Abstract
The development of augmented reality (AR) and its application in total joint arthroplasty aims at improving the accuracy and precision in implant components’ positioning, hopefully leading to increased outcomes and survivorship. However, this field is far from being thoroughly explored. We therefore performed a systematic review of the literature in order to examine the application, the results, and the different AR systems available in TJA. A systematic review of the literature according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines was performed. A comprehensive search of PubMed, MEDLINE, EMBASE, and the Cochrane Database of Systematic Reviews was conducted for English articles on the application of augmented reality in total joint arthroplasty using various combinations of keywords since the inception of the database to 31 March 2022. Accuracy was intended as the mean error from the targeted positioning angle and compared as mean values and standard deviations. In all, 14 articles met the inclusion criteria. Among them, four studies reported on the application of AR in total knee arthroplasty, six studies on total hip arthroplasty, three studies reported on reverse shoulder arthroplasty, and one study on total elbow arthroplasty. Nine of the included studies were preclinical (sawbones or cadaveric), while five of them reported results of AR’s clinical application. The main common feature was the high accuracy and precision when implant positioning was compared with preoperative targeted angles with errors ≤2 mm and/or ≤2°. Despite the promising results in terms of increased accuracy and precision, this technology is far from being widely adopted in daily clinical practice. However, the recent exponential growth in machine learning techniques and technologies may eventually lead to the resolution of the ongoing limitations including depth perception and their high complexity, favorably encouraging the widespread usage of AR systems.
1. Introduction
The development of new technologies in total joint arthroplasty (TJA) has gained increasing interest in the last two decades. Since the introduction of minimally invasive surgery (MIS), computer-assisted orthopaedic surgery (CAOS), and robotic surgery, computer navigation has become a powerful tool for the correct positioning of the implant components [1,2,3]. Conventionally, surgical imaging can be classified as off-line, taken before and after surgery (X-rays, computed tomography (CT), magnetic resonance imaging (MRI)), and on-line (intraoperative X-ray fluoroscopy, ultrasound (US)) [4,5]. On-line 2-dimensional (2D) intraoperative imaging can be supportive to enhance accuracy in TJA; however, despite the advantages, it is associated with several major problems including the necessity for video monitors, the interpretation and conversion of the 2D images into 3-dimensional (3D) images, the potential overlapping of anatomic structures, and increased radiation exposure [6].
The concept of augmented reality (AR) was introduced by Azuma et al. [7] as a variation of virtual reality (VR). The difference is that AR allows the user to see the real world with virtual objects superimposed upon it, supplementing the reality with virtual computer-generated sensory impression, enhancing the user’s ability to visualize a patient’s anatomy.
Several studies have evaluated the application of AR in orthopaedic surgery [8,9,10]; however, its use in TJA is still limited and mostly explored at preclinical levels. Therefore, there remains no consensus on the overall performance of these systems in the different settings. We performed a systematic review of the literature in order to examine (1) the applications of AR systems in TJA surgery, (2) their preclinical and clinical results in terms of accuracy and precision, and (3) the different systems currently available and their main characteristics. Moreover, to better understand the information provided in this study, the aim of the authors was to give a simple and thorough explanation of the AR systems used in orthopaedic surgery.
2. General Principles of Augmented Reality
The most common type of AR technology is based on the superimposition of a computer-generated image on the real world that is captured with a camera and then projected through screens [8,11,12,13,14,15,16,17]. Special head-mounted displays (HMD) or “smart glasses” have been successfully used to obtain a view of the surgical site without the need for any screens [8,18] that force the surgeon to look away from the surgical site, as usually happens with standard 2D intraoperative imaging.
A position tracking system, a display device, and a system control software are the basic elements required [9]. The first step is the registration phase that can be marker-based [19], marker-less (surface registration) [10], or through an augmented C-arm device [20]. The surface registration was introduced by Liebmann et al. [10] as a radiation-free approach with intraoperative surface digitization and navigation. Following this, the tracking phase allows the object to remain in the correct position during the surgical procedure, despite eventual motion, and adapt in a 3D space to the user and the instruments’ position. Therefore, a correct registration phase is essential for the success of the following phases [10]. After the registration and tracking are performed, the elaborated images are shown through three different approaches: HMD, monitors, and projectors [7]. Among those, video see-through headsets (HMD) have been introduced to avoid the necessity for a display; however, they are not free from complications including headaches and nausea being reported by users [21,22]. Alternatively, the virtual information can be directly projected onto the real environment, overlaying the information on a semi-transparent mirror [23].
