter study. Gastrointest. Endosc. 2018, 88, 283–291.e283. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ko, G.B.; Lee, T.H.; Park, S.H.; Lee, Y.N.; Cho, Y.S.; Jung, Y.; Chung, I.K.; Choi, H.J.; Cha, S.W.; et al. Partially Covered Metal Stents May Not Prolong Stent Patency Compared to Uncovered Stents in Unresectable Malignant Distal Biliary Obstruction. Gut Liver 2017, 11, 440–446. [Google Scholar] [CrossRef]
- Yang, M.J.; Kim, J.H.; Yoo, B.M.; Hwang, J.C.; Yoo, J.H.; Lee, K.S.; Kang, J.K.; Kim, S.S.; Lim, S.G.; Shin, S.J.; et al. Partially covered versus uncovered self-expandable nitinol stents with anti-migration properties for the palliation of malignant distal biliary obstruction: A randomized controlled trial. Scand. J. Gastroenterol. 2015, 50, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.J.; Heimbach, J.K.; Gores, G.J. Endoscopic ultrasound staging of cholangiocarcinoma. Curr. Opin. Gastroenterol. 2012, 28, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Sanchez, W.; Rosen, C.B.; Gores, G.J. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB 2011, 13, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Rerknimitr, R.; Angsuwatcharakon, P.; Ratanachu-ek, T.; Khor, C.J.; Ponnudurai, R.; Moon, J.H.; Seo, D.W.; Pantongrag-Brown, L.; Sangchan, A.; Pisespongsa, P.; et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J. Gastroenterol. Hepatol. 2013, 28, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Saluja, S.S.; Gulati, M.; Garg, P.K.; Pal, H.; Pal, S.; Sahni, P.; Chattopadhyay, T.K. Endoscopic or percutaneous biliary drainage for gallbladder cancer: A randomized trial and quality of life assessment. Clin. Gastroenterol. Hepatol. 2008, 6, 944–950.e943. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.T.; Gerdes, H.; Kurtz, R.C. Endoscopic management of occluded biliary Wallstents: A cancer center experience. Gastrointest. Endosc. 2003, 58, 879–884. [Google Scholar] [CrossRef]
- Katanuma, A.; Irisawa, A.; Itoi, T. Otaru consensus on biliary stenting for unresectable malignant hilar biliary obstruction. Dig. Endosc. 2013, 25 (Suppl. 2), 58–62. [Google Scholar] [CrossRef]
- Vienne, A.; Hobeika, E.; Gouya, H.; Lapidus, N.; Fritsch, J.; Choury, A.D.; Chryssostalis, A.; Gaudric, M.; Pelletier, G.; Buffet, C.; et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: The role of liver volume assessment. Gastrointest. Endosc. 2010, 72, 728–735. [Google Scholar] [CrossRef]
- Perdue, D.G.; Freeman, M.L.; DiSario, J.A.; Nelson, D.B.; Fennerty, M.B.; Lee, J.G.; Overby, C.S.; Ryan, M.E.; Bochna, G.S.; Snady, H.W.; et al. Plastic versus self-expanding metallic stents for malignant hilar biliary obstruction: A prospective multicenter observational cohort study. J. Clin. Gastroenterol. 2008, 42, 1040–1046. [Google Scholar] [CrossRef]
- Deviere, J.; Baize, M.; de Toeuf, J.; Cremer, M. Long-term follow-up of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest. Endosc. 1988, 34, 95–101. [Google Scholar] [CrossRef]
- Inal, M.; Akgul, E.; Aksungur, E.; Seydaoglu, G. Percutaneous placement of biliary metallic stents in patients with malignant hilar obstruction: Unilobar versus bilobar drainage. J. Vasc. Interv. Radiol. 2003, 14, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, J.K.; Yoon, W.J.; Lee, J.K.; Ryu, J.K.; Yoon, Y.B.; Kim, Y.T. Optimal biliary drainage for inoperable Klatskin’s tumor based on Bismuth type. World J. Gastroenterol. 2007, 13, 3948–3955. [Google Scholar] [CrossRef] [PubMed]