3. Materials and Methods
3.1. Search Strategy
This search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [24]. The US National Library of Medicine (PubMed/MEDLINE), EMBASE, and the Cochrane Database of Systematic Reviews were queried for publications utilizing various combinations of the search terms “augmented reality,” “orthopaedics surgery,” “total joint arthroplasty,” “total hip arthroplasty,” “THA,” “total hip replacement,” “THR,” “total knee arthroplasty,” “total knee replacement,” “TKA,” “TKR,” “total shoulder arthroplasty,” “TSA,” “reverse total shoulder arthroplasty,” “RTSA,” “TEA,” “total elbow arthroplasty,” and “intraoperative instability,” in combination with the Boolean operators (AND, OR, *) since inception of the database to 31 March 2022. No limit was set regarding the year of publication. Two authors (F.M. and R.S.) independently conducted all the searches and screened the titles and abstracts to identify relevant studies. Differences were resolved by consulting the senior author (S.M.P.R.). Only abstracts that evaluated the application of augmented reality technology in preclinical (sawbones or cadaveric) or clinical settings in total joint arthroplasty were included. If the title and abstract of each study contained insufficient information, the full manuscript was reviewed. An additional search was conducted by screening the reference list of each selected article. Studies were excluded if they exclusively reported on the use of extended reality technologies in non-arthroplasty procedures (e.g., spine surgery, trauma surgery, arthroscopy). Case reports and reviews were included. Articles that were not available in English were excluded.
3.2. Inclusion and Exclusion Criteria
Inclusion criteria were any original study in which augmented reality technology was used in preclinical or clinical setting in TJA. Type of system and its application were reported, procedure was thoroughly described, accuracy and precision, and implant survivorship were reported. Exclusion criteria were commentary reports, expert opinions, letters to editors, instructional course lectures, studies on animals, book chapters, abstracts from scientific meetings, unpublished reports, studies with no details on the AR system reported, and studies written in non-English language.
3.3. Data Extraction and Collection
Two independent reviewers (F.M. and R.S.) separately examined all the identified studies and extracted data. During initial review of the data, the following information was collected for each study: title, first author, year of publication, study design, number of patients/specimens, joint of application, AR system, additional hardware required, clinical application, feasibility, accuracy, and precision. Risk of bias among the studies was assessed using the risk-of-bias visualization (robvis) tool [25]. Accuracy was intended as the mean difference from the planned targeted angle and summarized in mean values and standard deviations (SD), so that the smaller the value with the smaller SD, the more precise the AR device was in hitting the target.
4. Results
4.1. Study Selection
The search query resulted in 157 abstracts that were then examined to determine if they met the inclusion criteria related to the application of AR to total joint arthroplasty (Figure 1). Following the elimination of duplicate articles, the predetermined inclusion and exclusion criteria were applied. Consensus on which articles would be analyzed in the present study was achieved by discussion between the reviewers based on the predetermined inclusion and exclusion criteria described above. In total, 14 articles met the inclusion criteria and were included in the final analysis [19,22,26,27,28,29,30,31,32,33,34,35,36,37] (Table 1). Among them, four studies reported on the application of AR in total knee arthroplasty (TKA) [26,27,28,29], six studies reported on total hip arthroplasty (THA) [19,22,30,31,32,33], one study reported on total elbow arthroplasty (TEA) [34], and three studies reported on reverse shoulder arthroplasty (RSA) [35,36,37]. The risk-of-bias, as calculated with the robvis tool, assessed for an overall moderate risk among the studies included (Figure 2).
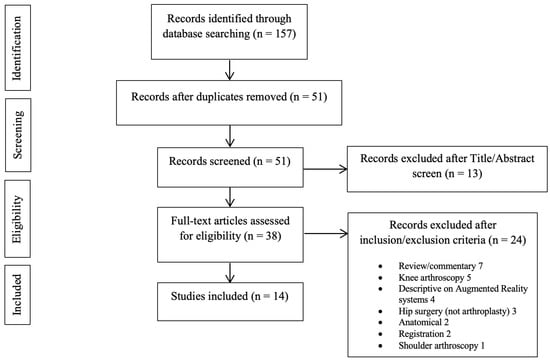
Figure 1.
PRISMA flow diagram outlining the systems review process.

Table 1.
Baseline information on studies on AR application in TJA.
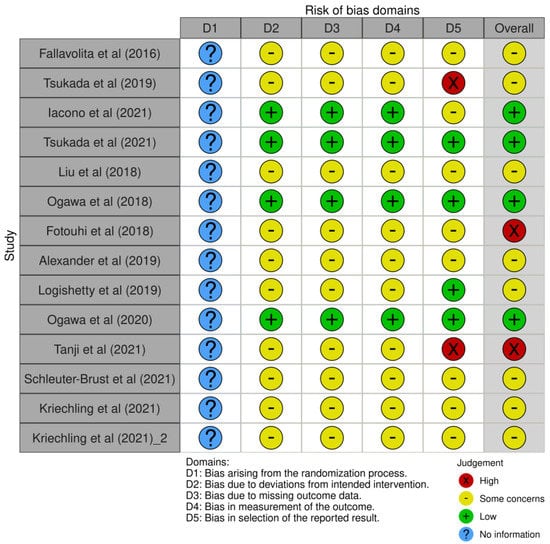
Figure 2.
Risk-of-bias visualization (robvis).
4.2. Total Knee Arthroplasty
Four studies reported on the application of AR in TKA [26,27,28,29], and among those, two were preclinical [26,27] and two were clinical [28,29].
Tsukada et al. [27,29] documented the results of the imageless navigation AR-KNEE system, firstly in a pilot study using 10 femoral sawbones specimens (Pacific Research Laboratories) to verify its accuracy and secondly in a clinical study of 74 patients to compare it with a conventional intramedullary guide [29]. No preoperative CT scan was required, and real-time information was provided on a smartphone screen, enabling the surgeon to visualize the aimed varus/valgus and posterior slope angles superimposed on the surgical field. The authors reported that the absolute values of the differences between the angles measured using CT images and the angles displayed on the smartphone screen were <1° in both the coronal and sagittal planes for tibial and femoral cuts [27,29]. Moreover, increased accuracy was detected in the AR-KNEE group compared with the conventional group when considering the absolute difference between the measured lateral distal femoral angle (LDFA) on a standing long-leg X-ray and the target (90°): 1.1° ± 1.0° vs. 2.2° ± 1.6°, respectively (p < 0.001) [29].
Similarly, Fallavollita et al. [26] developed a camera-augmented mobile C-arm (Cam-C) technology to intraoperatively assess lower limb alignment requiring only 3 X-ray images, and evaluated the accuracy, efficiency, and reliability by intraoperatively controlling the alignment of the mechanical axis deviations. The authors compared the results on 25 cadaveric specimens with the data obtained from a CT scan and reported no statistical difference between the augmented reality system and ground-truth CT (p > 0.05).
Finally, Iacono et al. [28] reported in a clinical pilot study (5 TKAs) the results of the Knee+ AR navigation system (Pixee medical company, Besancon, France) that allows the surgeon to view the tibial and femur axis superimposed on the surgical field through smart glasses without needing additional preoperative images. The authors reported a coronal error ≤1° for both tibial and femoral cuts, and a sagittal error ≤2° for tibial cuts. In addition, discrepancies between the reported angles measured on standard X-rays and the angles reported by the AR system were ≤1° (Table 2).

Table 2.
Characteristics and Outcomes of Studies on AR Application in TJA.
4.3. Total Hip Arthroplasty
Six studies reported on the application of AR in THA [19,22,30,31,32,33], and among those, one reported on hip resurfacing (HR) [22], and two were on clinical THA [19,31].
Liu et al. [22] explored the feasibility of an AR system in HR using optical see-through headsets during femoral preparation to simplify the procedure of locating and drilling the guide hole. The position and orientation of the drilled holes on the sawbones were compared with the preoperative plan, and the mean errors were approximately 2 mm and 2°. Similarly, Fotouhi et al. [30] reported in a preclinical study the results of an AR solution based on intraoperative planning for the easy and accurate placement of acetabular components during THA. The system is based on two C-arm X-ray images that are combined with 3D AR visualization that simplifies impactor and cup placement and provides a real-time red-green-blue-depth (RGBD) data overlay. The authors reported errors in translation, anteversion, and abduction of 2 mm, 1°, and 0.5°, respectively.
Alexander et al. [32] compared the accuracy of component placement, procedure time, radiation dose, and usability of a novel 3D AR guidance system compared with standard fluoroscopic guidance for acetabular component placement using a radiopaque foam pelvis (Sawbones, Pacific Research Laboratories, Vashon Island, WA) in a supine position to simulate the direct anterior approach. Fluoroscopic and 3D-images were obtained using a motorized C-arm scanner with cone-beam CT capability (Siemens Medical Solutions USA, Malvern, PA) and optical data were coregistered to create the AR environment from an RGBD camera (Intel Corporation, Santa Clara, CA). The authors reported that the AR technique was associated with significantly increased accuracy for target inclination (p = 0.01) and anteversion (p = 0.02), and increased precision for target anteversion (p < 001). In addition, the authors reported that the AR technique was faster (mean ± SD, 1.8 ± 0.25 vs. 3.9 ± 1.6 min; p < 0.01), and that it was considered easier to use by the participants. Moreover, no difference was documented in terms of the radiation dose to the patients between the two techniques (p = 0.48), while radiation exposure to the surgical team could be eliminated with the AR technique.
Regarding the clinical application, Ogawa et al. [19,31] reported in a pilot study the development of an acetabular cup measurement device (AR-HIP system) to investigate the accuracy of the acetabular placement angles in 56 primary THAs compared with a conventional goniometer. The AR-HIP system was based on a preoperative CT scan and allowed the surgeon to view an acetabular cup image superimposed on the real surgical field through the display of a smartphone that showed inclination and anteversion angles. Postoperatively, a second CT scan was required to calculate the placement angles and the difference with either the intraoperative measurements obtained with the goniometer or the AR system. The authors reported significantly better accuracy on the acetabular cup anteversion when the AR system was used (mean difference 4.1°, 95% confidence interval, 3.0–5.2; p < 0.0001). Subsequently, a randomized controlled trial was performed (41 hips) by the same authors [31] on acetabular cup placement using the AR marker-based portable navigation system and the conventional free-hand technique. Preoperative CT images were used for planning. The evaluation of inclination and anteversion was performed radiographically after surgery and at 3-month follow-up with a CT scan. The authors reported no difference in acetabular anteversion accuracy and no clinically important difference in acetabular inclination between the AR-based navigation system and the conventional technique.