- Rerknimitr, R.; Kladcharoen, N.; Mahachai, V.; Kullavanijaya, P. Result of endoscopic biliary drainage in hilar cholangiocarcinoma. J. Clin. Gastroenterol. 2004, 38, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Paik, W.H.; Park, Y.S.; Hwang, J.H.; Lee, S.H.; Yoon, C.J.; Kang, S.G.; Lee, J.K.; Ryu, J.K.; Kim, Y.T.; Yoon, Y.B. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: A percutaneous versus endoscopic approach. Gastrointest. Endosc. 2009, 69, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chahal, P.; Baron, T.H. Expandable metal stents for endoscopic bilateral stent-within-stent placement for malignant hilar biliary obstruction. Gastrointest. Endosc. 2010, 71, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Kogure, H.; Isayama, H.; Kawakubo, K.; Sasaki, T.; Yamamoto, N.; Hirano, K.; Sasahira, N.; Tsujino, T.; Tada, M.; Koike, K. Endoscopic bilateral metallic stenting for malignant hilar obstruction using newly designed stents. J. Hepatobiliary Pancreat. Sci. 2011, 18, 653–657. [Google Scholar] [CrossRef]
- Hwang, J.C.; Kim, J.H.; Lim, S.G.; Kim, S.S.; Yoo, B.M.; Cho, S.W. Y-shaped endoscopic bilateral metal stent placement for malignant hilar biliary obstruction: Prospective long-term study. Scand. J. Gastroenterol. 2011, 46, 326–332. [Google Scholar] [CrossRef]
- Kogure, H.; Isayama, H.; Nakai, Y.; Tsujino, T.; Ito, Y.; Yamamoto, K.; Mizuno, S.; Yagioka, H.; Kawakubo, K.; Sasaki, T.; et al. Newly designed large cell Niti-S stent for malignant hilar biliary obstruction: A pilot study. Surg. Endosc. 2011, 25, 463–467. [Google Scholar] [CrossRef]
- Saleem, A.; Baron, T.H.; Gostout, C.J. Large-diameter therapeutic channel duodenoscope to facilitate simultaneous deployment of side-by-side self-expandable metal stents in hilar cholangiocarcinoma. Gastrointest. Endosc. 2010, 72, 628–631. [Google Scholar] [CrossRef]
- Park, D.H.; Lee, S.S.; Moon, J.H.; Choi, H.J.; Cha, S.W.; Kim, J.H.; Seo, D.W.; Lee, S.K.; Park, S.H.; Lee, M.S.; et al. Newly designed stent for endoscopic bilateral stent-in-stent placement of metallic stents in patients with malignant hilar biliary strictures: Multicenter prospective feasibility study (with videos). Gastrointest. Endosc. 2009, 69, 1357–1360. [Google Scholar] [CrossRef]
- Dowsett, J.F.; Vaira, D.; Hatfield, A.R.; Cairns, S.R.; Polydorou, A.; Frost, R.; Croker, J.; Cotton, P.B.; Russell, R.C.; Mason, R.R. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology 1989, 96, 1180–1186. [Google Scholar] [CrossRef]
- Bismuth, H. Surgical anatomy and anatomical surgery of the liver. World J. Surg. 1982, 6, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Moy, B.T.; Birk, J.W. An Update to Hepatobiliary Stents. J. Clin. Transl. Hepatol. 2015, 3, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.J.; Knyrim, K.; Vakil, N.; Klose, K.J. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy 1993, 25, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Gupta, K.; Mallery, S.; Li, R.; Kinney, T.; Freeman, M.L. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: Cumulative experience at a single center. A case series. Endoscopy 2010, 42, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Dhir, V.; Bhandari, S.; Bapat, M.; Maydeo, A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos). Gastrointest. Endosc. 2012, 75, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Will, U.; Thieme, A.; Fueldner, F.; Gerlach, R.; Wanzar, I.; Meyer, F. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy 2007, 39, 292–295. [Google Scholar] [CrossRef]
- Park, D.H.; Song, T.J.; Eum, J.; Moon, S.H.; Lee, S.S.; Seo, D.W.; Lee, S.K.; Kim, M.H. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos). Gastrointest. Endosc. 2010, 71, 413–419. [Google Scholar] [CrossRef]
- Komaki, T.; Kitano, M.; Sakamoto, H.; Kudo, M. Endoscopic ultrasonography-guided biliary drainage: Evaluation of a choledochoduodenostomy technique. Pancreatology 2011, 11 (Suppl. 2), 47–51. [Google Scholar] [CrossRef]
- Dhir, V.; Itoi, T.; Khashab, M.A.; Park, D.H.; Yuen Bun Teoh, A.; Attam, R.; Messallam, A.; Varadarajulu, S.; Maydeo, A. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest. Endosc. 2015, 81, 913–923. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).