Finally, Logishetty et al. [33] evaluated how an enhanced AR headset capable of tracking bony anatomy would improve the accuracy of acetabular component positioning compared with hands-on training by an expert surgeon when used for novices training. Twenty-four participants were involved in four once-weekly simulated THA sessions, and the authors reported that AR guidance was associated with smaller mean errors in orientation than those receiving guidance from the experienced surgeon: 1° ± 1° versus AR 6° ± 4° (p < 0.001). (Table 2)
4.4. Reverse Shoulder Arthroplasty
Three studies reported on the application of AR systems in RSA [34,35,36], all of them in a preclinical setting.
Schlueter-Brust et al. [35] explored the feasibility of an AR system for the positioning of the glenoid baseplate. The system requires a standardized CT protocol, with 3D reconstruction, 3D planning, and the use of a commercial AR headset, to conduct AR-assisted k-wire placement in the glenoid. The postoperative outcome was measured using a high-resolution laser scanner on the patient-specific 3D printed bone. In this proof of concept study, the discrepancy between the planned and the achieved glenoid entry point and guidewire orientation was approximately 3 mm with a mean angulation error of 5°. A similar study based on a navigation technology of AR through HMD was reported by Kriechling et al. [36], where 3D models of ten human scapulae were printed from CT data and a hologram of the planned guidewire was projected onto the 3D models. After navigated placement of the central guidewires, another CT imaging was recorded, and the 3D model was superimposed with the preoperative planning to analyze the deviation from the planned guidewire, reporting a mean deviation of the trajectory of 2.7° ± 1.3° and a mean deviation of the entry point of 2.3 mm ±1.1 mm. Moreover, the same authors reproduced the same study on cadaveric specimens [37]. After obtaining CT images of 12 human cadaver shoulders, an RSA baseplate positioning was 3D planned, and an augmented reality hologram was superimposed using the HMD Microsoft HoloLense. Then, the shoulders were CT scanned a second time, postoperatively, to evaluate the deviation from the planning, reporting a mean deviation of the entry point of 3.5 mm ± 1.7 mm and a mean deviation of the trajectory of 3.8° ± 1.7° (Table 2).
4.5. Total Elbow Arthroplasty
One basic science study reported on the application of AR in TEA [34]. The authors used twelve frozen upper extremities and planned the implant’s placement sites using computer simulation on a previous CT scan, and then compared the AR technique (six cases) with the conventional technique (six cases). Postoperatively, a CT scan was repeated to evaluate the actual error of the ulnar and humeral components’ placement with respect to the preoperative plan. Regarding the humeral component, the mean positional error of the AR technique compared to the conventional method was 1.4° ± 0.6° (vs. 4.4° ± 0.9°; p = 0.002) in total rotation and 1.5 ± 0.6 mm (vs. 8.6 ± 1.3 mm; p = 0.002) in total translation. Regarding the ulnar component, the mean positional error was 5.5° ± 3.1° (vs. 19.5° ± 9.8°; p = 0.004) in total rotation and 1.5 ± 0.4 mm (vs. 6.9 ± 1.6 mm; p = 0.002) in total translation. Both rotational accuracy and translational accuracy were greater for joint components replaced using the AR technique compared with the conventional technique (p < 0.05). (Table 2)
5. Discussion
The application of AR in modern orthopaedic surgery and TJA is progressively finding wider approval with promising results in multiple settings. Indeed, it has been widely used in spine surgery, considering the complexity of the anatomical district and the risk of iatrogenic injury during instrumentation [10,38,39,40,41,42,43,44]; favorable outcomes have also been reported in complex corrective osteotomies and trauma surgery, increasing accuracy while reducing radiation exposure and surgery time [44,45,46].
The correct implantation of prosthetic components in TJA is a key element for success in terms of the functional outcomes, patient’s recovery and rehabilitation, and implant survivorship [47]. For those reasons, different technological innovations have been developed and introduced including robotic surgery [48], 3-D printed patient-specific instrumentation (PSI) [49], navigation devices [3], and, finally, AR [50]. To date, there are several different AR systems that have been applied in preclinical and clinical studies [26,27,28,29,30,31,32,33,34,35,36,37]; however, their application in TJA has not been thoroughly investigated yet. Despite that, a few studies have reported the potential benefits of this technology in terms of the reduced radiation exposure of patients and OR staff, reduced surgical time, and improved accuracy of surgical performance. In addition, studies have reported that, at least in Sawbones, AR navigation provides more accurate results in terms of implant positioning when compared to the conventional free-hand technique in total hip [19,22,23,24,25,30,31] and knee [26,27,28,29] arthroplasty.
Regarding the application in THA, according to the current literature, we can state that AR is able to provide several advantages, particularly with regards to increased accuracy in acetabular component positioning. Alexander et al. [32] compared an AR system with standard fluoroscopic guidance on pelvic sawbones reporting increased accuracy of the placement angles. Despite that, the study is limited by its application on sawbones, and clinically relevant differences with conventional techniques have not yet been reported to support this technique in daily practice. Similarly, Ogawa et al. [19,31] reported promising results when an AR system was used to improve the quality of the cup positioning considering that the conventional tools may not recognize determinant elements such as the pelvic tilt or the pelvic movement related to the retractors, leading to potential version errors. In spite of that, even though there was an absence of reported complications, there was no evaluation of the clinical outcomes, forcing us to suggest that additional studies are necessary to assess the cost-effectiveness and risk-effectiveness of the technique before its introduction in daily practice. This technology has also been explored in HR by Liu et al. [22] to evaluate the accuracy of the guide hole along the axis of the femoral neck in a sawbones model. The authors reported an error of 2 mm and 2° for position and direction compared to what was planned, providing good accuracy according to the Audenaert et al. criteria [51]. Nevertheless, despite the promising results, the biggest obstacle to its application in a realistic setting would be to identify suitable image processing algorithms to segment the target from the surrounding surgical scene in order to correctly register it, limiting its dissemination to date.
Similar results have been described in TKA surgery. Tsukada et al. [29], reported on the AR-KNEE system and suggested that it may provide reliable accuracy for the coronal, sagittal, and rotational alignment when used for tibial and femoral resection and that it may become a useful alternative to conventional navigation devices. In addition, the authors reported promising results in an experimental setting when analyzing the error in distal femoral resection (<1.0°) in both the coronal and sagittal planes. Their non-randomized comparative study showed that the AR system can provide significantly greater accuracy during distal femoral resection in TKA compared with the conventional intramedullary guide. Similarly, Fallavollita et al. [26], after the promising results obtained at preclinical level, aimed to extend their study to a clinical level to assess the technical efficacy of this AR technique. Indeed, it has the potential to quickly simplify the workflow of mechanical axis deviation measurement, by combining X-ray and video images into a single procedure while avoiding the exposure to ionizing radiation of patients and operators. However, considering the lack of available literature in clinical settings, further high-quality studies are probably necessary before we widely support the diffusion and application of AR systems in TKA.
Interesting results have also been reported regarding RSA and TEA, even though these fields are yet to be fully explored and thoroughly understood. In particular, baseplate glenoid component positioning has been studied by several authors [35,36,37] in sawbones and cadaveric scapulae specimens to evaluate the potential benefits of AR technology, and these studies reported that baseplate navigation in RSA using AR through an HMD seems feasible, showing high accuracy and precision at preclinical level. However, the application of this novel technology in such fields is still in its infancy and various technical problems still need to be addressed before it can be widely used in clinical practice.
Similarly, Tanji et al. [34] investigated the field of TEA stating that the AR technique can provide several advantages over the conventional surgical technique, with quick registration and easy application while being able to reproduce the preoperative placement plan onto the surgical field, potentially leading to more accurate component placement. Nevertheless, we still need valuable data to confirm these promising preliminary results in order to support the application of this technology in the clinical setting of TEA.
In spite of the promising preliminary results, we must remember that, recently, many surgical techniques have been introduced hoping to achieve improved results in total joint arthroplasty including MIS, PSI, CAOS, and, lastly, robotics. However, after an initial interest they can easily follow Scott’s description of the parabola of the rise and fall of surgical techniques [52], facing an exponential drop-off when the high costs of such innovative tools are not balanced by improved clinical outcomes.
There were a variety of limitations to this study. First, we were limited by the quality and the type of the original studies, most of the studies related to the application of AR in TJA are preclinical (sawbones), leading to the impossibility to define a clear conclusion on the efficacy of this technology and effectively compare the results between the different studies. Second, we encountered a high heterogeneity among the studies available in the literature, including different devices used and different clinical and preclinical settings, undermining the possibility to systematically analyze them. Third, different outcomes were often used across the reported studies, limiting the possibility to directly compare them among each other and assess overall results, and provide clear conclusions and potential suggestions. Fourth, very few clinical studies are currently available, and they often analyze different outcomes and different AR devices; moreover, limited data are available from comparative studies between AR and conventional techniques, probably due to the limited diffusion of this novel instrumentation. Fifth, the devices used to provide AR were often different or related to pilot studies, limiting the possibility to provide a clear and objective conclusion on each of them. Finally, many of these studies were industry funded, leading to potential selection, performance, and publication bias; therefore, the authors suggest interpreting the reported results with caution.
6. Conclusions
The development of AR is progressing fast, and its application in orthopedic surgery has gained increasing attention. However, multiple limitations are still encountered in the development of this technology including high costs and the complexity of these devices. The process of moving forward from preclinical promising results to daily application is still long, but the fast pace at which AR technology is moving makes us believe that, in the near future, this technology will become part of clinical practice.
Author Contributions
F.M. and S.M.P.R.: designing the work. F.M., R.S. and L.L.: acquisition and analysis of the data. F.M.: drafting the work. S.M.P.R., F.B. and L.P.: revised the work critically for important intellectual content. S.M.P.R. and F.B.: final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors have no conflict of interest to declare that are relevant to the content of this article. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Abbreviations
2-dimensional (2D), operating room (OR), augmented reality (AR), virtual reality (VR), head-mounted display (HMD), 3-dimensional (3D), patient-specific instrumentation (PSI), lateral distal femoral angle LDFA.
References
- Cheng, T.; Feng, J.G.; Liu, T.; Zhang, X.L. Minimally invasive total hip arthroplasty: A systematic review. Int. Orthop. 2009, 33, 1473–1481. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mancino, F.; Cacciola, G.; Malahias, M.-A.; De Filippis, R.; De Marco, D.; Di Matteo, V.; Gu, A.; Sculco, P.K.; Maccauro, G.; De Martino, I. What are the benefits of robotic-assisted total knee arthroplasty over conventional manual total knee arthroplasty? A systematic review of comparative studies. Orthop. Rev. 2020, 12, 8657. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.W.; Jerabek, S.A. Current Role of Computer Navigation in Total Knee Arthroplasty. J. Arthroplast. 2018, 33, 1989–1993. [Google Scholar] [CrossRef] [PubMed]
- Nikou, C.; Digioia, A.M.; Blackwell, M.; Jaramaz, B.; Kanade, T. Augmented reality imaging technology for orthopaedic surgery. Oper. Tech. Orthop. 2000, 10, 82–86. [Google Scholar] [CrossRef]
- Chen, T.K.; Abolmaesumi, P.; Pichora, D.R.; Ellis, R.E. A system for ultrasound-guided computer-assisted orthopaedic surgery. Comput. Aided Surg. 2005, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, J.; Boudissa, M.; Kerschbaumer, G.; Seurat, O. Role of 3D intraoperative imaging in orthopedic and trauma surgery. Orthop. Traumatol. Surg. Res. 2019, 106, S19–S25. [Google Scholar] [CrossRef]
- Azuma, R.T. A Survey of Augmented Reality. Presence Teleoperators Virtual Environ. 1997, 6, 355–385. [Google Scholar] [CrossRef]
- Tabrizi, L.B.; Mahvash, M. Augmented reality–guided neurosurgery: Accuracy and intraoperative application of an image projection technique. J. Neurosurg. 2015, 123, 206–211. [Google Scholar] [CrossRef]
- Blackwell, M.; Morgan, F.; DiGioia, A.M., III. Augmented Reality and Its Future in Orthopaedics. Clin. Orthop. Relat. Res. 1998, 354, 111–122. [Google Scholar] [CrossRef]
- Liebmann, F.; Roner, S.; von Atzigen, M.; Scaramuzza, D.; Sutter, R.; Snedeker, J.; Farshad, M.; Fürnstahl, P. Pedicle screw navigation using surface digitization on the Microsoft HoloLens. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1157–1165. [Google Scholar] [CrossRef]
- Vavra, P.; Roman, J.; Zonča, P.; Ihnát, P.; Němec, M.; Kumar, J.; Habib, N.; El-Gendi, A. Recent Development of Augmented Reality in Surgery: A Review. J. Heal. Eng. 2017, 2017, 4574172. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Yasuda, H.; Koda, K.; Suzuki, M.; Yamazaki, M.; Tezuka, T.; Kosugi, C.; Higuchi, R.; Watayo, Y.; Yagawa, Y.; et al. Image overlay navigation by markerless surface registration in gastrointestinal, hepatobiliary and pancreatic surgery. J. Hepato-Biliary-Pancreat. Sci. 2009, 17, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Gavaghan, K.A.; Peterhans, M.; Oliveira-Santos, T.; Weber, S. A Portable Image Overlay Projection Device for Computer-Aided Open Liver Surgery. IEEE Trans. Biomed. Eng. 2011, 58, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Gavaghan, K.; Oliveira-Santos, T.; Peterhans, M.; Reyes, M.; Kim, H.; Anderegg, S.; Weber, S. Evaluation of a portable image overlay projector for the visualisation of surgical navigation data: Phantom studies. Int. J. Comput. Assist. Radiol. Surg. 2011, 7, 547–556. [Google Scholar] [CrossRef]
- Wen, R.; Chui, C.-K.; Ong, S.-H.; Lim, K.-B.; Chang, S.K.-Y. Projection-based visual guidance for robot-aided RF needle insertion. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 1015–1025. [Google Scholar] [CrossRef]
- Kocev, B.; Ritter, F.; Linsen, L. Projector-based surgeon–computer interaction on deformable surfaces. Int. J. Comput. Assist. Radiol. Surg. 2013, 9, 301–312. [Google Scholar] [CrossRef]
- Pessaux, P.; Diana, M.; Soler, L.; Piardi, T.; Mutter, D.; Marescaux, J. Towards cybernetic surgery: Robotic and augmented reality-assisted liver segmentectomy. Langenbeck’s Arch. Surg. 2014, 400, 381–385. [Google Scholar] [CrossRef]
- Badiali, G.; Ferrari, V.; Cutolo, F.; Freschi, C.; Caramella, D.; Bianchi, A.; Marchetti, C. Augmented reality as an aid in maxillofacial surgery: Validation of a wearable system allowing maxillary repositioning. J. Cranio-Maxillofac. Surg. 2014, 42, 1970–1976. [Google Scholar] [CrossRef]
- Ogawa, H.; Hasegawa, S.; Tsukada, S.; Matsubara, M. A Pilot Study of Augmented Reality Technology Applied to the Acetabular Cup Placement During Total Hip Arthroplasty. J. Arthroplast. 2018, 33, 1833–1837. [Google Scholar] [CrossRef]
- Navab, N.; Heining, S.-M.; Traub, J. Camera Augmented Mobile C-Arm (CAMC): Calibration, Accuracy Study, and Clinical Applications. IEEE Trans. Med. Imaging 2009, 29, 1412–1423. [Google Scholar] [CrossRef]
- Shuhaiber, J.H. Augmented Reality in Surgery. Arch. Surg. 2004, 139, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Auvinet, E.; Giles, J.; Baena, F.R.Y. Augmented Reality Based Navigation for Computer Assisted Hip Resurfacing: A Proof of Concept Study. Ann. Biomed. Eng. 2018, 46, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Van Krevelen, D.; Poelman, R. A survey of augmented reality technologies, applications and limitations. Int. J. Virtual Real. 2010, 9, 1–20. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Fallavollita, P.; Brand, A.; Wang, L.; Euler, E.; Thaller, P.; Navab, N.; Weidert, S. An augmented reality C-arm for intraoperative assessment of the mechanical axis: A preclinical study. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 2111–2117. [Google Scholar] [CrossRef]
- Tsukada, S.; Ogawa, H.; Nishino, M.; Kurosaka, K.; Hirasawa, N. Augmented reality-based navigation system applied to tibial bone resection in total knee arthroplasty. J. Exp. Orthop. 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Iacono, V.; Farinelli, L.; Natali, S.; Piovan, G.; Screpis, D.; Gigante, A.; Zorzi, C. The use of augmented reality for limb and component alignment in total knee arthroplasty: Systematic review of the literature and clinical pilot study. J. Exp. Orthop. 2021, 8, 52. [Google Scholar] [CrossRef]
- Tsukada, S.; Ogawa, H.; Nishino, M.; Kurosaka, K.; Hirasawa, N. Augmented Reality-Assisted Femoral Bone Resection in Total Knee Arthroplasty. JBJS Open Access 2021, 6, e21.00001. [Google Scholar] [CrossRef]
- Fotouhi, J.; Alexander, C.P.; Unberath, M.; Taylor, G.; Lee, S.C.; Fuerst, B.; Johnson, A.; Osgood, G.; Taylor, R.H.; Khanuja, H.; et al. Plan in 2-D, execute in 3-D: An augmented reality solution for cup placement in total hip arthroplasty. J. Med. Imaging 2018, 5, 021205. [Google Scholar] [CrossRef]
- Ogawa, H.; Kurosaka, K.; Sato, A.; Hirasawa, N.; Matsubara, M.; Tsukada, S. Does An Augmented Reality-based Portable Navigation System Improve the Accuracy of Acetabular Component Orientation During THA? A Randomized Controlled Trial. Clin. Orthop. Relat. Res. 2020, 478, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Loeb, A.E.; Fotouhi, J.; Navab, N.; Armand, M.; Khanuja, H.S. Augmented Reality for Acetabular Component Placement in Direct Anterior Total Hip Arthroplasty. J. Arthroplast. 2020, 35, 1636–1641.e3. [Google Scholar] [CrossRef] [PubMed]
- Logishetty, K.; Western, L.; Morgan, R.; Iranpour, F.; Cobb, J.; Auvinet, E. Can an Augmented Reality Headset Improve Accuracy of Acetabular Cup Orientation in Simulated THA? A Randomized Trial. Clin. Orthop. Relat. Res. 2018, 477, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Tanji, A.; Nagura, T.; Iwamoto, T.; Matsumura, N.; Nakamura, M.; Matsumoto, M.; Sato, K. Total elbow arthroplasty using an augmented reality–assisted surgical technique. J. Shoulder Elb. Surg. 2021, 31, 175–184. [Google Scholar] [CrossRef]
- Schlueter-Brust, K.; Henckel, J.; Katinakis, F.; Buken, C.; Opt-Eynde, J.; Pofahl, T.; Baena, F.R.Y.; Tatti, F. Augmented-Reality-Assisted K-Wire Placement for Glenoid Component Positioning in Reversed Shoulder Arthroplasty: A Proof-of-Concept Study. J. Pers. Med. 2021, 11, 777. [Google Scholar] [CrossRef]
- Kriechling, P.; Roner, S.; Liebmann, F.; Casari, F.; Fürnstahl, P.; Wieser, K. Augmented reality for base plate component placement in reverse total shoulder arthroplasty: A feasibility study. Arch. Orthop. Trauma. Surg. 2020, 141, 1447–1453. [Google Scholar] [CrossRef]
- Kriechling, P.; Loucas, R.; Loucas, M.; Casari, F.; Fürnstahl, P.; Wieser, K. Augmented reality through head-mounted display for navigation of baseplate component placement in reverse total shoulder arthroplasty: A cadaveric study. Arch. Orthop. Trauma. Surg. 2021; published online ahead of print. [Google Scholar] [CrossRef]
- Molina, C.A.; Theodore, N.; Ahmed, A.K.; Westbroek, E.M.; Mirovsky, Y.; Harel, R.; Orru’, E.; Khan, M.; Witham, T.; Sciubba, D.M. Augmented reality–assisted pedicle screw insertion: A cadaveric proof-of-concept study. J. Neurosurg. Spine 2019, 31, 139–146. [Google Scholar] [CrossRef]
- Gibby, J.T.; Swenson, S.A.; Cvetko, S.; Rao, R.; Javan, R. Head-mounted display augmented reality to guide pedicle screw placement utilizing computed tomography. Int. J. Comput. Assist. Radiol. Surg. 2018, 14, 525–535. [Google Scholar] [CrossRef]
- Elmi-Terander, A.; Burström, G.; Nachabe, R.; Skulason, H.; Pedersen, K.; Fagerlund, M.; Ståhl, F.; Charalampidis, A.; Söderman, M.; Holmin, S.; et al. Pedicle Screw Placement Using Augmented Reality Surgical Navigation with Intraoperative 3D Imaging. Spine 2019, 44, 517–525. [Google Scholar] [CrossRef]
- Müller, F.; Roner, S.; Liebmann, F.; Spirig, J.M.; Fürnstahl, P.; Farshad, M. Augmented reality navigation for spinal pedicle screw instrumentation using intraoperative 3D imaging. Spine J. 2020, 20, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Chen, R.E.; Han, P.K.; Si, P.; Freeman, M.W.D.; Pirris, S.M. Technical feasibility and safety of an intraoperative head-up display device during spine instrumentation. Int. J. Med. Robot. Comput. Assist. Surg. 2016, 13, e1770. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-R.; Wang, M.-L.; Liu, K.-C.; Hu, M.-H.; Lee, P.-Y. Real-time advanced spinal surgery via visible patient model and augmented reality system. Comput. Methods Programs Biomed. 2014, 113, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Kosterhon, M.; Gutenberg, A.; Kantelhardt, S.R.; Archavlis, E.; Giese, A. Navigation and Image Injection for Control of Bone Removal and Osteotomy Planes in Spine Surgery. Oper. Neurosurg. 2017, 13, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ortega, G.; Wolff, A.; Baumgaertner, M.; Kendoff, D. Usefulness of a head mounted monitor device for viewing intraoperative fluoroscopy during orthopaedic procedures. Arch. Orthop. Trauma. Surg. 2007, 128, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Chen, B.; Guo, Q.; Qi, Y.; Shen, Y. Augmented reality patient-specific reconstruction plate design for pelvic and acetabular fracture surgery. Int. J. Comput. Assist. Radiol. Surg. 2012, 8, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Mancino, F.; Cacciola, G.; Di Matteo, V.; Perna, A.; Proietti, L.; Greenberg, A.; Malahias, M.; Sculco, P.K.; Maccauro, G.; De Martino, I. Surgical implications of the hip-spine relationship in total hip arthroplasty. Orthop. Rev. 2020, 12, 8656. [Google Scholar] [CrossRef]
- Kayani, B.; Konan, S.; Tahmassebi, J.; Pietrzak, J.R.T.; Haddad, F.S. Robotic-arm assisted total knee arthroplasty is associated with improved early functional recovery and reduced time to hospital discharge compared with conventional jig-based total knee arthroplasty. Bone Jt. J. 2018, 100-B, 930–937. [Google Scholar] [CrossRef]
- Hafez, M.A.; Moholkar, K. Patient-specific instruments: Advantages and pitfalls. SICOT-J. 2017, 3, 66. [Google Scholar] [CrossRef]
- Makhni, E.C.; Makhni, S.; Ramkumar, P.N. Artificial Intelligence for the Orthopaedic Surgeon: An Overview of Potential Benefits, Limitations, and Clinical Applications. J. Am. Acad. Orthop. Surg. 2020, 29, 235–243. [Google Scholar] [CrossRef]
- Audenaert, E.; De Smedt, K.; Gelaude, F.; Clijmans, T.; Pattyn, C.; Geebelen, B. A custom-made guide for femoral component positioning in hip resurfacing arthroplasty: Development and validation study. Comput. Aided Surg. 2011, 16, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.W. Scott’s parabola. BMJ 2001, 323, 1477. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).